Abstract
Background
COVID-19 infections are still at pandemic levels globally and there are currently no specific drugs to treat these infections. Previous studies have demonstrated that serum albumin levels were abnormally low in COVID-19 patients and might be used as a prognosis biomarker. Supplemental albumin has been used as an experimental therapeutic method. However, dynamic evaluation of albumin in patients with COVID-19 was limited and whether serum albumin could predict the prognosis of these patients is unknown.
Methods
We enrolled 79 COVID-19 patients in the present study and reviewed electronic medical laboratory records. Data was processed using SPSS software (Version 20.0) and correlation analysis was performed between serum albumin and other clinical and laboratory findings.
Results
Serum albumin levels were gradually decreased both in severe and non-severe COVID-19 patients. Moreover, 17.7% of the patients presented with hypoalbuminemia at least one time during 3 consecutive weekly time points. The hypoalbuminemia group displayed more severe disease and comorbidity that included fever, fatigue, headache, and dizziness on admission. Moreover, serum albumin levels were positively correlated with lymphocyte and RBC numbers, Hb and prealbumin levels as well as with total T cell numbers and the presence of CD4+ and CD8+ T cells. In contrast, there was a negative correlation with C-reactive protein levels and this was an indicator of patient recovery.
Conclusion
Our results demonstrated that hypoalbuminemia was common in COVID-19 patients and its levels were linked to disease severity. Patients with fever, fatigue and headache or dizziness on admission were more likely to experience hypoalbuminemia. Dynamic monitoring of serum albumin is therefore necessary and should be performed during COVID-19 patient treatments as a tool for evaluating the prognosis of COVID-19 infections.
Keywords: COVID-19, SARS-CoV-2, albumin, liver function, prognosis
Introduction
The recent outbreak of COVID-19 (SARS-CoV-2) has resulted in more than 100 million infections and 3.2 million deaths. Infection by this virus mimics the clinical phenotypes of SARS in its severity that includes pneumonia and high mortality.1,2 Additionally, the lungs are not unique targets of SARS-CoV-2 and other targets include the heart, liver, kidney and the male reproductive system.3–7 These studies have suggested that COVID-19 might cause systemic disease.
A large cohort analysis with 2623 patients demonstrated a marked hypoalbuminemia that occurred upon admission in patients that were non-critically ill (38.2%), critically ill (71.2%) and that had succumbed to the illness (82.4%). These numbers rose to 45.9, 77.7, and 95.6% during hospitalization, respectively.8 This study also indicated that hypoalbuminemia caused by COVID-19 infection was the result of a cytokine release syndrome or a “cytokine storm” that was associated with poor prognosis in these patients. Age and comorbidities were also associated with poor prognosis.9,10 In contrast, another study demonstrated that hypoalbuminemia was associated with COVID-19 infection outcomes that was age and comorbidity independent.11 Low albumin levels were also associated with poor prognosis of COVID-19 patients as measured by length of hospitalization and mortality.12 Importantly, the link between hypoalbuminemia and infection severity has not been followed-up and it is not known whether serum albumin is a useful biomarker to monitor infection severity. In this study, we retrospectively assessed serum albumin levels that were collected over time in patients diagnosed with COVID-19 disease to analyze the role of hypoalbuminemia in disease progression and prognosis.
Methods
Subjects
This study was a retrospective single-center analysis including patients suffered from COVID-19 disease hospitalized in Chongqing Public Health Medical Center from January 2020 to May 2020. Diagnosis of COVID-19 and clinical classification was made according to the new coronavirus pneumonia diagnosis and treatment plan (trial 5th version) developed by the National Health Committee of the People’s Republic of China (http://www.nhc.gov.cn/). Based on these guidelines, patients were defined as suspected COVID-19 when they met the following clinical criteria: (1) Mild: mild symptoms without pneumonia; (2) Typical: fever or respiratory tract symptoms with pneumonia; (3) Severe: fulfill any of the three criteria: respiratory distress, respiratory rate R 30 times/min; means oxygen saturation 93% in resting state; arterial blood oxygen partial pressure/oxygen concentration 300% mm Hg (1 mmHg = 0.133 kPa); (4) Critical: fulfill any of the three criteria: respiratory failure and require mechanical ventilation; shock incidence; admission to ICU with other organ failure. The SARS-CoV-2 virus nucleic acid detection in throat swab was positive for all patients in this study. In summary, we enrolled 79 patients that were divided into 17 severe and 62 of non-severe cases. This study was approved by the ethics committee of Chongqing People’s Hospital (S2020-021-01).
Data Collection
Electronic medical records were used as the data source and included epidemiological history, demographic characteristics, comorbidities and clinical symptoms. Laboratory tests included routine peripheral blood and urine screening, coagulation profiles and serum biochemical and immunological analyses. Additionally, therapeutic measures including use of antibiotics, oxygen therapy, fluid and nutritional support and application of glucocorticoids were also included in the present study. The serum albumin levels <35 g/L was defined as hypoalbuminemia. All laboratory findings were collected from COVID-19 patients at three time points including weeks 1, 2 and 3–4 from onset or the first positive nucleic acid test to blood collection. To ensure the accuracy and completeness of the data, all authors reviewed the extracted data and revised errors immediately by checking the original case data after the errors were found.
