Abstract
The ongoing COVID-19 pandemic made us re-realize the importance of environmental disinfection and sanitation in indoor areas, hospitals, and clinical rooms. UVC irradiation of high energy and short wavelengths, especially in the 200–290-nm range possesses the great potential for germicidal disinfection. These properties of UVC allow to damage or destruct the nucleic acids (DNA/RNA) in diverse microbes (e.g., bacteria, fungi, and viruses). UVC light can hence be used as a promising tool for prevention and control of their infection or transmission. The present review offers insights into the historical perspective, mode of action, and recent advancements in the application of UVC-based antiviral therapy against coronaviruses (including SARS CoV-2). Moreover, the application of UVC lights in the sanitization of healthcare units, public places, medical instruments, respirators, and personal protective equipment (PPE) is also discussed. This article, therefore, is expected to deliver a new path for the developments of UVC-based viricidal approach.
Keywords: Coronaviruses, COVID-19, Disinfection, Irradiation, Photo-inactivation, UVC germicidal activity
Graphical abstract
1. Introduction
Virus transmission between individuals typically occurs through contaminated media (including air, water, and food) and inanimate surfaces called fomites (Kutter et al., 2018; Stephens et al., 2019). Airborne and water-borne pathogenic viruses are among the most important global risks faced by mankind. Such viruses can cause disease in human, animal, and plant systems (Artika et al., 2020; Matthews, 2019). These viruses have been the foremost players in causing the deadliest pandemics, as evidenced throughout history. Several viruses, including influenza, Ebola, Hepatitis, human immunodeficiency virus (HIV), and coronaviruses, have contributed to the development of dangerous disease outbreaks (CDCP, 2009; Jakhesara et al., 2014; Spengler et al., 2016). For the effective control of a pandemic, it is desirable to gain detailed information in various respects (e.g., the virus structure, the mechanism of infection in the host organism, and its epidemiology).
Inactivation of the viruses is one of the safest goals to prevent the spread of infections. Such inactivation makes viruses incapable of infection/multiplication by either altering their structural core (DNA/RNA) or by denaturing viral proteins (capsid) in the viral assembly (Guo et al., 2018; Majiya et al., 2018; Zhang et al., 2019a). Broadly, virus inactivation techniques are classified into physical and chemical methods. The former can be further divided into two types: thermal treatment (including pasteurization and dry heating) and non-thermal treatment (such as application of high pressure). The latter includes virus disinfection using detergents, solvents, acidic pH, ethanol, and sodium hypochlorite (WHO, 2004; Zhang et al., 2019b). Recently, the application of non-thermal plasma for viral inactivation is gaining attraction as a chemical-free technique (Pradeep and Chulkyoon, 2016; Xia et al., 2019). The inactivation or disinfection technique largely depends upon the virus composition, structure, and biological function as a better knowledge of these parameters can facilitate virus disinfection at the primary level (WHO, 2004; Wigginton and Kohn, 2012). For example, virus composition and structure data can help predict the reactivity of viral components treated with disinfectants. Moreover, the information about the biological function of viral domains, including virus-host cell interaction, virus assembly, etc. can play an important role in the identification of regions that should be targeted during virus inactivation strategies.
In recent years, several novel approaches have been developed for virus inactivation. These include light-based inactivation (UV/gamma rays), ozone gas treatment, and chemical disinfection using iodine, H2O2, cold plasma, etc. (Feng et al., 2011; Filipić et al., 2020; Hadi et al., 2020; Sunnen, 2003; Wolf et al., 2018). Ultra-violet (particularly UVC) irradiation has particularly attracted the researcher's attention as one of the most effective anti-viral strategies. UV light is capable of destroying a broad range of microbes, including bacteria, fungi, yeasts, and viruses. UV light has many other diverse applications (e.g., water disinfection, food sterilization, and surface decontamination) (Caillet-Fauquet et al., 2004; Dai et al., 2012; Kowalski, 2010; Narita et al., 2020; Weiss and Horzinek, 1986). UVC rays can disintegrate the genetic material (DNA/RNA) of microbes by dimerizing the pyrimidines (particularly thymine/uracil) present in the nucleic acids (as discussed further in Section 2.1) (Dai et al., 2012). With respect to viral inactivation, UV irradiation provides several advantages such as broader virus inactivation, manageable costs, and practical applicability. As such, UV irradiation is also applicable as a supplement to the existing techniques.
Across the complete UV spectrum, UVC wavelength at around 260 nm is the most effective for germicidal applications toward harmful viruses in the air, water, and on any kind of environmental surfaces. Likewise, low-pressure mercury vapor lamps also have a strong emission at 254 nm (UV254) with a strong disinfection effect (Daryany et al., 2009; de Roda Husman et al., 2004). However, UVC radiation from the pulsed xenon lamps (around a 230-nm wavelength) can provide more instantaneous energy than conventional mercury lamps. Conventional mercury lamps used for UV emissions are now being replaced by UVC-LED, which has higher virus inactivation efficiency and germicidal wavelengths of 269–276 nm (Kim and Kang, 2020; Kim and Kang, 2018). The most important factors that must be considered before using UV irradiation are UV dose uniformity with respect to time and area, UV flow rate, and the nature of the material being treated (Araud et al., 2020; Welch et al., 2018; WHO, 2004). In the natural environment, viruses may be inactivated (including coronaviruses) simply after sunlight exposure (Fujioka and Yoneyama, 2002; Ratnesar-Shumate et al., 2020; Sagripanti and Lytle, 2020; Silverman et al., 2013). In contrast, ultraviolet germicidal irradiation (UVGI/UVC) can be employed for virus inactivation in public environments, such as healthcare settings, dentistry offices, and hospitals (Lindblad et al., 2019; McDevitt et al., 2008; Tseng and Li, 2007). The use of UVC light has already been accepted for use in water disinfection, medical sanitation, and the generation of sterile conditions. UVGI can be used to disinfect surfaces, air, rooms, and even liquids. It is preferred over heat sterilization and chemical disinfectants. However, the practical use of UV light in public settings has been quite limited, because UV light may pose hazards to human health as a carcinogen (Löfgren, 2017; Schulman and Fisher, 2009).
At present, one of the most desirable applications of UV irradiation appears to be the inactivation of coronaviruses. Coronaviruses are positive-directional and enveloped single-stranded RNA (30–35 kb) viruses that belong to the family Coronaviridae (Ortiz-Prado et al., 2020; Weiss and Leibowitz, 2011). Coronaviruses cause different diseases in humans and animals (Chen et al., 2020). The transmission of coronaviruses through contaminated surfaces and aerosols has proven to be of great significance in the outbreaks of severe acute respiratory syndrome (SARS) coronavirus, middle east respiratory syndrome (MERS) coronavirus, and the severe acute respiratory syndrome coronavirus 2 (SARS CoV-2) pandemic (Acter et al., 2020; Bradley and Bryan, 2019; Harapan et al., 2020; Liu et al., 2020a). Recently, the outbreak of coronavirus disease 2019 (COVID-19) has affected the entire world by causing nearly 3.5 million deaths worldwide. The outbreak of COVID-19, which is caused by a novel SARS CoV-2 strain, was declared to be a global pandemic by the WHO in early March 2020. Human to human transmission of SARS CoV-2 has been identified in hospitals, healthcare units, and personal households via respiratory droplets that are released during speaking, coughing, and sneezing. SARS CoV-2 has a high transmission rate, high reproduction number, and large incubation period. Therefore, the most urgent issue in controlling COVID-19 has been to prevent further infection in public places (Ortiz-Prado et al., 2020; Shereen et al., 2020).
UVC light exposure can be used as a highly effective direct anti-viral approach against coronaviruses. To combat viral spread, UVC (290–200 nm) radiation can be effectively employed to decontaminate surfaces infected with coronaviruses. Prior studies have already evaluated the use of UVC light to inactivate enteric viruses, polioviruses, and noroviruses (Bosshard et al., 2013; Jean et al., 2011). In the last few decades, several reports have addressed the inactivation of coronavirus strains using UVC light. However, relatively little is known about the UVC sensitivity of the novel Cov-2 strain. All human coronaviruses have similar genomic sizes. Therefore, the CoV-2 strain is thought to be susceptible to UVC light just as are other coronaviruses. The present article describes the application of UVC light as an effective treatment option against coronaviruses, including the novel SARS CoV-2 virus. The motive behind this work is to estimate the SARS CoV-2 sensitivity toward UVC radiation and to identify the optimal wavelength to inactivate the SARS CoV-2 strain. We have compiled and reviewed the prior studies regarding the UVC-based inactivation of coronavirus (CoV). These results can be used in a primary database for researchers and scientists to develop an effective anti-viral strategy against the ongoing pandemic.
2. UVC irradiation
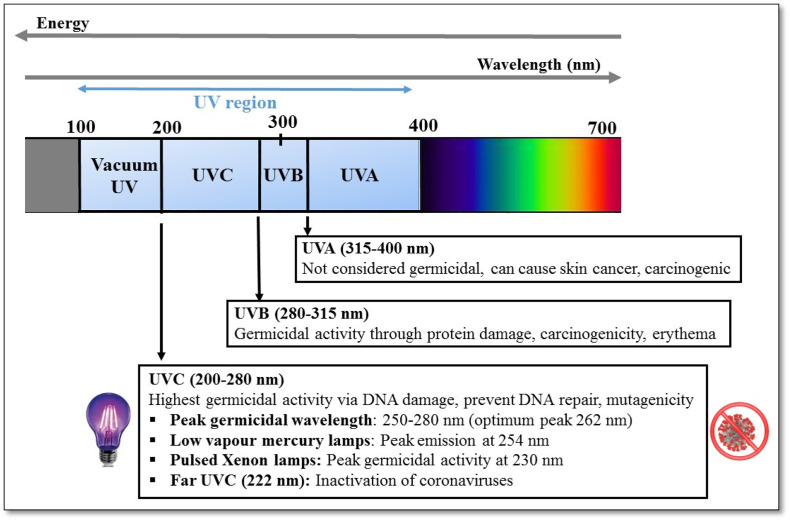
Ultraviolet (UV) rays make up a region of the electromagnetic spectrum (100–400 nm) that occurs between the extreme of the visible region and the X-ray bands. The UV region can be systematically divided into three different spectral regions according to their wavelength and energy: UVA region (315–400 nm), UVB region (280–315 nm), and UVC region (100–280 nm). Electromagnetic spectra for the three UV spectral regions are presented in Fig. 1 .
Fig. 1.
A diagram showing the ultra-violet (UV) spectrum (100–400 nm), the corresponding wavelength ranges, their germicidal activity, and hazards for human health.
2.1. Hazardous effects of UVC radiation on human health
Ultraviolet radiation makes up just 8% of the total solar radiation. The amount of UV radiations reaching the Earth's surface can vary widely around the globe with time. Natural UV exposure from sunlight depends upon many environmental factors, as follows: the position and height of the sun, aerosols, latitude, altitude, cloud coverage, haze formation, and the reflection of sunlight from surfaces (Turner et al., 2020). The natural UV forms a small proportion of sunlight although it is generally known to be strong enough to cause skin defects in humans. At the same time, many people are also exposed to artificial UV sources such as vapor lamps, halogen, and fluorescent lamps, tanning beds in the industry, commerce, and recreation.
