Abstract
Background
A vaccine against SARS-CoV-2 for children and adolescents will play an important role in curbing the COVID-19 pandemic. Here we aimed to assess the safety, tolerability, and immunogenicity of a candidate COVID-19 vaccine, CoronaVac, containing inactivated SARS-CoV-2, in children and adolescents aged 3–17 years.
Methods
We did a double-blind, randomised, controlled, phase 1/2 clinical trial of CoronaVac in healthy children and adolescents aged 3–17 years old at Hebei Provincial Center for Disease Control and Prevention in Zanhuang (Hebei, China). Individuals with SARS-CoV-2 exposure or infection history were excluded. Vaccine (in 0·5 mL aluminum hydroxide adjuvant) or aluminum hydroxide only (alum only, control) was given by intramuscular injection in two doses (day 0 and day 28). We did a phase 1 trial in 72 participants with an age de-escalation in three groups and dose-escalation in two blocks (1·5 μg or 3·0 μg per injection). Within each block, participants were randomly assigned (3:1) by means of block randomisation to receive CoronaVac or alum only. In phase 2, participants were randomly assigned (2:2:1) by means of block randomisation to receive either CoronaVac at 1·5 μg or 3·0 μg per dose, or alum only. All participants, investigators, and laboratory staff were masked to group allocation. The primary safety endpoint was adverse reactions within 28 days after each injection in all participants who received at least one dose. The primary immunogenicity endpoint assessed in the per-protocol population was seroconversion rate of neutralising antibody to live SARS-CoV-2 at 28 days after the second injection. This study is ongoing and is registered with ClinicalTrials.gov, NCT04551547.
Findings
Between Oct 31, 2020, and Dec 2, 2020, 72 participants were enrolled in phase 1, and between Dec 12, 2020, and Dec 30, 2020, 480 participants were enrolled in phase 2. 550 participants received at least one dose of vaccine or alum only (n=71 for phase 1 and n=479 for phase 2; safety population). In the combined safety profile of phase 1 and phase 2, any adverse reactions within 28 days after injection occurred in 56 (26%) of 219 participants in the 1·5 μg group, 63 (29%) of 217 in the 3·0 μg group, and 27 (24%) of 114 in the alum-only group, without significant difference (p=0·55). Most adverse reactions were mild and moderate in severity. Injection site pain was the most frequently reported event (73 [13%] of 550 participants), occurring in 36 (16%) of 219 participants in the 1·5 μg group, 35 (16%) of 217 in the 3·0 μg group, and two (2%) in the alum-only group. As of June 12, 2021, only one serious adverse event of pneumonia has been reported in the alum-only group, which was considered unrelated to vaccination. In phase 1, seroconversion of neutralising antibody after the second dose was observed in 27 of 27 participants (100·0% [95% CI 87·2–100·0]) in the 1·5 μg group and 26 of 26 participants (100·0% [86·8-100·0]) in the 3·0 μg group, with the geometric mean titres of 55·0 (95% CI 38·9–77·9) and 117·4 (87·8–157·0). In phase 2, seroconversion was seen in 180 of 186 participants (96·8% [93·1–98·8]) in the 1·5 μg group and 180 of 180 participants (100·0% [98·0–100·0]) in the 3·0 μg group, with the geometric mean titres of 86·4 (73·9–101·0) and 142·2 (124·7–162·1). There were no detectable antibody responses in the alum-only groups.
Interpretation
CoronaVac was well tolerated and safe and induced humoral responses in children and adolescents aged 3–17 years. Neutralising antibody titres induced by the 3·0 μg dose were higher than those of the 1·5 μg dose. The results support the use of 3·0 μg dose with a two-immunisation schedule for further studies in children and adolescents.
Funding
The Chinese National Key Research and Development Program and the Beijing Science and Technology Program.
Introduction
The ongoing COVID-19 pandemic, caused by SARS-CoV-2, has led to more than 174·5 million infections and more than 3·8 million deaths worldwide as of June 11, 2021.1 Children and adolescents infected with SARS-CoV-2 are mainly mild or asymptomatic compared with adults, but a relatively small number of children and adolescents might be at risk for severe COVID-19, especially those with underlying health comorbidities.2, 3, 4, 5 Studies have also found that the SARS-CoV-2 infection can lead to a serious complication called multisystem inflammatory syndrome in children, which includes myocardial dysfunction, shock, and respiratory failure requiring intensive care.3, 6, 7 Furthermore, children and adolescents can be important transmitters of SARS-CoV-2 in communities.8, 9 Therefore, testing the effectiveness of COVID-19 vaccines in this population is important. As of June 11, 2021, a total of 287 candidate vaccines are in clinical or preclinical development.10 The results from phase 3 trials of multiple vaccines across three platforms, including mRNA, viral vector, and inactivated virus, have confirmed that the vaccines are effective in preventing SARS-CoV-2 infection in adults,11, 12 and more than ten vaccines have been rolled out in many countries for general population use. No COVID-19 vaccines are authorised for use among children under the age of 12 years, but vaccine companies have been started to assess the safety and efficacy of various vaccine platforms among the population aged 6 months to 17 years.13, 14 The mRNA vaccine developed by Pfizer has shown 100% efficacy and robust antibody responses in adolescents aged 12–15 years.15
Research in context.
Evidence before this study
We searched PubMed on Apr 29, 2021, for published research articles, with no language or date restrictions, using the search terms of “SARS-CoV-2”, “COVID-19”, “vaccine”, and “clinical trial”. We identified several clinical trials of COVID-19 vaccines across different platforms, including mRNA, viral vector, protein subunit, and inactivated virus. The results from phase 1–3 studies have confirmed that different vaccines were safe, effective, and induced humoral antibody responses in adults. As of April 19, 2020, more than ten COVID-19 candidate vaccines have been rolled out in many countries for general population use. Although vaccine companies have started to assess the safety and efficacy of COVID-19 vaccines in populations of 6 months to 17 years of age, there are currently no authorised vaccines for use among children and adolescents under the age of 16. We previously assessed CoronaVac, an inactivated vaccine developed by Sinovac Life Sciences, in adults aged 18–59 years and those aged 60 years and older, and showed that it was safe and well tolerated. Seroconversion rates ranged from 92% to 100% after two doses of CoronaVac (3·0 μg and 6·0 μg) with two immunisation schedules (on days 0 and 14, or on days 0 and 28) in adults aged 18–59 years. Seroconversion rates were higher than 98% after two doses of CoronaVac (3 μg and 6 μg) with the 0–28 days schedule in patients aged 60 years and older.