Statistical Analysis
Continuous variables were described as mean ± SD or median [IQR]. The Student’s t test and Mann–Whitney U-test were used to analyze continuous variables based on normal distributed data or non-normal distributed data, respectively. Categorical variables were presented as frequency rates and percentages and analyzed using the χ2 test or Fisher’s exact test as appropriate. All of the data was performed in SPSS 22.0 software (IBM, Chicago, Ill, USA) and a two-sided p value of 0.05 or less was considered statistically significant.
Results
The Clinical Characteristics of Patients
Our group of 79 patients included 38 male and 41 female with a median age of 45 (range 4 to 80). Non-severe cases included 17 (21.5%) patients and 62 (78.5%) were defined as severe cases. All of the patients were further divided into a normal albumin group and hypoalbuminemia group to evaluate the association of albumin with disease severities. We identified 65 normal and 14 patients with hypoalbuminemia and in the latter, albumin levels were significantly less in the severe group (71.4%) than in the non-severe group (10.8%). This revealed that hypoalbuminemia was more common in COVID-19 patients with severe disease (Table 1).
Table 1.
Clinical Features of Patients with COVID-19
| Indicators | Albumin | p value | ||
|---|---|---|---|---|
| Total (n=79, 39.2±4.1) | Normal (n=65, 40.7±2.9) | Hypoalbuminemia (n=14, 32.6±1.5) | ||
| Gender | ||||
| Male | 38(48.1%) | 30(46.2%) | 8(57.1%) | 0.455 |
| Female | 41(51.9%) | 35(53.8%) | 6(42.9%) | |
| Severity | ||||
| Non-severe | 62(78.5%) | 58(89.2%) | 4(28.6%) | 0.000 |
| Severe | 17(21.5%) | 7(10.8%) | 10(71.4%) | |
| Cigarette smoking | 17(21.5%) | 14(21.5%) | 3(21.4%) | 1.000 |
| Alcohol consumption | 21(26.6%) | 17(81.0%) | 4(19.0%) | 1.000 |
| Oxygen inhalation | 44(55.7%) | 31(47.7%) | 13(92.9%) | 0.002 |
| Days from Onset/first nucleic acid positive to Admission | ||||
| M±S.D. | 3.5±2.1 | 3.4±2.1 | 3.8±1.8 | 0.440 |
| Median (IQR) | 3.0(2.0–5.0) | 3.0(2.0–5.0) | 4.0(2.3–5.8) | |
| Range | 0–9.0 | 0–9.0 | 1.0–6.0 | |
| Days from Onset/first nucleic acid positive to First consecutive nucleic acid negative | ||||
| M±SD. | 20.9±9.9 | 20.9±10.3 | 20.9±8.1 | 0.919 |
| Median (IQR) | 20.0(14.0–27.0) | 20(14.0–28.0) | 19.0(16.5–23.0) | |
| Range | 2.0–44.0 | 2.0–44.0 | 10.0–44.0 | |
| Days from Admission to Discharge | ||||
| M±SD. | 22.4±8.9 | 21.4±8.7 | 26.7±8.2 | 0.056 |
| Median (IQR) | 21.0(14.0–29.0) | 20(13.0–28.0) | 26.0(20.5–33.8) | |
| Range | 6.0–42.0 | 6.0–42.0 | 13.0–42.0 | |
| Days from Onset/first nucleic acid positive to Discharge | ||||
| M±SD. | 25.9±9.1 | 24.9±8.9 | 30.5±8.4 | 0.073 |
| Median (IQR) | 25.0(17.5–32.0) | 23.0(17.0–31.0) | 31.0(23.5–36.8) | |
| Range | 7.0–47.0 | 7.0–46.0 | 14.0–47.0 | |
| Symptoms | ||||
| Fever | 47(59.5%) | 34(52.3%) | 13(92.9%) | 0.005 |
| Cough | 41(51.9%) | 32(49.2%) | 9(64.3%) | 0.306 |
| Sputum | 25(31.6%) | 21(32.3%) | 4(28.6%) | 1.000 |
| Pharyngalgia | 12(15.2%) | 11(16.9%) | (7.1%) | 0.607 |
| Headache or dizzy giddy | 16(19.0%) | 10(15.4%) | 6(42.9%) | 0.051 |
| Fatigue | 21(26.6%) | 13(20.0%) | 8(57.1%) | 0.012 |
| Diarrhea | 7(8.9%) | 5(7.7%) | 2(14.3%) | 0.431 |
| Chest tightness | 22(27.8%) | 14(21.5%) | 8(57.1%) | 0.018 |
