Abstract
Melioidosis is an infectious disease that is initiated by a bacteria recognized as Burkholderia pseudomallei. Despite the high fatality rate from melioidosis, there is a minimal published study about the disease in Malaysia.
This study aimed to identify the prognostic factors of mortality among melioidosis patients in northern Malaysia.
All inpatient patients who were admitted to Hospital Sultanah Bahiyah, Kedah and Hospital Tuanku Fauziah, Perlis with culture-confirmed melioidosis during the period 2014 to 2017 were included in the study. The study retrospectively collected 510 melioidosis patients from the Melioidosis Registry. Hazard ratio (HR) used in advanced multiple Cox regression was used to obtain the final model of prognostic factors of melioidosis. The analysis was performed using STATA/SE 14.0 for Windows software.
From the results, among the admitted patients, 50.1% died at the hospital. The mean age for those who died was 55 years old, and they were mostly male. The most common underlying disease was diabetes mellitus (69.8%), followed by hypertension (32.7%). The majority of cases (86.8%) were bacteremic. The final Cox model identified 5 prognostic factors of mortality among melioidosis patients. The factors were diabetes mellitus, type of melioidosis, platelet count, white blood cell count, and urea value. The results showed that bacteremic melioidosis increased the risk of dying by 3.47 (HR: 3.47, 95% confidence intervals [CI]: 1.67–7.23, P = .001) compared to non-bacteremic melioidosis. Based on the blood investigations, the adjusted HRs from the final model showed that all 3 blood investigations were included as the prognostic factors for the disease (low platelet: HR = 1.76, 95% CI: 1.22–2.54, P = .003; high white blood cell: HR = 1.49, 95% CI 1.06–2.11, P = .023; high urea: HR = 2.92, 95% CI: 1.76–4.85, P < .001; and low level of urea: HR = 2.69, 95% CI: 1.69–4.29, P < .001). By contrast, melioidosis patients with diabetic had 30.0% lower risk of dying from melioidosis compared to those with non-diabetic (HR = 0.70, 95% CI: 0.52–0.94, P = .016).
Identifying the prognostic factors of mortality in patients with melioidosis allows a guideline of early management in these patients, which may improve patient's survival.
Keywords: Burkholderia pseudomallei, Cox model, infectious diseases, melioidosis, mortality, prognostic factors, survival
Key Points
The cases of melioidosis are considered an emerging infectious disease as it is increasingly reported worldwide. The fatality from melioidosis was higher compared to other viral contagious diseases like dengue and tuberculosis. The previous study highlighted the significance of increased mortality in melioidosis. However, limited research has been conducted to determine the prognostics factors of mortality from melioidosis in Malaysia. Although the disease is only infected with certain areas worldwide, the appropriate management must be taken to prevent sporadic cases. The current study addresses the extension of the study population to the 2 hospitals in Malaysia. The advanced multiple Cox regression was applied to identify the prognostic factors of melioidosis, and the hazard ratio was interpreted to measure the risk of each factor toward the mortality of melioidosis. From the study, the Cox model of mortality from melioidosis was diabetes mellitus, type of melioidosis, platelet count, white blood cell count, and urea value. These findings represent the need for thorough and efficient laboratory investigations to prevent mortality among melioidosis patients.
1. Introduction
Melioidosis is endemic in Southeast Asia, Northern Australia, Africa, India, and China.[1] Affected individuals usually have direct contact with the soil that is exposed to the bacteria known as Burkholderia pseudomallei.[2] Around the 1980s in Malaysia, this disease was associated with hospitals’ high mortality rate, particularly in the septicaemic form, which is 65%.[3] Melioidosis is undisclosed because of minimal research done even though the cases reported all over Malaysia approximately approaching over a thousand cases.[4] The incidence of mortality from melioidosis was much higher than other infectious viral diseases like dengue and tuberculosis infection, in which more than 2000 patients die from this disease every year in Malaysia.[5] The mortality rate among melioidosis patients ranges from 14% to 40%, among which >80% of the cases are untreated patients.[6] Based on 2014 to 2017, a dead patient's direct medical cost in Kedah, Malaysia, was USD 210.42.[7]
Melioidosis is not notifiable compared to other diseases due to misdiagnosed.[5] The common presentation after the bacteria attacks the human body, including inaccessible mediastinal lymphadenopathy and abscess in organ lymphoma, is similar to tuberculosis or lymphoma,[8] leading to the diagnosis of this disease rather than melioidosis. Additionally, due to the lower demand to diagnose the illness than dengue or tuberculosis, the tools used in detecting melioidosis are low sensitivity.[5] There was also no evidence reported on the best clinical diagnosis's cost-effectiveness to diagnose and manage the disease in Malaysia.[5] A study reported based on the Laos population, the gold standard to detect melioidosis is by culture[9] with 60% sensitivity and 100% specificity.[10] Nevertheless, because of culture taking at least 48 to 72 hours to diagnose melioidosis, this problem leads to delayed treatment that causes fatal among the patients.[10] The lack of awareness of melioidosis among the physicians, healthcare personnel, and the general public is a critical issue in Malaysia,[5] and it should be promoted. More research on melioidosis should be reported to develop and validate the more firm diagnostic workflow in managing this disease.[5]
Previous studies in Malaysia defined melioidosis's risk factors, including preexisting comorbid conditions, older age, occupational exposure, inappropriate treatment, and male; these factors also lead to mortality and recurrence of melioidosis.[11] In Malaysia, Hassan et al.[12] had identified the incidence and risk factors of melioidosis in Kedah from 2005 to 2008 retrospectively. The present study performed in the same hospital and added another hospital (Hospital Tuanku Fauziah [HTF]). This study also updates the current status of melioidosis, expanding the survey from 2014 to 2017, and added additional reports on the risk factors of mortality from melioidosis using hazard ratio (HR). The study was conducted to identify the prognostic factors of mortality in melioidosis patients from 2014 to 2017 at HSB and HTF.
2. Materials and methods
A retrospective cohort study research design was used to review 453 melioidosis patients in HSB and HTF between 2014 and 2017. Both hospitals were the main hospital in Kedah and Perlis is serving melioidosis patients. The data was collected from the Melioidosis Registry based on patients’ demographics, blood investigations, antibiotics received during the admission, and comorbidities. Figure 1 illustrates the flow in extracting the final sample size.
Figure 1.