Added value of this study
This is, we believe, the first report of an inactivated SARS-CoV-2 vaccine, CoronaVac, tested in children and adolescents aged 3–17 years. CoronaVac was found to be well tolerated and safe in this population. The seroconversion rates of neutralising antibody with both doses (1·5 μg and 3·0 μg) were over 96% after two-dose vaccination and the neutralising antibody titres induced by the 3·0 μg dose were higher than those induced by the 1.5 μg dose. Taken together, the 3·0 μg dose of CoronaVac induced higher immune responses compared with 1·5 μg dose.
Implications of all the available evidence
While a small number of children and adolescents with SARS-CoV-2 infection might be at risk for severe COVID-19 and complicated illnesses, they usually have mild or asymptomatic symptoms compared with adults. Nevertheless, children and adolescents can be important transmitters of SARS-CoV-2 in communities. Therefore, testing the effectiveness of COVID-19 vaccines in this population is important. CoronaVac was well tolerated and immunogenic in healthy children and adolescents aged 3–17 years in this trial, which supports the use of CoronaVac for further studies in this population.
Purified inactivated viruses have traditionally been used for vaccine development. CoronaVac is an inactivated SARS-CoV-2 vaccine developed by Sinovac Life Sciences (Beijing, China), which provided partial or complete protection in macaques following SARS-CoV-2 challenge, without observable antibody-dependent enhancement of infection.16 The analyses from phase 1–3 trials have shown that CoronaVac was effective, immunogenic, and safe in adults aged 18 years and older.12, 17, 18, 19 Furthermore, another 11 inactivated COVID-19 candidate vaccines are in clinical evaluation, and several studies have also shown that the inactivated vaccines can induce neutralising antibody responses and have good safety profiles.20, 21, 22, 23, 24
The phase 1/2 trial of CoronaVac in children and adolescents was launched in October, 2020 to assess the safety, tolerability, and immunogenicity. Here we report the results of CoronaVac among healthy participants aged 3–17 years old.
Method
Study design and participants
We have done two phase 1/2 clinical trials of CoronaVac in participants aged 18–59 years and aged 60 years and older.17, 18 The preliminary immunogenicity and safety results supported the expansion of the trial to children and adolescents. We subsequently did a single-centre, randomised, double-blind, controlled, phase 1/2 trial to evaluate the safety, tolerability, and immunogenicity of CoronaVac in children and adolescents aged 3–17 years. On the basis of the results of previous trials and considering the low weight of this population, two different doses—1·5 μg and 3·0 μg—were adopted in this study. This trial was run at Hebei Provincial Center for Disease Control and Prevention in Zanhuang (Hebei, China).
The phase 1 trial was an age de-escalation and dose-escalation study of 72 participants. Participants in each age group (3–5 years, 6–11 years, and 12–17 years) were recruited in order from the low-dose stage (block 1) to the high-dose stage (block 2). In block 1, participants were randomly assigned to receive either 1·5 μg vaccine or aluminum hydroxide adjuvant only (alum only, control) and participants in block 2 were randomly assigned to receive either 3·0 μg vaccine or alum only. In phase 1, 7 days of follow-up for safety were required before entering the next stage. The phase 2 trial was initiated only after all the participants in phase 1 had finished and passed a 7-days safety observation period after the first dose, as confirmed by the data monitoring committee. The required safety criteria were: no-life threatening vaccine-related adverse events (adverse reactions), no more than 15% of vaccinated participants reporting severe adverse reactions, and no other safety concerns in the opinion of the data monitoring committee. A total of 480 participants were recruited in phase 2, including 120 aged 3–5 years, 180 aged 6–11 years, and 180 aged 12–17 years.
Eligible participants were healthy children and adolescents aged 3–17 years. The key exclusion criteria included high-risk epidemiology history within 14 days before enrolment (eg, travel or residence history in communities with case reports, or contact history with someone infected with SARS-CoV-2), history of severe acute respiratory syndrome or SARS-CoV-2 infection (as reported by participants), axillary temperature of more than 37·0°, and history of allergy to any vaccine component. A complete list of exclusion criteria is listed in the protocol, which is available online.
Parents provided written informed consents, and participants 8–17 years of age also provided written assents before enrolment. The clinical trial protocol and informed consent form were approved by the Ethics Committee of Hebei CDC (IRB2020-005). The study was done in accordance with the requirements of Good Clinical Practice of China and the International Conference on Harmonisation.
Randomisation and masking
In phase 1, participants of block 1 and block 2 were randomly assigned (3:1) to either vaccine or alum only, and in phase 2, participants were randomly assigned (2:2:1) to either 1·5 μg, 3·0 μg of vaccine, or alum only. The randomisation codes for the phase 1 and phase 2 were generated by the randomisation statistician by means of block randomisation using SAS software (version 9.4). The randomisation code was assigned to each participant in sequence in the order of enrolment, and then the participants received the study vaccine labelled with the same code. The vaccine and alum only were completely identical in appearance, and all participants, investigators, and laboratory staff were masked to group allocation.
Procedures
CoronaVac is an inactivated vaccine candidate against SARS-CoV-2 infection. To prepare the vaccine, SARS-CoV-2 (CN02 strain) was propagated in African green monkey kidney cells (WHO Vero 10-87 Cells). At the end of the incubation period, the virus was harvested, inactivated with β-propiolactone, concentrated, purified, and finally adsorbed onto aluminum hydroxide. The aluminium hydroxide complex was then diluted in sodium chloride, phosphate-buffered saline, and water, before being sterilised and filtered for injection. The control was aluminum hydroxide adjuvant (alum only) with no virus. Both the vaccine and alum only were prepared in the Good Manufacturing Practice-accredited facility of Sinovac Life Science that was periodically inspected by the National Medical Products Administration committee for compliance. The production process of the vaccine in this trial was a highly automated bioreactor (ReadyToProcess WAVE 25, GE, Umea, Sweden), which was consistent with the production process of vaccine used in the phase 2 trial of adults aged 18–59 years and in the phase 1/2 trial of older adults aged at least 60 years.17, 18 Vaccine doses of 1·5 μg, or 3·0 μg in 0·5 mL of aluminium hydroxide diluent per dose and alum only in ready-to-use syringes were administered intramuscularly to participants on day 0 and day 28.