| Comorbidity | ||||
| Hypertension | 9(11.4%) | 6(9.2%) | 3(21.4%) | 0.401 |
| Diabetes | 5(6.3%) | 3(4.6%) | 2(14.3%) | 0.458 |
| Hematological System diseases | 4(5.1%) | 2(3.1%) | 2(14.3%) | 0.288 |
| Digestive system diseases | 7(8.9%) | 5(7.7%) | 2(14.3%) | 0.788 |
| Other Cardiovascular systemdiseases | 2(2.5%) | 1(1.5%) | 1(7.1%) | 0.785 |
| Respiratory system diseases | 1(1.3%) | 1(1.5%) | 0(0.0%) | 1.000 |
| Other Metabolicdiseases | 3(3.8%) | 2(3.1%) | 1(7.1%) | 1.000 |
| Complications | ||||
| Respiratory system | 21(26.6%) | 12(18.5%) | 9(64.3%) | 0.001 |
| Digestive system | 11(13.9%) | 7(10.8%) | 4(28.6%) | 0.187 |
| Metabolicdiseases | 3(3.8%) | 3(4.6%) | 0(0.0%) | 0.961 |
| Hematological System | 4(5.1%) | 1(1.5%) | 3(21.4%) | 0.016 |
| Immune system | 4(5.1%) | 2(3.1%) | 2(14.3%) | 0.288 |
| Urinary system | 1(1.3%) | 0(0.0%) | 1(7.1%) | 0.395 |
| Neuropsychological system | 1(1.3%) | 1(1.5%) | 0(0.0%) | 1.000 |
| Skin diseases | 3(3.8%) | 0(0.0%) | 3(21.4%) | 0.002 |
| Cardiovascular system | 3(3.8%) | 1(1.5%) | 2(14.3%) | 0.136 |
| Chest CT-no (%) | ||||
| Involvement of chest radiographs | 58(73.4%) | 45(69.2%) | 13(92.9%) | 0.138 |
| Treatment | ||||
| Antiviral | 78(98.7%) | 64(98.5%) | 14(100%) | 1.000 |
| Traditional Chinese medicine | 67(84.8%) | 54(83.1%) | 13(92.9%) | 0.607 |
| Corticosteroid | 13(16.5%) | 4(6.2%) | 9(64.3%) | 0.000 |
| Immunomodulator | 17(21.5%) | 8(12.3%) | 9(64.3%) | 0.000 |
| Antibiotic | 18(22.8%) | 9(13.8%) | 9(64.3%) | 0.000 |
Patients that required oxygen inhalation therapies were also more common in the hypoalbuminemia group and these patents also experienced higher incidences of fever (13, 92.9%), fatigue (8, 57.1%), and chest tightness (8, 57.1%). Overall, the most common comorbidities found in the infected patients were hypertension, diabetes, hematological, digestive and respiratory system diseases. These comorbidities were not significantly between the normal albumin and hypoalbuminemia groups. Cause of the limited case of each subgroup, the results should be further validated in other cohorts. Furthermore, the most significant complications for the hypoalbuminemia group were respiratory and hematological system involvement and skin disease. All 79 patients received antiviral treatment and more patients received corticosteroids, an immunomodulator and antibiotic treatment in the hypoalbuminemia (Table 1).
Association of Serum Albumin with Hematological Parameters
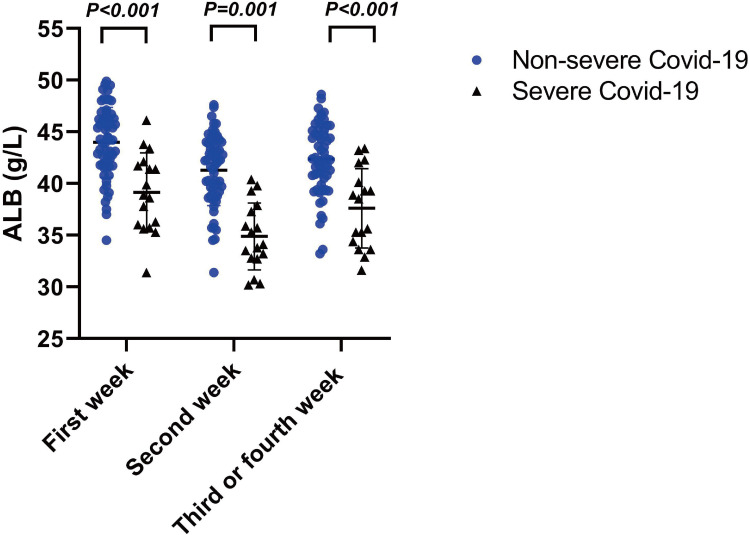
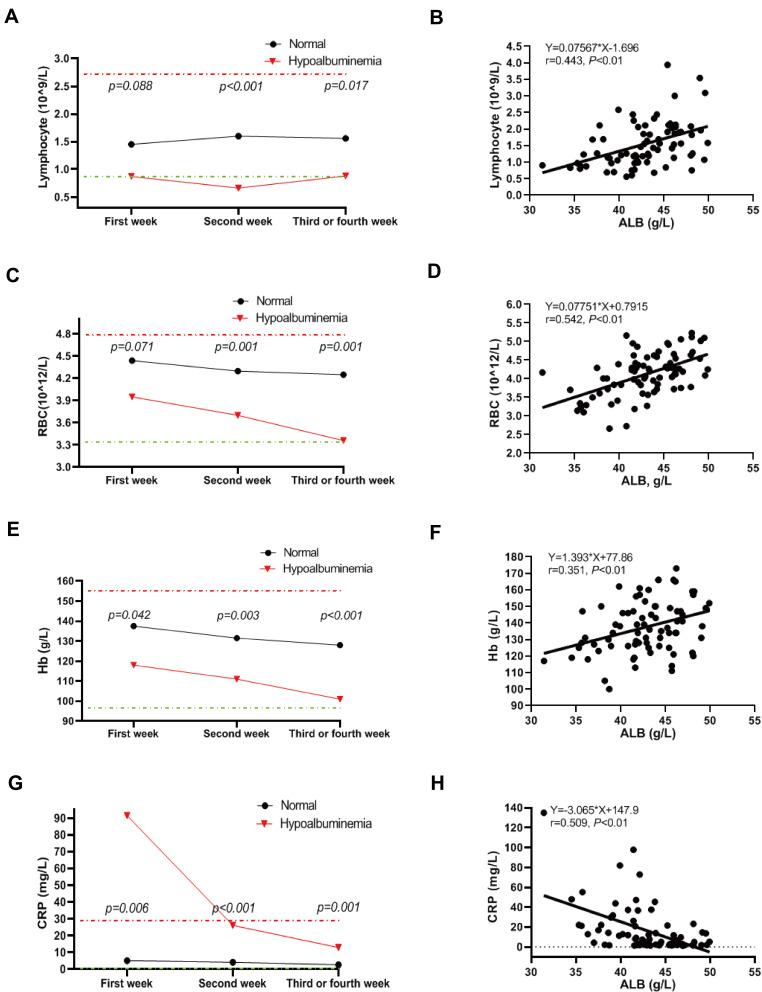
During 3–4 weeks of hospitalization and treatment, serum albumin levels for all patients had risen compared with week 2. However, severe COVID-19 infections resulted in lower serum albumin levels overall for the severe patients at the 3 time points (Figure 1). Hemoglobin levels were lower in the hypoalbuminemia group within the 3 time points while C reactive protein (CRP) levels were higher compared with the normal controls (Table 2). Lymphocyte counts for the hypoalbuminemia group were elevated by week 2 and gradually decreased by weeks 3 and 4 (Figure 2A). Moreover, erythrocyte counts and hemoglobin levels for the hypoalbuminemia group were significantly different within the first