Abstract
Background
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is the cause of the ongoing global pandemic known as COVID-19. Based on the potential antiviral role of quercetin, and on its described anti-blood clotting, anti-inflammatory and antioxidant properties, we hypothesize that subjects with mild COVID-19 treated with Quercetin Phytosome® (QP), a novel bioavailable form of quercetin, may have a shorter time to virus clearance, a milder symptomatology, and higher probabilities of a benign earlier resolution of the disease.
Methods
In our 2-week, randomized, open-label, and controlled clinical study, we have enrolled 42 COVID-19 outpatients. Twenty-one have been treated with the standard of care (SC), and 21 with QP as add-on supplementation to the SC. Our main aims were to check virus clearance and symptoms.
Results
The interim results reveal that after 1 week of treatment, 16 patients of the QP group were tested negative for SARS-CoV-2 and 12 patients had all their symptoms diminished; in the SC group, 2 patients were tested SARS-CoV-2 negative and 4 patients had their symptoms partially improved. By 2 weeks, the remaining 5 patients of the QP group tested negative for SARS-CoV-2, whereas in the SC group out of 19 remaining patients, 17 tested negatives by week 2, one tested negative by week 3 and one patient, still positive, expired by day 20. Concerning blood parameters, the add on therapy with QP, reduced LDH (−35.5%), Ferritin (−40%), CRP (−54.8%) and D-dimer (−11.9%).
Conclusion
QP statistically shortens the timing of molecular test conversion from positive to negative, reducing at the same time symptoms severity and negative predictors of COVID-19.
Keywords: SARS-CoV-2, RT-PCR, LDH, ferritin, CRP, D-dimer, coronavirus, phytosome®
Introduction
The ongoing COVID-19 (coronavirus disease 2019) pandemic has affected the lives of all human beings on earth. Since December 2019, the disease has killed over 3 million people worldwide and severely changed our way of living and social interactions. Despite attempts at controlling the spread of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) this pandemic continues unabated and keep on taking more and more lives worldwide.1 To date, several apparently effective vaccines have been developed. But, due to limitations in manufacturing and distribution capacities, the desired global “herd immunity” still appears to be a distant mirage, if ever achievable at all, not to mention the fact that the constant occurrence of new viral mutations puts the effectiveness of the vaccination campaign at serious risk regardless of its speed.2 Furthermore, while enormous advancements have been made in understanding the biology of this virus and the spectrum of diseases it causes, therapeutic interventions to date have had a limited effect on the skyrocketing increase in deaths.3 While it is unlikely that we will discover a single “magic bullet” that cures COVID-19, it is more likely that a combination of agents targeting different pathways will prove to be effective.4 Furthermore, there is an urgent need for therapeutic agents that are effective across the wide spectrum of COVID-19 “infections” including pre-exposure prophylaxis, post-exposure prophylaxis, during the symptomatic phase and during the severe pneumonia and multi organ failure.5,6 The clinical experiences accumulated so far also indicate that time is of essence in treatment of the disease, and even milder treatment (like NSAIDs) - if administered at the right time, as early as possible and well before hyperinflammation is established - can have a dramatically positive impact in steering the evolution of the disease toward a benign resolution. It has been recently demonstrated that the implementation of an early home treatment protocol (based on cyclooxygenase-2 inhibitors, given early in the course of the disease at the very beginning of the onset of symptoms, even before the nasopharyngeal swab) almost eliminated the risk of hospitalization and related treatment costs.7 The development of an effective adaptive immune response can limit the SARS-CoV-2 viral infection, but the uncontrolled activation of innate immune cells results in an aggressive hyperinflammatory response with the release of an excessive amount of pro-inflammatory cytokines in a process known as “cytokine storm”.8 The cytokine storm leads to increased risk of vascular hyperpermeability, acute respiratory distress syndrome (ARDS), multiorgan failure, and eventually death when the high cytokine concentrations are unabated over time. The cytokine storm is believed to be the major underlying cause of large number of COVID-19 deaths. Therefore, a timely intervention of well tolerated agents that can modulate or prevent the cytokine storm and the viral replication imbalance are potentially the strategies to prevent the severe COVID-19 disease development.9 Quercetin (Figure 1), a flavonoid with an excellent safety profile, has powerful antioxidant, anti-inflammatory, immunomodulatory and antiviral properties, and can potentially help in the early stage of SARS-CoV-2 viral infection to prevent disease development and progression.10,11 Its excellent safety profile allows widespread use in the early phase of the disease or when it is suspected, starting even before a confirmatory nasal swab is obtained. Quercetin acts as a free radical scavenger, and both in vitro and in vivo studies showed quercetin as a potent antioxidant.12 Quercetin supplementation of the diet of mice infected with the influenza virus significantly reduced the levels of both superoxide radicals and lipid peroxidation products, suggesting that quercetin may be useful as an anti-viral therapy to alleviate the cyto-pathological effects of virus infections.13 In addition, quercetin has anti-inflammatory properties which include inhibition of lipid peroxidation, and inhibitory effects on pro-inflammatory mediators such as lipoxygenase and phospholipase A2. This anti-inflammatory effect is mediated in part by flavonoid activity on arachidonic acid metabolism and the associated leukotriene/prostaglandin pathways. Furthermore, quercetin has been shown to downregulate LPS stimulated release of tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6) and IL-1 from macrophages.14 The inhibition of pro-inflammatory cytokines may be particularly important in the pulmonary phase of COVID-19 (cytokine storm).15 C reactive protein (CRP) is an inflammatory biomarker reflective of pro-inflammatory cytokine levels, particularly for IL-6. CRP is one of the most important prognostic markers in patients with COVID-19 disease.16 In a metagenomics analysis, supplementation with quercetin significantly reduced CRP levels, supporting its role as an anti-inflammatory agent.17 Quercetin exhibits important immunomodulatory properties, which could be important in subjects with SARS-CoV-2 infection. Quercetin stimulates T-helper cells to produce (Th-1)-derived Interferon-γ (IFN- γ) and down-regulates Th2-derived IL-4 when added to cultured blood peripheral mononuclear cells.18 Studies in mice demonstrate that quercetin induced enhanced NK cell lytic activity, neutrophil chemotaxis, and lymphocyte proliferation.19 This is likely of critical importance in patients with COVID-19 infection as SARS-CoV-2 inhibits NK cell activity at multiple steps.20 Finally, although quercetin’s precise antiviral mechanisms are not yet completely understood, there is a considerable body of evidence supporting the broad antiviral properties of quercetin, demonstrated both in in vitro and in vivo studies through inhibition of viral replication of several respiratory viruses, including influenza virus, parainfluenza virus, respiratory syncytial virus, adenovirus, and rhinovirus.21 As for most of polyphenols, the practical use of quercetin is limited by its low solubility and reduced oral absorption.22,23 Recently, quercetin formulated with sunflower lecithin has been demonstrated in humans to attain high plasma levels, up to 20 times greater than those usually obtained following the administration of an equal dose of unformulated quercetin, without any notable side effects.24 Since the bioavailable form of quercetin showed promising potential as an anti-COVID-19 candidate in symptomatic patients,25 we have carried out a second prospective, randomized, controlled, open-label study at the King Edward Medical University, Lahore, Pakistan, on 42 outpatients who had been infected with SARS-CoV-2 prior to the start of the study but were not severely symptomatic at enrollment. We hypothesized that subjects with mild COVID-19, treated with QP plus the standard of care (SC), would have had a shorter time to virus clearance, a reduced symptomatology than the control group and better reduction of some blood parameters (Lactate dehydrogenase, Ferritin, CRP, D-dimer) considered to be possible negative predictors of COVID-19.
Figure 1.
Chemical structure of quercetin.
Materials and Methods
Participants
Between December 2020 and March 2021, 42 outpatients not severely affected by COVID-19 were enrolled at the Department of Medicine, King Edward University, Lahore, Pakistan. The inclusion criteria include 18 years or older of either gender, tested positive for COVID-19 shown by polymerase chain reaction (PCR), with oxygen saturation higher than 93%, and demonstrating symptoms associated with COVID-19 including fever, dyspnea, persistent dry cough, sore throat, myalgia, weakness, cold, rhinorrhea, and conjunctivitis of mild/moderate grade to be manageable with pharmacological therapy at home. The exclusion criteria were receiving anti-retroviral therapy or immune system booster medications in the last 3 months, glucose-6-phosphate dehydrogenase (G6PD) deficiency, end-stage renal disease, and terminal cancer.