Participants were observed in the study site for at least 30 min after vaccination. For the first 7 days after each dose, parents or guardians of participants were required to record any injection-site adverse events (eg, pain, swelling, erythema), or systemic adverse events (eg, allergic reaction, cough, fever) on the diary cards. From day 8 to day 28 after each dose, safety data were collected by spontaneous report from the participants combined with the regular visit (which occurred on day 3, day 8 and day 28 after each dose in phase 1, and on day 8 and day 28 in phase 2). Solicited adverse events were recorded for 7 days after each dose and unsolicited adverse events for 28 days. The serious adverse events are recorded throughout the study and follow-up will continue until 12 months after the second dose. The reported adverse events were graded according to the China National Medical Products Administration guidelines.25 The causal relationship between adverse events and vaccination was established by the investigators.
In the phase 1 trial, blood and urine samples were taken on day 3 after each dose and tested to investigate any abnormal changes of the haematology, biochemistry, and urine routine indexes. Blood samples were collected on day 0, 28, and 56 from participants in phase 1, and on day 0 and 56 in phase 2 to evaluate the neutralising antibody titres. The neutralising antibody titres to live SARS-CoV-2 (virus strain: SARS-CoV-2/human/CHN/CN1/2020, genebank number MT407649.1) was quantified by means of the microcytopathogenic effect assay.26 Serum samples were inactivated at 56° for 30 min and serially diluted with cell culture medium in two-fold steps. The diluted serum samples were incubated with equal volume (50 μL) of the live SARS-CoV-2 virus suspension, with a 50% cell culture infective dose of 100 for 2 h at 37·0°. Vero cells (1·0–2·0 × 105 cells per mL) were then added to the serum–virus suspensions in microplates in duplicate and incubated at 36·5° for 5 days. Cytopathic effects were recorded under microscopes and the neutralising antibody titre was calculated by the dilution number of 50% protective condition. Detection was done by the National Institute for Food and Drug Control. Further information on the method has been provided in the appendix (p 1).
Outcomes
The primary safety endpoint was any vaccine-related adverse events (adverse reactions) within 28 days after the administration of each dose of the study vaccine or alum only. Secondary safety endpoints were serious adverse events and any abnormal changes in laboratory measurements at day 3 after each dose. Laboratory index tests were prespecified only in the phase 1 trial. The primary immunogenic endpoint was the seroconversion rate of neutralising antibodies to live SARS-CoV-2 at day 28 after the second dose. Secondary immunogenic endpoints were geometric mean titre (GMT) of neutralising antibodies to live SARS-CoV-2, as well as seropositive rates and geometric mean increase. Seroconversion was defined as a change from seronegative at baseline to seropositive or a four-fold titre increase if the participant was seropositive at baseline. The positive cutoff of the titre for neutralising antibodies to live SARS-CoV-2 was 1/8.
Statistical analysis
We assessed the safety endpoints in the safety population, which included all participants who had received at least one dose of vaccine or alum only. We assessed the immunogenicity endpoints in the per-protocol population, which included all participants who had randomly received two doses of vaccine or alum only, had antibody results available, and did not violate the trial protocol.
We did not determine the sample sizes on the basis of a statistical power calculation, but followed the requirements of the China National Medical Products Administration and Chinese Technical Guidelines for Clinical Trials of Vaccines—ie, recruitment of at least 20–30 participants in phase 1 and 300 participants in phase 2 trial.
We used the Pearson χ2 test or Fisher's exact test for the analysis of categorical outcomes. We calculated the 95% CIs for all categorical outcomes using the Clopper-Pearson method. We calculated GMTs and corresponding 95% CIs on the basis of the standard normal distribution of the log-transformation antibody titre. We used the ANOVA method to compare the log-transformed antibody titres. When the comparison among all groups showed significant difference, we then did pairwise comparisons. Hypothesis testing was two-sided and we considered a p value of less than 0·05 to be significant.
An independent data monitoring committee consisting of one independent statistician, one clinician, and one epidemiologist was established before commencement of the study. Safety data were assessed and reviewed by the committee to ensure further proceeding of the study. We used SAS (version 9.4) for all analyses. This trial is registered with ClinicalTrials.gov, NCT04551547.
Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. Employees of Sinovac Life Sciences and Sinovac Biotech, listed as the authors, contributed to the study design, data interpretation, clinical trial monitoring, writing or revising the manuscript.
Results
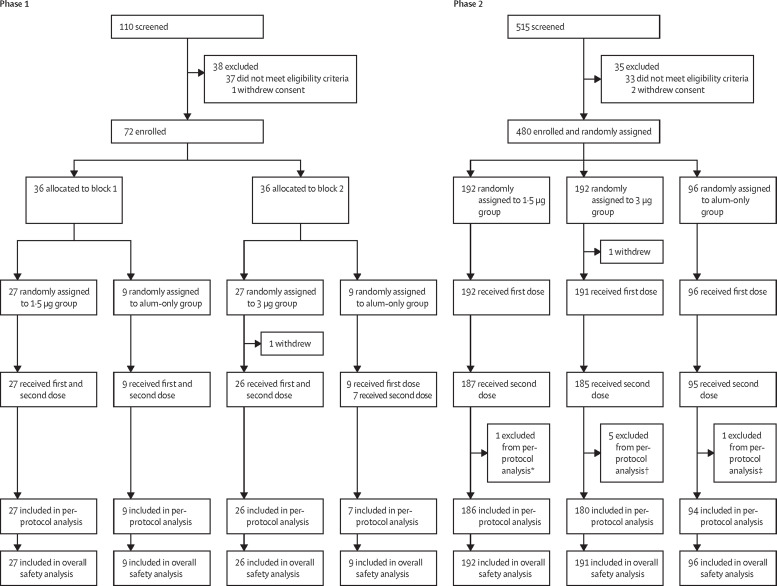
Between Oct 31, 2020, and Dec 2, 2020, 110 individuals were screened and 72 were enrolled in phase 1. Between Dec 12 and Dec 30, 2020, 515 individuals were screened and 480 were enrolled in phase 2. 550 (>99%) of 552 enrolled participants received the first dose of vaccine or alum only (71 in phase 1 and 479 in phase 2) and were included in the safety population (figure 1 ). 69 (96%) participants in phase 1 received the second dose and all were eligible for the immunogenic evaluation at day 28 after the second dose (per-protocol population; figure 1). In phase 2, 467 (97%) participants received the second dose and 460 (96%) were included in the per-protocol population (figure 1). Seven participants were excluded because one received tetanus immunoglobulin at day 14 after the second dose, five did not have a blood sample taken at 28 days after the second dose, and one took a blood sample outside of the specified time window. The demographic characteristics of the participants were similar in terms of sex, mean age, height, weight, and ethnicity among groups. The mean age of study participants was 8·3 years (SD 4·0) in phase 1, including 24 (34%) of 71 participants aged 3–5 years, 24 (34%) aged 6–11 years, and 23 (32%) aged 12–17 years. The mean age of study participants was 9·2 years (3·9) in phase 2, including 119 (25%) of 479 participants aged 3–5 years, 180 (38%) aged 6–11 years, and 180 (38%) aged 12–17 years (table 1 ).