UV rays (particularly UVB) present in the sunlight can stimulate the body to produce vitamin D3, which is essential for bone health and normal metabolism (Wacker and Holick, 2013). In addition, moderate UV exposure in humans have beneficial effects, such as regulating endorphin levels; providing protection against sclerosis and melatonin biosynthesis; maintaining normal body rhythm; preventing many seasonal disorders; and reducing the risk of skin diseases such as scleroderma and dermatitis (Juzeniene and Moan, 2012; Kreuter et al., 2006). However, the over-exposure of humans to UVC light can also pose certain adverse implications for individuals and public health. The evidence of damage caused by UV light overexposure to humans has been investigated in some reports and documents (MacKie, 2000; SCHEER, 2017; Schulman and Fisher, 2009; Tenkate et al., 2019; WHO, 1994).
UVC radiation is a known cause of skin cancer, skin aging, and eye damage with a potential to affect the immune system. Human eyes are particularly sensitive to UVC light. Prolonged UVC exposure can cause temporary damage to the eyes and even corneal injuries (Welch et al., 2018; Young, 2006). Examples of different eye disorders resulting from high UV exposure are flash burns, ground-glass eyeballs, welder's flash, and snow blindness depending upon the source of the UV light. Exposure to UVC light is common during welding operations at construction sites and workplaces. Ocular hazards experienced by welders include photo-phthalmia (welder's flash), conjunctivitis, photokeratitis, cataracts, and eye melanoma if exposed to UVR emitted from welding arcs.
There is a line of evidence that chronic exposure to high-intensity UV light can lead to the development of age-related macular degeneration of the retina and cortical cataracts (SCHEER, 2017; WHO, 2003). Apart from causing destructive effects on the eyes, short-term UVC exposure can pose high damage to human skin (e.g., skin burns, erythema (reddening of the skin), swelling, and skin darkening). On the other hand, prolonged and long-term exposure to UV light can induce skin cancers such as basal cell carcinomas, squamous cell carcinomas, and even malignant melanoma (Liu-Smith et al., 2017; Narayanan et al., 2010; Pfeifer and Besaratinia, 2012).
There are certain guidelines framed by the World Health Organisation (WHO) or Occupational Safety and Health Association (OSHA) related to the environmental safety and health aspects of UVC radiation. To protect the humans from harmful effects of UV light, ISO 15858, 2016 guidelines specify minimum human safety requirements for the use of UVC lamp devices with the maximum limit of exposure limit value (ELV) as 60 J/m2 at 254 nm for 8 h in a day. Note that this guideline complies of the ones recommended by the National Institute of Occupational Safety and Health (NIOSH). The European Commission health agency has framed several guidelines regarding the biological effects of UV-A, B, and C used in the cosmetics industry. In addition, the European Commission (Directive 2006/25/EC) has set the exposure limit of UVC irradiation for workers to be 30 J m−2 as ELV for 8 h a day. The “American Conference of Governmental Industrial Hygienists” (ACGIH) has issued guidelines to protect the skin and eyes from UVC exposure by setting certain limits. The threshold value for UVC (UV254) exposure cannot exceed 6 mJ cm−2 for 8 h, while the upper limit of UVC irradiation is set at 0.2 μW cm−2 (Nardell et al., 2008). As discussed above, UVC light can be a potential health hazard due to its destructive and carcinogenic effects. Consequently, the application of UVC light in public settings is a highly controversial issue.
2.2. Effectiveness of UVC on virus inactivation
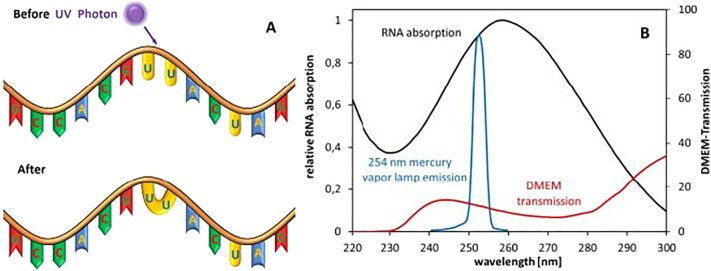
Among the three UV spectral regions, the UVC bandwidth exhibits high potential in inactivating microorganisms due to its strong antiviral properties (Kowalski, 2010; Reed, 2010; Yin et al., 2013). The main mechanism behind UVC toxicity to microbes lies in its ability to damage the genetic material present in the cell nucleus. The wavelength range of 250–270 nm is considered to be one of the most lethal ranges because its energy is intensely absorbed by nucleic acids (DNA/RNA) in microbial cells and viruses (Gurzadyan et al., 1995; Wang et al., 2019). DNA/RNA usually absorbs wavelengths ranging from 200 to 300 nm, with a peak absorbance at 260 nm. Genetic damage occurs due to the photo-dimerization (formation of dimers) between the pyrimidine nucleotide molecules (uracil dimers) in the DNA/RNA strands (Fig. 2 ). Subsequently, the formation of cyclobutane pyrimidine dimers (CPDs) results in DNA disassembly and ultimately disrupts cellular replication and other cellular functions (Chevremont et al., 2012a; Chevremont et al., 2012b). The prevention of cell replication may lead to cell death and prevention of viral reproduction/infection (González-Ramírez et al., 2011; Mouret et al., 2006). Further, the UVC light denatures enzymes that are required during DNA repair via a photo-reactivation mechanism (Horikawa et al., 2013). Thus, the UVC induced photolysis in viruses produces different photoproducts (i.e. CPDs) and some other non-toxic byproducts. These mutagenic DNA lesions are the most common and abundant damage products caused by UV photolysis (Sinha and Häder, 2002).
Fig. 2.
Schematic of the RNA damage mechanism through the formation of a dimer with UVC light. Relative absorption spectra of RNA, relative emission spectrum of a low-pressure mercury vapor lamp, and transmission of a typical (Eagle) cell culture medium (Heßling et al., 2020).
Several research teams have discussed the application of UVC light against various animal and human viruses such as HIV, chikungunya virus, parvovirus, human enteric virus, human papilloma virus, SARS coronavirus, hemorrhagic fever virus, pancreas necrosis virus, and erythrovirus B-19 (Caillet-Fauquet et al., 2004; Eickmann et al., 2020; Keil et al., 2020; Kim et al., 2017; Marx et al., 1996; Mathew et al., 2018; Meyers et al., 2017; Øye and Rimstad, 2001; Sugawara et al., 2001). Also, UVC radiation has been reported to successfully inactivate non-enveloped viruses such as hepatitis A virus, feline calicivirus, porcine circovirus type 2 (PCV-2), and Senecavirus A (SVA) in biological samples such as platelets and animal plasma (Blázquez et al., 2019; Gravemann et al., 2018). The key factors that must be taken into consideration for virus inactivation with UVC irradiation are the wavelength, the UV dosage, and the virus inactivation factor (k) (summarized in Table 1 ). The UVC effectiveness varies across different viruses because they require different irradiation doses for complete inactivation of the microorganism. The UV sensitivity of viruses can be described by inactivation kinetics. Applying the first order Chick Watson model, virus disinfection mechanisms by UVC light can be expressed as follows (Hijnen et al., 2006):
where N/N0 indicates the ratio between the number of microbes (viruses) after and before UVC irradiation. K indicates the virus inactivation rate constant (cm2/mJ) to describe inactivation. UV dose (D) indicates the radiant exposure per unit area or fluence (mJ cm−2) at a particular wavelength (λ). The inactivation kinetics can be used to measure the anti-viral efficiency of the UVC irradiation process. Virus inactivation is usually defined in the terms of ‘log inactivation,’ which basically reflects the number of reductions expressed in the order of magnitude of virus concentration (Kane, 2018; Song et al., 2016). A log inactivation of 1 means that 90% of the desired viruses are inactivated, while a log inactivation of 2 implies that 99% of viruses are inactivated. Similarly, a log inactivation of 3 means that 99.9% of viruses are inactivated by UVC irradiation. A higher value of k displays the increased sensitivity of the virus at a particular wavelength. For example, a high value of k (0.045 cm2/mJ) was obtained with 260-nm UVC LEDs for human adenovirus C (Beck et al., 2017). In another report, k of MS2 was measured as 0.066 cm2/mJ in 260-nm UVC LEDs, while a combination of UVC LEDs of 260 and 280 nm yielded a k value of 0.61 cm2/mJ (Rattanakul et al., 2014).
Table 1.
Various parameters influencing the inactivation of a virus in a UVC irradiated process.
| Order | Factor affecting virus inactivation | Characteristics/Remarks | References |
|---|---|---|---|
| 1. | UVC sources and wavelength |
|
(Barnard et al., 2020; Buonanno et al., 2020; Eickmann et al., 2020; Kitagawa et al., 2021; Welch et al., 2018) |
| 2. | Applied UVC dose (E) |
|
(Chevrefils et al., 2006; Heßling et al., 2020; Malayeri et al., 2016) |
| 3. | Inactivation rate constant (k) |
|
(Rattanakul and Oguma, 2018; Rattanakul et al., 2014) |
| 4. | Inherent viral characteristics |
|
(Raeiszadeh and Adeli, 2020; Wang et al., 2020) |
The UV wavelength is also considered to be the most influential factor in anti-viral disinfection in the UVC irradiation process since the nucleic acids (DNA) have the largest absorbance at 262 nm and strong inactivation efficiency between 250 and 280 nm. Studies have confirmed that a wavelength of 254 nm is considered the most effective for the maximum germicidal activity for viruses (Bolton and Cotton, 2011). Also, other UV wavelengths such as 255 nm, 275 nm, and 280 nm have been reported for the inactivation of viruses (Bowker et al., 2011; Rattanakul and Oguma, 2018).
The efficacy of shorter wavelengths (UVC255) has also been assessed for virus inactivation relative to longer wavelengths (UVC280) on bacteriophages. Currently, far UVC wavelengths (222 nm) are considered the most effective for inactivating air-borne viruses, including coronaviruses. The far-UVC light being strongly absorbed by the proteins in peptide bonds generally show limited penetration depth in biological materials as compared to conventional UVGI. However, this limited penetration is sufficient enough (in terms of depth) to inactivate the viruses and bacteria which are still smaller than the penetration size. Hence, far-UVC light is found to be as efficient in killing the pathogens as conventional germicidal UV light. Far-UVC is considered to be safer than other UVC ranges and is therefore unlikely to cause any harm to eyes and skin as well (Buonanno et al., 2020; Welch et al., 2018). It was reported that 222-nm far-UVC light (between 1.7 and 1.2 mJ/cm2) could inactivate 99.9% of aerosolized human coronaviruses such as alpha HCoV-229E and beta HCoV (Buonanno et al., 2020). The effect of far-UVC light was also tested in public places at the exposure limit of ~3 mJ/cm2/h to show 90% viral inactivation in 8 min and 99.9% inactivation in ~25 min. Recently, far UVC was reported to result in 88.5 and 99.7% reduction of viable SARS-CoV-2 based on the TCID50 assay for 10 and 30 s, respectively (Kitagawa et al., 2021).