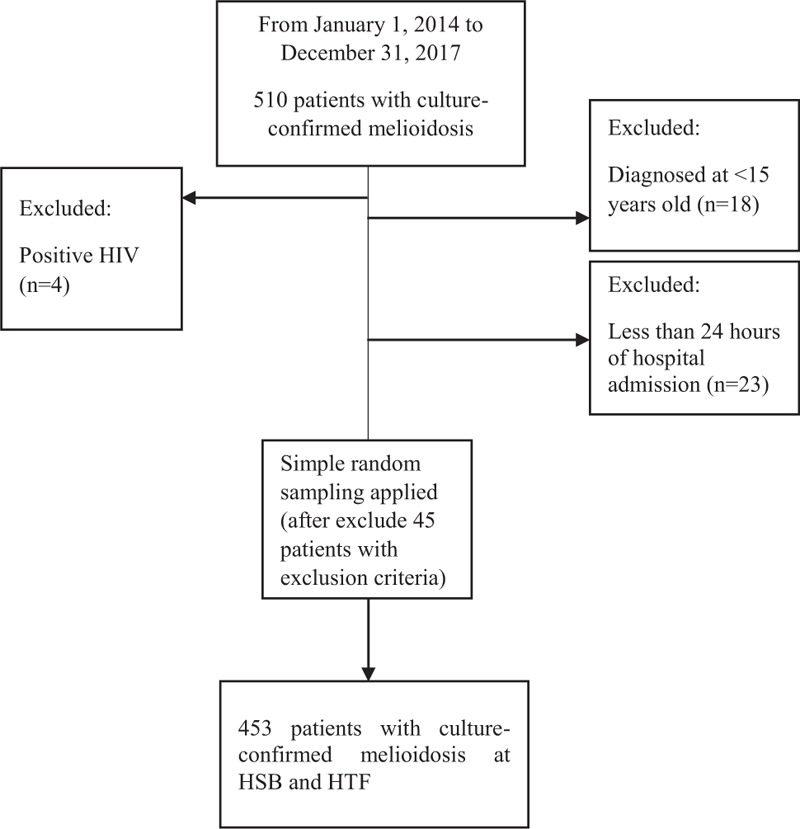
The flow chart of selection of the patients.
2.1. Selection of study participants
All the culture-confirmed melioidosis cases admitted to HSB and HTF were included in the study. Patients admitted with community-acquired septicemia caused by bacteria other than B pseudomallei and positive human immunodeficiency virus were excluded from the study. Patients that were diagnosed by the serology only and less than 24 hours of hospital admission were also excluded from the study. All the patients included in the study were aged more than 15 years old at the time of diagnosis.
The calculation of sample size was done using Power and Sample Size Calculation (PS) software, with the significance level (alpha) 0.05 and the power of the study (1 − β) of 90%. The final sample size calculated was 453 patients. The missing data for the continuous variables, less than 20%, was replaced by multiple imputations.
2.2. Definitions
Bacteremic melioidosis was defined as patients with blood culture-positive for B pseudomallei. In contrast, non-bacteremic melioidosis was patients with positive B pseudomallei when the organism was isolated from other than blood culture. Data collection based on the prognostic factors was divided into socio-demographic, antibiotic received, comorbidities, previous history of melioidosis, and blood investigations. Occupations, which were reported as risk factors for melioidosis, were classified into low and high risk according to the chances of exposure to B pseudomallei from the soil and water. The race of patients was reported as Malay, Chinese, Indian, or others. The patient's outcome was also recorded as discharged well, AOR discharge, transfer to other hospitals, or died.
2.3. Statistical analysis for socio-demographic data and risk factors
To summarize the demographic and clinical data, the percentage (%) used for the categorical data, while mean and standard deviation used for the numerical data. Pearson's chi-square or Fisher's exact test was applied to compare the association between each independent variable and the outcome.
2.4. Advanced multiple Cox regression analysis
All analyses were conducted using the STATA/SE 14.0 for Windows (SAS Institute, Inc., Cary, NC). Analyses were based on time-to-event, with time measured from the start of diagnosis of culture-confirmed melioidosis. Events were defined as a patient who died from melioidosis. The date of admission with culture-confirmed melioidosis was set as the time of entry (T0), while the last date at the hospital (death or discharge or transfer to another hospital) was set as T1. The follow-up time was between the difference of T1 and T0. The primary analysis aimed to determine the risk factors associated with mortality from melioidosis. This analysis was censored for death due to causes other than culture-confirmed melioidosis and lived until the study ended.
The 25 independent variables (potential prognostic factors) included the patient's demographic characteristics, premorbid conditions, antibiotics received, and blood investigations were tested. Kaplan–Meier survival analysis was used to compare the median survival time between bacteremic and non-bacteremic melioidosis. Multivariate Cox proportional hazard models were used to adjust for interactions between prognostic factors of mortality from melioidosis. The final Cox proportional hazard models were concluded after possible multicollinearity and interactions were tested for the model, checking the proportional hazards assumption, Hosmer-Lemeshow test, classification table, area under the curve, and applied the regression diagnostic test and remedial measure. Statistical results were reported as crude hazard ratios (HRs), adjusted HRs, and 95% confidence intervals (CIs).
2.5. Ethics statement
This study was approved by Universiti Zainal Abidin Human Research Ethics Committee (UHREC) (protocol code: UniSZA/UHREC/2019/119) and Medical Research and Ethics Committee, Ministry of Health Malaysia (MOH) (NMRR-19-3090-46158 [IIR]). For the ethics approval, the consent form was not mandatory for the secondary data. The data was collected based on reviewing the Melioidosis Registry; the researcher did not have to access the patients’ personal information. All procedures performed in this study were in accordance with the institution's ethical standards and the Malaysian research committee, and the 1964 Helsinki declaration.
3. Results
3.1. Overview of melioidosis patients admitted to HSB and HSF
Overall, a total of 227 (50.1%) patients died from melioidosis, 159 (35.1%) patients were discharged well, 16 (3.5%) patients were AOR discharge, and 51 (11.3%) patients were transferred to another hospital (Table 1). For the study, 98.2% were culture-confirmed melioidosis patients, and 1.8% of patients were diagnosed by a combination of culture and serology. The number of patients who were culture-positive for each sample type was as follows: blood, 393 (86.8%); tissue, 32 (84.2%); urine, 5 (4.8%); wound, 9 (56.3%); sputum, 3 (9.1%); and stool, 0 (0.0%) (Table 2). Many patients were tested positive for more than 1 sample type. The results indicate that out of the 453 culture-positive cases, a large number (393 patients representing 86.8% of total culture positives) were bacteremic. For the treatment history based on Table 3, most patients received ceftazidime during the intensive phase, with 210 (46.4%) patients. During the maintenance phase, 253 (55.8%) patients received trimethoprim-sulfamethoxazole.
