Abstract
Intravenous leiomyomatosis is a rare nonmalignant tumor, which originates from the uterine smooth muscle cells and is usually confined to the pelvic venous system. Sometimes it can extend from the pelvis through the veins into the right side of the heart; this condition is named intracardiac leiomyomatosis (ICLM). To date few cases of these conditions have been described, the treatment is surgical, often challenging and usually multidisciplinary. In this paper are described the clinical presentation, the full radiologic study and surgical treatment of a case of ICLM that authors treated at their institution with thoraco-abdominal approach.
Surgical removal of the ICLM is strongly recommended, because no recurrence has been reported, in our case at 7 years we did not observe recurrence of the disease.
INTRODUCTION
Uterine leiomyoma is a benign disease, which originates from the uterine smooth muscle cells that in some cases can be complicated in their clinical course by a rare behavioral change of the disease with intravascular embolization. Uterine intravenous leiomyomatosis (IVL) is an uncommon tumor, arising from the uterus and extending intravascularly over variable distances and may reach the inferior vena cava (IVC), right atrium and pulmonary arteries [1].
Intracardiac leiomyomatosis (ICLM) is a condition rarely seen that represent a cardiac emergency as the prolapse into the right ventricle can interfere with cardiac function causing sudden death [2, 3].
Patients are female with an average age of 45 years. Less than 300 cases have been reported and fewer than 100 cases with cardiac involvement; extra-uterine involvement is seen in 30 to 80% of cases of uterine IVL, with cardiac involvement in 10 to 30% [4].
Surgery is the only treatment including a combined multidisciplinary thoraco-abdominal surgical approach. The treatment of IVL has two goals: to remove the intracardiac burden on right heart and prevent recurrence [5].
There are two pathways for IVL extension into the IVC and RA. The first pathway is left and right uterine veins–internal iliac veins–common iliac veins–IVC–right heart. The second pathway extends to the left ovary vein–left renal vein–IVC– right heart or right ovarian vein–IVC–right heart [6].
In this paper, we report a case of IVL extended through the second pathway and excised with a thoraco-abdominal approach.
CASE REPORT
A 52-year-old woman was transferred to our department with a diagnosis of right atrial thrombus and mild right cardiac insufficiency. Her complaint was due to a mass in her IVC extending through the right atrium and abutting in the right ventriculum, highly suspect for a thrombus. CT scan revealed a mass arising from the right adnexa that via the right densely enhancing vascular structures of the ovarian veins reached the IVC and right atrium forming the intracardiac mass (Fig. 1). The preoperative work-up consisted of transoesophageal ultrasonography that showed a free-floating echogenic right intraatrial mass measuring 70 × 50 mm originating from the IVC and protruding in the right ventriculum through the tricuspid valve. Cardiac MRI confirmed the presence and features of the mass (Fig. 2).
Figure 1 .
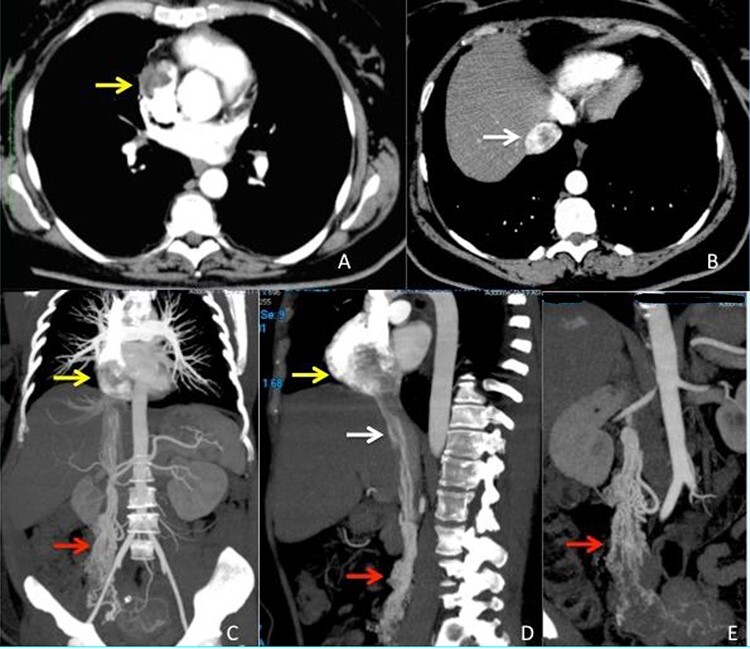
CT scan images (A–B) arterious phase showing the defect inside right atrium (yellow arrow) and intrahepatic inferior vena cava (white arrow) due to the presence of the intravascular leimyoma. (C–D) vascular CT reconstruction of the ovarian veins (red arrow), inferior vena cava (white arrow) and right atrium (yellow arrow) stored with the intravascular leiomyoma. (E) Particular view of the ovarian veins (red arrow) with the origin from the right uterine aspect.
Figure 2 .