week and dropped more rapidly as time progressed compared with the normal albumin group (Figure 2C and E). CRP was elevated in most of the severe COVID-19 patients while the normal albumin group maintained low CRP levels throughout the course of study. The high CRP levels in the hypoalbuminemia group declined over weeks 2–4 (Figure 2G). Furthermore, serum albumin in COVID-19 patients was positively correlated with lymphocyte count, Hb levels and RBC counts and negatively correlated with CRP levels (Figure 2B, D, F, H). These results indicated that serum albumin might be a predicator of hematological parameters for COVID-19 patients.
Figure 1.
Serum albumin levels between severe and non-severe patients at 3 time-points. Statistically significant at P value less than 0.05.
Table 2.
Hematological Parameters of COVID-19 Patients
| Indicators | Total | Within the First Week | p value | Total | Within the Second Week | p value | Total | Within the Third or Fourth Week | p value | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n=79 | Normal Albumin (n=77) | Hypoalbuminemia (n=2) | n=79 | Normal Albumin (n=67) | Hypoalbuminemia (n=12) | n=79 | Normal Albumin (n=72) | Hypoalbuminemia (n=7) | ||||
| WBC | 5.32(4.25–6.73) | 4.25(5.36–6.76) | 5.11(5.05–5.16) | 0.384 | 5.93(4.80–7.24) | 5.74(4.76–7.09) | 6.94(5.86–8.72) | 0.108 | 6.22(5.29–6.85) | 6.16(5.33–6.76) | 7.06(4.67–7.35) | 0.552 |
| Neutrophil count (×10/L) | 3.09(2.43–4.40) | 3.09(2.46–4.40) | 2.56(1.88–3.23) | 0.450 | 3.86(2.75–4.97) | 3.53(2.63–4.36) | 5.55(4.50–7.82) | 0.000 | 3.90(3.13–4.72) | 3.88(3.17–4.57) | 5.28(3.51–5.59) | 0.216 |
| Lymphocyte count (×10/L) | 1.43(1.04–2.01) | 1.45(1.07–2.04) | 0.87(0.85–0.88) | 0.088 | 1.44(0.94–1.89) | 1.60(1.13–1.94) | 0.66(0.56–0.78) | 0.000 | 1.54(1.09–1.99) | 1.56(1.18–2.01) | 0.88(0.85–1.24) | 0.017 |
| NLR (Neutrophil to lymphocyte ratio) | 2.17(1.59–3.27) | 2.17(1.60–3.20) | 2.90(2.17–3.62) | 0.912 | 2.51(1.84–4.09) | 2.26(1.71–3.04) | 7.28(5.83–13.46) | 0.000 | 2.57(1.92–3.40) | 2.48(1.84–3.08) | 5.35(4.18–6.01) | 0.003 |
| Monocyte count (×10 /L) | 0.38(0.31–0.50) | 0.39(0.32–0.50) | 0.27(0.23–0.30) | 0.140 | 0.41(0.32–0.51) | 0.41(0.34–0.51) | 0.32(0.28–0.64) | 0.760 | 0.44(0.34–0.53) | 0.43(0.33–0.53) | 0.56(0.45–0.69) | 0.203 |
| Hemoglobin (g/L) | 136.50(126.00–148.75) | 137.50(126.75–149.00) | 118.00(117.50–118.50) | 0.042 | 130.00(121.00–139.00) | 131.50(123.50–141.00) | 111.00(101.50–127.50) | 0.003 | 125.00(112.75–136.75) | 128.00(116.00–137.50) | 101.00(100.00–105.50) | 0.000 |
| Erythrocyte count (×10 /L) | 4.43(4.20–4.80) | 4.44(4.23–4.81) | 3.95(3.93–3.98) | 0.071 | 4.26(3.95–4.61) | 4.30(4.06–4.63) | 3.70(3.22–4.01) | 0.001 | 4.20(3.66–4.44) | 4.25(3.81–4.50) | 3.36(3.15–3.56) | 0.001 |
| Platelet count (×10/L) | 177.50(137.75–216.50) | 178.50(139.0–219.00) | 148.00(142.5–153.5) | 0.439 | 222.00(191.00–305.00) | 225.00(195.25–308.00) | 203.00(149.50–259.50) | 0.233 | 236.00(204.00–283.00) | 236.00(205.50–291.00) | 239.50(180.00–280.25) | 0.567 |
| C-reactive protein (mg/L) | 5.31(1.98–21.18) | 5.08(1.93–17.89) | 91.45(69.69–113.23) | 0.006 | 5.58(2.26–15.41) | 4.18(2.06–14.32) | 26.10(12.05–30.45) | 0.000 | 2.97(1.80–6.84) | 2.67(1.79–4.00) | 12.89(6.93–39.56) | 0.001 |
Figure 2.