Table 1.
The outcome of patients with culture-confirmed melioidosis in HSB and HTF (n = 453).
| Outcome | n (%) |
| Discharge well | 159 (35.1) |
| AOR discharge | 16 (3.5) |
| Transfer to other hospitals | 51 (11.3) |
| Dead | 227 (50.1) |
HSB = Hospital Sultanah Bahiyah; HTF = Hospital Tuanku Fauziah.
Table 2.
The number of patients who were culture-positive for each sample type.
| Type of sample | Tested n (%) | Positive n (%) |
| Blood | 453 (100.0) | 393 (86.8) |
| Tissue | 38 (8.4) | 32 (84.2) |
| Urine | 105 (23.2) | 5 (4.8) |
| Wound | 16 (30.2) | 9 (56.3) |
| Sputum | 33 (7.3) | 3 (9.1) |
| Stool | 10 (2.2) | 0 (0.0) |
| Others | 131 (28.9) | Not available |
A few of type of sample included in the “Others” classification are bone, ear, eye, knee, pus, rectal, TASP, tracheal aspirate, and HVS.
Table 3.
Type of treatment received by melioidosis patients admitted to HSB and HTF.
| Treatment history | n (%) |
| Intensive phase | |
| Ceftazidime | 210 (46.4) |
| Amoxycilline-clavulinic | 23 (5.1) |
| Cefoperanzone-sulbactam | 3 (0.7) |
| Doxycycline | 7 (1.5) |
| Others | 145 (32.0) |
| Maintenance phase | |
| Amoxycilline-clavulinic | 5 (1.1) |
| Trimethoprim-sulfamethoxazole | 253 (55.8) |
| Doxycycline | 14 (0.2) |
| Ciprofloxacin | 3 (0.7) |
| Others | 21 (4.6) |
HSB = Hospital Sultanah Bahiyah; HTF = Hospital Tuanku Fauziah.
3.2. Characteristics of patients based on demographic data and risk factors
Table 4 shows the socio-demographic data and underlying disease of patients with melioidosis. The results were based on 453 patients from HSB and HTF from 2014 to 2017. The mean (SD) age of melioidosis patients who survived was 49.20 (15.63) years and 54.55 (14.27) years for those who died (Table 4). Overall, 357 (78.8%) patients were males and 96 (21.2%) were females. Almost all subjects were Malay, with 399 (88.1%) patients. In the job context, 96 (21.2%) patients exposed to high-risk jobs died. Four (1.8%) patients who had a previous history of melioidosis died and 10 (4.4%) patients survived. A total of 219 (96.5%) bacteremic patients died from melioidosis. Based on comorbidities, most of the patients had diabetes mellitus, followed by hypertension and chronic kidney disease. There was a statistically significant association between age, type of melioidosis, white blood cell, platelet, urea, and albumin between the groups (P < .05).
Table 4.
Characteristics of melioidosis patients in HSB and HTF (n = 453).
| Censored (no. = 226) (%‡) | Event (no. = 227) (%‡) | P | X2 | |
| Mean age (in yr)∗,† | 49.20 (15.63) | 54.55 (14.27) | <.001 | 3.79 (451) |
| Gender | ||||
| Male | 177 (78.3) | 180 (79.3) | .799 | 0.07 |
| Female | 49 (21.7) | 47 (20.7) | ||
| Race | ||||
| Malay | 201 (88.9) | 198 (87.2) | .418 | 2.84 |
| Chinese | 9 (4.1) | 9 (4.0) | ||
| Indian | 10 (4.4) | 17 (7.5) | ||
| Others | 6 (2.7) | 3 (1.3) | ||
| Nationality | ||||
| Malaysian | 223 (98.7) | 223 (98.2) | >.95 | 0.14 |
| Non-Malaysian | 3 (1.3) | 4 (1.8) | ||
| Occupation | ||||
| Unknown | 84 (37.2) | 96 (42.3) | .538 | 1.24 |
| High risk | 50 (22.1) | 46 (20.3) | ||
| Low risk | 92 (40.7) | 85 (37.4) | ||
| Smoking status | ||||
| Unknown | 207 (91.5) | 212 (93.4) | .589 | 1.06 |
| Yes | 9 (4.1) | 9 (4.0) | ||
| No | 10 (4.4) | 6 (2.6) | ||
| Antibiotics received | ||||
| No | 149 (65.9) | 161 (70.9) | .253 | 1.31 |
| Yes | 77 (34.1) | 66 (29.1) | ||
| Type of melioidosis∗ | ||||
| Non-bacteremic | 52 (23.0) | 8 (3.5) | <.001 | 37.42 |
| Bacteremic | 174 (77.0) | 219 (96.5) | ||
| Previous history of melioidosis | ||||
| Yes | 10 (4.4) | 4 (1.8) | .102 | 2.68 |
| No | 216 (95.6) | 223 (98.2) | ||
| Comorbidity | ||||
| Diabetes mellitus | 163 (72.1) | 153 (67.4) | .307 | 1.20 |
| Chronic kidney disease | 25 (11.1) | 35 (15.4) | .212 | 1.87 |
| Chronic lung disease | 6 (2.7) | 10 (4.4) | .446 | 1.02 |
| Hypertension | 65 (28.8) | 82 (36.1) | .089 | 3.14 |
| Asthma | 6 (2.7) | 6 (2.64) | >.95 | <0.001 |
| Heart disease | 10 (4.4) | 12 (5.3) | .828 | 0.18 |
| Gout | 4 (1.8) | 6 (2.6) | .751 | 0.40 |
| Thalassemia | 2 (0.9) | 2 (0.9) | >.95 | <0.001 |
| Liver disease | 1 (0.4) | 4 (1.8) | .372 | 1.81 |
| Other diseases | 6 (2.7) | 8 (3.5) | .248 | 3.01 |
| Blood investigations | ||||
| Abnormal hemoglobin | 164 (72.6) | 162 (71.4) | .834 | 0.08 |
| Low and high white blood cell∗ | 140 (66.4) | 178 (78.4) | <.001 | 19.61 |
| Low and high platelet∗ | 41 (18.1) | 97 (42.7) | <.001 | 38.85 |
| High and Intermediate level urea∗ | 83 (36.7) | 175 (77.1) | <.001 | 79.31 |
| Abnormal albumin | 210 (92.9) | 224 (98.7) | .002 | 9.34 |
| Abnormal AST | 206 (91.2) | 214 (94.3) | .211 | 1.64 |
| Abnormal ALT | 129 (57.1) | 136 (59.9) | .568 | 0.37 |
A few of the illnesses included in the “Other Diseases” classification are hepatitis B and C, thalassemia, and tuberculosis. Comorbidity is comparable to “no”; blood investigations are comparable to “normal.” ALT = alanine aminotransferase; AST = aspartate aminotransferase; HSB = Hospital Sultanah Bahiyah; HTF = Hospital Tuanku Fauziah.