Study Arms, Tested Product, and Treatment Plans
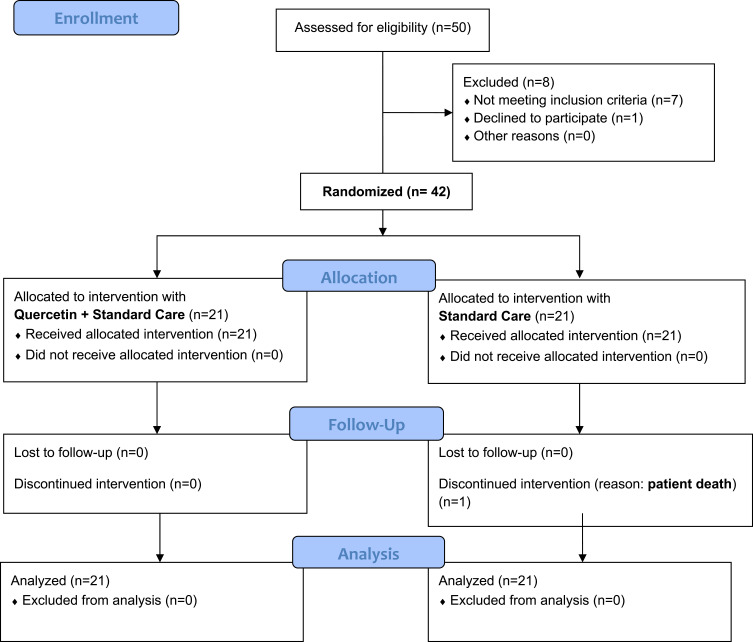
After randomization process (Block Randomization Algorithm), the 42 subjects were divided into two groups of 21 subjects each. A detailed flow diagram of the study is reported in Figure 2. The first group were prescribed with a SC, to be performed at home, constituted by analgesics/anti-fevers and antibiotics, as established by the hospital guidelines (acetaminophen 500–1000 mg/dose if body temperature was higher than 37.5 °C with a maximum daily dosage of 3 g/die; azithromycin 500 mg/die for 3 consecutive days). This group was indicated as SC. The second group were prescribed, along with the same standard of care foreseen for the first group, with an adjunctive daily supplementation, lasting 14 days, constituted in the first 7 days by 3 tablets (morning, afternoon, evening) containing quercetin formulated with sunflower lecithin. Each tablet (Quevir®, a dietary supplement notified by Pharmextracta SpA, Italy, to the Italian Health Authorities on August 3rd, 2020 with document number I.5.i.h.2/2020/103806) contained 500 mg of a novel lecithin-based delivery form of quercetin, named Quercetin Phytosome® (QP) developed by Indena SpA, Milan, Italy. In each tablet, 500 mg of QP corresponded to 200 mg of quercetin; therefore, each daily supplementation corresponded to 600 mg of quercetin. The following 7 days, the outpatients were treated daily with 2 tablets/day. This group was indicated as QP. After being visited, diagnosed, and prescribed with the therapeutic scheme, SC, or SC plus QP, each subject was sent back home. Following first visit, finalized to enrollment, the following visits were established to happen after 7 and 14 days. Along the study, all enrolled patients were provided with a telephone number to be in a daily direct contact with the medical doctors involved in the study and to inform them about their health conditions (body temperature, saturimetry, and general conditions like short breath, cough, asthenia, fatigue, myalgia, insomnia, flu-like symptoms). Patients were also invited to immediately require further cures, including hospitalization, in case of minimal worsening or side effects occurrence.
Figure 2.
Flow Diagram of the study.
Aim and Endpoints of the Study
The main aim of our pilot, 2-week, prospective, randomized, controlled and open-label study was to highlight the global impact of QP on COVID-19 outpatients. According to this aim, primary endpoints were: 1) time needed to become negative at the RT-PCR for SARS-CoV-2; 2) course of C-reactive protein (CRP), lactate dehydrogenase (LDH), ferritin, D-dimers, hemoglobin, white blood cells (WBC), platelets (PL), neutrophils (N) and lymphocytes (L); 3) course of COVID-19 related symptoms; 4) need of hospitalization. Secondary endpoints were: 1) adherence to therapy, 2) tolerability, and 3) side effects as a direct consequence of the therapy.
Ethical Considerations
In accordance with the Declaration of Helsinki (7th revision, October 2013), written informed consent was obtained from all participants before initiation of the study. The patients were assured that declining to participate in the study or leaving the study at any time would not affect the quality of their treatment and that they would thereafter receive the best care possible. The study protocol was approved by the Department of Medicine, King Edward University, Lahore, Pakistan, via document number 192/RC/KEMU. The trial has been registered on clinicaltrial.gov with identifier number as NCT04861298.
Statistical Analysis
The statistical evaluation of data was performed by Between-Within (B-W) Two-Factor Mixed Design Anova (Split-Plot Design), a Mixed Analysis of Variance Design for the difference in mean, the only probabilistic model correctly applicable to the type of experimental design to be evaluated between groups (SC versus QP) and within observation periods (Day 1 and Day 7). In presence of significant interaction, it was applied Tukey’s Multiple HSD Comparison Test for non-confounded means to identify, among the observed mean, any differences within the planned observation periods and between the two groups to be compared. All comparisons were conducted at p<0.05 level. The variables analyzed with this procedure were CPR, LDH, Ferritin and D-Dimers. For RT-PCR, Pearson’s Chi-Square statistic was used. To study the variation in frequency of symptoms after seven days of treatment, the pseudo discrete-time Markov chain (DTMC) was applied to examine the “permanence” or “state migration” of the subject’s and symptoms during the programmed study period. At the end of the trial, the experimental times Day 1 and Day 7, we created a contingency table illustrating the changes in frequency of the number of symptoms that each patient had suffered. The analysis then allowed us to define the subjects; healed: these are patients who show one or more symptoms at Day 1, but no symptoms (0) at Day 7; improved: these are patients who show fewer symptoms at Day 7 than those revealed at Day 1; unchanged: these are patients who are not affected by changes in their symptom frequency between the two periods; worsened: these are patients with a higher number of symptoms at Day 7 than at Day 1. We considered asymptomatic those patients with no symptoms at either Day 1 or Day 7. The results obtained from this methodology were compared, between groups, using Chi-Square Test (Pearson and Likelihood Ratio). The statistical report is available on request.