Dynamic analysis of peripheral blood parameters for COVID-19 patients. (A, C, E, G) Levels of Lymphocytes count (A), RBC count (C), Hb concentration (E), and CRP concentration (G) in COVID patients with albumin reduction and normal albumin at three time points, including acute phase, advanced phase, and recovery phase. (B, D, F, H) The correlation of serum albumin with Lymphocytes count (B), RBC (D), Hb concentration (F), and CRP concentration (H) in COVID patients. Statistically significant at P value less than 0.05.
Association of Serum Albumin with Biomarkers from Serum Biochemical Tests
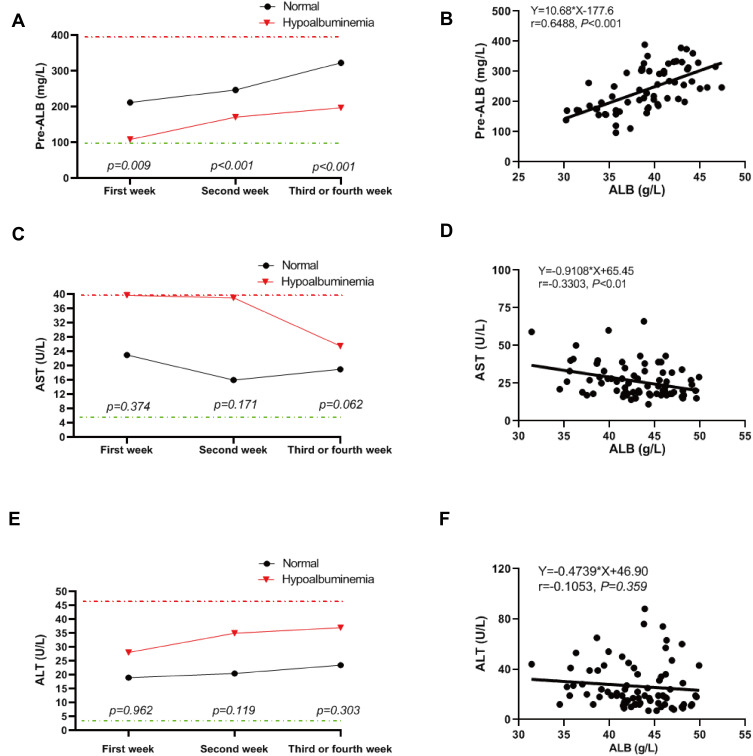
Our group of patients with hypoalbuminemia also displayed prealbumin levels that were significantly lower than the normal albumin controls over the whole study period. In addition, AST but not ALT levels were higher in patients with hypoalbuminemia and these levels were significantly reduced within 3–4 weeks of treatment. Kidney functional parameters including urea nitrogen, uric acid, creatinine, β2-microglobulin and cystatin C were not markedly different over time between the normal albumin and hypoalbuminemia groups (Table 3). These results indicated that liver function, especially those that were involved in protein synthesis functions were damaged in patients with hypoalbuminemia. A dynamic analysis of these parameters also indicated that in both our test groups, prealbumin levels gradually increased over hospitalization time but recovery for patients with hypoalbuminemia was significantly slower than the normal albumin group (Figure 3A). This indicated that liver functional recovery was still weak in the hypoalbuminemia group. Moreover, AST levels were recovered in the latter group over time while ALT levels showed no significant change (Figure 3C and E). Furthermore, prealbumin was positively correlated and AST was negatively correlated with albumin concentration while ALT was not (Figure 3B, D, F). These results demonstrated that serum albumin could be used as a biomarker to evaluate liver damage in COVID-19 patients.
Table 3.