Figure 1.
Trial profile
*One participant in the 1·5 μg group was excluded from the per-protocol analysis because he received tetanus immunoglobulin at day 14 after the second dose. †One participant in the 3 μg group was excluded from the per-protocol analysis because blood collection after vaccination was outside of the specified time window, and four did not have a blood sample taken 28 days after the second dose. ‡One participant in the alum only group was excluded from the per-protocol analysis because he did not have a blood sample taken 28 days after the second dose.
Table 1.
Baseline characteristics
|
Phase 1 |
Phase 2 |
||||||
|---|---|---|---|---|---|---|---|
| 1·5 μg group (n=27) | 3 μg group (n=26) | Aluminium hydroxide only group (n=18) | 1·5 μg group (n=192) | 3·0 μg group (n=191) | Aluminium hydroxide only group (n=96) | ||
| Age, years | 8·4 (4·2) | 8·2 (4·0) | 8·3 (4·0) | 9·3 (3·9) | 9·2 (3·8) | 9·1 (4·0) | |
| 3–5 | 9 (33%) | 9 (35%) | 6 (33%) | 48 (25%) | 47 (25%) | 24 (25%) | |
| 6–11 | 9 (33%) | 9 (35%) | 6 (33%) | 72 (38%) | 72 (38%) | 36 (38%) | |
| 12–17 | 9 (33%) | 8 (31%) | 6 (33%) | 72 (38%) | 72 (38%) | 36 (38%) | |
| Sex | |||||||
| Male | 10 (37%) | 12 (46%) | 8 (44%) | 105 (55%) | 108 (57%) | 54 (56%) | |
| Female | 17 (63%) | 14 (54%) | 10 (56%) | 87 (45%) | 83 (43%) | 42 (44%) | |
| Han ethnicity | 27 (100%) | 26 (100%) | 18 (100%) | 192 (100%) | 191 (100%) | 96 (100%) | |
| Height, m | 1·3 (0·2) | 1·3 (0·3) | 1·3 (0·3) | 1·4 (0·2) | 1·4 (0·2) | 1·4 (0·2) | |
| Weight, kg | 34·3 (15·7) | 35·0 (14·9) | 34·9 (17·7) | 40·4 (19·0) | 37·9 (16·9) | 39·2 (18·9) | |
Data are mean (SD) or n (%).
The safety data of the phase 1 and phase 2 trial were combined for analysis because the same batches of the vaccine and alum only and the same safety observation method were used. 146 (27%) of 550 participants reported at least one adverse reaction within 28 days of either vaccination, and the proportions of participants with any adverse reactions were similar across groups. Most adverse reactions were mild (grade 1) and moderate (grade 2) in severity. Only two (<1%) of 550 had grade 3 adverse reactions. Most adverse reactions occurred within 7 days after vaccination and participants recovered within 48 h. The most common reactions were injection site pain (73 [13%] participants) and fever (25 [5%]). Except for a higher prevalence of injection site pain in two vaccine groups than that in alum-only group, there were no significant differences in the prevalence of other solicited or unsolicited reactions among the three groups (table 2 ). In an exploratory analysis by age, the prevalence of adverse reactions was highest in participants aged 12–17 years (72 [35%] of 203 participants) followed by 3–5 years (37 [26%] of 143 participants) and 6–11 years (37 [18%] of 204 participants; appendix pp 8–10). As of June 12, 2021, only one participant in the alum-only group has reported one serious adverse event (pneumonia; appendix p 15), which was considered to be unrelated to vaccination. Additionally, only two (3%) of 71 participants at day 3 after the first dose and two (3%) of 69 participants after the second dose in phase 1 had a significant increase of laboratory indicator (appendix p 11).
Table 2.