The UV dose is also a significant factor in UVC irradiation-based virus inactivation. The UV dose (E) is determined by the radiant energy falling per unit area. It can be calculated as the product of UV radiant flux (I) and contact time (Bolton et al., 2015; Wigginton et al., 2012). The UV radiant flux (radiant power) is the radiant energy passing in a particular unit of time (t). The equation for the UV dose can be expressed as follows:
A large value of radiant flux will generate a higher UV dose that will provide a more efficient virus inactivation process. The optimal UV dose range for 4 log inactivation (99.99% reduction) varies with the type of virus (LeChevallier and Au, 2004). For example, rotaviruses need approx. 25 mJ/cm2 dose of UVC (254 nm: mercury lamp) for a log inactivation of 3 (Kowalski, 2010). In contrast, for the same amount of log inactivation, adenoviruses require 140 mJ/cm2 of UVC irradiation (Malayeri et al., 2016). The relationship between the incremental log inactivation of various pathogens (including viruses) and UV irradiation doses using different UVC sources was also studied (Chevrefils et al., 2006).
3. Importance of UVC during COVID-19 pandemic
UVC exposure is a direct anti-viral approach with proven efficiency against various airborne and other viruses (Zhang et al., 2019a). The importance of UVC radiation in the current pandemic is therefore recognized because UVC irradiation has already been used for the prevention of airborne virus transmission and infection (Budowsky et al., 1981; Hijnen et al., 2006). Based on earlier studies regarding the inactivation of coronaviruses using UVC, it is possible to predict the reactivity and susceptibility of the SARS CoV-2 virus to UVC irradiation (Blázquez et al., 2019; Heßling et al., 2020; Shirbandi et al., 2020). However, little is known regarding the UVC dose required to inactivate SARS CoV-2. This section is therefore organized to emphasize the efficiency of UVC light against coronavirus transmission and to establish a primary database for its effectiveness against SARS CoV-2.
3.1. COVID-19 pandemic
COVID-19 is an ongoing infectious disease outbreak that was declared a global pandemic by the WHO in March 2020 (Sohrabi et al., 2020). The pandemic has caused more than 3.5 million deaths and more than 169 million confirmed cases in over 200 countries since its first report in Wuhan, China in December 2019 (WHO Coronavirus dashboard accessed on 28 May 2021). The causative agent of COVID-19 is an enveloped positive-sense RNA virus (SARS CoV-2) that belongs to the coronavirus family. Among its diverse family, several coronaviruses have previously caused pandemics. These include severe acute respiratory syndrome coronavirus (SARS CoV-1) in 2003, and the Middle East respiratory syndrome coronavirus (MERS CoV) in 2012 (Lu et al., 2020; Oboho et al., 2015; Zhong et al., 2003). The SARS CoV-2 virus transmits between individuals in close contact through respiratory droplets that are produced through talking, coughing, and sneezing, or through contaminated surfaces/aerosols (Lai et al., 2020; Van Doremalen et al., 2020). Recently, the WHO reported that there is a high probability of airborne transmission of SARS CoV-2 through fine aerosol forms, which can be suspended in the air for a long period of time and over large distances (Morawska and Cao, 2020).
The average incubation period of COVID-19 is approximately 2–12 days (Guo et al., 2020; Li et al., 2020). Since the basic R0 number of SARS CoV-2 is between 2.4 and 3.3. On average, a person can infect 2–3 other people after the onset of infection (Kucharski et al., 2020; Liu et al., 2020b). COVID-19 causes flu-like symptoms (fever, cough, throat pain, etc.), mild respiratory disorders, and lethargy in humans. Its genomic structure is quite similar to all other human coronaviruses, which includes viral RNA that is enclosed by crown-like glycoprotein spikes on its surface (McIntosh et al., 2020). Although only a few vaccines for COVID-19 are available, their efficacy is not yet validated to the full extent. Moreover, the ongoing mutations in the COVID-19 virus further pose a challenge to the vaccination program. Therefore, it has become a highly global threat to humankind. The next section will provide a detailed review of reports to date regarding the inactivation and control of human coronaviruses using UVC irradiation.
3.2. UVC irradiation for coronavirus inactivation
In order to contain coronavirus multiplication and transmission in the environment, UVC has been suggested as one of the most cost-effective germicidal solutions for the ongoing COVID-19 pandemic (Derraik et al., 2020; Heimbuch and Harnish, 2019; Ianevski et al., 2020; Li et al., 2020). After the SARS outbreak in 2002–3, some studies focused on the germicidal activity of UV light against SARS CoV-1 (Ansaldi et al., 2004; Rabenau et al., 2005). Animal models of coronaviruses have also been studied to predict the susceptibility of human coronaviruses to UVC (Pratelli, 2008; Saknimit et al., 1988; Walker and Ko, 2007). The virucidal action of UVC irradiation has been explored with respect to the stability of human coronaviruses in cell cultures, aerosols, and even biological fluids. Besides the transmission of coronaviruses through direct contact and respiratory droplets, there is an increasing concerns on the transmission of SARS CoV-2 via water, wastewater, and aerosols. This section discusses the tests conducted on UVC sensitivity against three human coronaviruses (SARS CoV-1, MERS CoV, and SARS CoV-2), as summarized in Table 2 . The complete information on the relationship between applied UV dosage and the log inactivation of coronaviruses is also evaluated, along with the inactivation efficiency of UVC sterilization. Despite the lack of uniformity in the research methods for human coronavirus inactivation studies, the critical review of all prior reports are discussed and compared in the following sections.
Table 2.
A compilation of different published studies and reports regarding the photo-inactivation of coronaviruses using UVC irradiation.
| Order | UVC exposure wavelength | UVC light intensity/irradiance | Distance from UVC source/illumination | Inactivation time/conditions | Sample/media | Reference |
|---|---|---|---|---|---|---|
| (a) SARS CoV-1 | ||||||
| 1. | 260 nm | 90 μW cm−2 | 80 cm | 60 min | Vero-E6 cells | Dong, 2003 |
| 2. | 254 nm | 4016 μW cm−2 | 3 cm | 15 min | Vero-E6 cells | Darnell et al., 2004 |
| 3. | 254 nm | 4016 μW cm−2 | 3 cm | 40 min | Non-cellular blood products PBS solution, BSA protein solutions | Darnell and Taylor, 2006 |
| 4. | 254 nm | 200 mJ cm−2 | – | Log reduction factor of ≥3.1 | Platelets concentrates/ plasma | Eickmann et al., 2020 |
| 5. | 222 nm | 3 mJ cm−2 | 22 cm | 25 min (99.9% inactivation) | Aerosols | Buonanno et al., 2020 |
| (b) MERS CoV | ||||||
| 6. | 254 nm | – | 1.22 m | 5 min | Platelets concentrates/ plasma | Bedell et al., 2016 |
| 7. | 254 nm | 200 mJ cm−2 | – | Log reduction factor of ≥3.7 | Platelets concentrates/ plasma | Eickmann et al., 2018 |
| (c) SARS CoV-2 | ||||||
| 8. | 265 nm | 6.2 J/m2 | 75 × 45 × 50 cm UVC chamber | log reduction of ≥3.4 | Platelets concentrates/ plasma | Keil et al., 2020 |
| 9. | 280 ± 5 nm | 3.75 mW cm−2 | 2 cm | 1–60 s | Aerosols | Inagaki et al., 2020 |
| 10. | 254 nm | 70 mJ cm−2 (estimated) | – | 1 log inactivation (estimated) | Aerosols | Sagripanti and Lytle, 2020 |
| 11. | 254 nm | 3.7 mJ cm−2 | – | 3 log inactivation for low virus concentration. | Vero-E6 cells | Bianco et al., 2020 |
| 12. | 254 nm | 1940 mW cm−2 | 3 cm | Complete inactivation in 9 min | Liquid suspension | (Heilingloh et al., 2020) |
| 13. | 222 nm | 0.1 mW cm−2 | 24 cm | 2.51 log reduction (Undetectable levels) in 30 s | Liquid suspension | (Kitagawa et al., 2021) |
| 14. | 254 nm | 2.2 mW cm−2 | 30 cm | Inactivation, by lethal dose (viral inactivation 99.999%) | Liquid suspension | (Sabino et al., 2020) |
| 15. | PX-UV robot model PXUV4D | – | 1 m | 99.992% reduction in 5 min | Liquid suspension and dried samples | (Simmons et al., 2021) |
| 16. | 254 nm | 1.082 mW cm−2 | – | more than 3-log inactivation and inhibition of SARS CoV-2 replication | Vero-E6 cells | (Biasin et al., 2021) |
3.2.1. Severe acute respiratory syndrome coronavirus (SARS CoV-1)
SARS CoV-1 was the infectious agent responsible for the SARS outbreak in 2003. This disease was transmissible from person to person and causes clusters of disease in healthcare workers (Peiris et al., 2004; Zhong et al., 2003). Studies showed that UVC treatment of SARS CoV-1 in culture medium was able to eliminate the viral infectivity. The stability of the SARS CoV-1 strain subjected to UVC irradiation was investigated in Vero-E6 cell lines (Dong, 2003). When irradiated with UVC light, the virus-infected cells were inactivated within 60 min, and their viral infectivity reached an undetectable limit as tested by the cytopathic effect (CPE). A year later, another study reported the inactivation of SARS CoV-1 by employing UVC irradiation of 254 nm (Darnell et al., 2004). Inactivation of SARS CoV-1 was observed within 15 min of irradiation, while UVA irradiation was unable to show any effect on virus viability. In this study, a quick increase in viral inactivation was observed (15 min), which may be attributed to a high dosage due to close proximity (3 cm) of the UV light to the viral aliquots. The same group of researchers successfully examined the inactivation efficiency of UVC light on SARS CoV-1 in blood and plasma products (Darnell and Taylor, 2006). This group found that the UVC treatment could inactivate SARS CoV-1 to the limit of detection (TCID50 at 1 log/ml) after 40 min of exposure in PBS solution. Recently, a group of researchers conducted a study on the UVC based inactivation of emerging SARS CoV viruses in plasma concentrates (Eickmann et al., 2020). The plasma concentrates spiked with viruses were exposed to UVC irradiation. The TCID50 assay was used to assess viral infectivity before and after UVC treatment. The infectivity assays suggested that a UVC dose of even 100 mJ cm−2 was able to inactivate SARS CoV-1 to an undetectable level. A similar experiment was performed to demonstrate the treatment efficiency of blood plasma and platelets by employing UVC light in combination with methylene blue (Eickmann et al., 2020). The results indicated 3.4 log reduction of SARS CoV (in plasma concentrates) at UVC dose of 200 mJ cm−2. Accordingly, UVC treatment was demonstrated as an effective option for reducing virus infectivity in blood products.
