Abstract
We identified seasonal human coronaviruses, influenza viruses and rhinoviruses in the exhaled breath and coughs of children and adults with acute respiratory illness. Surgical face masks significantly reduced detection of influenza virus RNA in respiratory droplets and coronavirus RNA in aerosols, with a marginally significant reduction in coronavirus RNA in respiratory droplets. Our results indicate that surgical facemasks could prevent transmission of human coronaviruses and influenza viruses from symptomatic individuals.
Keywords: influenza virus, coronavirus, aerosol, face mask, public health
INTRODUCTION
Respiratory virus infections cause a broad and overlapping spectrum of symptoms collectively referred to as acute respiratory virus illnesses (ARIs), or more commonly the “common cold”. Although mostly mild, these ARIs can sometimes cause severe disease and death1. These viruses spread between humans through direct or indirect contact, respiratory droplets (including larger droplets that fall rapidly near the source as well as coarse aerosols with aerodynamic diameter >5μm) and fine particle aerosols (droplets and droplet nuclei with aerodynamic diameter ≤5μm)2,3. Although hand hygiene and use of face masks, primarily targeting contact and respiratory droplet transmission, have been suggested as important mitigation strategies against influenza virus transmission4, little is known about the relative importance of these modes in the transmission of other common respiratory viruses2,3,5. Uncertainties similarly apply to the modes of transmission of COVID-196,7.
Some health authorities recommend that masks are worn by ill individuals to prevent onwards transmission (i.e. source control)4,8. Surgical face masks were originally introduced to protect participants from wound infection and contamination from surgeons (the wearer) during surgical procedures, and were later adopted to protect healthcare workers against acquiring infection from their patients. However, most of the existing evidence on the filtering efficacy of face masks and respirators comes from in vitro experiments with non-biological particles9,10 which may not be generalizable to infectious respiratory virus droplets. There is little information on the efficacy of face masks in filtering respiratory viruses and reducing viral release from an individual with respiratory infections8, with most research focusing on influenza11,12.
Here we aimed to explore the importance of respiratory droplet and aerosol routes of transmission with a particular focus on coronaviruses, influenza viruses and rhinoviruses, by quantifying the amount of respiratory viruses in exhaled breath of participants with medically-attended ARI and determining the potential efficacy of surgical face masks to prevent respiratory virus transmission.
RESULTS
We screened 3,363 individuals in two study phases, ultimately enrolling 246 individuals who provided exhaled breath samples (Supporting Figure 1). Among these 246 participants, 122 (50%) participants were randomized to not wearing a face mask during the first exhaled breath collection and 124 (50%) participants randomized to wearing a face mask. 49 (20%) voluntarily provided a second exhaled breath collection of the alternate type.
Infections by at least one respiratory virus were confirmed by RT-PCR in 123/246 (50%) participants. Of these 123 participants, 111 (90%) were infected by human (seasonal) coronavirus (n=17), influenza virus (n=43), or rhinovirus (n=54) ( Supporting Figure 1, Supporting Figure 2), including one participant co-infected by both coronavirus and influenza virus, and another two participants co-infected by both rhinovirus and influenza virus. These 111 participants were the focus of our analyses.
There were some minor differences in characteristics of the 111 participants with the different viruses (Table 1a). 24% of participants had a measured fever ≥37.8°C, with influenza patients more than twice as likely than coronavirus and rhinovirus-infected patients to have a measured fever. Coronavirus-infected participants coughed the most with an average of 17 (SD 30) coughs during the 30-minute exhaled breath collection. The profiles of the participants randomized to with-mask vs without-mask groups were similar (Supplementary Table 1).
Table 1a.