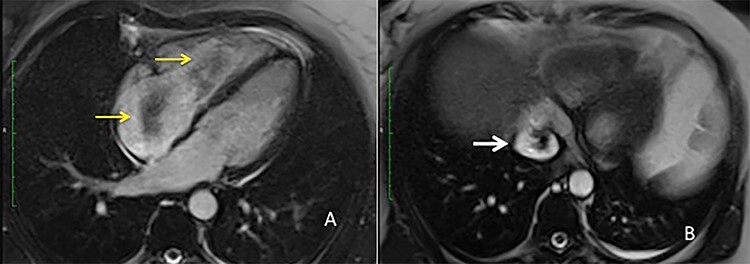
MRI view (A) right atrium with the intravascular leiomyoma prolapsing into the right ventricle (yellow arrow) (B) intrahepatic inferior vena cava stored with the intravascular leiomyoma (white arrow).
Surgery was conducted via sternolaparotomy, extracorporeal circulation and moderate hypothermia (30°C); then a combined right atriotomy and suprarenal IVC longitudinal venotomy was made, and the intravascular mass was successfully and easily removed. The length of the specimen measured 30 cm (Fig. 3). A right salpingoophorectomy was performed and the right ovarian veins were also removed. The postoperative course was uneventful. The patient was discharged from the hospital 15 days later with the only complaint of a mild anemia. Histology showed a proliferation of spindled cells leiomuscular delimited by regular endothelial line. Neither mythoses nor necrosis was observed. The images were consistent with the diagnosis of intravenous leimyomatosis (Fig. 3).
Figure 3 .
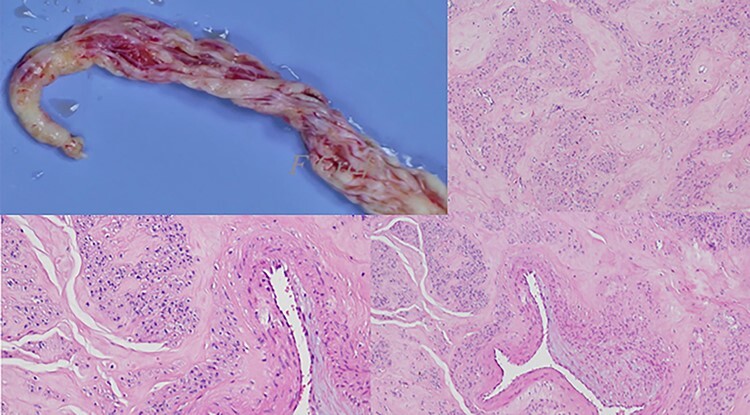
The surgical and pathological aspect of the extracted specimen.
The patients 2 months later underwent total abdominal histerectomy, and pathology confirmed the diagnosis of uterine miomatosis.
At 7 years follow-up no neoplasm relapses and no recurrent cardiac symptoms are recorded.
DISCUSSION
Dürck [7] and Hörmann [8] in 1907 described the initial cases of ICLM; Basso et al. [9] reported the first complete removal of an ICLM using a two-stage procedure in 1984.
There are two main theories which attempt to explain the etiology of uterine IVL: mural origin of the tumor from the venous walls and direct vascular invasion by a primary tumor arising from an uterine leiomyoma [10]; the second theory is favored in the literature for the following reasons: (a) patients have a history of uterine myoma or have undergone myomectomy or hysterectomy; (b) tumor bases were often connected to the uterine wall on pathological and imaging examination; (c) tumor cells tested positive for estrogen- and progesterone-receptors [11].
The clinical presentation of ICLM usually depends on the extension and size of the tumor. Asymptomatic manifestations were reported in 13% of ICLM patients because of little or no compromise of venous return. Symptomatic patients presented with various symptoms, the most common being dyspnoea, syncope, oedema of the lower extremities and palpitations [12].
Space-occupying lesions in the venous system or right heart and pulmonary system are considered in the differential diagnosis. Examples of such conditions include intravenous thrombus, Budd-Chiari syndrome, right atrial myxoma, primary leiomyosarcoma, endometrial stromal sarcoma.
In most cases a two-stage surgical approach was adopted with resection of the intracardiac component first followed by a second stage to resect the remaining intraabdominal tumor. Recently, a single-stage surgery [13] under deep hypothermic and using a multidisciplinary team has been adopted as a safe and effective technique. The advantages of a single-stage approach include a complete resection, a reconstruction of the vascular structures, a control of bleeding and reduction of the risk of tumor progression and embolism caused by incomplete resection [14].
In our case we adopted a one-stage approach with a sternolaparotomy to better achieve intraabdominal vascular control and to remove the sites of venous invasion (ovarian venous system). The distal part of the tumor was controlled through the atriotomy to prevent embolism of the distal portion, while the proximal part of the tumor was loosely attached to the wall of the IVC at the orifice of the gonadic vessels and was easily extracted via the IVC.
Simply pulling out the tumor from the right atrium is not feasible because the blind traction of the tumor could be dangerous. The site of the attachment is located in the pelvic veins, and attempted removal just from the thoracic approach results in either the failure of the complete retrieval of the tumor or tearing of the vein at the point of attachment.
In conclusion a complete surgical removal of the ICLM is curative and strongly recommended, because no recurrence has been reported; in our case at 7 years of follow-up we did not observe recurrence of the disease.