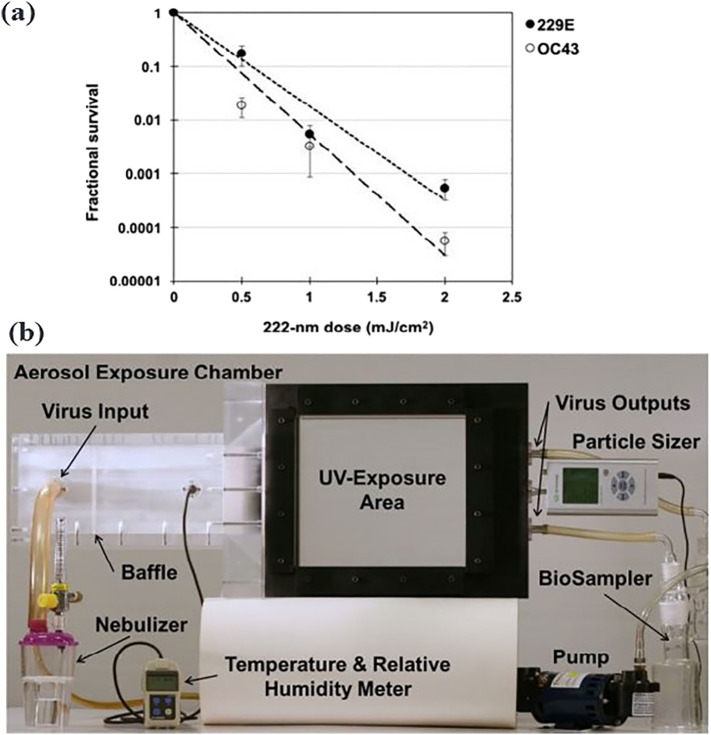
Far UVC light (wavelength range 207–222 nm) commonly produced by excimer lamps has also gained attention in the place of conventional 254-nm UVC light (Narita et al., 2020). One of the most important characteristics of far UVC light is its viral disinfection without causing any harm to human tissues, such as mammalian skin (Barnard et al., 2020; Buonanno et al., 2017; Buonanno et al., 2013). Far UVC light (222 nm) has been shown to produce 95% inactivation of aerosolized influenza virus (H1N1) at a low dose of just 2 mJ cm−2 (Welch et al., 2018). Recently, far UVC light was also studied for its activity against two airborne coronaviruses strains of SARS CoV-1 (HCoV-229E and HCoV-OC43) (Buonanno et al., 2020). The viral infectivity was measured using a 50% tissue culture infectious dose assay TCID50 assay. A low UV intensity of 3 mJ cm−2 resulted in 1 log inactivation in 8 min, 2 log inactivation in 16 min, and 3 log inactivation in 25 min (Fig. 3a). Far-UVC light-based fiber optics have been proposed in the direct and selective treatment of COVID-19 patients, while far-UVC excimer lamps replace conventional overhead lamps (Haider et al., 2020). Therefore, UVC irradiation treatment may be a tool to treat aerosols and blood-related products (e.g. plasma concentrates, plasma, etc.). Still, the effect of far-UVC on human health is not fully elucidated.
Fig. 3.
A chamber system built for UV irradiation testing of the survival of coronaviruses: (a) The survival of two coronaviruses HCoV-229E and HCoV-OC43 as a function of far-UVC (222 nm) dose. The inactivation rate constant (k) was found to be 4.1 cm2/mJ and 5.9 cm2/mJ for both strains, respectively (Buonanno et al., 2020). (b) Photograph of the custom UV irradiation chamber (Welch et al., 2018).
3.2.2. Middle east respiratory syndrome coronavirus (MERS CoV)
UVC treatment was also used to treat surfaces to inactivate MERS coronaviruses. For example, one study proposed using UVC radiation to disinfect entire rooms (Bedell et al., 2016). The disinfection setup consisted of a sensor controlling three UVC emitters in a biosafety level-3 (BSL-3) facility. The virus samples (MERS CoV) were loaded on glass slips and then exposed to UVC. Next, their infectivity was evaluated by incubating the slips in Vero E6 cell lines. UVC treatment for only 5 min resulted in 2.71 log inactivation of the virus, with a 6 log inactivation after 30 min (Bedell et al., 2016). UVC irradiation may also be used as an effective strategy to disinfect blood-related products (such as plasma, plasma concentrates, and platelets units) to prevent virus transmission during blood transfusion (Terpstra et al., 2008). In this context, the inactivation of MERS CoV was described by varying doses of UVC (using THERAFLEX UV-Platelets system) (Eickmann et al., 2018). The platelet concentrates were spiked with MERS CoV titers extracted from cell cultures. According to the infectivity assays (TCID50), MERS CoV was inactivated using UVC irradiation in a dose-dependent relationship. A UV dosage of 150 mJ cm−2 reduced the infectivity level of the virus to a non-detectable range. The high virus inactivation constant (k) (> 3.7) obtained after the assay indicated that the radiation was sufficient to lower the risk of infection through blood products.
3.2.3. Severe acute respiratory syndrome coronavirus (SARS CoV-2)
The novel SARS CoV-2 has caused a deadly pandemic in 2020 (Guo et al., 2020; Harapan et al., 2020). In some recent studies, the high susceptibility of the SARS CoV-2 virus to UVC light has been demonstrated (Heilingloh et al., 2020; Simmons et al., 2021). Efforts have also been made to inactivate SARS CoV-2 in the blood (plasma/platelet units) by means of UVC irradiation and riboflavin treatment (Keil et al., 2020). SARS CoV-2 cultured in monolayer Vero-E6 cell lines were inoculated into plasma/platelets units. The plasma or platelets units were suspended in illumination (storage bags) and mixed with riboflavin solution to make a final product. After the units were spiked with the virus, they were subjected to UVC treatment using a Mirasol illuminator under biosafety level-3. The infectious titers of SARS CoV-2 were determined using a plaque assay of Vero-E6 cells. The UVC dose resulted in ≥3.4 log reduction of the average viral titer of 4.62 PFU/ml. A similar setup consisting of deep-UV LEDs (280 ± 5 nm) was displayed for SARS CoV-2 inactivation in aerosols and contaminated surfaces (Inagaki et al., 2020). The viral aliquots containing virus-infected Vero-E6 cell lines were irradiated from a short distance of 2 cm, after which the infectivity assay was determined using CPE. These authors were able to achieve 87.4% of SARS CoV-2 inactivation within 1 s, while 3 log inactivation was achieved after 10 s of UV exposure. (Inagaki et al., 2020). In a separate set of studies, the UVC sensitivity of the novel SARS CoV-2 strain was evaluated (Sagripanti and Lytle, 2020). The UVC254 sensitivity and genomic characteristics of SARS CoV-2 were compared with that of other ssRNA coronaviruses (e.g. SARS CoV-1 and MERS CoV) and the influenza virus. The virus survival of 37% (D37s) was calculated during testing. Therefore, a UVC fluence of 70 mJ cm−2 was estimated for SARS CoV-2 inactivation corresponding to 1 log inactivation.
The potential effect of UVC radiation on the viricidal properties of SARS CoV-2 was tested using different irradiation doses and viral concentrations (Bianco et al., 2020). UVC was generated using low vapor mercury lamps for uniform illumination. Three concentrations of SARS CoV-2 were prepared. Their viability post UVC exposure was determined using RT-PCR and CPE. The UVC treatment inhibited viral replication at lower concentrations of virus (multiplicity of infection - 0.5) for the first two days. However, the complete inhibition of replication was observed after 6 days at a dose of 3.7 mJ cm−2. The virus inhibition was observed as a function of both UVC intensity and viral concentration (Bianco et al., 2020).
Among all studies of human coronavirus inactivation, log inactivation studies have been considered to be important tools for measurements of viral infectivity. Many studies on UVC treatment for SARS CoV-2 have achieved log inactivation constants of greater than 3 (Bianco et al., 2020; Keil et al., 2020). It may be inferred that the existing UVC disinfection methods and procedures should be sufficient to inactivate human coronaviruses, including SARS-CoV-2. The exposure of UVC to humans limits its practical applications, while there is a need for more research on far UVC for future applications.
3.3. Disinfection of N95 respiratory masks and clinical settings
A global pandemic such as COVID-19 poses an extensive concern for community and medical health. Healthcare workers are at the largest risk of infection given their particular exposure. COVID-19 response measures include the disinfection of filtering facemasks respirators (FFRs). These FFRs can filter the viral burden in air droplets, and prevent viral transmission. Numerous techniques, such as the use of heat, steam, microwaves, chemicals (e.g., H2O2 and bleach), and gases, have been evaluated for respiratory mask decontamination (Lindsley et al., 2015). Accordingly, the UVC disinfection of respiratory masks and face pieces has been considered to be the best studied and effective method for germicidal control. Several studies have addressed the germicidal properties of UVC radiation in the decontamination of N95 respirators (EloiseáTorres, 2020; Fisher and Shaffer, 2011; Hamzavi et al., 2020). For instance, a UVC dose of 1 J cm−2 was enough to eliminate six strains of viruses, including SARS CoV and MERS CoV, from facepiece respirators (Heimbuch and Harnish, 2020). Likewise, the complete decontamination of N95 respirators infected with influenza H1N1 virus was achieved at a UVC254 dose of nearly 1 J cm−2 (Fisher and Shaffer, 2011; Mills et al., 2018). It is important to stress that a minimum UVC dose of 1 J cm−2 is required for the decontamination of respirators to ensure the safety of healthcare workers (Narla et al., 2020).
It is important to use valid dosimetry not only to provide effective decontamination but also to prevent any impairment in the mask's efficacy and safety (Liao et al., 2020; O'Hearn et al., 2020). High and inappropriate doses of UVC irradiation were reported to lower the efficiency and structural integrity of face pieces and N95 respiratory masks (Huber et al., 2020). For example, the treatment of germicidal UVC (950 J cm−2) was able to increase virus penetration through the masks (Lindsley et al., 2015). In the same report, the UVC irradiation of 2360 J cm−2 reduced the breaking strength of materials (used in making respiratory masks) by approximately 51%. The maximum number of decontamination cycles for FFRs for reuse primarily depends on the model of the respiratory face piece and the UVC dose intensity to inactivate viruses (Lindsley et al., 2015). In addition to these studies, UVC irradiation has been used as a novel technology for inactivating airborne coronaviruses in healthcare places, hospitals, nursing homes, laboratories, and personal households (Botta et al., 2020; Gurzawska-Comis et al., 2020; Lindblad et al., 2019; Memarzadeh et al., 2010).
4. Perspectives and technology evaluation
In this review, a comprehensive survey was conducted to evaluate the scientific investigations made on the photo-inactivation of different coronaviruses using UVC radiation. Because the high energy of UVC radiation is strongly absorbed by organic molecules (including viral DNA/RNA), it has become an important tool in germicidal and disinfection applications. Recently, in a number of news reporting and scientific articles, the application of UVC technology has been recommended for the disinfection of coronaviruses (Table 3 ). Most studies investigated in this survey employed germicidal UVC254 radiation for virus inactivation because 254 nm radiation is near the absorption band of RNA present in ssRNA viral genomes such as coronaviruses. UVC radiation at 254 nm is able to inactivate all types of coronaviruses in almost all examined studies. However, exposure to UVC254 can also cause harm to mammalian skin and eyes. Therefore, its direct applications in indoor and outdoor environments are highly discouraged. Instead of conventional UVC light, far UVC (222 nm) is proving to be useful in coronavirus inactivation without causing any harm to human health. Most prior studies have employed a low intensity of UVC radiant energy (between 2 and 200 mJ cm−2) to efficiently inactivate coronaviruses. The determined upper limit for the log-reduction median dose for inactivating coronaviruses is estimated to be 10.6 mJ cm−2 (Heßling et al., 2020). These estimations are primarily based on studies of different coronaviruses, including SARS and MERS CoV. The ongoing COVID-19 pandemic and threat to FFRs safety have led to the development of UVC-based devices and instruments by certain manufacturers. Here, some of the commercialized devices and for disinfection of laboratories and FFRs are summarized in Table 4 . The disinfection of indoor places, including hospitals, healthcare rooms, and clinical settings, has been already achieved using such devices. However, the large-scale application of UVC-based filtration devices in various public domains (like airports and bus stations) is limited until operation cost and human health safety issues are convincingly addressed and resolved. Some commercial devices manufactured for UVC disinfection of coronaviruses in healthcare areas and FFRs are shown in Fig. 4 .