Characteristics of individuals with symptomatic coronavirus, influenza virus or rhinovirus infection.
| All who provided exhaled breath | Coronavirus | Influenza virus | Rhinovirus | |||||
|---|---|---|---|---|---|---|---|---|
| (n = 246) | (n = 17) | (n = 43) | (n = 54) | |||||
| n (%) | n (%) | n (%) | n (%) | |||||
| Female | 144 (59) | 13 (76) | 22 (51) | 30 (56) | ||||
| Age group (in years) | ||||||||
| 11–17 | 12 (5) | 0 (0) | 8 (19) | 4 (7) | ||||
| 18–34 | 114 (46) | 10 (59) | 11 (26) | 24 (44) | ||||
| 35–50 | 79 (32) | 2 (12) | 16 (37) | 18 (33) | ||||
| 51–64 | 35 (14) | 4 (24) | 8 (19) | 5 (9) | ||||
| ≥ 65 | 6 (2) | 1 (6) | 0 (0) | 3 (6) | ||||
| Chronic medical conditions | ||||||||
| Any | 49 (20) | 5 (29) | 5 (12) | 10 (19) | ||||
| Respiratory | 18 (7) | 0 (0) | 4 (9) | 3 (6) | ||||
| Influenza vaccination | ||||||||
| Ever | 94 (38) | 6 (35) | 15 (35) | 20 (37) | ||||
| Current season | 23 (9) | 2 (12) | 1 (2) | 4 (7) | ||||
| Prior season only | 71 (29) | 4 (24) | 14 (33) | 16 (30) | ||||
| Ever smoker | 31 (13) | 1 (6) | 6 (14) | 6 (11) | ||||
| Time since illness onset, hours | ||||||||
| <24 | 22 (9) | 0 (0) | 5 (12) | 2 (4) | ||||
| 24–48 | 100 (41) | 9 (53) | 13 (30) | 25 (46) | ||||
| 48–72 | 85 (35) | 8 (47) | 18 (42) | 20 (37) | ||||
| 72–96 | 39 (16) | 0 (0) | 7 (16) | 7 (13) | ||||
| History of measured fever ≥37.8ºC | 58 (24) | 3 (18) | 17 (40) | 8 (15) | ||||
| Measured fever ≥37.8ºC at presentation | 36 (15) | 2 (12) | 18 (42) | 2 (4) | ||||
| Measured body temperature (˚C) at enrolment (Mean, SD) | 36.8 (0.8) | 36.9 (0.8) | 37.4 (0.9) | 36.6 (0.7) | ||||
| Symptoms at presentation | ||||||||
| Feverishness | 111 (45) | 10 (59) | 27 (63) | 16 (30) | ||||
| Cough | 198 (80) | 15 (88) | 40 (93) | 44 (81) | ||||
| Sore throat | 211 (86) | 15 (88) | 31 (72) | 49 (91) | ||||
| Runny nose | 200 (81) | 17 (100) | 36 (84) | 48 (89) | ||||
| Headache | 186 (76) | 13 (76) | 30 (70) | 38 (70) | ||||
| Myalgia | 176 (72) | 12 (71) | 31 (72) | 34 (63) | ||||
| Phlegm | 176 (72) | 9 (53) | 34 (79) | 41 (76) | ||||
| Chest tightness | 64 (26) | 3 (18) | 12 (28) | 9 (17) | ||||
| Shortness of breath | 103 (42) | 6 (35) | 14 (33) | 25 (46) | ||||
| Chills | 100 (41) | 8 (47) | 29 (67) | 16 (30) | ||||
| Sweats | 95 (39) | 5 (29) | 18 (42) | 20 (37) | ||||
| Fatigue | 218 (89) | 16 (94) | 38 (88) | 48 (89) | ||||
| Vomiting | 19 (8) | 2 (12) | 5 (12) | 2 (4) | ||||
| Diarrhea | 17 (7) | 2 (12) | 1 (2) | 6 (11) | ||||
| Number of cough during exhaled breath collection (Mean, SD) | 8 (14) | 17 (30) | 8 (11) | 5 (9) |
Seasonal coronavirus (n = 17), seasonal influenza virus (n = 43) and rhinovirus (n = 54) infection were confirmed in individuals with acute respiratory symptoms by RT-PCR in any samples (nasal swab, throat swab, respiratory droplets and aerosols) collected.