Data shown as no. (%); p-value < .001.
Data is shown as mean (standard deviation). Data were analyzed using an independent t test. X2 = differences between groups were analyzed by either the Pearson Chi-Square Test or Fisher's exact test when appropriate.
Percentages calculated based on total subjects (no.) for each disease group.
3.3. Univariable analysis of prognostic factors of melioidosis
The results of simple Cox regression were shown in Table 5. The possible prognostic factor of melioidosis patients that was included in the univariable analysis was socio-demographic, type of melioidosis, previous history of melioidosis, antibiotic received, comorbidities, and blood investigations. The factors that P < .25 included age, type of melioidosis, previous history of melioidosis, diabetes mellitus, chronic kidney disease, chronic lung disease, hypertension, asthma, thalassemia, liver disease, white blood cell, platelet, urea, creatinine, albumin, and aspartate aminotransferase then proceeded into the multivariable analysis.
Table 5.
Univariable analysis of prognostic factors of melioidosis using simple Cox regression analysis (n = 453).
| Univariable analysis | ||||
| β | Crude HR | 95% CI | P | |
| Age | 0.01 | 1.01 | 1.005–1.023 | .002 |
| Gender | ||||
| Female | 0 | 1 | ||
| Male | 0.05 | 1.05 | 0.76–1.45 | .748 |
| Race | ||||
| Malay | 0 | 1 | ||
| Chinese | 0.02 | 1.02 | 0.40–2.61 | .975 |
| Indian | 0.55 | 1.73 | 0.77–3.86 | .184 |
| Others | −0.68 | 0.51 | 0.13–2.06 | .342 |
| Occupation | ||||
| Low risk | 0 | 1 | ||
| High risk | 0.10 | 1.11 | 0.86–1.42 | .429 |
| Type of melioidosis | ||||
| Non-bacteremia | 0 | 1 | ||
| Bacteremia | 1.69 | 5.40 | 2.66–10.93 | <.001 |
| Antibiotics received | ||||
| Yes | 0 | 1 | ||
| No | −0.08 | 0.92 | 0.69–1.23 | .578 |
| Previous history of melioidosis | ||||
| No | 0 | 1 | ||
| Yes | −0.72 | 0.49 | 0.18–1.31 | .155 |
| Comorbid§ | ||||
| Diabetes mellitus | ||||
| No | 0 | 1 | ||
| Yes | −0.29 | 0.75 | 0.57–0.99 | .045 |
| Chronic kidney disease | ||||
| No | 0 | 1 | ||
| Yes | 0.20 | 1.22 | 0.85–1.75 | .283 |
| Chronic lung disease | ||||
| No | 0 | 1 | ||
| Yes | 0.59 | 0.59 | 0.95–3.41 | .069 |
| Hypertension | ||||
| No | 0 | 1 | ||
| Yes | 0.19 | 1.21 | 0.93–1.59 | .161 |
| Asthma | ||||
| No | 0 | 1 | ||
| Yes | −0.09 | 0.91 | 0.40–2.05 | .821 |
| Heart disease | ||||
| No | 0 | 1 | ||
| Yes | 0.13 | 1.14 | 0.64–2.04 | .655 |
| Gout | ||||
| No | 0 | 1 | ||
| Yes | 0.59 | 1.68 | 0.75–3.78 | .212 |
| Thalassemia | ||||
| No | 0 | 1 | ||
| Yes | 0.07 | 1.07 | 0.27–4.32 | .920 |
| Liver disease | ||||
| No | 0 | 1 | ||
| Yes | 0.62 | 1.86 | 0.69–5.02 | .217 |
| Other diseases | ||||
| No | 0 | 1 | ||
| Yes | −0.11 | 0.89 | 0.69–1.16 | .396 |
| Blood investigations§ | ||||
| Haemoglobin | ||||
| Normal (13–17 g/dL) | 0 | 1 | ||
| Abnormal | −0.09 | 0.92 | 0.69–1.22 | .552 |
| White blood cell | ||||
| Normal (4–10 × 1000/mL) | 0 | 1 | ||
| Low (<4 × 1000/mL) | 0.96 | 2.61 | 1.52–4.49 | <.001 |
| High (>10 × 1000/mL) | 0.42 | 1.51 | 1.11–2.10 | .010 |
| Platelet | ||||
| Normal (>158 × 1000/mL) | 0 | 1 | ||
| Intermediate (100–149 × 1000/mL) | 0.54 | 1.77 | 1.25–2.52 | .001 |
| Low (<100 × 1000/mL) | 0.95 | 2.58 | 1.88–3.53 | <.001 |
| Urea | ||||
| Normal (2.5–7.8 mmol/L) | 0 | 1 | ||
| Low (<2.5 mmol/L) | 1.15 | 3.15 | 2.20–4.51 | <.0001 |
| High (>7.8 mmol/L) | 1.36 | 3.90 | 2.79–4.46 | <.001 |
| Creatinine | ||||
| Normal (65–125 μmol/L) | 0 | 1 | ||
| Low (<65 μmol/L) | −0.27 | 0.77 | 4.35–1.35 | .352 |
| High (>125 μmol/L) | 0.91 | 0.91 | 1.80–3.42 | <.001 |
| Albumin | ||||
| Normal (37–51 g/L) | 0 | 1 | ||
| Abnormal | 1.47 | 4.37 | 1.40–13.66 | .011 |
| AST | ||||
| Normal (10–45 U/L) | 0 | 1 | ||
| Abnormal | 0.50 | 1.65 | 0.94–2.90 | .079 |
| ALT | ||||
| Normal (10–55 U/L) | 0 | 1 | ||
| Abnormal | 0.11 | 1.12 | 0.86–1.46 | .404 |
ALT = alanine aminotransferase; AST = aspartate aminotransferase; CI = confidence Interval; HR = hazard ratio.