Results
The study (Figure 2) compared two groups of treatment constituted by 21 outpatients each: a SC group and a second group (QP) where, in addition to the SC, the subjects were supplemented for the first 7 days with 3 tablets/day and for the following 7 days with 2 tablets/day of a product containing, as active ingredient, 500 mg of formulated quercetin per tablet (for further details, see MM section). In both groups, all the participants who were randomly assigned, received the intended treatment, and were analyzed for the primary and secondary outcomes. Moreover, for each group, no patients quit or was excluded after randomization. During the study, in both groups no harms and/or unintended effects occurred. The sample under examination, 42 patients, has a mean age of 49.3 ± 16.5 years (45.8 ± 14.9 for the 22 females and 53.2 ± 17.7 for the 20 males). The modal age group is between 50–60 years with 24% of the total cases (data not shown). As reported in Table 1, at enrollment the two groups were shown to be overlapping for all parameters considered, including number of subjects, sex, comorbidities (hypertension, type 2 diabetes, heart disease, kidney disease, hypercholesterolemia, allergy), and observed symptoms, except for the age that in the SC group demonstrated to be, on average, higher than in the QP one. At enrollment, the most common observed symptoms were fever, cough, myalgia, and sore throat. In all patients, these were not severe, and in no case was fever higher than 39.5 °C with an average value for both groups of about 38.3 °C (data not shown). Less common symptoms were shortness of breath, headache, flu-like symptoms, weakness, and fatigue. At enrollment, D-dimer, Ferritin, LDH and CPR showed, between groups, not significant differences (data not shown). At enrollment, patients under therapy with antihistamine drugs, statins, metformin, proton pump inhibitors, acetyl salicylic acid, and antihypertensive drugs, were 2, 4, 1, 3, 2, 2 for SC group, and 2, 4, 1, 3, 3, 3 for QP group, respectively (data not shown). As shown in Table 2, the adjuvant treatment with QP significantly reduced the virus persistence by 76% in the first week (versus 9.5% in the SC group). After 2 weeks, all subjects of the QP group tested negative, whereas 2 patients of the SC group were shown to still be positive. One week later, all living patients (20) of the SC group tested negative as well. Regarding symptoms (see Table 1 for the complete list), we considered healed those patients who manifested on Day 1 one or more symptoms, but no symptoms on Day 7, improved those patients who show fewer symptoms on Day 7 than on Day 1, and unchanged those patients not affected, between the two periods, by variations in their symptoms’ frequency. In this case as well, we observed, after just 1 week of treatment, better clinical outcomes in the QP group than in the SC one, with 57% of symptoms-free patients (versus 19%). After 2 weeks of treatment the two groups showed not significant difference as regards to symptoms, most of patients of both groups being symptoms-free (data not shown). As regards to potential biomarkers for predicting disease severity, including CRP, LDH, ferritin, D-dimer, hemoglobin, white blood cells, platelets, neutrophils and lymphocytes, no significant difference were observed at enrollment (data not shown). A highly significant reduction was observed after 1 week of supplementation with QP for lactate dehydrogenase (LDH) and ferritin (see Table 2). All other blood parameters resulted in no significantly difference between the two groups, although in the QP group a light but not significant increase of neutrophils and lymphocytes was observed after 1 week of treatment (data not shown). One patient (sex: female; age: 69; affected by hypertension, type 2 diabetes, and chronic kidney disease), died (day 20 from enrollment) after being hospitalized and admitted in intensive care unit. As regards to secondary endpoints, adherence to add-on supplementation was more than 95%; QP was generally well tolerated with no apparent toxicity; no peculiar side effects were reported by the patients and the few cases of gastric pain and reflux, constipation, diarrhea, meteorism, flatulence and sleep disorders were self-resolving in few days and similarly occurred in the SC group demonstrating that they likely could not be attributed to the use of QP (data not shown). Conversely, most patients of the QP group orally reported to investigators clear beneficial effects like a reduction of fatigue and tiredness and appetite improvement.
Table 1.
Outpatients’ Sex, Age, Comorbidities, and Symptoms at Time of Enrollment
| Group SC | Group QP | p | |
|---|---|---|---|
| N | 21 | 21 | n. s. |
| Sex: Male/Female | 10/11 | 10/11 | n. s. |
| Age | 56.2 ± 3.3 | 42.5 ± 3.3 | 0.0056 |
| Comorbidities* | |||
| > 2 | 2 | 2 | n. s. |
| > 1 | 5 | 4 | n. s. |
| 1 | 5 | 4 | n. s. |
| 0 | 9 | 11 | n. s. |
| Symptoms | |||
| Fever | 19 | 17 | n. s. |
| Cough | 12 | 11 | n. s. |
| Myalgia | 10 | 12 | n. s. |
| Dyspnoea | 3 | 3 | n. s. |
| Sore throat | 8 | 11 | n. s. |
| Headache | 0 | 1 | n. s. |
| Flu-like | 2 | 2 | n. s. |
| Weakness | 1 | 1 | n. s. |
Notes: Age is expressed as mean ± standard deviation; *Observed comorbidities were: hypertension, type 2 diabetes, heart disease, kidney disease, hypercholesterolemia, asthma/allergy.