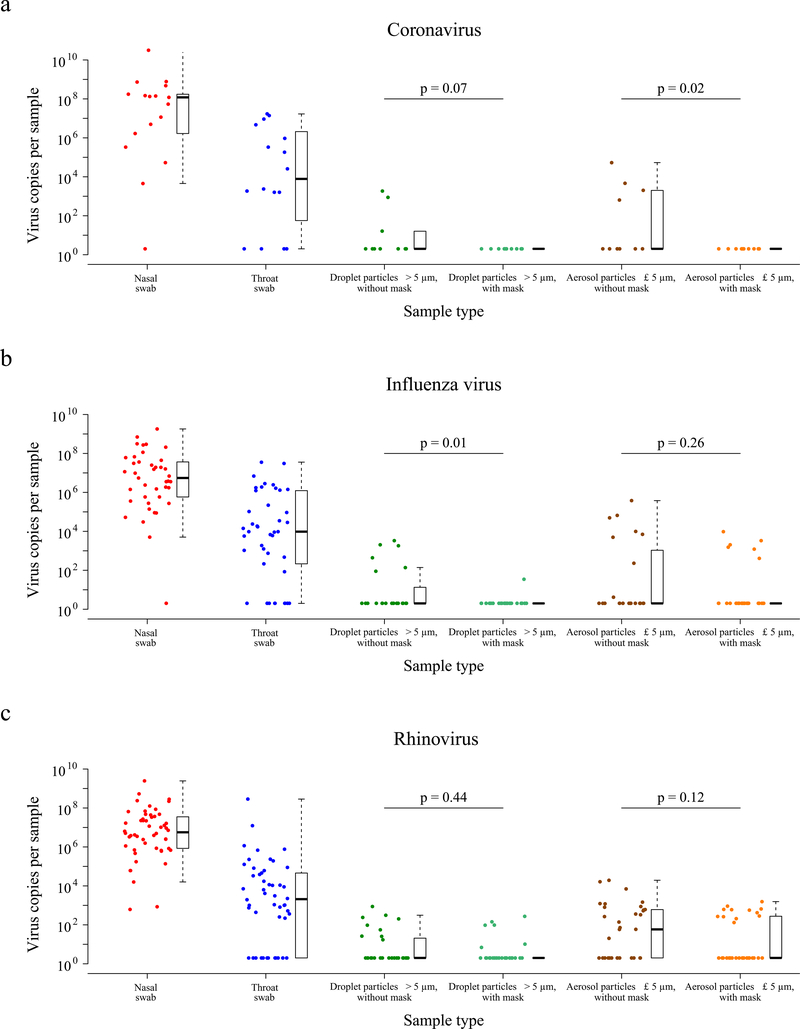
We tested viral shedding (in terms of viral copies per sample) in nasal swabs, throat swabs, respiratory droplet samples, and aerosol samples, and compared the latter two between the samples collected with or without a face mask (Figure 1a–c). On average the viral shedding was higher in nasal swabs than in throat swabs for each of coronavirus (median 8.1 log10 virus copies per sample vs. 3.9), influenza virus (6.7 vs. 4.0) and rhinovirus (6.8 vs. 3.3) respectively. Viral RNA was identified from both respiratory droplets and aerosols for all three viruses, including 30%, 26% and 28% of the respiratory droplets, and 40%, 35% and 56% of the aerosols collected while not wearing a face mask, from coronavirus, influenza virus and rhinovirus-infected participants respectively (Table 1b). In particular for coronavirus, we identified OC43 and HKU1 from both respiratory droplets and aerosols, but only identified NL63 from aerosols and not from respiratory droplets (Supplementary Table 2, Supporting Figure 3).
Figure 1. Efficacy of surgical face masks in reducing respiratory virus shedding in respiratory droplets and aerosols of symptomatic individuals with (a) coronavirus, (b) influenza virus or (c) rhinovirus infection.
The figure showed the virus copies per sample collected in nasal swab (red), throat swab (blue), respiratory droplets collected for 30 minutes while not wearing (dark green) or wearing (light green) a surgical face mask, and aerosols collected for 30 minutes while not wearing (brown) or wearing (orange) a face mask, collected from individuals with acute respiratory symptoms who were RT-PCR positive for coronavirus, influenza virus and rhinovirus in any samples. P-values for mask intervention as predictor of log10virus copies per sample in an unadjusted univariate Tobit regression model which allowed for censoring at the lower limit of detection of the RT-PCR assay were shown, with significant difference in bold. For nasal swabs and throat swabs, all infected individuals were included (coronavirus, n = 17; influenza virus, n = 43; rhinovirus, n = 54). For respiratory droplets and aerosols, numbers of infected individuals who provided exhaled breath samples while not wearing, or wearing, a surgical face mask respectively were: coronavirus (n = 10, 11), influenza virus (n = 23, 28), rhinovirus (n = 36, 32). A subset of participants provided exhaled breath samples for both mask intervention (coronavirus, n = 4; influenza virus, n = 8; rhinovirus, n = 14). The box plots indicated the median with the interquartile range (lower/ upper hinge) and ± 1.5*interquartile range from the first/ third quartile (lower/ upper whisker).