3.4. The Cox model of prognostic factors of mortality among melioidosis patients
The prognostic factors of mortality based on the final Cox model are shown in Table 6. The determinants were the type of melioidosis, diabetes mellitus, white blood cell count, platelet count, and urea. From the results, bacteremic melioidosis increased the risk of dying from melioidosis by 3.47 (HR: 3.47, 95% CI: 1.67–7.23, P = .001) compared to non-bacteremic melioidosis. Kaplan–Meier graph for time to mortality was constructed to illustrate the group's difference (Fig. 2). Based on laboratory test, the adjusted HRs from the final model showed that all 3 blood investigations were included as the prognostic factors of mortality for the disease (low platelet: HR = 1.76, 95% CI: 1.22–2.54, P = .003; high white blood cell: HR = 1.49, 95% CI 1.06–2.11, P = .023; high urea: HR = 2.92, 95% CI: 1.76–4.85, P < .001; and intermediate level of urea: HR = 2.69, 95% CI: 1.69–4.29, P < .001). By contrast, melioidosis patients with diabetic had 30.0% lower risk of dying from melioidosis compared to those with non-diabetic (HR = 0.70, 95% CI: 0.52–0.94, P = .016).
Table 6.
The final model of prognostic factors of melioidosis in HSB and HTF using Multiple Cox Regression analysis (n = 453).
| Multivariable analysis | ||||
| B | Adjusted HR | 95% CI | Pa | |
| Type of melioidosis | ||||
| Non-bacteremia | 0 | 1 | ||
| Bacteremia | 1.25 | 3.47 | 1.67–7.23 | .001 |
| Diabetes mellitus | ||||
| No | 0 | 1 | ||
| Yes | −0.36 | 0.70 | 0.52–0.94 | .016 |
| White blood cell | ||||
| Normal | 0 | 1 | ||
| Low | 0.49 | 1.64 | 0.92–2.93 | .095 |
| High | 0.40 | 1.49 | 1.06–2.11 | .023 |
| Platelet | ||||
| Normal | 0 | 1 | ||
| Intermediate | 0.29 | 1.33 | 0.92–1.94 | .134 |
| Low | 0.56 | 1.76 | 1.22–2.54 | .003 |
| Urea | ||||
| Normal | 0 | 1 | ||
| Intermediate | 0.99 | 2.69 | 1.69–4.29 | <.001 |
| High | 1.07 | 2.92 | 1.76–4.85 | <.001 |
Multicollinearity and interaction were checked and not found. The preliminary final model was properly specified (_hat: P < .001) (_hatsq: P = .356). Hazard function plot, Log-minus-log plot, Schoenfeld partial residuals plot, scaled, and non-scaled. Schoenfeld residual test (Globat test:0.525) and C-statistics (70.6%) were run to check model assumptions. Hosmer-Lemeshow test (P = .321), classification table (overall correctly classified percentage = 73.0%), and area under the curve (76.2%) were applied to check the model fitness. Regression diagnostics were performed by Cox-snell residual Martingale residual, Deviance residual, and influential analysis. Remedial measures were applied, and no influential observations were detected in the model. CI = confidence intervals; HR = hazard ratio; HSB = Hospital Sultanah Bahiyah; HTF = Hospital Tuanku Fauziah.
Forward stepwise Cox proportional hazards regression model applied.
Figure 2.
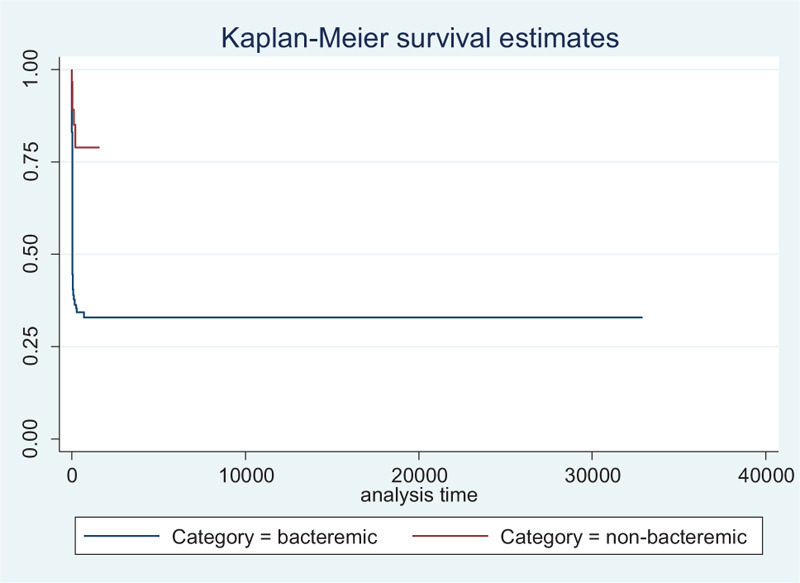
The comparison of median survival time between the type of melioidosis.
3.5. Further validation on the survivor of melioidosis in the diabetic patient
Table 7 shows the patient characteristic, and blood investigations were compared descriptively to validate further details on the contradictory finding on the variable diabetes mellitus. The result showed that those who survived among diabetic patients had a higher percentage than those who survived in non-diabetic patients. Overall, a total of 94 (68.6%) patients and 270 (68.4%) patients did not receive any antibiotics during the admission among those without and with diabetes mellitus, respectively. Among those who died and not receiving any antibiotics in a non-diabetic group, 74.3% died than 69.3% who died and not receiving any antibiotics among diabetic groups. The survival percentage from melioidosis in bacteremic and those with more than 1 risk factor among the diabetic patients were also higher than those with non-diabetic. The portion of the dead patient among non-diabetic with the renal disease was 15 (16.2%) patients and 23 (15.0%) patients who died among diabetic patients with renal disease.
Table 7.