Abbreviations: SC, standard care; QP, formulated quercetin (+Standard Care); n. s., not significant.
Table 2.
Clinical Outcomes in the Two Groups
| Group SC | Group QP | p | |
|---|---|---|---|
| RT-PCR (positive subjects) | 0.0002 | ||
| At enrollment | 21/21 (100%) | 21/21 (100%) | |
| At day 7 | 19/21 (90.5%) | 5/21 (24%) | |
| At day 14 | 4/21 (19%) | 0/21 (0%) | |
| At day 21 | 0/20 (0%) | 0/21 (0%) | |
| Symptoms variation° | 0.0118 | ||
| Healed | 4/21 (19%) | 12/21 (57%) | |
| Improved | 17/21 (81%) | 8/21 (38%) | |
| Unchanged | 0/21 (0%) | 1/21 (5%) | |
| CRP* (mg/L) | n. s. | ||
| At enrollment | 30.5± 27.9 | 27.2 ± 27.0 | |
| Day 7 | 18.1± 22.9 | 12.3 ± 16.5 | |
| LDH* (U/L) | 0.0001 | ||
| At enrollment | 364.9± 139.9 | 418.6 ± 192.9 | |
| Day 7 | 327.6± 128.9 | 270.0 ± 119.6 | |
| Ferritin* (ng/mL) | 0.0029 | ||
| At enrollment | 687.8± 879.1 | 532.7 ± 264.9 | |
| Day 7 | 557.5± 642.6 | 319.7 ± 151.6 | |
| D-dimer* (ng/mL) | n. s. | ||
| At enrollment | 282.0 ± 240.0 | 211.5 ± 65.7 | |
| Day 7 | 183.6 ± 111.8 | 186.3 ± 50.8 | |
| Hospitalized patients | 1/21 (4.8%) | 0/21 (0%) | n. s. |
| Patients in ICU | 1/21 (4.8%) | 0/21 (0%) | n. s. |
| Deaths | 1/21 (4.8%) | 0/21 (0%) | n. s. |
Notes: °Regarding to symptoms, “healed” are those patients who manifest on Day 1 one or more symptoms, but no symptoms on Day 7; “improved” are those patients who show fewer symptoms on Day 7 than on Day 1; “unchanged” are those patients not affected, between the two periods, by variations in their symptoms’ frequency. *Values are expressed as mean ± standard deviation.
Abbreviations: SC, standard care; QP, formulated quercetin (+Standard Care); RT-PCR, real-time reverse-transcriptase polymerase chain reaction; LDH, lactate dehydrogenase; ICU, intensive care unit; n. s., not significant.
Discussion
The pandemic of SARS-CoV-2 is rapidly growing all over the world with infection’s frequency, hospitalization, and death developing very often different epidemiologic pattern. Containment of the viral spread and reducing mortality have certainly been the two major areas of commitments against the pandemic. But these well-justified efforts have not addressed the ambulatory patient who is at risk for hospitalization and death. A proper focus must be directed to the attempt of treatment in the days or weeks before hospitalization could occur. Indeed, most patients who arrive at the hospital by emergency medical services with COVID-19 do not initially require forms of advanced medical care.26 Differently, once hospitalized, about 25% of them require mechanical ventilation, advanced circulatory support, or renal replacement therapy. Hence, it is conceivable that some, if not a majority, of hospitalizations could be avoided with a treat-at-home first approach.27 In outpatients with confirmed infection, the following principles could be deployed depending on the clinical manifestations of illness: 1) reduction of risk of transmission, 2) possible use of antiviral, antibiotic and corticosteroid therapies, and 3) use of antiplatelet/antithrombotic drugs. The use of immunomodulating nutraceuticals, including zinc, vitamin C, lactoferrin and quercetin, as adjuvant, have been also suggested.28–31 Quercetin is a natural substance that has multiple pharmacological properties, such as anti-inflammatory action, and is worldwide used as a dietary supplement. There is some recent evidence of the anti-coronavirus activities of this compound, including against SARS-CoV-2 main proteases and S-protein.25 Its assumed ability to inhibit coronavirus and its well-described anti-inflammatory role make quercetin a possible new candidate for outpatients’ treatment of COVID-19.32 We have therefore tested as adjuvant supplementation an orally bioavailable form of quercetin in a pilot, randomized, controlled and open-label clinical study in which we have enrolled 42 ambulatory COVID-19 patients. Our results demonstrated the beneficial role played by QP after only 1 week of add-on therapy. In particular, the use of QP at the dose of 1500 mg/day for 1 week followed using 1000 mg/day for another week (corresponding to 600 and 400 mg of quercetin per day, respectively), has demonstrated to significantly: 1) increase the clearance of the virus, 2) reduce the symptoms occurrence, 3) improve disease biomarkers. We do not know the reason why the QP group has shown a better clearance of the virus. Recent data on a large cohort of well-characterized patients hospitalized at a single University Hospital in Milano (Italy), have correlated the viral clearance with clinical and biochemical features of COVID-19 patients.33 Indeed, in our study we have got both clinical and biochemical improvement in the QP group. Moreover, in a previous clinical trial performed in COVID-19 patients, we have got important clinical improvement in patients treated with QP.34 Of course, we cannot establish which observed effect precedes the other, but we can assume that the antiviral and immunomodulating effect of quercetin