Adverse reactions reported within 28 days after the first and the second dose of vaccine or alum only in phase 1 and phase 2
| 1·5 μg group (n=219) | 3·0 μg group (n=217) | Aluminium hydroxide only group (n=114) | Total (n=550) | p value* | ||
|---|---|---|---|---|---|---|
| Solicited adverse reactions within 0–7 days | ||||||
| Any | 51 (23%) | 59 (27%) | 22 (19%) | 132 (24%) | 0·28 | |
| Grade 1 | 39 (18%) | 51 (24%) | 15 (13%) | 105 (19%) | 0·065 | |
| Grade 2 | 16 (7%) | 19 (9%) | 9 (8%) | 44 (8%) | 0·82 | |
| Grade 3 | 2 (1%) | 0 | 0 | 2 (<1%) | 0·36 | |
| Injection site adverse reactions | ||||||
| Pain | 36 (16%) | 35 (16%) | 2 (2%) | 73 (13%) | <0·0001 | |
| Grade 1 | 34 (16%) | 35 (16%) | 2 (2%) | 71 (13%) | <0·0001 | |
| Grade 2 | 2 (1%) | 0 | 0 | 2 (<1%) | 0·36 | |
| Swelling | 3 (1%) | 6 (3%) | 1 (1%) | 10 (2%) | 0·50 | |
| Grade 1 | 0 | 4 (2%) | 0 | 4 (1%) | 0·053 | |
| Grade 2 | 3 (1%) | 3 (1%) | 1 (1%) | 7 (1%) | 1·0 | |
| Induration | 0 | 2 (1%) | 0 | 2 (<1%) | 0·20 | |
| Grade 1 | 0 | 2 (1%) | 0 | 2 (<1%) | 0·20 | |
| Erythema | 0 | 1 (<1%) | 0 | 1 (<1%) | 0·60 | |
| Grade 1 | 0 | 1 (<1%) | 0 | 1 (<1%) | 0·60 | |
| Pruritus | 3 (1%) | 2 (1%) | 0 | 5 (1 %) | 0·64 | |
| Grade 1 | 3 (1%) | 2 (1%) | 0 | 5 (1%) | 0·64 | |
| Systematic adverse reactions | ||||||
| Fever | 9 (4%) | 11 (5%) | 5 (4%) | 25 (5%) | 0·93 | |
| Grade 1 | 3 (1%) | 2 (1%) | 2 (2%) | 7 (1%) | 0·89 | |
| Grade 2 | 4 (2%) | 10 (5%) | 3 (3%) | 17 (3%) | 0·22 | |
| Grade 3 | 2 (1%) | 0 | 0 | 2 (<1%) | 0·36 | |
| Cough | 5 (2%) | 8 (4%) | 5 (4%) | 18 (3%) | 0·47 | |
| Grade 1 | 1 (<1%) | 4 (2%) | 3 (3%) | 8 (1%) | 0·19 | |
| Grade 2 | 4 (2%) | 4 (2%) | 2 (2%) | 10 (2%) | 1·0 | |
| Headache | 6 (3%) | 4 (2%) | 3 (3%) | 13 (2%) | 0·82 | |
| Grade 1 | 3 (1%) | 3 (1%) | 1 (1%) | 7 (1%) | 1·0 | |
| Grade 2 | 4 (2%) | 1 (<1%) | 2 (2%) | 7 (1%) | 0·39 | |
| Anorexia | 3 (1%) | 4 (2%) | 2 (2%) | 9 (2%) | 0·92 | |
| Grade 1 | 1 (<1%) | 3 (1%) | 2 (2%) | 6 (1%) | 0·52 | |
| Grade 2 | 3 (1%) | 1 (<1%) | 0 | 4 (1%) | 0·54 | |
| Diarrhoea | 2 (1%) | 2 (1%) | 4 (4%) | 8 (1%) | 0·16 | |
| Grade 1 | 2 (1%) | 2 (1%) | 4 (4%) | 8 (1%) | 0·16 | |
| Nausea | 3 (1%) | 2 (1%) | 2 (2%) | 7 (1%) | 0·89 | |
| Grade 1 | 3 (1%) | 2 (1%) | 2 (2%) | 7 (1%) | 0·89 | |
| Mucocutaneous eruption | 2 (1%) | 2 (1%) | 1 (1%) | 5 (1%) | 1·0 | |
| Grade 1 | 1 (<1%) | 1 (<1%) | 0 | 2 (<1%) | 1·0 | |
| Grade 2 | 1 (<1%) | 1 (<1%) | 1 (1%) | 3 (1%) | 1·0 | |
| Vomiting | 3 (1%) | 1 (<1%) | 1 (1%) | 5 (1%) | 0·85 | |
| Grade 1 | 3 (1%) | 1 (<1%) | 1 (1%) | 5 (1%) | 0·85 | |
| Muscle pain | 4 (2%) | 0 | 0 | 4 (1%) | 0·078 | |
| Grade 1 | 2 (1%) | 0 | 0 | 2 (<1%) | 0·36 | |
| Grade 2 | 2 (1%) | 0 | 0 | 2 (<1%) | 0·36 | |
| Fatigue | 1 (<1%) | 1 (<1%) | 1 (1%) | 3 (1%) | 1·0 | |
| Grade 1 | 1 (<1%) | 1 (<1%) | 1 (1%) | 3 (1%) | 1·0 | |
| Grade 2 | 1 (<1%) | 0 | 0 | 1 (<1%) | 1·0 | |
| Hypersensitivity | 0 | 0 | 1 (1%) | 1 (<1%) | 0·21 | |
| Grade 1 | 0 | 0 | 1 (1%) | 1 (<1%) | 0·21 | |
| Unsolicited adverse reactions within 0–28 days | ||||||
| Any | 11 (5%) | 15 (7%) | 9 (8%) | 35 (6%) | 0·52 | |
| Grade 1 | 2 (1%) | 3 (1%) | 3 (3%) | 8 (1%) | 0·43 | |
| Grade 2 | 10 (5%) | 12 (6%) | 7 (6%) | 29 (5%) | 0·75 | |
| Overall adverse reactions within 0–28 days | ||||||
| Any | 56 (26%) | 63 (29%) | 27 (24%) | 146 (27%) | 0·55 | |
| Grade 1 | 40 (18%) | 52 (24%) | 18 (16%) | 110 (20%) | 0·16 | |
| Grade 2 | 22 (10%) | 24 (11%) | 15 (13%) | 61 (11%) | 0·67 | |
| Grade 3 | 2 (1%) | 0 | 0 | 2 (<1%) | 0·36 | |
Data are n (%), representing the total number of participants who had adverse reactions (ie, adverse events related to vaccination). Results are broken down by dose and age group in the appendix (pp 2–10).
For differences across all groups.