Comparison of survival based on patient characteristics and blood investigation between those with diabetic and non-diabetic (n = 453).
| Non-diabetic n = 137 | Diabetic n = 316 | |||
| Characteristics | Survived n (%) | Died n (%) | Survived n (%) | Died n (%) |
| Mean age (in yr)∗ | 42.16 (19.10) | 53.61 (16.67) | 51.93 (13.13) | 55.00 (12.99) |
| Sex | ||||
| Male | 47 (74.6) | 63 (85.1) | 130 (79.8) | 117 (76.5) |
| Female | 16 (25.4) | 11 (14.9) | 33 (20.2) | 36 (23.5) |
| Received antibiotics | ||||
| No | 39 (61.9) | 55 (74.3) | 110 (67.5) | 106 (69.3) |
| Yes | 24 (38.1) | 19 (25.7) | 53 (32.5) | 47 (30.7) |
| Bacteremic | ||||
| Yes | 46 (73.0) | 71 (95.9) | 128 (78.5) | 148 (96.7) |
| No | 17 (24.0) | 3 (4.1) | 35 (21.5) | 5 (3.3) |
| Renal disease | ||||
| Yes | 7 (11.1) | 12 (16.2) | 18 (11.0) | 23 (15.0) |
| No | 56 (88.9) | 62 (83.8) | 145 (89.0) | 130 (85.0) |
| ≥1 risk factor | ||||
| Yes | 9 (14.3) | 15 (20.3) | 70 (42.9) | 86 (56.2) |
| No | 54 (85.7) | 59 (79.7) | 93 (57.1) | 67 (43.8) |
| Mean white blood cell∗ | 11.85 (7.03) | 15.62 (13.20) | 14.12 (8.85) | 15.37 (8.67) |
| Mean platelet∗ | 267.56 (125.83) | 185.94 (133.33) | 273.40 (136.04) | 194.82 (109.72) |
| Mean urea∗ | 9.07 (9.31) | 17.98 (13.74) | 9.56 (8.96) | 17.45 (11.43) |
Data is shown as mean (standard deviation).
4. Discussion
The risk factors associated with mortality from melioidosis in this study are similar to those in the published literature. However, our results for diabetes mellitus should be interpreted with caution because it becomes a protective factor toward mortality from melioidosis. A diabetic patient was 30.0% less likely to die compared to a non-diabetic patient. In this study, diabetes mellitus founded the highest percentage of comorbid that affected both survived and dead melioidosis patients. A total of 316 (69.8%) patients were found to have diabetes mellitus. In our setting, the percentage of surviving who had diabetes mellitus was higher than those who died from melioidosis (72.1% vs 67.4%), which led to protective HR = 0.70. This result appeared to be contradicted by several studies showing a significant relationship between diabetes mellitus and mortality among melioidosis patients.[12–14]
The study was in line with a study conducted in Thailand that reported a protective effect of diabetes against mortality from melioidosis with an odds ratio of 0.57.[15] The lower rate of mortality among those with diabetes mellitus may be attributable to glyburide use that acts as an anti-inflammatory effect.[15–17] The use of this medication before the hospital admission can reduce the inflammatory reaction.[3,16]
The first study supporting the results proved that the CX3CR1 expression in diabetic patients increases the survival of infectious disease.[18] Diabetes mellitus altered the immune response toward melioidosis producing high antibody titers and double-negative T cells against B pseudomallei. The antibody acts to protect the human body during the infection from melioidosis by eliminating the infected host cells.[18] A study that was conducted in Australia and Thailand found that the high antibody titers were detected in diabetic patients in particular endemic regions.[19,20] The production of antibodies toward B pseudomallei was higher in this group but still unclear.[18] It is recommended to compare the neutrophils and H1ABC in survivor diabetic patients versus survivor non-diabetic patients to see any differences in this test.
A study in Thai compared the interferon-gamma between melioidosis patients with and without diabetes found that the frequency of interferon-gamma to fight the B pseudomallei was similar in both groups.[21] From the finding, it is strongly suggested to validate more aspects based on the relation of antibody production with the correlation of multiple underlying diseases and bacteremic to determine the causes of higher survival in diabetic patients. In this study, among those with renal disease, 15 non-diabetic patients (16.2%) and 23 diabetic patients (15.0%) died from melioidosis.
The recommendation from journals to treat those with risk factors such as diabetes mellitus with an appropriate antibiotic was highlighted in most of the published papers on melioidosis. Early management toward this group can be why patients with diabetes mellitus lower the risk of getting died from this disease.[3] Based on the percentage reported, it found that the diabetic patients in the study who had received the antibiotic were higher than non-diabetic patients, and maybe the results why the survival from this group much higher compared to those in the non-diabetic group. Based on the study in Thailand that was conducted among the tuberculosis patient, the anti-tuberculosis treatment in those with diabetes significantly faster the disease progression in this patient.[22] A study conducted among 72 recovered melioidosis patients reported that T cells’ induction in those who received the treatment was better than those who did not receive any treatment.[23] Many previous studies reported that diabetes mellitus is a significant risk factor in developing melioidosis,[4,24–27] but it was not the factor contributing to the mortality from melioidosis.[28]
The results found the highly significant risk factors of platelet level in mortality of melioidosis patients (P < .001). The mean of platelet for those who died from melioidosis was much lower than those who survived, with 174.15 (112.91) versus 288.01 (148.85). This finding was supported by a study conducted by Birnie et al., both in animals and humans that was published in 2019. The research presented the association between thrombocytopenia and mortality among melioidosis cases.[29] Those who had low platelet increased the odds of dying from melioidosis by 7.90 compared to those who had normal platelet.[29] The association between the level of platelet and mortality can be caused by disease severity. Those who had low platelet levels tend to develop more hypotension and failure in the respiratory system and renal, leading to mortality.[30] Another study in Australia compared the outcome of melioidosis patients with low and normal platelet during admission.[31] Patients with more severe illness, usually bacteremia and septic shock, had lower platelet levels than those with less severe disease. After admission, the increased platelet level also correlated with a higher chance of survival among the cases.[31]
Several studies reported that statistically significant laboratory factors for mortality in melioidosis were white blood cells count and blood urea nitrogen value.[11,28–30] The study reported the adjusted ORs (95% CI) for white blood cells and blood urea nitrogen were 0.772 (0.540–0.966) and 1.110 (1.026–1.201), respectively.[32] From our results, patients with high white blood cell count increased the risk of dying from melioidosis by 1.49 compared to patients with a standard range of white blood cell count. From another updated study in Thailand, 60% of bacteremic melioidosis patients had abnormal white blood cell count (>11,000 or <3000 cells/mL), while 43% had a standard range of white blood cell count.[33] The study also reported poor clinical outcomes among these patients and died at discharge.[33] Zueter et al. reported the relationship of the higher level of white blood cell, which was more than 11 × 103 cell/μL in patients with sepsis. The presence of sepsis can be why high white blood cell counts toward the risk of dying from melioidosis. Future studies are recommended to identify sepsis in high white blood cell patients and the correlation toward mortality from melioidosis.