have promoted a better viral clearance, and that early viral elimination have determined the better course of symptoms. Concerning disease biomarkers, a recent study has demonstrated that laboratory parameters such as CRP, LDH or Ferritin could be considered as predictors of worsening and/or death in COVID-19 patients.35 The study demonstrated that these parameters were higher in patients who did not survive to COVID-19 disease. In fact, a statistically significant increase was observed in the group of 72 non-survivors among the 1270 patients evaluated with increase in CRP (+76.2 mg/L, +76.0%), LDH (+175 U/L, +39.5%) and Ferritin (+878.5 ng/mL, +61.5%). Our study showed, as far as we know for the first time, that a nutraceutical, QP, significantly reduced LDH (−148.6 U/L, −35.5%) and Ferritin (−213 ng/mL, −40%) and reduced, although not significantly, CRP (−14.9 mg/L, −54.8%) and D-dimer (−25.2 ng/mL, −11.9%). Of course, we can assume that also the disease biomarkers reduction observed in the QP group follow the viral clearance. As regards to safety, the use of QP was very well tolerated and not inducing side effects different from those observed in the SC group. Its use was also reported by the subjects of the QP group to promote anti-fatigue and pro-appetite effects. Unfortunately, during the drafting of the protocol we did not foresee the possible anti-asthenic and pro-appetite effect of QP. We therefore did not envisage the use of an appropriate questionnaire that could have highlighted these two aspects, not only in a “narrative” but more “objective” way. It is therefore not possible for us to quantify these two results. These findings, evaluated in their entirety and complexity, suggest for quercetin an important adjuvant role capable to attenuate and likely slow the disease progression and indirectly underline once more the importance of herbal medicine in counteracting COVID-19.36
Limitations
We are perfectly aware of the pragmatic nature of our study and of the possible limits of our results, having a small number of patients per group, and not being obtained in double-blind and in placebo-controlled conditions. Anyway, these results are preliminary, and the study is still ongoing. Indeed, we guess to be able to enroll about 250 outpatients per group by the end of September 2021. Our desire to anticipate these results, despite their evident preliminary nature, is linked to their relevance for outpatient management strategies for non-seriously ill patients. In fact, we believe that significantly reducing the time to get negative swab, as confirmed by the molecular test, as well as the evident and early reduction in the frequency of symptoms typically characterizing the initial stages of COVID-19, are results that must be communicated as soon as observed. Our findings seem also to suggest that some, but not all, COVID-19 biomarkers could be positively affected by the QP supplementation as we have observed for LDH and ferritin. We do not know the reason why other biomarkers were not significantly modified by the treatment. A possible assumption could rely on the small number of the so far enrolled patients along with their not severe conditions. We hope that enlargement of the number of participants could widen the impact of the treatment to other biomarkers. Last, notwithstanding the randomization process at enrollment, the average age of subjects resulted to be different in the two groups with older people being in the SC one. We do know that age is an important parameter in COVID-19 studies. However, analysis of the statistical relationship between the course of symptoms and the age, suggest these two as not correlated but independent variables. We guess that the enlargement of clinical sample of our study likely will reduce the age differences between the groups.
Conclusions
According to the preliminary results obtained in our 2-week, still-ongoing, prospective, randomized, controlled, and open-label study, in which we have enrolled 42 COVID-19 symptomatic outpatients, the use as adjuvant supplementation of a daily dose of 1500 mg/day (first week) and of 1000 mg/day (second week) of QP, significantly improves some of the clinical outcomes considered (virus clearance, symptoms frequency, LDH, ferritin) at the same time being very-well tolerated by users.
Acknowledgments
We wish to thank Prof. Martino Recchia for the contribution given to the execution of the statistical analysis and Indena S.p.A. and Pharmextracta S.p.A. for supporting the study.
Data Sharing Statement
Data related to this manuscript can be made available from the corresponding author upon reasonable request.
Disclosure
FDP belongs to the Scientific Board of Pharmextracta. AB is a Pharmextracta consultant. PA, ST and AR belong to the Scientific Board of Indena. ST & PA are employees of Indena SpA, producer of Quercetin Phytosome (ingredient used in the trial). AR reports a patent WO2019016146A1 pending. The other authors do not claim possible conflicts of interest.