Contributor Information
Antonio Miro, Oncological and General Surgery Unit, “St. Giuseppe Moscati” Hospital of National Relevance and High Specialty, Avellino, Italy.
Enrico Coppola Bottazzi, Oncological and General Surgery Unit, “St. Giuseppe Moscati” Hospital of National Relevance and High Specialty, Avellino, Italy.
Serafino Vanella, Oncological and General Surgery Unit, “St. Giuseppe Moscati” Hospital of National Relevance and High Specialty, Avellino, Italy.
Tommaso Palma, Oncological and General Surgery Unit, “St. Giuseppe Moscati” Hospital of National Relevance and High Specialty, Avellino, Italy.
Adele Noviello, Oncological and General Surgery Unit, “St. Giuseppe Moscati” Hospital of National Relevance and High Specialty, Avellino, Italy.
Ivano Apicella, Oncological and General Surgery Unit, “St. Giuseppe Moscati” Hospital of National Relevance and High Specialty, Avellino, Italy.
Giulio Lombardi, Department of Radiology, “St. Giuseppe Moscati” Hospital of National Relevance and High Specialty, Avellino, Italy.
Brenno Fiorani, Cardiac Surgery Unit, “St. Giuseppe Moscati” Hospital of National Relevance and High Specialty, Avellino, Italy.
Francesco Crafa, Oncological and General Surgery Unit, “St. Giuseppe Moscati” Hospital of National Relevance and High Specialty, Avellino, Italy.
CONFLICT OF INTEREST STATEMENT
None declared.
FUNDING
None.
References
- 1. Ma SQ, Bai CM, Yu XH, Huang OP, Lang JH, Li J. Clinical and pathological analyses of intravenous leiomyomatosis. Chin J Obstet Gynecol 2005;40:34–7. [PubMed] [Google Scholar]
- 2. Castelli P, Caronno R, Piggaretti G, Tozzi M. Intravenous uterine leiomyomatosis with right heart extension: successful two-stage surgical removal. Ann Vasc Surg 2006;20:405–7. [DOI] [PubMed] [Google Scholar]
- 3. Mulvany NJ, Slavin JL, Ostor AG, Fortune D. Intravenous leiomyomatosis of the uterus: a clinicopathologic study of 22 cases. Int J Gynecol Pathol 1994;13:1–9. [DOI] [PubMed] [Google Scholar]
- 4. Marrone G, Crinò F, Morsolini M, Caruso S, Miraglia R. Multidisciplinary approach in the management of uterine intravenous leiomyomatosis with intracardiac extension: case report and review of literature. J Radiol Case Rep 2019;13:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Price JD, Anagnostopoulos C, Benvenisty A, Kothuru RK, Balaram SK. Intracardiac Extension of Intravenous Leiomyomatosis. Ann Thorac Surg 2017;103:e145–7. [DOI] [PubMed] [Google Scholar]
- 6. Lam PM, Lo KW, Yu MY, Wong WS, Lau JY, Arifi AA, et al. Intravenous leiomyomatosis: two cases with different routes of tumor extension. J Vasc Surg 2004;39:465–9. [DOI] [PubMed] [Google Scholar]
- 7. Dürck H. Ueber ein kontinvierlich durch die learned Hohlvene in das Herz vorwachsendes Fibromyom des Uterus. München Med Wochenschr 1907;54:1154. [Google Scholar]
- 8. Hörmann K. Über einen Fall von myomatosem Uterus Tumor. Zentralbl Gynakol 1907;51:1604–5. [Google Scholar]
- 9. Basso LV, Gradman M, Finkelstein S, Gonzalez-Lavin L. Tricuspid valve obstruction due to intravenous leiomyomatosis. Clin Nucl Med 1984;9:152–5. [DOI] [PubMed] [Google Scholar]
- 10. Nakai G, Maeda K, Yamamoto K, Yamada T, Hirose Y, Terai Y, et al. Uterine Intravenous Leiomyomatosis with Cardiac Extension: Radiologic Assessment with Surgical and Pathologic Correlation. Case Rep Obstet Gynecol 2015;2015:576743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gui T, Qian Q, Cao D, Yang J, Peng P, Shen K. Computerized tomography angiography in preoperative assessment of intravenous leiomyomatosis extending to inferior vena cava and heart. BMC Cancer 2016;16:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Li B, Chen X, Chu YD, Li RY, Li WD, Ni YM. Intracardiac leiomyomatosis: a comprehensive analysis of 194 cases. Interact Cardiovasc Thorac Surg 2013;17:132–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rispoli P, Santovito D, Tallia C, Varetto G, Conforti M, Rinaldi M. A one-stage approach to the treatment of intravenous leiomyomatosis extending to the right heart. J Vasc Surg 2010;52:212–5. [DOI] [PubMed] [Google Scholar]
- 14. Deng Y, Song B. Three Case Reports of Intravenous Leiomyomatosis with Intracardiac Extensions. Thorac Cardiovasc Surg Rep 2020;9:e40–3. [DOI] [PMC free article] [PubMed] [Google Scholar]