Another significant prognostic factor toward mortality from melioidosis was urea. The hazard ratios of dying from melioidosis in patients with intermediate and high urea levels were 2.69 and 2.92, respectively. The results were similar to several studies that reported the association between elevated urea toward mortality from melioidosis.[31,34,35] Kirby et al. (2018) stated that the risk factors of mortality from melioidosis were platelets, serum urea, age, bilirubin, and bicarbonate. The high serum urea value level was 1 indicator of organ failure, predominantly renal dysfunction.[32]
In this study, 75.0% of bacteremic melioidosis patients died from the disease. Many studies reported the high mortality rate among bacteremic cases.[36–39] A study conducted in Hospital Universiti Sains Malaysia compared the risk factors of early mortality in melioidosis patients who reported high bacteremic melioidosis in the early mortality group.[36] Based on that study, early mortality was defined as died within less than 48 hours of hospital admission.[36] The mean length of stay in the hospital for those who died from bacteremic melioidosis patients was 3.1 days.[36] From the total deaths, 60% of melioidosis patients died within less than 48 hours of admission.[36] Mostly, bacteremic patients will have high white blood cell counts due to bacteria's presence in the blood. A study conducted in South Indian state Kerala reported that the overall mortality was 24.3%, and it was 40% among bacteremic cases and 13.6% among non-bacteremic melioidosis.[40]
4.1. Limitation
In this study, several limitations should be considered for future enhancement. The data were retrospectively collected from the registry, data likely to have information bias that influenced the data quality. From the collected data, we cannot identify the real timing of diagnosis other than from the date of admission; either the patients received the delayed diagnosis/treatment or not, which lead the patients to die before the diagnosis. The lack of this information leads to information bias and underestimates the hazard ratio value that we calculated for this study. Since we cannot identify the lack of clinical suspicion leading to delay in treatment, it is recommended to conduct a prospective study for future research. Identifying all these uncertainties will provide a good result in assessing the HRs to promote early therapy and reduce the mortality rate among melioidosis patients. Secondly, 189 patients were reported with an unknown occupation. We cannot identify the real effect of patients’ occupation on the mortality of melioidosis due to this variable's missing records.
5. Conclusion
The findings from the study have provided an updated major review of factors affecting mortality among melioidosis patients in HSB and the extension of results from HTF. The findings can be represented for the whole Kedah and Perlis state as both hospitals are the major hospital for the patients to get treatment. The precautions toward patients with bacteremic melioidosis need to be targeted with high monitoring and received early and appropriate treatment during the admission. An effort to monitor patients with diabetes mellitus by controlling their food intake and lifestyle is also important because most of those who have diabetes have bacteremic melioidosis that leads to death.[11] The proper management also needs to focus on patients without diabetes mellitus with other health issues to prevent this group from not being treated well. Disease management in terms of early clinical laboratory diagnosis and early melioidosis diagnosis needs to be done at the hospital level. The platelet, white blood cell, and urea level can be early predictors to detect the state of melioidosis patients.
Author contributions
Conceptualization: Nadiah Wan-Arfah, Nyi Nyi Naing.
Formal analysis: Kamaruddin Mardhiah, Nadiah Wan-Arfah, Nyi Nyi Naing.
Funding acquisition: Nadiah Wan-Arfah.
Investigation: Kamaruddin Mardhiah.
Project administration: Muhammad Radzi Abu Hassan, Huan-Keat Chan.
Resources: Muhammad Radzi Abu Hassan, Huan-Keat Chan.
Software: Kamaruddin Mardhiah.
Supervision: Nadiah Wan-Arfah, Nyi Nyi Naing, Muhammad Radzi Abu Hassan, Huan-Keat Chan.
Writing – original draft: Kamaruddin Mardhiah.
Writing – review & editing: Nadiah Wan-Arfah, Nyi Nyi Naing, Muhammad Radzi Abu Hassan, Huan-Keat Chan, Kamaruddin Mardhiah.
Footnotes
Abbreviations: CI = confidence intervals, HR = hazard ratio, HSB = Hospital Sultanah Bahiyah, HTF = Hospital Tuanku Fauziah.
How to cite this article: Mardhiah K, Wan-Arfah N, Naing NN, Hassan MR, Chan HK. The Cox model of predicting mortality among melioidosis patients in Northern Malaysia: a retrospective study. Medicine. 2021;100:25(e26160).
The project was supported by the internal grant of Universiti Sultan Zainal Abidin (grant number: UniSZA/2018/DPU/16).
The author reports that no conflicts of interest are involved in this research.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- [1].Chakravorty A, Heath CH. AJGP-05-2019-Professional-Chakravorty-Melioidosis-WEB (1). 2019; 48: 327–32 [DOI] [PubMed] [Google Scholar]
- [2].Kim SW, Kwon GY, Kim B, Kwon D, Shin J, Bae GR. Imported melioidosis in South Korea: a case series with a literature review. Osong Public Heal Res Perspect 2015;6:363–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Wiersinga WJ, Virk HS, Torres AG, et al. HHS Public Access. 2019;DOI 10.1038/nrdp.2017.107.Melioidosis. [Google Scholar]
- [4].How SH, Ng KH, Jamalludin AR, Shah A, Rathor Y. Melioidosis in Pahang, Malaysia. Med J Malaysia 2005;60:606–13. [PubMed] [Google Scholar]
- [5].Nathan S, Chieng S, Kingsley PV, et al. Melioidosis in Malaysia: incidence, clinical challenges, and advances in understanding pathogenesis. Trop Med Infect Dis 2018;3:01–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Volpe-Chaves CE, Rodrigues ACS, Lacerda MLGG, et al. Melioidosis, an emerging infectious disease in the Midwest Brazil: a case report. Medicine (Baltimore) 2019;98:01–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Mardhiah K, Wan-Arfah N, Naing NN, Abu Hassan MR, Chan HK, Hasan H. The trend of direct medical cost of meliodiosis patients in Kedah: a retrospective study from 2014 to 2017. ClinicoEcon Outcomes Res 2017;13:155–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Mohapatra PR, Behera B, Mohanty S, Bhuniya S, Mishra B. Melioidosis. Lancet Infect Dis 2019;19:1056–7. [DOI] [PubMed] [Google Scholar]