Table 3.
A summary of newspaper reports, articles and various independent researchers that tested the UVC inactivation of coronaviruses.
Table 4.
Commercial instruments and devices developed for UVC inactivation of coronaviruses during the COVID-19 pandemic.
| Order | Product | Developer | Product specifications | Source/company website |
|---|---|---|---|---|
| 1. | UVC disinfection chamber | Skytron technologies |
|
https://www.skytron.com/products/infection-prevention/uvc-light-disinfection-robots/ |
| 2. | THOR UVC™ | Finsen technologies |
|
https://www.finsentech.com/uvc-disinfection-robots |
| 3. | Connor UVC disinfection robot | RobotLAB technologies |
|
https://www.robotlab.com/store/connor-uvc-disinfection-robot |
| 4. | ChargeMax and UV-C Wand Sterilizer | Cetrix Technologies Ltd. |
|
https://www.cetrixtablets.com/coronavirus/ |
| 5. | DONTICS UVC towerTM | Dr. Ajay Bajaj, Bombay Dental, Mumbai |
|
https://in.dental-tribune.com/news/how-to-use-ultraviolet-light-uvc-to-fight-covid-19-effectively-in-dental-clinics-dr-ajay-bajaj/ |
| 6. | UVC disinfection robotTM | UVD robots technology |
|
http://www.uvd-robots.com/ |
| 7. | UVC Scanz Plus sanitizing machine | Eurotek Environmental Private Limited |
|
https://eurotekindia.com/ |
| 8. | Handsfree UVC decontamination device | UVC cleaning systems Inc. |
|
https://www.uvccleaningsystems.com/ |
| 9. | UV air sanitizers and germicidal UV lamps | Atlantic ultraviolet corporation |
|
https://ultraviolet.com/ |
| 10. | UV room disinfection system | ICROCHEM laboratories |
|
http://microchemlab.com/test/uv-room-disinfection-devices |
Fig. 4.
Pictures of several germicidal devices that are based on UVC radiation for deployment in indoor places and respiratory masks (a) A UVC-disinfection based trolley prototype developed by Mekins Industries, Hyderabad, India for the rapid disinfection of hospitals (b) UV-C device working developed for disinfection of rooms (Bentancor and Vidal, 2018) (c) A portable UVC disinfection lamp developed for daily sterilization of essential items, toys, electronics and small areas. (d) UV device developed by Daavlin International for decontaminating respirators and other essential items (Hamzavi et al., 2020).
5. Conclusion
We recognize the importance of response measures for the effective control of coronaviruses under the unprecedented situation of the COVID-19 pandemic. To treat diverse forms of coronaviruses, multiple strategies have been employed, such as dry heating, chemical disinfection, and microwave irradiation. Among such options, UVC-based irradiation has proven to be a potential agent against coronaviruses such as MERS CoV, SARS CoV-1, and SARS CoV-2. However, the application of UVC light for the routine disinfection of airborne viruses has technical challenges. Virus inactivation kinetics across different environmental conditions (such as temperature and humidity) have not been fully investigated. The UVC exposure doses must be quantified in relation to the virus inactivation kinetics. Moreover, the delivery of uniform UVC illumination doses over large volumes of air is a challenging task. UVC irradiation has been employed successfully to treat blood and plasma products to prevent virus transmission during a blood transfusion. Recently, far-UVC was explored as a potential alternative to conventional UVC germicidal wavelengths. The potential utility of UVC-based inactivation approaches has been validated in many case studies (including disinfection of surgical instruments, respiratory masks, and indoor environments like healthcare facilities and clinical settings). The utility of UVC has been gained attention for the disinfection of microbes in water systems and food products over the past few years. However, it has not been shown to be sufficient in the treatment of airborne viral transmission. Therefore, this technology requires more development and investigation before it is used widely. The main challenge with aerosols is the provision of sufficient UVC irradiation doses to disinfect large quantities of air under different environmental parameters such as temperature and relative humidity. The routine use of UVC for virus inactivation may eventually be used to target viruses such as SARS CoV-2.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
SKB and JB are thankful to DBT-CIAB (Department of Biotechnology-Centre of Innovative and Applied Bioprocessing). Neha Bhardwaj acknowledges DST (Department of Science and Technology) for the INSPIRE FACULTY grant (Reg. no. IFA18-LSPA 127) for this research. MK is thankful to Wellcome trust/DBT IA for their early career fellowship. KHK acknowledges support from a grant from the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT, & Future Planning (Grant No: 2016R1E1A1A01940995).
Editor: Ewa Korzeniewska
References
- Acter T., Uddin N., Das J., Akhter A., Choudhury T.R., Kim S. Evolution of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) as coronavirus disease 2019 (COVID-19) pandemic: a global health emergency. Sci. Total Environ. 2020:138996. doi: 10.1016/j.scitotenv.2020.138996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansaldi F., Banfi F., Morelli P., Valle L., Durando P., Sticchi L., et al. SARS-CoV, influenza A and syncitial respiratory virus resistance against common disinfectants and ultraviolet irradiation. J. Prev. Med. Hyg. 2004;45:5–8. [Google Scholar]
- Araud E., Fuzawa M., Shisler J.L., Li J., Nguyen T.H. UV inactivation of rotavirus and Tulane virus targets different components of the virions. Appl. Environ. Microbiol. 2020;86 doi: 10.1128/AEM.02436-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artika I.M., Wiyatno A., Ma’roef C.N. Pathogenic viruses: molecular detection and characterization. Infect. Genet. Evol. 2020;81 doi: 10.1016/j.meegid.2020.104215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnard I.R.M., Eadie E., Wood K. 2020. Far-UVC Wavelengths for Disinfection Are Unlikely to Harm Skin. [Google Scholar]
- Beck S.E., Ryu H., Boczek L.A., Cashdollar J.L., Jeanis K.M., Rosenblum J.S., et al. Evaluating UV-C LED disinfection performance and investigating potential dual-wavelength synergy. Water Res. 2017;109:207–216. doi: 10.1016/j.watres.2016.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedell K., Buchaklian A.H., Perlman S. Infection Control & Hospital Epidemiology. Vol. 37. 2016. Efficacy of an automated multiple emitter whole-room ultraviolet-C disinfection system against coronaviruses MHV and MERS-CoV; pp. 598–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentancor M., Vidal S. Programmable and low-cost ultraviolet room disinfection device. HardwareX. 2018;4 [Google Scholar]
- Bianco A., Biasin M., Pareschi G., Cavalleri A., Cavatorta C., Fenizia F., et al. Inactivating and Inhibiting SARS-CoV-2 Replication (June 5, 2020) 2020. UV-C irradiation is highly effective in inactivating and inhibiting SARS-CoV-2 replication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biasin M., Bianco A., Pareschi G., Cavalleri A., Cavatorta C., Fenizia C., et al. UV-C irradiation is highly effective in inactivating SARS-CoV-2 replication. Sci. Rep. 2021;11:1–7. doi: 10.1038/s41598-021-85425-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blázquez E., Rodríguez C., Ródenas J., Navarro N., Riquelme C., Rosell R., et al. Evaluation of the effectiveness of the SurePure Turbulator ultraviolet-C irradiation equipment on inactivation of different enveloped and non-enveloped viruses inoculated in commercially collected liquid animal plasma. PLoS One. 2019;14 doi: 10.1371/journal.pone.0212332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton J.R., Cotton C.A. American Water Works Association; 2011. The Ultraviolet Disinfection Handbook. [Google Scholar]
- Bolton J.R., Mayor-Smith I., Linden K.G. Rethinking the concepts of fluence (UV dose) and fluence rate: the importance of photon-based units–a systemic review. Photochem. Photobiol. 2015;91:1252–1262. doi: 10.1111/php.12512. [DOI] [PubMed] [Google Scholar]
- Bosshard F., Armand F., Hamelin R., Kohn T. Mechanisms of human adenovirus inactivation by sunlight and UVC light as examined by quantitative PCR and quantitative proteomics. Appl. Environ. Microbiol. 2013;79:1325–1332. doi: 10.1128/AEM.03457-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botta S.B., de Sá Teixeira F., Hanashiro F.S., de Araújo W.W.R., Cassoni A., da Silveira Salvadori M.C.B. Brazilian Dental Science. Vol. 23. 2020. Ultraviolet-C decontamination of a dental clinic setting: required amount of UV light; p. 10. [Google Scholar]
- Bowker C., Sain A., Shatalov M., Ducoste J. Microbial UV fluence-response assessment using a novel UV-LED collimated beam system. Water Res. 2011;45 doi: 10.1016/j.watres.2010.12.005. [DOI] [PubMed] [Google Scholar]
- Bradley BT, Bryan A. Emerging respiratory infections: the infectious disease pathology of SARS, MERS, pandemic influenza, and legionella. Semin. Diagn. Pathol.. 36. Elsevier, 2019, pp. 152–159. [DOI] [PMC free article] [PubMed]
- Budowsky E., Bresler S., Friedman E., Zheleznova N. Principles of selective inactivation of viral genome. Arch. Virol. 1981;68:239–247. doi: 10.1007/BF01314577. [DOI] [PubMed] [Google Scholar]
- Buonanno M., Randers-Pehrson G., Bigelow A.W., Trivedi S., Lowy F.D., Spotnitz H.M., et al. 207-nm UV light-a promising tool for safe low-cost reduction of surgical site infections. I: in vitro studies. PLoS One. 2013;8 doi: 10.1371/journal.pone.0076968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buonanno M., Ponnaiya B., Welch D., Stanislauskas M., Randers-Pehrson G., Smilenov L., et al. Germicidal efficacy and mammalian skin safety of 222-nm UV light. Radiat. Res. 2017;187:493–501. doi: 10.1667/RR0010CC.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buonanno M., Welch D., Shuryak I., Brenner D.J. 2020. Far-UVC Light Efficiently and Safely Inactivates Airborne Human Coronaviruses. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caillet-Fauquet P., Di Giambattista M., Draps M.-L., Sandras F., Branckaert T., De Launoit Y., et al. Continuous-flow UVC irradiation: a new, effective, protein activity-preserving system for inactivating bacteria and viruses, including erythrovirus B19. J. Virol. Methods. 2004;118:131–139. doi: 10.1016/j.jviromet.2004.02.002. [DOI] [PubMed] [Google Scholar]
- CDCP Centre for Disease Control and Prevention: outbreak of swine-origin influenza A (H1N1) virus infection-Mexico, March-April 2009. MMWR. Morb. Mortal. Wkly Rep. 2009;58:467. [PubMed] [Google Scholar]