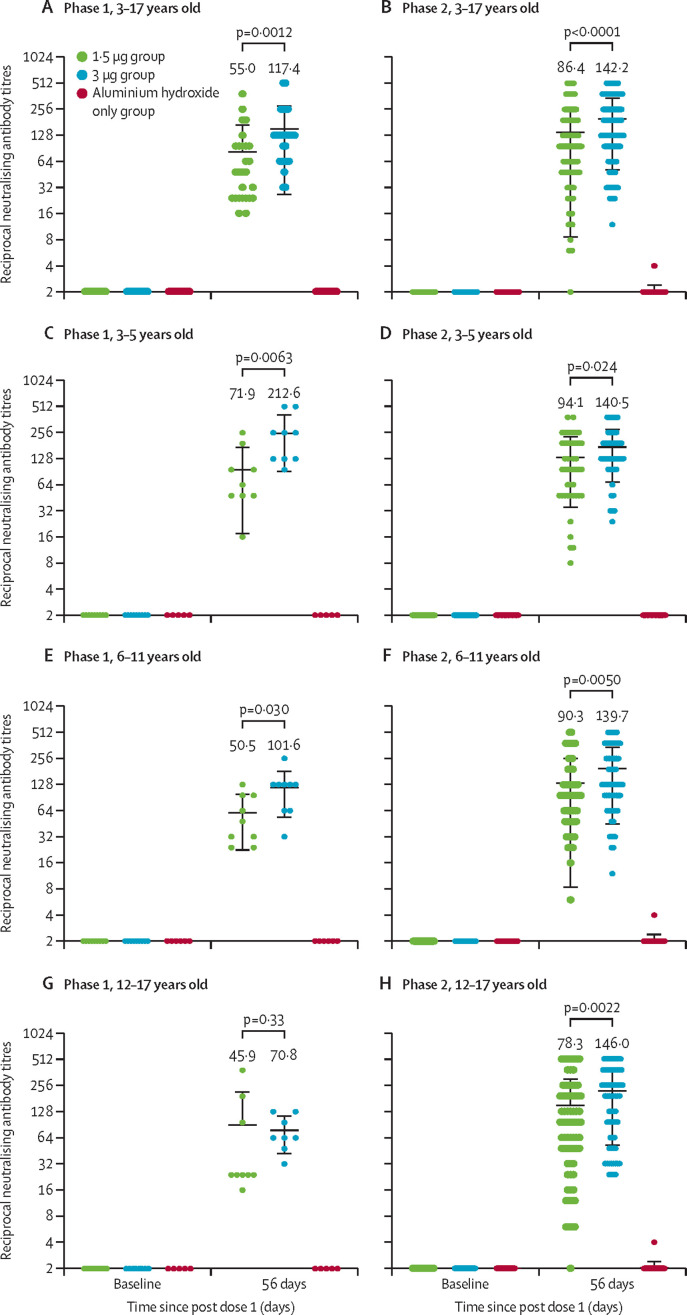
In phase 1, none of the participants had any detectable neutralising antibody response against live SARS-CoV-2 at baseline (appendix p 12). The seroconversion rates at day 28 after the second dose were 27 (100%) of 27 participants in the 1·5 μg group (GMT 55·0 [95% CI 38·9–77·9]) and 26 (100%) of 26 in the 3·0 μg group (117·4 [87·8–157·0]). The GMT of the 3·0 μg group was significantly higher than that of the 1·5 μg group (p=0·0012; table 3 , figure 2 , appendix p 12). Testing for neutralising antibodies in all alum-only recipients was negative after vaccination (appendix p 12). In an exploratory analysis by age, seroconversion rates at day 28 after the second dose of 1·5 μg or 3·0 μg vaccine were all 100% in participants aged 3–5 years, 6–11 years, and 12–17 years, with the GMTs ranging from 45·9 to 212·6 (figure 2, appendix p 14).
Table 3.
Seroconversion rates of neutralising antibody responses to live SARS-CoV-2 28 days after the second dose
|
1·5 μg group |
3·0 μg group |
Aluminium hydroxide only group |
p value |
|||||
|---|---|---|---|---|---|---|---|---|
| Rate | % (95%) CI | Rate | % (95%) CI | Rate | % (95%) CI | Three groups | 1·5-μg vs 3·0-μg group | |
| Phase 1 | ||||||||
| Total | 27/27 | 100·0% (87·2–100·0) | 26/26 | 100·0% (86·8–100·0) | 0/16 | 0·0% (0·0–20·6) | <0·0001 | 1·0 |
| 3–5 years | 9/9 | 100·0% (66·4–100·0) | 9/9 | 100·0% (66·4–100·0) | 0/5 | 0·0% (0·0–52·2) | <0·0001 | 1·0 |
| 6–11 years | 9/9 | 100·0% (66·4–100·0) | 9/9 | 100·0% (66·4–100·0) | 0/6 | 0·0% (0·0–45·9) | <0·0001 | 1·0 |
| 12–17 years | 9/9 | 100·0% (66·4–100·0) | 8/8 | 100·0% (63·1–100·0) | 0/5 | 0·0% (0·0–52·2) | <0·0001 | 1·0 |
| Phase 2 | ||||||||
| Total | 180/186 | 96·8% (93·1–98·8) | 180/180 | 100·0% (98·0–100·0) | 0/94 | 0·0% (0·0–3·9) | <0·0001 | 0·030 |
| 3–5 years | 46/46 | 100·0% (92·3–100·0) | 45/45 | 100·0% (92·1–100·0) | 0/24 | 0·0% (0·0–14·2) | <0·0001 | 1·0 |
| 6–11 years | 68/69 | 98·6% (92·2–100·0) | 68/68 | 100·0% (94·7–100·0) | 0/35 | 0·0% (0·0–10·0) | <0·0001 | 1·0 |
| 12–17 years | 66/71 | 93·0% (84·3–97·7) | 67/67 | 100·0% (94·6–100·0) | 0/35 | 0·0% (0·0–10·0) | <0·0001 | 0·059 |
Data are n/N (% [95% CI]).
Figure 2.
Antibody titres of neutralising antibodies to live SARS-CoV-2 induced after two doses of CoronaVac or aluminium hydroxide diluent only in phase 1 and phase 2 trials
GMT=geometric mean titre. The error bars indicate the 95% CI of the GMT and the spots indicate the individual antibody titres, with the number above the spots showing the GMT estimate. Only p values between 1·5 μg and 3·0 μg groups after the second vaccination are shown in the figure. All p values for all data are in the appendix (pp 12–13)
In phase 2, none of the participants had any detectable neutralising antibody response at baseline (appendix p 13). After the second dose of vaccination, the seroconversion rates were 180 (95% CI 96·8% [93·1–98·8]) of 186 participants in the 1·5 μg group (GMT 86·4 [73·9–101·0]) and 180 (100·0% [98·0–100·0]) of 180 participants in the 3·0 μg group (142·2 [124·7–162·1]). The seroconversion rate and GMT of the 3·0 μg group were higher than those of the 1·5 μg group (p=0·030 and p<0·0001; table 3, figure 2, appendix p 13). Neutralising antibodies in all alum-only recipients were negative after vaccination (appendix p 13). In an exploratory analysis by age, the seroconversion rates at day 28 after the second dose were higher than 93% in the 1·5 μg and 3·0 μg groups for participants aged 3–5 years, 6–11 years, and 12–17 years, with the GMTs ranging from 78·3 to 146·0 (figure 2, appendix p 14).