Clinical Biochemical Tests of COVID-19 Patients
| Indicators | Total | Within the First Week | p value | Total | Within the Second Week | p value | Total | Within the Third or Fourth Week | p value | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n=79 | Normal Albumin (n=77) | Hypoalbuminemia (n=2) | n=79 | Normal Albumin (n=67) | Hypoalbuminemia (n=12) | n=79 | Normal Albumin (n=72) | Hypoalbuminemia (n=7) | ||||
| Prealbumin (g/L) | 209.00(161.00–280.0) | 212.00(162.00–283.50) | 108.50(105.75–111.25) | 0.009 | 225.50(176.00–274.75) | 247.00(201.75–282.50) | 171.00(157.25–183.50) | 0.000 | 311.50(238.00–351.00) | 323.00(272.50–357.00) | 197.00(181.00–216.00) | 0.000 |
| Alanine aminotransferase (ALT) | 19.00(13.00–37.50) | 19.00(13.00–36.00) | 28.10(20.05–36.15) | 0.962 | 21.00(16.00–33.00) | 20.50(15.25–30.75) | 35.00(18.50–61.50) | 0.119 | 24.00(14.25–30.00) | 23.50(14.75–29.25) | 37.00(15.00–83.75) | 0.303 |
| Aspartate aminotransferase (AST) | 23.00(18.00–32.50) | 23.00(18.00–32.00) | 39.75(30.38–49.13) | 0.374 | 20.00(17.00–28.00) | 16.00(16.00–25.25) | 39.00(21.00–42.00) | 0.171 | 19.00(15.00–23.00) | 19.00(15.00–22.00) | 25.50(21.25–38.75) | 0.062 |
| PCT | 0.02(0.02–0.04) | 0.02(0.02–0.04) | 0.14(0.14–0.15) | 0.003 | 0.02(0.02–0.05) | 0.02(0.02–0.02) | 0.05(0.02–0.09) | 0.067 | 0.04(0.02–0.05) | 0.03(0.02–0.05) | 0.04(0.03–0.04) | 0.661 |
| Globulin | 25.80(23.30–29.90) | 25.90(23.50–30.00) | 22.40(22.30–22.50) | 0.146 | 25.30(22.90–29.00) | 25.20(22.50–28.40) | 26.95(24.20–33.58) | 0.085 | 25.80(22.80–28.50) | 25.70(22.75–28.50) | 28.55(25.43–32.43) | 0.210 |
| Total bilirubin | 14.60(10.60–19.90) | 14.60(10.70–19.60) | 22.50(14.05–30.95) | 0.987 | 15.40(10.80–21.00) | 14.20(10.55–19.15) | 21.35(13.85–27.43) | 0.041 | 12.15(9.38–12.15) | 12.15(9.53–14.93) | 12.70(8.45–15.68) | 0.874 |
| Urea nitrogen | 3.92(3.11–4.69) | 3.92(3.12–4.65) | 4.04(3.52–4.55) | 0.818 | 3.68(2.79–4.41) | 3.67(2.79–4.34) | 4.30(2.62–5.34) | 0.352 | 3.75(3.07–4.23) | 3.75(3.10–4.22) | 3.07(3.05–4.06) | 0.916 |
| Uric acid | 287.50(237.75–334.75) | 288.00(237.50–335.50) | 259.00(256.00–262.00) | 0.711 | 321.00(263.00–359.00) | 321.00(264.00–358) | 293.00(260.00–326.00) | 0.011 | 317.50(246.25–413.25) | 333.00(252.00–414.75) | 198.50(120.75–292.75) | 0.011 |
| Creatinine | 67.90(54.63–77.28) | 67.90(54.38–77.75) | 67.30(63.75–71.25) | 0.865 | 64.60(56.20–75.10) | 64.70(56.15–75.88) | 62.60(56.90–74.10) | 0.958 | 64.80(54.80–80.30) | 64.5(54.75–80.65) | 69.65(64.38–73.43) | 0.763 |
| β2-microglobulin | 2.35(1.58–2.93) | 2.35(1.58–2.90) | 3.10(2.72–3.49) | 0.107 | 1.82(1.58–2.31) | 1.82(1.57–2.31) | 2.53(2.40–2.66) | 0.423 | 1.72(1.39–2.13) | 1.71(1.39–2.04) | 2.31(1.92–2.66) | 0.203 |
| CystatinC | 0.92(0.84–1.10) | 0.92(0.84–1.10) | 0.65(0.37–0.93) | 0.267 | 0.91(0.85–1.07) | 0.90(0.84–1.06) | 1.06(1.00–1.13) | 0.017 | 1.00(0.84–1.10) | 0.98(0.84–1.10) | 1.14(0.99–1.19) | 0.311 |
Figure 3.
Dynamic analysis of prealbumin, ALT, and AST for COVID-19 patients. (A, C, E) Levels of prealbumin (A), ALT (C), andAST (E) in COVID-19 patients with albumin reduction and normal albumin at the indicated three time points.(B, D, F) The correlation of serum albumin with prealbumin (B), ALT (D), and AST (F) in COVID-19 patients. Statistically significant at P value less than 0.05.