- Chen Y., Liu Q., Guo D. Emerging coronaviruses: genome structure, replication, and pathogenesis. J. Med. Virol. 2020;92:418–423. doi: 10.1002/jmv.25681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevrefils G., Caron É., Wright H., Sakamoto G., Payment P., Barbeau B., et al. UV dose required to achieve incremental log inactivation of bacteria, protozoa and viruses. IUVA News. 2006;8:38–45. [Google Scholar]
- Chevremont A.-C., Farnet A.-M., Coulomb B., Boudenne J.-L. Effect of coupled UV-A and UV-C LEDs on both microbiological and chemical pollution of urban wastewaters. Sci. Total Environ. 2012;426:304–310. doi: 10.1016/j.scitotenv.2012.03.043. [DOI] [PubMed] [Google Scholar]
- Chevremont A.-C., Farnet A.-M., Sergent M., Coulomb B., Boudenne J.-L. Multivariate optimization of fecal bioindicator inactivation by coupling UV-A and UV-C LEDs. Desalination. 2012;285:219–225. [Google Scholar]
- Dai T., Vrahas M.S., Murray C.K., Hamblin M.R. Ultraviolet C irradiation: an alternative antimicrobial approach to localized infections? Expert Rev. Anti-Infect. Ther. 2012;10:185–195. doi: 10.1586/eri.11.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnell M.E., Taylor D.R. Evaluation of inactivation methods for severe acute respiratory syndrome coronavirus in noncellular blood products. Transfusion. 2006;46:1770–1777. doi: 10.1111/j.1537-2995.2006.00976.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnell M.E., Subbarao K., Feinstone S.M., Taylor D.R. Inactivation of the coronavirus that induces severe acute respiratory syndrome, SARS-CoV. J. Virol. Methods. 2004;121:85–91. doi: 10.1016/j.jviromet.2004.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daryany M.K.A., Hosseini S.M., Raie M., Fakharie J., Zareh A. Study on continuous (254 nm) and pulsed UV (266 and 355 nm) lights on BVD virus inactivation and its effects on biological properties of fetal bovine serum. J. Photochem. Photobiol. B Biol. 2009;94:120–124. doi: 10.1016/j.jphotobiol.2008.10.009. [DOI] [PubMed] [Google Scholar]
- Derraik J.G., Anderson W.A., Connelly E.A., Anderson Y.C. MedRxiv. 2020. Rapid evidence summary on SARS-CoV-2 survivorship and disinfection, and a reusable PPE protocol using a double-hit process. [Google Scholar]
- Dong J.H.X.-P. 2003. Stability of SARS Coronavirus in Human Specimens and Environment and its Sensitivity to Heating and UV Irradiation. [PubMed] [Google Scholar]
- Eickmann M., Gravemann U., Handke W., Tolksdorf F., Reichenberg S., Müller T.H., et al. Inactivation of Ebola virus and Middle East respiratory syndrome coronavirus in platelet concentrates and plasma by ultraviolet C light and methylene blue plus visible light, respectively. Transfusion. 2018;58:2202–2207. doi: 10.1111/trf.14652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickmann M., Gravemann U., Handke W., Tolksdorf F., Reichenberg S., Müller T.H., et al. Inactivation of three emerging viruses–severe acute respiratory syndrome coronavirus, Crimean–Congo haemorrhagic fever virus and Nipah virus–in platelet concentrates by ultraviolet C light and in plasma by methylene blue plus visible light. Vox Sang. 2020;115:146–151. doi: 10.1111/vox.12888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EloiseáTorres A. Ultraviolet-C and other methods of decontamination of filtering facepiece N-95 respirators during the COVID-19 pandemic. Photochem. Photobiol. Sci. 2020;19:746–751. doi: 10.1039/d0pp00131g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng K., Divers E., Ma Y., Li J. Inactivation of a human norovirus surrogate, human norovirus virus-like particles, and vesicular stomatitis virus by gamma irradiation. Appl. Environ. Microbiol. 2011;77:3507–3517. doi: 10.1128/AEM.00081-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filipić A., Gutierrez-Aguirre I., Primc G., Mozetič M., Dobnik D. Cold plasma, a new hope in the field of virus inactivation. Trends Biotechnol. 2020;11:1278–1291. doi: 10.1016/j.tibtech.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher E.M., Shaffer R.E. A method to determine the available UV-C dose for the decontamination of filtering facepiece respirators. J. Appl. Microbiol. 2011;110:287–295. doi: 10.1111/j.1365-2672.2010.04881.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujioka R., Yoneyama B. Sunlight inactivation of human enteric viruses and fecal bacteria. Water Sci. Technol. 2002;46:291–295. [PubMed] [Google Scholar]
- González-Ramírez I., Roca-Sanjuán D., Climent T., Serrano-Pérez J.J., Merchán M., Serrano-Andrés L. On the photoproduction of DNA/RNA cyclobutane pyrimidine dimers. Theor. Chem. Accounts. 2011;128:705–711. [Google Scholar]
- Gravemann U., Handke W., Lambrecht B., Schmidt J.P., Müller T.H., Seltsam A. Ultraviolet C light efficiently inactivates nonenveloped hepatitis A virus and feline calicivirus in platelet concentrates. Transfusion. 2018;58:2669–2674. doi: 10.1111/trf.14957. [DOI] [PubMed] [Google Scholar]
- Guo L., Xu R., Gou L., Liu Z., Zhao Y., Liu D., et al. Mechanism of virus inactivation by cold atmospheric-pressure plasma and plasma-activated water. Appl. Environ. Microbiol. 2018;84 doi: 10.1128/AEM.00726-18. (e00726-18) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y.-R., Cao Q.-D., Hong Z.-S., Tan Y.-Y., Chen S.-D., Jin H.-J., et al. Military Medical Research. Vol. 7. 2020. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak–an update on the status; pp. 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurzadyan G.G., Görner H., Schulte-Frohlinde D. Ultraviolet (193, 216 and 254 nm) photoinactivation of Escherichia coli strains with different repair deficiencies. Radiat. Res. 1995;141:244–251. [PubMed] [Google Scholar]
- Gurzawska-Comis K., Becker K., Brunello G., Gurzawska A., Schwarz F. Recommendations for dental care during COVID-19 pandemic. J. Clin. Med. 2020;9:1833. doi: 10.3390/jcm9061833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadi J., Dunowska M., Wu S., Brightwell G. Control measures for SARS-CoV-2: a review on light-based inactivation of single-stranded RNA viruses. Pathogens. 2020;9:737. doi: 10.3390/pathogens9090737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haider I., Ali A., Arifeen T., Hassan A.S. 2020. Far UV-C Lights and Fiber Optics Induced and Selective Far UV-C Treatment Against COVID-19 for Fatality-Survival Tradeoff. [Google Scholar]
- Hamzavi I.H., Lyons A.B., Kohli I., Narla S., Parks-Miller A., Gelfand J.M., et al. Ultraviolet germicidal irradiation: possible method for respirator disinfection to facilitate reuse during COVID-19 pandemic. J. Am. Acad. Dermatol. 2020;82:1511–1512. doi: 10.1016/j.jaad.2020.03.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harapan H., Itoh N., Yufika A., Winardi W., Keam S., Te H., et al. Coronavirus disease 2019 (COVID-19): a literature review. J. Infect. Public Health. 2020;13:667–673. doi: 10.1016/j.jiph.2020.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilingloh C.S., Aufderhorst U.W., Schipper L., Dittmer U., Witzke O., Yang D., et al. Susceptibility of SARS-CoV-2 to UV irradiation. Am. J. Infect. Control. 2020;48:1273–1275. doi: 10.1016/j.ajic.2020.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimbuch B., Harnish D. Applied Research Associates. 2019. Research to mitigate a shortage of respiratory protection devices during public health emergencies; p. 275. [Google Scholar]
- Heimbuch B., Harnish D. 2020. Research to Mitigate a Shortage of Respiratory Protection Devices During Public Health Emergencies; p. 2020. [Google Scholar]
- Heßling M., Hönes K., Vatter P., Lingenfelder C. GMS Hygiene and Infection Control. 2020. Ultraviolet irradiation doses for coronavirus inactivation–review and analysis of coronavirus photoinactivation studies; p. 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hijnen W., Beerendonk E., Medema G.J. Inactivation credit of UV radiation for viruses, bacteria and protozoan (oo) cysts in water: a review. Water Res. 2006;40:3–22. doi: 10.1016/j.watres.2005.10.030. [DOI] [PubMed] [Google Scholar]
- Horikawa D.D., Cumbers J., Sakakibara I., Rogoff D., Leuko S., Harnoto R., et al. Analysis of DNA repair and protection in the Tardigrade Ramazzottius varieornatus and Hypsibius dujardini after exposure to UVC radiation. PLoS One. 2013;8 doi: 10.1371/journal.pone.0064793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber T., Goldman O., Epstein A.E., Stella G., Sakmar T.P. Principles and practice of SARS-CoV-2 decontamination of N95 masks with UV-C. MedRxiv. 2020 doi: 10.1016/j.bpj.2021.02.039. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ianevski A., Yao R., Fenstad M.H., Biza S., Zusinaite E., Reisberg T., et al. Potential antiviral options against SARS-CoV-2 infection. Viruses. 2020;12:642. doi: 10.3390/v12060642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki H., Saito A., Sugiyama H., Okabayashi T., Fujimoto S. Rapid inactivation of SARS-CoV-2 with deep-UV LED irradiation. bioRxiv. 2020;9(1):1744–1747. doi: 10.1080/22221751.2020.1796529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakhesara S.J., Bhatt V.D., Patel N.V., Prajapati K.S., Joshi C.G. Isolation and characterization of H9N2 influenza virus isolates from poultry respiratory disease outbreak. Springerplus. 2014;3 doi: 10.1186/2193-1801-3-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jean J., Morales-Rayas R., Anoman M.-N., Lamhoujeb S. Inactivation of hepatitis A virus and norovirus surrogate in suspension and on food-contact surfaces using pulsed UV light (pulsed light inactivation of food-borne viruses) Food Microbiol. 2011;28:568–572. doi: 10.1016/j.fm.2010.11.012. [DOI] [PubMed] [Google Scholar]
- Juzeniene A., Moan J. Beneficial effects of UV radiation other than via vitamin D production. Dermato-endocrinology. 2012;4:109–117. doi: 10.4161/derm.20013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane T. 2018. Taking Credit: Case Study of a UV System to Expand Methods of Virus Inactivation and to Quantify the Enhanced Public Health and Sustainability. [Google Scholar]
- Keil S.D., Ragan I., Yonemura S., Hartson L., Dart N.K., Bowen R. Inactivation of severe acute respiratory syndrome coronavirus 2 in plasma and platelet products using a riboflavin and ultraviolet light-based photochemical treatment. Vox Sang. 2020;115:495–501. doi: 10.1111/vox.12937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D.-K., Kang D.H. UVC-LED irradiation effectively inactivates aerosolized viruses, bacteria, and 3 fungi in a chamber -type air disinfection system. Appl. Environ. Microbiol. 2018;84 doi: 10.1128/AEM.00944-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D.-k., Kang D.-H. Effect of surface characteristics on the bactericidal efficacy of UVC LEDs. Food Control. 2020;108 [Google Scholar]