References
- 1.Bulut C, Kato Y. Epidemiology of COVID-19. Turk J Med Sci. 2020;50(SI–1):563–570. doi: 10.3906/sag-2004-172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sharma O, Sultan AA, Ding H, Triggle CR. A Review of the progress and challenges of developing a vaccine for COVID-19. Front Immunol. 2020;11:585354. doi: 10.3389/fimmu.2020.585354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Singh SP, Pritam M, Pandey B, Yadav TP. Microstructure, pathophysiology, and potential therapeutics of COVID-19: a comprehensive review. J Med Virol. 2021;93(1):275–299. doi: 10.1002/jmv.26254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jean SS, Lee PI, Hsueh PR. Treatment options for COVID-19: the reality and challenges. J Microbiol Immunol Infect. 2020;53(3):436–443. doi: 10.1016/j.jmii.2020.03.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shanmugaraj B, Siriwattananon K, Wangkanont K, Phoolcharoen W. Perspectives on monoclonal antibody therapy as potential therapeutic intervention for Coronavirus disease-19 (COVID-19). Asian Pac J Allergy Immunol. 2020;38(1):10–18. doi: 10.12932/AP-200220-0773 [DOI] [PubMed] [Google Scholar]
- 6.Toniati P, Piva S, Cattalini M, et al. Tocilizumab for the treatment of severe COVID-19 pneumonia with hyper-inflammatory syndrome and acute respiratory failure: a single center study of 100 patients in Brescia, Italy. Autoimmun Rev. 2020;19(7):102568. doi: 10.1016/j.autrev.2020.102568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Suter F, Consolaro E, Pedroni S, et al. A simple, home-therapy algorithm to prevent hospitalization for covid-19 patients: a retrospective observational matched-cohort study. medRxiv Preprint. doi: 10.1101/2021.03.25.21254296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hu B, Huang S, Yin L. The cytokine storm and COVID-19. J Med Virol. 2021;93(1):250–256. doi: 10.1002/jmv.26232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jamilloux Y, Henry T, Belot A, et al. Should we stimulate or suppress immune responses in COVID-19? Cytokine and anti-cytokine interventions. Autoimmun Rev. 2020;19(7):102567. doi: 10.1016/j.autrev.2020.102567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Derosa G, Maffioli P, D’Angelo A, Di Pierro F. A role for quercetin in coronavirus disease 2019 (COVID-19). Phytother Res. 2021;35(3):1230–1236. doi: 10.1002/ptr.6887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.D’Andrea G. Quercetin: a flavonol with multifaceted therapeutic applications? Fitoterapia. 2015;106:256–271. doi: 10.1016/j.fitote.2015.09.018 [DOI] [PubMed] [Google Scholar]
- 12.Taslidere E, Dogan Z, Elbe H, Vardi N, Cetin A, Turkoz Y. Quercetin protection against ciprofloxacin induced liver damage in rats. Biotech Histochem. 2016;91(2):116–121. doi: 10.3109/10520295.2015.1085093 [DOI] [PubMed] [Google Scholar]
- 13.Ullah A, Munir S, Badshah SL, et al. Important flavonoids and their role as a therapeutic agent. Molecules. 2020;25(22):5243. doi: 10.3390/molecules25225243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li Y, Yao J, Han C, et al. Quercetin, inflammation and immunity. Nutrients. 2016;8(3):167. doi: 10.3390/nu8030167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moradian N, Gouravani M, Salehi MA, et al. Cytokine release syndrome: inhibition of pro-inflammatory cytokines as a solution for reducing COVID-19 mortality. Eur Cytokine Netw. 2020;31(3):81–93. doi: 10.1684/ecn.2020.0451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen W, Zheng KI, Liu S, Yan Z, Xu C, Qiao Z. Plasma CRP level is positively associated with the severity of COVID-19. Ann Clin Microbiol Antimicrob. 2020;19(1):18. doi: 10.1186/s12941-020-00362-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mohammadi-Sartang M, Mazloom Z, Sherafatmanesh S, Ghorbani M, Firoozi D. Effects of supplementation with quercetin on plasma C-reactive protein concentrations: a systematic review and meta-analysis of randomized controlled trials. Eur J Clin Nutr. 2017;71(9):1033–1039. doi: 10.1038/ejcn.2017.55 [DOI] [PubMed] [Google Scholar]
- 18.Tanaka Y, Furuta A, Asano K, Kobayashi H. Modulation of Th1/Th2 cytokine balance by quercetin in vitro. Medicines (Basel). 2020;7(8):46. doi: 10.3390/medicines7080046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bae JH, Kim JY, Kim MJ, et al. Quercetin enhances susceptibility to NK cell-mediated lysis of tumor cells through induction of NKG2D ligands and suppression of HSP70. J Immunother. 2010;33(4):391–401. doi: 10.1097/CJI.0b013e3181d32f22 [DOI] [PubMed] [Google Scholar]