- [9].Woods KL, Boutthasavong L, NicFhogartaigh C, et al. Evaluation of a rapid diagnostic test for detection of Burkholderia pseudomallei in the Lao people's democratic republic. J Clin Microbiol 2018;56:e02002–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Hoffmaster AR, Aucoin D, Baccam P, et al. Melioidosis diagnostic workshop, 20131. Emerg Infect Dis 2015;21:01–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Zueter AR, Yean CY, Abumarzouq M, Rahman ZA, Deris ZZ, Harun A. The epidemiology and clinical spectrum of melioidosis in a teaching hospital in a North-Eastern state of Malaysia: a fifteen-year review. BMC Infect Dis 2016;16:01–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Hassan MRA, Pani SP, Peng NP, et al. Incidence, risk factors and clinical epidemiology of melioidosis: a complex socio-ecological emerging infectious disease in the Alor Setar region of Kedah, Malaysia. BMC Infect Dis 2010;10:1471–2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Pang L, Harris PNA, Seiler RL, et al. Melioidosis, Singapore, 2003–2014. Vol 24. 2018;0–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Stewart JD, Smith S, Binotto E, McBride WJ, Currie BJ, Hanson J. The epidemiology and clinical features of melioidosis in Far North Queensland: implications for patient management. PLoS Negl Trop Dis 2017;11:01–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Hantrakun V, Kongyu S, Klaytong P, et al. Clinical epidemiology of 7126 melioidosis patients in Thailand and the implications for a national notifiable diseases surveillance system. Open Forum Infect Dis 2019;6:01–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Koh GCKW, Maude RR, Schreiber MF, et al. Glyburide is anti-inflammatory and associated with reduced mortality in melioidosis. Clin Infect Dis 2011;52:717–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Kaewarpai T, Ekchariyawat P, Phunpang R, et al. Longitudinal profiling of plasma cytokines in melioidosis and their association with mortality: a prospective cohort study. Clin Microbiol Infect 2020;26:783.e1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Kronsteiner B, Chaichana P, Sumonwiriya M, et al. Diabetes alters immune response patterns to acute melioidosis in humans. Eur J Immunol 2019;49:1092–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Harris PNA, Ketheesan N, Owens L, Norton RE. Clinical features that affect indirect-hemagglutination-assay responses to Burkholderia pseudomallei. Clin Vaccine Immunol 2009;16:924–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Kanaphun P, Thirawattanasuk N, Suputtamongkol Y, et al. Serology and carriage of pseudomonas pseudomallei: a prospective study in 1000 hospitalized children in Northeast Thailand. J Infect Dis 1993;167:230–3. [DOI] [PubMed] [Google Scholar]
- [21].Tippayawat P, Saenwongsa W, Mahawantung J, et al. Phenotypic and functional characterization of human memory T cell responses to Burkholderia pseudomallei. PLoS Negl Trop Dis 2009;3:02–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Kumar Nathella P, Babu S. Influence of diabetes mellitus on immunity to human tuberculosis. Immunology 2017;152:13–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Suwannasaen D, Mahawantung J, Chaowagul W, et al. Human immune responses to Burkholderia pseudomallei characterized by protein microarray analysis. J Infect Dis 2011;1002–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Kingsley PV, Leader M, Nagodawithana NS, Tipre M, Sathiakumar N. Melioidosis in Malaysia: a review of case reports. PLoS Negl Trop Dis 2016;10:01–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Pande K, Kadir KAA, Asli R, Chong VH. Melioidosis in Brunei Darussalam. Trop Med Infect Dis 2018;3:01–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Currie BJ, Jacups SP, Cheng AC, et al. Melioidosis epidemiology and risk factors from a prospective whole-population study in northern Australia. Trop Med Int Heal 2004;9:1167–74. [DOI] [PubMed] [Google Scholar]
- [27].Holland DJ, Wesley A, Drinkovic D, Currie BJ. Cystic Fibrosis and Burkholderia pseudomallei Infection: an emerging problem? Clin Infect Dis 2002;35:e138–40. [DOI] [PubMed] [Google Scholar]
- [28].Shaw T, Tellapragada C, Kamath A, Kalwaje Eshwara V, Mukhopadhyay C. Implications of environmental and pathogen-specific determinants on clinical presentations and disease outcome in melioidosis patients. PLoS Negl Trop Dis 2019;13:01–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Birnie E, Claushuis TAM, Koh GCKW, et al. Thrombocytopenia impairs host defense against Burkholderia pseudomallei (Melioidosis). J Infect Dis 2019;219:648–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Claushuis TAM, Van Vught LA, Scicluna BP, et al. Thrombocytopenia is associated with a dysregulated host response in critically ill sepsis patients. Blood 2016;127:3062–72. [DOI] [PubMed] [Google Scholar]
- [31].Kirby P, Smith S, Ward L, Hanson J, Currie BJ. Clinical utility of platelet count as a prognostic marker for melioidosis. Am J Trop Med Hyg 2019;100:1085–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Domthong P, Chaisuksant S, Sawanyawisuth K. What clinical factors are associated with mortality in septicemic melioidosis? A report from an endemic area. J Infect Dev Ctries 2016;10:404–9. [DOI] [PubMed] [Google Scholar]
- [33].Jatapai A, Gregory CJ, Thamthitiwat S, et al. Hospitalized bacteremic melioidosis in rural Thailand: 2009–2013. Am J Trop Med Hyg 2018;98:1585–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Cheng AC, Jacups SP, Anstey NM, Currie BJ. A proposed scoring system for predicting mortality in melioidosis. Trans R Soc Trop Med Hyg 2003;97:577–81. [DOI] [PubMed] [Google Scholar]
- [35].Rajendran M, Anand S, Aravind K. Melioidosis: a multiple disease imposter. Int J Res Med Sci 2018;6:2872–4. [Google Scholar]
- [36].Deris ZZ, Hasan H, Suraiya MNS. Clinical characteristics and outcomes of bacteraemic melioidosis in a teaching hospital in a northeastern state of Malaysia: a five-year review. J Infect Dev Ctries 2010;4:430–5. [DOI] [PubMed] [Google Scholar]
- [37].Sam IC, Puthucheary SD. Melioidosis in children from Kuala Lumpur, Malaysia. Ann Trop Paediatr 2006;26:219–24. [DOI] [PubMed] [Google Scholar]
- [38].Sukauichai S, Pattarowas C. Melioidosis with septic shock and disseminated infection in a neutropenic patient receiving chemotherapy. Case Rep Infect Dis 2020;2020:01–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Le Tohic S, Montana M, Koch L, Curti C, Vanelle P. A review of melioidosis cases imported into Europe. Eur J Clin Microbiol Infect Dis 2019;38:1395–408. [DOI] [PubMed] [Google Scholar]
- [40].Jose R, Valsan C, Sathiavathy KA. Clinical and epidemiological profile of melioidosis in a tertiary care teaching hospital from South India. IP Int J Med Microbiol Trop Dis 2019;5:107–11. [Google Scholar]