- Kim D.-K., Kim S.-J., Kang D.-H. Inactivation modeling of human enteric virus surrogates, MS2, Qβ, and ΦX174, in water using UVC-LEDs, a novel disinfecting system. Food Res. Int. 2017;91:115–123. doi: 10.1016/j.foodres.2016.11.042. [DOI] [PubMed] [Google Scholar]
- Kitagawa H., Nomura T., Nazmul T., Omori K., Shigemoto N., Sakaguchi T., et al. Effectiveness of 222-nm ultraviolet light on disinfecting SARS-CoV-2 surface contamination. Am. J. Infect. Control. 2021;49:299–301. doi: 10.1016/j.ajic.2020.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowalski W. Springer science & business media; 2010. Ultraviolet Germicidal Irradiation Handbook: UVGI for Air and Surface Disinfection. [Google Scholar]
- Kreuter A., Hyun J., Skrygan M., Sommer A., Tomi N.S., Breuckmann F., et al. Ultraviolet A1 phototherapy decreases inhibitory SMAD7 gene expression in localized scleroderma. Arch. Dermatol. Res. 2006;298:265–272. doi: 10.1007/s00403-006-0695-8. [DOI] [PubMed] [Google Scholar]
- Kucharski A.J., Russell T.W., Diamond C., Liu Y., Edmunds J., Funk S., et al. Early dynamics of transmission and control of COVID-19: a mathematical modelling study. Lancet Infect. Dis. 2020;20(5):553–558. doi: 10.1016/S1473-3099(20)30144-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutter J.S., Spronken M.I., Fraaij P.L., Fouchier R.A., Herfst S. Transmission routes of respiratory viruses among humans. Curr. Opin. Virol. 2018;28:142–151. doi: 10.1016/j.coviro.2018.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai C.-C., Shih T.-P., Ko W.-C., Tang H.-J., Hsueh P.-R. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and corona virus disease-2019 (COVID-19): the epidemic and the challenges. Int. J. Antimicrob. Agents. 2020;55(3) doi: 10.1016/j.ijantimicag.2020.105924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeChevallier M.W., Au K.-K. Iwa Publishing; 2004. Water Treatment and Pathogen Control. [Google Scholar]
- Li R., Pei S., Chen B., Song Y., Zhang T., Yang W., et al. Substantial undocumented infection facilitates the rapid dissemination of novel coronavirus (SARS-CoV-2) Science. 2020;368:489–493. doi: 10.1126/science.abb3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao L., Xiao W., Zhao M., Yu X., Wang H., Wang Q., et al. Can N95 respirators be reused after disinfection? How many times? ACS Nano. 2020;14(5):6348–6356. doi: 10.1021/acsnano.0c03597. [DOI] [PubMed] [Google Scholar]
- Lindblad M., Tano E., Lindahl C., Huss F. Ultraviolet-C decontamination of a hospital room: amount of UV light needed. Burns. 2019;46(4):842–849. doi: 10.1016/j.burns.2019.10.004. [DOI] [PubMed] [Google Scholar]
- Lindsley W.G., Martin S.B., Jr., Thewlis R.E., Sarkisian K., Nwoko J.O., Mead K.R., et al. Effects of ultraviolet germicidal irradiation (UVGI) on N95 respirator filtration performance and structural integrity. J. Occup. Environ. Hyg. 2015;12:509–517. doi: 10.1080/15459624.2015.1018518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y.-C., Kuo R.-L., Shih S.-R. COVID-19: the first documented coronavirus pandemic in history. Biom. J. 2020;43(4):328–333. doi: 10.1016/j.bj.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Gayle A.A., Wilder-Smith A., Rocklöv J. The reproductive number of COVID-19 is higher compared to SARS coronavirus. J. Travel Med. 2020;27(2):1–4. doi: 10.1093/jtm/taaa021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu-Smith F., Jia J., Zheng Y. Ultraviolet Light in Human Health, Diseases and Environment. 2017. UV-induced molecular signaling differences in melanoma and non-melanoma skin cancer; pp. 27–40. [DOI] [PubMed] [Google Scholar]
- Löfgren S. Solar ultraviolet radiation cataract. Exp. Eye Res. 2017;156:112–116. doi: 10.1016/j.exer.2016.05.026. [DOI] [PubMed] [Google Scholar]
- Lu R., Zhao X., Li J., Niu P., Yang B., Wu H., et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKie R. Effects of ultraviolet radiation on human health. Radiat. Prot. Dosim. 2000;91:15–18. [Google Scholar]
- Majiya H., Adeyemi O.O., Stonehouse N.J., Millner P. Photodynamic inactivation of bacteriophage MS2: the A-protein is the target of virus inactivation. J. Photochem. Photobiol. B Biol. 2018;178:404–411. doi: 10.1016/j.jphotobiol.2017.11.032. [DOI] [PubMed] [Google Scholar]
- Malayeri A.H., Mohseni M., Cairns B., Bolton J.R., Chevrefils G., Caron E., et al. Fluence (UV dose) required to achieve incremental log inactivation of bacteria, protozoa, viruses and algae. IUVA News. 2016;18:4–6. [Google Scholar]
- Marx G., Mou X., Freed R., Ben-Hur E., Yang C., Horowitz B. Protecting fibrinogen with rutin during UVC irradiation for viral inactivation. Photochem. Photobiol. 1996;63:541–546. doi: 10.1111/j.1751-1097.1996.tb03081.x. [DOI] [PubMed] [Google Scholar]
- Mathew A.M., Mun A.B., Balakrishnan A. Ultraviolet inactivation of chikungunya virus. Intervirology. 2018;61:36–41. doi: 10.1159/000490567. [DOI] [PubMed] [Google Scholar]
- Matthews R.E.F. CRC Press; 2019. Diagnosis of Plant Virus Diseases. [Google Scholar]
- McDevitt J.J., Milton D.K., Rudnick S.N., First M.W. Inactivation of poxviruses by upper-room UVC light in a simulated hospital room environment. PLoS One. 2008;3 doi: 10.1371/journal.pone.0003186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh K., Hirsch M.S., Bloom A. UpToDate Hirsch MS Bloom. 2020. Coronavirus disease 2019 (COVID-19) p. 5. [Google Scholar]
- Memarzadeh F., Olmsted R.N., Bartley J.M. Applications of ultraviolet germicidal irradiation disinfection in health care facilities: effective adjunct, but not stand-alone technology. Am. J. Infect. Control. 2010;38:S13–S24. doi: 10.1016/j.ajic.2010.04.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers C., Milici J., Robison R. UVC radiation as an effective disinfectant method to inactivate human papillomaviruses. PLoS One. 2017;12 doi: 10.1371/journal.pone.0187377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills D., Harnish D.A., Lawrence C., Sandoval-Powers M., Heimbuch B.K. Ultraviolet germicidal irradiation of influenza-contaminated N95 filtering facepiece respirators. Am. J. Infect. Control. 2018;46:e49–e55. doi: 10.1016/j.ajic.2018.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morawska L., Cao J. 2020. Airborne Transmission of SARS-CoV-2: The World Should Face the Reality. Environment International; p. 105730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouret S., Baudouin C., Charveron M., Favier A., Cadet J., Douki T. Cyclobutane pyrimidine dimers are predominant DNA lesions in whole human skin exposed to UVA radiation. Proc. Natl. Acad. Sci. 2006;103:13765–13770. doi: 10.1073/pnas.0604213103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan D.L., Saladi R.N., Fox J.L. Ultraviolet radiation and skin cancer. Int. J. Dermatol. 2010;49:978–986. doi: 10.1111/j.1365-4632.2010.04474.x. [DOI] [PubMed] [Google Scholar]
- Nardell E.A., Bucher S.J., Brickner P.W., Wang C., Vincent R.L., Becan-McBride K., et al. Safety of upper-room ultraviolet germicidal air disinfection for room occupants: results from the Tuberculosis Ultraviolet Shelter Study. Public Health Rep. 2008;123:52–60. doi: 10.1177/003335490812300108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narita K., Asano K., Naito K., Ohashi H., Sasaki M., Morimoto Y., et al. 222-nm UVC inactivates a wide spectrum of microbial pathogens. J. Hosp. Infect. 2020;105(3):459–467. doi: 10.1016/j.jhin.2020.03.030. [DOI] [PubMed] [Google Scholar]
- Narla S., Lyons A.B., Kohli I., Torres A.E., Parks-Miller A., Ozog D.M., et al. The importance of the minimum dosage necessary for UVC decontamination of N95 respirators during the COVID-19 pandemic. Photodermatol. Photoimmunol. Photomed. 2020;36:324–325. doi: 10.1111/phpp.12562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oboho I.K., Tomczyk S.M., Al-Asmari A.M., Banjar A.A., Al-Mugti H., Aloraini M.S., et al. 2014 MERS-CoV outbreak in Jeddah—a link to health care facilities. N. Engl. J. Med. 2015;372:846–854. doi: 10.1056/NEJMoa1408636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Hearn K., Gertsman S., Sampson M., Webster R., Tsampalieros A., Ng R., et al. 2020. Decontaminating N95 Masks with Ultraviolet Germicidal Irradiation (UVGI) Does Not Impair Mask Efficacy and Safety: A Systematic Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz-Prado E., Simbaña-Rivera K., Gómez-Barreno L., Rubio-Neira M., Guaman L.P., Kyriakidis N.C., et al. Clinical, molecular and epidemiological characterization of the SARS-CoV2 virus and the coronavirus disease 2019 (COVID-19), a comprehensive literature review. Diagn. Microbiol. Infect. Dis. 2020;98(1) doi: 10.1016/j.diagmicrobio.2020.115094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Øye A.K., Rimstad E. Inactivation of infectious salmon anaemia virus, viral haemorrhagic septicaemia virus and infectious pancreatic necrosis virus in water using UVC irradiation. Dis. Aquat. Org. 2001;48:1–5. doi: 10.3354/dao048001. [DOI] [PubMed] [Google Scholar]
- Peiris J., Guan Y., Yuen K. Severe acute respiratory syndrome. Nat. Med. 2004;10:S88–S97. doi: 10.1038/nm1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifer G.P., Besaratinia A. UV wavelength-dependent DNA damage and human non-melanoma and melanoma skin cancer. Photochem. Photobiol. Sci. 2012;11:90–97. doi: 10.1039/c1pp05144j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradeep P., Chulkyoon M. Non-thermal plasmas (NTPs) for inactivation of viruses in abiotic environment. Res. J. Biotechnol. 2016;11:6. [Google Scholar]
- Pratelli A. Canine coronavirus inactivation with physical and chemical agents. Vet. J. 2008;177:71–79. doi: 10.1016/j.tvjl.2007.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabenau H., Cinatl J., Morgenstern B., Bauer G., Preiser W., Doerr H. Stability and inactivation of SARS coronavirus. Med. Microbiol. Immunol. 2005;194:1–6. doi: 10.1007/s00430-004-0219-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raeiszadeh M., Adeli B. A critical review on ultraviolet disinfection systems against COVID-19 outbreak: applicability, validation, and safety considerations. ACS Photonics. 2020;7:2941–2951. doi: 10.1021/acsphotonics.0c01245. [DOI] [PubMed] [Google Scholar]
- Ratnesar-Shumate S., Williams G., Green B., Krause M., Holland B., Wood S., et al. Simulated sunlight rapidly inactivates SARS-CoV-2 on surfaces. J. Infect. Dis. 2020;222(2):214–222. doi: 10.1093/infdis/jiaa274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rattanakul S., Oguma K. Inactivation kinetics and efficiencies of UV-LEDs against Pseudomonas aeruginosa, legionella pneumophila, and surrogate microorganisms. Water Res. 2018;130:31–37. doi: 10.1016/j.watres.2017.11.047. [DOI] [PubMed] [Google Scholar]