Table 1b.
Efficacy of surgical face masks in reducing respiratory virus frequency of detection and viral shedding in respiratory droplets and aerosols of symptomatic individuals with coronavirus, influenza virus or rhinovirus infection.
| Droplet particles >5μm | Aerosol particles ≤5μm | |||||||
|---|---|---|---|---|---|---|---|---|
| Virus type | Without surgical face mask | With surgical face mask | p | Without surgical face mask | With surgical face mask | p | ||
| DETECTION OF VIRUS | ||||||||
| No. Positive / No. Total (%) | No. Positive / No. Total (%) | No. Positive / No. Total (%) | No. Positive / No. Total (%) | |||||
| Coronavirus | 3/10 (30) | 0/11 (0) | 0.09 | 4/10 (40) | 0/11 (0) | 0.04 | ||
| Influenza virus | 6/23 (26) | 1/27 (4) | 0.04 | 8/23 (35) | 6/27 (22) | 0.36 | ||
| Rhinovirus | 9/32 (28) | 6/27 (22) | 0.77 | 19/34 (56) | 12/32 (38) | 0.15 | ||
| VIRAL LOAD (log10 virus copies per sample) | ||||||||
| Median (IQR) | Median (IQR) | Median (IQR) | Median (IQR) | |||||
| Coronavirus | 0.3 (0.3, 1.2) | 0.3 (0.3, 0.3) | 0.07 | 0.3 (0.3, 3.3) | 0.3 (0.3, 0.3) | 0.02 | ||
| Influenza virus | 0.3 (0.3, 1.1) | 0.3 (0.3, 0.3) | 0.01 | 0.3 (0.3, 3.0) | 0.3 (0.3, 0.3) | 0.26 | ||
| Rhinovirus | 0.3 (0.3, 1.3) | 0.3 (0.3, 0.3) | 0.44 | 1.8 (0.3, 2.8) | 0.3 (0.3, 2.4) | 0.12 | ||
P-values for comparing the frequency of respiratory virus detection between the mask intervention were obtained by two-sided Fisher’s exact test, and (two-sided) p-values for mask intervention as predictor of log10 virus copies per sample were obtained by an unadjusted univariate Tobit regression model which allowed for censoring at the lower limit of detection of the RT-PCR assay, with significant difference in bold. Undetectable values were imputed as 0.3 log10 virus copies per sample.
We detected coronavirus in respiratory droplets and aerosols in 3/10 (30%) and 4/10 (40%) of the samples collected without face masks, respectively, but did not detect any virus in respiratory droplets or aerosols either collected from participants wearing face masks, this difference was significant in aerosols and marginally significant in respiratory droplets (Table 1b). For influenza virus, we detected virus in 6/23 (26%) and 8/23 (35%) of the respiratory droplet and aerosol samples collected without face masks, respectively. There was a significant reduction by wearing face masks to 1/27 (4%) in detection of influenza virus in respiratory droplets, but no significant reduction in detection in aerosols (Table 1b). Moreover, among the 8 participants who had influenza virus detected by RT-PCR from without-mask aerosols, 5 were tested by viral culture and 4 were culture positive. Among the 6 participants who had influenza virus detected by RT-PCR from with-mask aerosols, 4 were tested by viral culture and 2 were culture positive. For rhinovirus, there were no significant differences between detection of virus with or without face masks, both in respiratory droplets and in aerosols (Table 1b). Conclusions were similar in comparisons of viral shedding (Table 1b). In addition, we found a significant reduction in viral shedding (Supplementary Table 2) in respiratory droplets for OC43 (Supporting Figure 4) and influenza B virus (Supporting Figure 5), and in aerosols for NL63 (Supporting Figure 4).