Discussion
To our knowledge, this is the first report of immunogenicity and safety of COVID-19 candidate vaccine among children as low as 3 years old. We found that two doses of the CoronaVac were safe and well tolerated at doses of 1·5 μg and 3·0 μg among children and adolescents aged 3–17 years old. The prevalence of adverse reactions in different dose groups was similar, indicating that there was no dose-related concern on safety. Most reactions were mild to moderate in severity and transient. Injection-site pain was the most reported symptom. The results were similar to our study of adults and elderly.17, 18 Furthermore, the higher grade 1 injection site pain reported by adolescents aged 12–17 years was the main reason for the higher prevalence of adverse reactions in this population compared with children aged 3–5 years and 6–11 years. None of the serious adverse events reported during the trial was related to vaccination.
CoronaVac was immunogenic in children and adolescents aged 3–17 years. The seroconversion rates of neutralising antibody in children and adolescents with both doses were over 96% after the two-dose vaccination. The GMTs of 142·2 in the 3·0 μg groups were higher than that of 86·4 in the 1·5 μg group in phase 2; however, even the GMT of 86·4 induced better immunogenicity compared with adults aged 18–59 years (44·1) and those aged 60 years and older (42·2) who received a 3·0 μg dose of vaccine with the same immunisation schedule.17, 18 Age plays an important role in antibody response to vaccine.27 Decreasing responses to vaccination with increasing age have been shown in other vaccines, such as hepatitis B vaccine, seasonal influenza, pneumococcal disease, tetanus, pertussis, and diphtheria.27, 28 The results implied that a lower dose of vaccine could induce higher immune response in children and adolescents.
In an exploratory analysis stratified by age, we did not observe significant differences in neutralising antibody responses between age groups (3–5 years, 6–11 years, and 12–17 years) after the second vaccination (appendix p 14). GMTs in phase 1 decreased with age in recipients of the same vaccine, whereas they were similar in phase 2. Small sample size might account for the change trends of GMT in phase 1. In each age group, there were significant differences in GMTs between the 1·5 μg and 3·0 μg groups after the second dose, except in the group aged 12–17 years old in phase 1. Taken together, the 3·0 μg dose of CoronaVac induced higher immune responses in all age groups compared with the 1·5 μg dose.
Evidence from various studies supports the important role of T-cell responses to SARS-CoV-2 infection,29 and such responses have been found with use of different vaccine platforms, including mRNA, viral vectors, and recombinant proteins.30 In this study, T cell responses were not assessed, which was a limitation of the study design. However, a study in Chile found a significant induction of a T-cell response characterised by the secretion of interferon-gamma following vaccination of CoronaVac in a population aged 18 years and older,19, which was different from the lower response observed in our phase 1 trial among adults aged 18–59 years.17 Another inactivated SARS-CoV-2 vaccine, BBV152, has also been reported to induced a Th1-biased response.21, 24 Future studies are needed to assess the responses of type 1 and type 2 T-helper cells by inactivated vaccines.
This study has some further limitations. First, the sample size of this study is relatively small per age group and all study populations were of Han ethnicity. Further studies will be done in different regions and multiethnic populations to collect more data to provide scientific evidence for immune strategy. Second, at the time of the report, long-term immunogenicity and safety could not be available, although the participants will be followed up for at least 1 year. Finally, the calculated p values cannot support any powerful statistical conclusions in this study, which are only for reference and should be interpreted with caution.
In conclusion, CoronaVac was well tolerated and safe, and induced humoral responses in children and adolescents aged 3–17 years. Among the two doses evaluated, the neutralising antibody titres induced by a 3·0 μg dose were higher than those of the 1·5 μg dose. The results support the use of 3·0 μg dose with a two-immunisation schedule for further studies in children and adolescents.
Data sharing
The individual participant-level data that underlie the results reported in this Article will be shared after de-identification (text, tables, figures, and appendices). This clinical trial is ongoing, and all the individual participant data will not be available until the immune persistence evaluation is completed. The data will be available immediately after publication and finalisation of the completed clinical study report for at least 6 months. Supporting clinical documents including the study protocol and statistical analysis plan and the informed consent form will be available immediately following publication of this Article for at least 1 year. Information on how to access the supporting clinical documents is available online. Researchers who provide a scientifically sound proposal will be allowed to access to the de-identified individual participant data. Proposals should be sent to the corresponding author. These proposals will be reviewed and approved by the sponsor, investigators, and collaborators on the basis of scientific merit. To gain access, data requestors will need to sign a data access agreement.
Declaration of interests
QG and XL are employees of Sinovac Life Sciences. YS, WY, and LW are employees of Sinovac Biotech. All other authors declare no competing interests.
Acknowledgments
Acknowledgments
This study was funded by the National Key Research and Development Program and Beijing Science and Technology Program.
Contributors
QL, QG, YZ, BH, and YS designed the trial and study protocol. BH, WY, and ML contributed to the literature search. All authors had access to data, and YS and QL verified the data. BH and WY wrote the first draft manuscript. QG, QL, YS, ML, XL, and YZ contributed to the data interpretation and revision of the manuscript. ZJ and QS contributed to data analysis. LW monitored the trial. QM and WJ were responsible for the site work including the recruitment, follow-up, and data collection, and ZW was the site coordinator. CL were responsible for the laboratory analysis. All the authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Supplementary Material
References
- 1.WHO WHO coronavirus disease (COVID-19) dashboard. 2021. https://covid19whoint/
- 2.Zimmermann P, Curtis N. Coronavirus infections in children including COVID-19: An overview of the epidemiology, clinical features, diagnosis, treatment and prevention options in children. Pediatr Infect Dis J. 2020;39:355–368. doi: 10.1097/INF.0000000000002660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.European Centre for Disease Prevention and Control Paediatric inflammatory multisystem syndrome and SARS-CoV-2 infection in children. May 15, 2020. https://www.ecdc.europa.eu/sites/default/files/documents/covid-19-risk-assessment-paediatric-inflammatory-multisystem-syndrome-15-May-2020.pdf