Association of Serum Albumin with T Lymphocytes
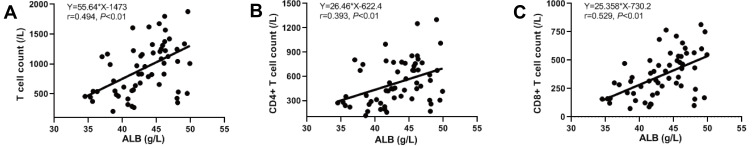
In our hypoalbuminemia test group, total numbers of T cells as well as CD4+ and CD8+ positive T cells were lower in patients with hypoalbuminemia compared to normal albumin controls (Table 4). Correlation analysis demonstrated that serum albumin was positively associated with the numbers for all 3 of these cell types (Figure 4). However, data points for weeks 1 and 3–4 were absent so conclusions of a correlation with patient recovery could not be made. Our results indicated that serum albumin levels in COVID-19 patients might be used as a biomarker to evaluate cellular immune functions.
Table 4.
T Cell Counts in COVID-19 Patients with Normal Albumin or Hypoalbuminemia
| Indicators | Total | Albumin | p value | |
|---|---|---|---|---|
| n=79 | Normal (n=65) | Hypoalbuminemia (n=14) | ||
| Total T cells | 737.00(481.25–1061.00) | 970.50(589.00–1122.00) | 271.00(186.00–511.00) | 0.004 |
| CD4+ T cells | 416.00(241.50–658.50) | 522.00(351.00–676.00) | 147.00(109.00–296.00) | 0.007 |
| CD8+ T cells | 276.50(154.75–350.75) | 289.00(213.00–448.00) | 103.00(76.5.00–202.50) | 0.004 |
Figure 4.
Correlation of serum albumin with total T lymphocytes count, CD4+ T lymphocytes, and CD8+ T lymphocytes. (A) Correlation of serum albumin with total T lymphocytes count. (B) Correlation of serum albumin with CD4+ T lymphocytes. (C) Correlation of serum albumin with CD8+ T lymphocytes. Statistically significant at P value less than 0.05.
Association of Serum Albumin with Coagulation Function
We also assessed coagulation functions in our patient groups and D dimer levels were significantly elevated in patients with hypoalbuminemia compared with patients with normal albumin. In contrast, PT, APTT and Fib levels were not significantly different from normal albumin controls (Table 5). These results revealed that serum albumin might be used as a predictor of coagulation function for COVID-19 patients.
Table 5.
The Coagulation Function of COVID-19 Patients with Normal Albumin or Hypoalbuminemia
| Indicators | Albumin | p value | ||
|---|---|---|---|---|
| Total n=79 | Normal (n=61) | Hypoalbuminemia (n=18) | ||
| PT | 11.60 (10.88–12.13) | 11.70(10.50–12.20) | 11.40(11.20–11.95) | 0.899 |
| APTT | 35.35(28.65–38.00) | 35.30(28.80–37.10) | 35.50(28.15–41.15) | 0.824 |
| FIB | 3.69(2.92–4.71) | 3.24(2.68–4.37) | 4.29(4.08–4.86) | 0.053 |
| DD | 0.23(0.12–1.00) | 0.20(0.09–0.28) | 2.37(1.92–5.37) | 0.003 |
Discussion
In the present study, we found that hypoalbuminemia was common in COVID-19 patients with severe disease. Moreover, the serum albumin levels gradually increased after treatment both in patients with normal serum albumin and hypoalbuminemia. We also found that serum albumin levels were positively correlated with lymphocyte and RBC numbers, Hb levels and total numbers of T cells and CD4+ and CD8+ cells and negatively correlated with CRP and AST. These results indicated that serum albumin concentrations might be used as a predicator of COVID-19 disease severity.
The half-life of serum albumin is 21 days13 and a previous study indicated that when considering the period from onset to admission, hypoalbuminemia was less likely to be a result of decreased albumin synthesis in severe COVID-19 cases.11 Although this was a reasonable assumption it has been challenged and the latter concluded that essential amino acid consumption due to viral replication, transcriptional inhibition and albumin clearance might be responsible for hypoalbuminemia in COVID-19 patients.14 In our cohort, renal functional biomarkers including creatinine, β2-microglobulin and cystatin C were not evidently different between patients with normal albumin and hypoalbuminemia. This indicated that albumin loss through the kidneys was not a likely reason for hypoalbuminemia in COVID-19 patients. In addition, ALT and AST were not elevated in patients with hypoalbuminemia but prealbumin levels were significantly decreased in the latter group. This indicated that SARS-CoV-2 infection impaired liver protein synthesis functions and might be one of the causes of hypoalbuminemia in COVID-19 patients. We also found a negative correlation between albumin and CRP suggesting that hypoalbuminemia may be associated with the increased microvascular permeability and consequent redistribution of albumin into extravascular compartments. This would result in a decrease in albumin in the blood vessels. Whether other factors contributed to the decrease of albumin in COVID-19 patients are unknown from these limited data and further studies are needed to elucidate the exact mechanisms.
The physiological functions of serum albumin include maintenance of osmotic pressure and transport of a variety of substances.13 After the outbreak of COVID-19, serum albumin was found significantly reduced in patients suffering from SARS-CoV-2. A recent study demonstrated that albumin binds to SARS-CoV-2 virions and this process might inhibit the formation of the endothelial glycocalyx by inhibition of albumin transport-binding sites. These authors suggested that albumin therapy should be tested as a matter of urgency in patients presenting with disseminated COVID-19 disease.15 In contrast, other reports indicated a cautious approach to albumin transfusion for COVID-19 treatment before the exact mechanism of hypoalbuminemia was clarified. A summary study of 9 publications attempted to clarify the underlying role of serum albumin in patients with COVID-19 infections. They concluded that albumin transfusion treatment for COVID-19 patients might have a limited benefit through the existing evidence based on ARDS and sepsis. A recent preliminary report demonstrated that albumin supplementation dampens hypercoagulability in COVID-19 patients and prevented ischemic events.16 Overall, whether albumin supplementation to COVID-19 patients is beneficial is currently unknown.