- 20.Zhou L, Huntington K, Zhang S, et al. Natural Killer cell activation, reduced ACE2, TMPRSS2, cytokines G-CSF, M-CSF and SARS-CoV-2-S pseudovirus infectivity by MEK inhibitor treatment of human cells. bioRxiv [Preprint]. 2020;2020. doi: 10.1101/2020.08.02.230839. [DOI] [Google Scholar]
- 21.Brito JCM, Lima WG, Cordeiro LPB, da Cruz Nizer WS. Effectiveness of supplementation with quercetin-type flavonols for treatment of viral lower respiratory tract infections: systematic review and meta-analysis of preclinical studies. Phytother Res. 2021. doi: 10.1002/ptr.7122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gao L, Liu G, Wang X, Liu F, Xu Y, Ma J. Preparation of a chemically stable quercetin formulation using nanosuspension technology. Int J Pharm. 2011;404(1–2):231–237. doi: 10.1016/j.ijpharm.2010.11.009 [DOI] [PubMed] [Google Scholar]
- 23.Wang W, Sun C, Mao L, et al. The biological activities, chemical stability, metabolism, and delivery systems of quercetin: a review. Trends Food Sci Technol. 2016;56:21–38. doi: 10.1016/j.tifs.2016.07.004 [DOI] [Google Scholar]
- 24.Riva A, Ronchi M, Petrangolini G, Bosisio S, Allegrini P. Improved Oral Absorption of quercetin from quercetin phytosome®, a new delivery system based on food grade lecithin. Eur J Drug Metab Pharmacokinet. 2019;44(2):169–177. doi: 10.1007/s13318-018-0517-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Di Pierro F, Khan A, Bertuccioli A, et al. Quercetin Phytosome® as a potential candidate for managing COVID-19. Minerva Gastroenterol Dietol. 2020. doi: 10.23736/S1121-421X.20.02771-3 [DOI] [PubMed] [Google Scholar]
- 26.Yang BY, Barnard LM, Emert JM. Clinical characteristics of patients with coronavirus disease 2019 (COVID-19) receiving emergency medical services in King County, Washington. JAMA Netw Open. 2020;3(7):7. doi: 10.1001/jamanetworkopen.2020.14549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Argenziano MG, Bruce SL, Slater CL. Characterization and clinical course of 1000 patients with coronavirus disease 2019 in New York: retrospective case series. BMJ. 2020;369:m1996. doi: 10.1136/bmj.m1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McCullough PA, Kelly RJ, Ruocco G, et al. Pathophysiological basis and rationale for early outpatient treatment of SARS-CoV-2 (COVID-19) infection. Am J Med. 2021;134(1):16–22. doi: 10.1016/j.amjmed.2020.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Holford P, Carr AC, Jovic TH, et al. Vitamin C-an adjunctive therapy for respiratory infection, sepsis and COVID-19. Nutrients. 2020;12(12):3760. doi: 10.3390/nu12123760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chang R, Ng TB, Sun WZ. Lactoferrin as potential preventative and adjunct treatment for COVID-19. Int J Antimicrob Agents. 2020;56(3):106118. doi: 10.1016/j.ijantimicag.2020.106118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Colunga Biancatelli RML, Berrill M, Catravas JD, Marik PE. Quercetin and vitamin C: an experimental, synergistic therapy for the prevention and treatment of SARS-CoV-2 related disease (COVID-19). Front Immunol. 2020;11:1451. doi: 10.3389/fimmu.2020.01451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Diniz LRL, Souza MTS, Duarte ABS, Sousa DP. Mechanistic aspects and therapeutic potential of quercetin against COVID-19-associated acute kidney injury. Molecules. 2020;25(23):5772. doi: 10.3390/molecules25235772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Farina N, Ramirez GA, De Lorenzo R, et al. COVID-19: pharmacology and kinetics of viral clearance. Pharmacol Res. 2020;161:105114. doi: 10.1016/j.phrs.2020.105114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Di Pierro F, Derosa G, Maffioli P, et al. Possible therapeutic effects of adjuvant quercetin supplementation against early stage COVID-19 infection: a prospective, randomized, controlled, and open-label study. Int J Gen Med. 2021;14:1–8. doi: 10.2147/IJGM.S318720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guan X, Zhang B, Fu M, et al. Clinical and inflammatory features-based machine learning model for fatal risk prediction of hospitalized COVID-19 patients: results from a retrospective cohort study. Ann Med. 2021;53(1):257–266. doi: 10.1080/07853890.2020.1868564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vicidomini C, Roviello V, Roviello GN. Molecular basis of the therapeutical potential of clove (Syzygium aromaticum L.) and clues to its anti-COVID-19 utility. Molecules. 2021;26(7):1880. doi: 10.3390/molecules26071880 [DOI] [PMC free article] [PubMed] [Google Scholar]