- 4.Maltezou HC, Magaziotou I, Dedoukou X, et al. Children and adolescents with SARS-CoV-2 Infection: epidemiology, clinical course and viral loads. Pediatr Infect Dis J. 2020;39:e388–e392. doi: 10.1097/INF.0000000000002899. [DOI] [PubMed] [Google Scholar]
- 5.Snape MD, Viner RM. COVID-19 in children and young people. Science. 2020;370:286–288. doi: 10.1126/science.abd6165. [DOI] [PubMed] [Google Scholar]
- 6.Kamidani S, Rostad CA, Anderson EJ. COVID-19 vaccine development: a pediatric perspective. Curr Opin Pediatr. 2021;33:144–151. doi: 10.1097/MOP.0000000000000978. [DOI] [PubMed] [Google Scholar]
- 7.Ebina-Shibuya R, Namkoong H, Shibuya Y, Horita N. Multisystem inflammatory syndrome in children (MIS-C) with COVID-19: insights from simultaneous familial Kawasaki disease cases. Int J Infect Dis. 2020;97:371–373. doi: 10.1016/j.ijid.2020.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kao CM, Orenstein WA, Anderson EJ. The importance of advancing SARS-CoV-2 vaccines in children. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang HS, Costa V, Racine-Brzostek SE, et al. Association of age with SARS-CoV-2 antibody response. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.4302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.WHO Draft landscape of COVID-19 candidate vaccines. 2021. https://wwwwhoint/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines
- 11.Creech CB, Walker SC, Samuels RJ. SARS-CoV-2 vaccines. JAMA. 2021;325:1318–1320. doi: 10.1001/jama.2021.3199. [DOI] [PubMed] [Google Scholar]
- 12.Palacios R, Batista AP, Albuquerque CSN, et al. Efficacy and safety of a COVID-19 inactivated vaccine in healthcare professionals in Brazil: the PROFISCOV study. SSRN. 2021 https://papers.ssrn.com/sol3/papers.cfm?abstract_id=3822780 published online April 14, 2021. (preprint). [Google Scholar]
- 13.Miller NS. COVID-19 vaccines in children: research to guide your news coverage. 2021. https://journalistsresourceorg/health/covid-19-vaccine-in-children/
- 14.National Institutes of Health Safety and immunogenicity study of inactivated vaccine for prevention of COVID-19. 2020. https://clinicaltrialsgov/ct2/show/NCT04551547?cond=NCT04551547&draw=2&rank=1
- 15.Pfizer Pfizer-Biontech announce positive topline results of pivotal COVID-19 vaccine study in adolescents. March 31, 2021. https://www.pfizer.com/news/press-release/press-release-detail/pfizer-biontech-announce-positive-topline-results-pivotal
- 16.Gao Q, Bao L, Mao H, et al. Development of an inactivated vaccine candidate for SARS-CoV-2. Science. 2020;369:77–81. doi: 10.1126/science.abc1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang Y, Zeng G, Pan H, et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18–59 years: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect Dis. 2021;21:181–192. doi: 10.1016/S1473-3099(20)30843-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu Z, Hu Y, Xu M, et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine (CoronaVac) in healthy adults aged 60 years and older: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect Dis. 2021;21:803–812. doi: 10.1016/S1473-3099(20)30987-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bueno SM, Abarca K, González PA, et al. Interim report: safety and immunogenicity of an inactivated vaccine against SARS-CoV-2 in healthy Chilean adults in a phase 3 clinical trial. medRxiv. 2021 doi: 10.1101/2021.03.31.21254494. published online April 1. (preprint). [DOI] [Google Scholar]
- 20.Che Y, Liu X, Pu Y, et al. Randomized, double-blinded and placebo-controlled phase II trial of an inactivated SARS-CoV-2 vaccine in healthy adults. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ella R, Vadrevu KM, Jogdand H, et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBV152: a double-blind, randomised, phase 1 trial. Lancet Infect Dis. 2021;21:637–646. doi: 10.1016/S1473-3099(20)30942-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xia S, Duan K, Zhang Y, et al. Effect of an inactivated vaccine against SARS-CoV-2 on safety and immunogenicity outcomes: interim analysis of 2 randomized clinical trials. JAMA. 2020;324:951–960. doi: 10.1001/jama.2020.15543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xia S, Zhang Y, Wang Y, et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBIBP-CorV: a randomised, double-blind, placebo-controlled, phase 1/2 trial. Lancet Infect Dis. 2021;21:39–51. doi: 10.1016/S1473-3099(20)30831-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ella R, Reddy S, Jogdand H, et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBV152: interim results from a double-blind, randomised, multicentre, phase 2 trial, and 3-month follow-up of a double-blind, randomised phase 1 trial. Lancet Infect Dis. 2021 doi: 10.1016/S1473-3099(21)00070-0. published online March 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chine National Medical Products Administration Guidelines for grading standards of adverse events in clinical trials of preventive vaccines. https://www.nmpa.gov.cn/xxgk/ggtg/qtggtg/20191231111901460html
- 26.Li YP, Liang ZL, Gao Q, et al. Safety and immunogenicity of a novel human enterovirus 71 (EV71) vaccine: a randomized, placebo-controlled, double-blind, phase I clinical trial. Vaccine. 2012;30:3295–3303. doi: 10.1016/j.vaccine.2012.03.010. [DOI] [PubMed] [Google Scholar]
- 27.Kang G, Chen H, Ma F, et al. Comparison of the effect of increased hepatitis B vaccine dosage on immunogenicity in healthy children and adults. Hum Vaccin Immunother. 2016;12:2312–2316. doi: 10.1080/21645515.2016.1172757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Van Der Meeren O, Crasta P, Cheuvart B, De Ridder M. Characterization of an age-response relationship to GSK's recombinant hepatitis B vaccine in healthy adults: an integrated analysis. Hum Vaccin Immunother. 2015;11:1726–1729. doi: 10.1080/21645515.2015.1039758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sauer K, Harris T. An effective COVID-19 vaccine needs to engage T cells. Front Immunol. 2020;11 doi: 10.3389/fimmu.2020.581807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rha MS, Kim AR, Shin EC. SARS-CoV-2-Specific T cell responses in patients with COVID-19 and unexposed individuals. Immune Netw. 2021;21:e2. doi: 10.4110/in.2021.21.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The individual participant-level data that underlie the results reported in this Article will be shared after de-identification (text, tables, figures, and appendices). This clinical trial is ongoing, and all the individual participant data will not be available until the immune persistence evaluation is completed. The data will be available immediately after publication and finalisation of the completed clinical study report for at least 6 months. Supporting clinical documents including the study protocol and statistical analysis plan and the informed consent form will be available immediately following publication of this Article for at least 1 year. Information on how to access the supporting clinical documents is available online. Researchers who provide a scientifically sound proposal will be allowed to access to the de-identified individual participant data. Proposals should be sent to the corresponding author. These proposals will be reviewed and approved by the sponsor, investigators, and collaborators on the basis of scientific merit. To gain access, data requestors will need to sign a data access agreement.