In addition to the therapeutic role of albumin, a number of studies have used albumin levels as a prognostic tool in patients with SARS-CoV-2 infections. For instance, serum albumin levels <35 g/L were found in 74% of the COVID-19 patients and this prevalence was higher for ICU cases and those that suffered ischemic events.17 In our cohort, we found that D dimer levels were significantly elevated in patients with hypoalbuminemia, while no ischemic events were observed. Taken together, we collected data from three time points including within the first, second and third/fourth weeks. The dynamic analysis of serum albumin indicated that albumin levels were elevated after patients were recovering and indicated that serum albumin might be used as a biomarker to predict the progress of COVID-19 disease.
Data Sharing Statement
The data used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics Approval and Informed Consent
The study protocol has been approved by the Medical Ethical Committee of the Chongqing General Hospital, University of Chinese Academy of Sciences with an approval number [S2020-021-01] and performed according to the Declaration of Helsinki. Due to the nature of this retrospective study without any additional interventions, patients’ informed consents were exempted. All personally identifiable records were kept confidential.
Disclosure
The authors declare that they have no competing interests.
References
- 1.Arentz M, Yim E, Klaff L, et al. characteristics and outcomes of 21 critically ill patients with Covid-19 in Washington State. JAMA. 2020;323(16):1612–1614. doi: 10.1001/jama.2020.4326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Matthay MA, Aldrich JM, Gotts JE. Treatment for severe acute respiratory distress syndrome from Covid-19. Lancet Respir Med. 2020;8(5):433–434. doi: 10.1016/S2213-2600(20)30127-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roychoudhury S, Das A, Jha NK, et al. Viral pathogenesis of SARS-CoV-2 infection and male reproductive health. Open Biol. 2021;11(1):200347. doi: 10.1098/rsob.200347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mayet V, Mousseaux C, Petit-Hoang C, et al. Covid-19-associated acute kidney injury: after the tubule and the glomerulus, now the vessel? Clin Kidney J. 2020;13(6):1105–1106. doi: 10.1093/ckj/sfaa210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iqbal QZ, Haider MA, Sattar SBA, Hanif M, Javid I. Covid-19 induced myocarditis: a rare cause of heart failure. Cureus. 2020;12(11):e11690. doi: 10.7759/cureus.11690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yao Y, Yuan X, Wu L, Guo N, Yin L, Li Y. Covid-19 and male reproduction: current research and unknown factors. Andrology. 2021. doi: 10.1111/andr.12970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mameli S, Marcialis MA, Bassareo PP, Fanos V. Covid-19 and hepatic damage: what we know? A systematic review. Panminerva Med. 2021. doi: 10.23736/S0031-0808.21.04239-7 [DOI] [PubMed] [Google Scholar]
- 8.Huang W, Li C, Wang Z, et al. Decreased serum albumin level indicates poor prognosis of Covid-19 patients: hepatic injury analysis from 2623 hospitalized cases. Sci China Life Sci. 2020;63(11):1678–1687. doi: 10.1007/s11427-020-1733-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guan WJ, Liang WH, Zhao Y, et al. Comorbidity and its impact on 1590 patients with Covid-19 in China: a nationwide analysis. Eur Respir J. 2020;55(5):2000547. doi: 10.1183/13993003.00547-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang J, Cheng A, Kumar R, et al. Hypoalbuminemia predicts the outcome of Covid-19 independent of age and co-morbidity. J Med Virol. 2020;92(10):2152–2158. doi: 10.1002/jmv.26003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de la Rica R, Borges M, Aranda M, et al. Low albumin levels are associated with poorer outcomes in a case series of Covid-19 patients in Spain: a retrospective cohort study. Microorganisms. 2020;8(8):1106. doi: 10.3390/microorganisms8081106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rothschild MA, Oratz M, Schreiber SS. Serum albumin. Hepatology. 1988;8(2):385–401. doi: 10.1002/hep.1840080234 [DOI] [PubMed] [Google Scholar]
- 14.Ambade V. Biochemical rationale for hypoalbuminemia in Covid-19 patients. J Med Virol. 2021;93(3):1207–1209. doi: 10.1002/jmv.26542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnson AS, Fatemi R, Winlow W. SARS-CoV-2 Bound Human Serum Albumin and Systemic Septic Shock. Front Cardiovasc Med. 2020;7:153. doi: 10.3389/fcvm.2020.00153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Violi F, Ceccarelli G, Loffredo L, et al. Albumin supplementation dampens hypercoagulability in Covid-19: a preliminary report. Thromb Haemost. 2021;121(1):102–105. doi: 10.1055/s-0040-1721486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Violi F, Ceccarelli G, Cangemi R, et al. Hypoalbuminemia, coagulopathy, and vascular disease in Covid-19. Circ Res. 2020;127(3):400–401. doi: 10.1161/CIRCRESAHA.120.317173 [DOI] [PubMed] [Google Scholar]