- Rattanakul S., Oguma K., Sakai H., Takizawa S. Inactivation of viruses by combination processes of UV and chlorine. J. Water Environ. Technol. 2014;12:511–523. [Google Scholar]
- Reed N.G. The history of ultraviolet germicidal irradiation for air disinfection. Public Health Rep. 2010;125:15–27. doi: 10.1177/003335491012500105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Roda Husman A.M., Bijkerk P., Lodder W., Van Den Berg H., Pribil W., Cabaj A., et al. Calicivirus inactivation by nonionizing (253.7-nanometer-wavelength [UV]) and ionizing (gamma) radiation. Appl. Environ. Microbiol. 2004;70:5089–5093. doi: 10.1128/AEM.70.9.5089-5093.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabino C.P., Sellera F.P., Sales-Medina D.F., Machado R.R.G., Durigon E.L., Freitas-Junior L.H., et al. UV-C (254 nm) lethal doses for SARS-CoV-2. Photodiagn. Photodyn. Ther. 2020;32 doi: 10.1016/j.pdpdt.2020.101995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagripanti J.L., Lytle C.D. Estimated inactivation of coronaviruses by solar radiation with special reference to COVID-19. Photochem. Photobiol. 2020;96:731–737. doi: 10.1111/php.13293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saknimit M., Inatsuki I., Sugiyama Y., Yagami K.-i. Virucidal efficacy of physico-chemical treatments against coronaviruses and parvoviruses of laboratory animals. Exp. Anim. 1988;37:341–345. doi: 10.1538/expanim1978.37.3_341. [DOI] [PubMed] [Google Scholar]
- SCHEER . Scientific Committee on Health, Environmental and Emerging Risks, European Commission; 2017. Opinion on Biological Effects of UV-C Radiation Relevant to Health with Particular Reference to UVC Lamps. [Google Scholar]
- Schulman J.M., Fisher D.E. Indoor UV tanning and skin cancer: health risks and opportunities. Curr. Opin. Oncol. 2009;21:144. doi: 10.1097/CCO.0b013e3283252fc5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shereen M.A., Khan S., Kazmi A., Bashir N., Siddique R. COVID-19 infection: origin, transmission, and characteristics of human coronaviruses. J. Adv. Res. 2020;24:91–98. doi: 10.1016/j.jare.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirbandi K., Barghandan S., Mobinfar O., Rahim F. 2020. Inactivation of Coronavirus with Ultraviolet Irradiation: What? How? Why? [Google Scholar]
- Silverman A.I., Peterson B.M., Boehm A.B., McNeill K., Nelson K.L. Sunlight inactivation of human viruses and bacteriophages in coastal waters containing natural photosensitizers. Environ. Sci. Technol. 2013;47:1870–1878. doi: 10.1021/es3036913. [DOI] [PubMed] [Google Scholar]
- Simmons S.E., Carrion R., Alfson K.J., Staples H.M., Jinadatha C., Jarvis W.R., et al. Deactivation of SARS-CoV-2 with pulsed-xenon ultraviolet light: implications for environmental COVID-19 control. Infect. Control Hosp. Epidemiol. 2021;42:127–130. doi: 10.1017/ice.2020.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R.P., Häder D.-P. UV-induced DNA damage and repair: a review. Photochem. Photobiol. Sci. 2002;1:225–236. doi: 10.1039/b201230h. [DOI] [PubMed] [Google Scholar]
- Sohrabi C., Alsafi Z., O’Neill N., Khan M., Kerwan A., Al-Jabir A., et al. World Health Organization declares global emergency: a review of the 2019 novel coronavirus (COVID-19) Int. J. Surg. 2020;76:71–76. doi: 10.1016/j.ijsu.2020.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song K., Mohseni M., Taghipour F. Application of ultraviolet light-emitting diodes (UV-LEDs) for water disinfection: a review. Water Res. 2016;94:341–349. doi: 10.1016/j.watres.2016.03.003. [DOI] [PubMed] [Google Scholar]
- Spengler J.R., Ervin E.D., Towner J.S., Rollin P.E., Nichol S.T. Perspectives on West Africa Ebola virus disease outbreak, 2013–2016. Emerg. Infect. Dis. 2016;22:956. doi: 10.3201/eid2206.160021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens B., Azimi P., Thoemmes M.S., Heidarinejad M., Allen J.G., Gilbert J.A. Microbial exchange via fomites and implications for human health. Curr. Pollut. Rep. 2019;5:198–213. doi: 10.1007/s40726-019-00123-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugawara H., Motokawa R., Abe H., Yamaguchi M., Yamada-Ohnishi Y., Hirayama J., et al. Inactivation of parvovirus B19 in coagulation factor concentrates by UVC radiation: assessment by an in vitro infectivity assay using CFU–E derived from peripheral blood CD34+ cells. Transfusion. 2001;41:456–461. doi: 10.1046/j.1537-2995.2001.41040456.x. [DOI] [PubMed] [Google Scholar]
- Sunnen G.V. 2003. SARS and Ozone Therapy: Theoretical Considerations. [Google Scholar]
- Tenkate T., Adam B., Al-Rifai R.H., Chou B.R., Gobba F., Ivanov I.D., et al. WHO/ILO work-related burden of disease and injury: protocol for systematic reviews of occupational exposure to solar ultraviolet radiation and of the effect of occupational exposure to solar ultraviolet radiation on cataract. Environ. Int. 2019;125:542–553. doi: 10.1016/j.envint.2018.10.001. [DOI] [PubMed] [Google Scholar]
- Terpstra F.G., Van’t Wout A.B., Schuitemaker H., Van Engelenburg F.A., Dekkers D.W., Verhaar R., et al. Potential and limitation of UVC irradiation for the inactivation of pathogens in platelet concentrates. Transfusion. 2008;48:304–313. doi: 10.1111/j.1537-2995.2007.01524.x. [DOI] [PubMed] [Google Scholar]
- Tseng C.-C., Li C.-S. Inactivation of viruses on surfaces by ultraviolet germicidal irradiation. J. Occup. Environ. Hyg. 2007;4:400–405. doi: 10.1080/15459620701329012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner J., Igoe D., Parisi A.V., McGonigle A.J., Amar A., Wainwright L. A review on the ability of smartphones to detect ultraviolet (UV) radiation and their potential to be used in UV research and for public education purposes. Sci. Total Environ. 2020;706 doi: 10.1016/j.scitotenv.2019.135873. [DOI] [PubMed] [Google Scholar]
- Van Doremalen N., Bushmaker T., Morris D.H., Holbrook M.G., Gamble A., Williamson B.N., et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N. Engl. J. Med. 2020;382:1564–1567. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wacker M., Holick M.F. Sunlight and vitamin D: a global perspective for health. Dermato-endocrinology. 2013;5:51–108. doi: 10.4161/derm.24494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker C.M., Ko G. Effect of ultraviolet germicidal irradiation on viral aerosols. Environ. Sci. Technol. 2007;41:5460–5465. doi: 10.1021/es070056u. [DOI] [PubMed] [Google Scholar]
- Wang C., Lu S., Zhang Z. Inactivation of airborne bacteria using different UV sources: performance modeling, energy utilization, and endotoxin degradation. Sci. Total Environ. 2019;655:787–795. doi: 10.1016/j.scitotenv.2018.11.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Shen J., Ye D., Yan X., Zhang Y., Yang W., et al. Disinfection technology of hospital wastes and wastewater: suggestions for disinfection strategy during coronavirus disease 2019 (COVID-19) pandemic in China. Environ. Pollut. 2020;262 doi: 10.1016/j.envpol.2020.114665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss M., Horzinek M. Resistance of Berne virus to physical and chemical treatment. Vet. Microbiol. 1986;11:41–49. doi: 10.1016/0378-1135(86)90005-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss S.R., Leibowitz J.L. vol. 81. Elsevier; 2011. Coronavirus Pathogenesis. Advances in Virus Research; pp. 85–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch D., Buonanno M., Grilj V., Shuryak I., Crickmore C., Bigelow A.W., et al. Far-UVC light: a new tool to control the spread of airborne-mediated microbial diseases. Sci. Rep. 2018;8:1–7. doi: 10.1038/s41598-018-21058-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO . World Health Organization; 1994. World Health Organization Ultraviolet Radiation: An Authoritative Scientific Review of Environmental and Health Effects of UV, with Reference to Global Ozone Layer Depletion. [Google Scholar]
- WHO . WHO; Geneva, CH: 2003. Ultraviolet Radiation as a Hazard in the Workplace. [Google Scholar]
- WHO . vol. 924. 2004. Guidelines on Viral Inactivation and Removal Procedures Intended to Assure the Viral Safety of Human Blood Plasma Products; pp. 150–224. (WHO Technical Report, Series). [Google Scholar]
- Wigginton K.R., Kohn T. Virus disinfection mechanisms: the role of virus composition, structure, and function. Curr. Opin. Virol. 2012;2:84–89. doi: 10.1016/j.coviro.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigginton K.R., Pecson B.M., Sigstam Tr, Bosshard F., Kohn T. Virus inactivation mechanisms: impact of disinfectants on virus function and structural integrity. Environ. Sci. Technol. 2012;46:12069–12078. doi: 10.1021/es3029473. [DOI] [PubMed] [Google Scholar]
- Wolf C., von Gunten U., Kohn T. Kinetics of inactivation of waterborne enteric viruses by ozone. Environ. Sci. Technol. 2018;52:2170–2177. doi: 10.1021/acs.est.7b05111. [DOI] [PubMed] [Google Scholar]
- Xia T., Kleinheksel A., Lee E.M., Qiao Z., Wigginton K.R., Clack H.L. Inactivation of airborne viruses using a packed bed non-thermal plasma reactor. J. Phys. D. Appl. Phys. 2019;52 doi: 10.1088/1361-6463/ab1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin R., Dai T., Avci P., AES Jorge, de Melo W.C., Vecchio D., et al. Light based anti-infectives: ultraviolet C irradiation, photodynamic therapy, blue light, and beyond. Curr. Opin. Pharmacol. 2013;13:731–762. doi: 10.1016/j.coph.2013.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young A.R. Acute effects of UVR on human eyes and skin. Prog. Biophys. Mol. Biol. 2006;92:80–85. doi: 10.1016/j.pbiomolbio.2006.02.005. [DOI] [PubMed] [Google Scholar]
- Zhang C., Li Y., Shuai D., Shen Y., Wang D. Progress and challenges in photocatalytic disinfection of waterborne viruses: a review to fill current knowledge gaps. Chem. Eng. J. 2019;355:399–415. [Google Scholar]
- Zhang Y., Qu S., Xu L. Progress in the study of virus detection methods: the possibility of alternative methods to validate virus inactivation. Biotechnol. Bioeng. 2019;116:2095–2102. doi: 10.1002/bit.27003. [DOI] [PubMed] [Google Scholar]
- Zhong N., Zheng B., Li Y., Poon L., Xie Z., Chan K., et al. Epidemiology and cause of severe acute respiratory syndrome (SARS) in Guangdong, People’s Republic of China, in February, 2003. Lancet. 2003;362:1353–1358. doi: 10.1016/S0140-6736(03)14630-2. [DOI] [PMC free article] [PubMed] [Google Scholar]