We identified correlations between viral loads in different samples (Supporting Figures 6–8) and some evidence of declines in viral shedding by time since onset for influenza virus but not for coronavirus or rhinovirus (Supporting Figure 9). In univariable analyses of factors associated with detection of respiratory viruses in various sample types, we did not identify significant association in viral shedding with days since symptom onset (Supplementary Table 3) for respiratory droplets or aerosols (Supplementary Tables 4–6).
A subset of participants (72/246, 29%) did not cough at all during at least one exhaled breath collection, including 37/147 (25%) during the without-mask and 42/148 (28%) during the with-mask breath collection. In this subset for coronavirus (n=4), we did not detect any virus in respiratory droplets or aerosols from any participants. In the subset for influenza virus (n=9), we detected virus in aerosols but not respiratory droplets from one participant. For rhinovirus (n=17), we detected virus in respiratory droplets from 3 participants, and we detected virus in aerosols in 5 participants.
DISCUSSION
Our results indicate that aerosol transmission is a potential mode of transmission for coronaviruses as well as influenza viruses and rhinoviruses. Published studies detected respiratory viruses13,14 such as influenza12,15 and rhinovirus16 from exhaled breath, and the detection of SARS-CoV17 and MERS-CoV18 from air samples (without size fractionation) collected from hospitals treating SARS and MERS patients, but ours is the first to demonstrate detection of human seasonal coronaviruses in exhaled breath, including the detection of OC43 and HKU1 from respiratory droplets, and NL63, OC43 and HKU1 from aerosols.
Our findings indicate that surgical masks can efficaciously reduce the emission of influenza virus particles into the environment in respiratory droplets, but not in aerosols12. Both the previous and current study used a bioaerosol collecting device, the Gesundheit-II (G-II)12,15,19, to capture exhaled breath particles and differentiated into two size fractions, where exhaled breath coarse particles >5μm (respiratory droplets) were collected by impaction with a 5.0μm slit inertial Teflon impactor and the remaining fine particles ≤5μm (aerosols) were collected by condensation in buffer. We also demonstrated the efficacy of surgical masks to reduce coronavirus detection and viral copies in large respiratory droplets and in aerosols (Table 1b). This has important implications for control of COVID-19, suggesting that surgical face masks could be used by ill persons to reduce onwards transmission.
Among the samples collected without a face mask, we found that the majority of participants with influenza virus and coronavirus infection did not shed detectable virus in respiratory droplets or aerosols, while for rhinovirus we detected virus in aerosols in 19/34 (56%) participants (compared to 4/10 (40%) for coronavirus and 8/23 (35%) for influenza). For those who did shed virus in respiratory droplets and aerosols, viral load in both tended to be low (Figure 1). Given the high collection efficiency of the G-II19, and given that each exhaled breath collection was done for 30 minutes, this might imply that prolonged close contact would be required for transmission to occur, even if transmission was primarily via aerosols as has been described for rhinovirus colds20. Our results also indicate that there could be considerable heterogeneity in contagiousness of individuals with coronavirus and influenza virus infections.
The major limitation of our study was the large proportion of participants with undetectable viral shedding in exhaled breath for each of the viruses studied. We could have increased the sampling duration beyond 30 minutes to increase the viral shedding being captured, at the cost of acceptability in some participants. An alternative approach would be to invite participants to perform forced coughs during exhaled breath collection12. However, it was the aim of our present study to focus on recovering respiratory virus in exhaled breath in a real-life situation, and we expected some individuals during an acute respiratory illness would not cough much or at all. Indeed, we identified virus RNA in a small number of participants who did not cough at all during the 30-minute exhaled breath collection, which would suggest droplet and aerosol routes of transmission are possible from individuals with no obvious signs or symptoms. Another limitation is that we did not confirm infectivity of coronavirus or rhinovirus detected in exhaled breath. While the G-II was designed to preserve viability of viruses in aerosols, and in the present study we were able to identify infectious influenza virus in aerosols, we did not attempt to culture coronavirus or rhinovirus from the corresponding aerosol samples.
METHODS
Study design
Participants were recruited year-round from March 2013 through May 2016 in a general outpatient clinic of a private hospital in Hong Kong. As routine practice, clinic staff screened all individuals attending the clinics for respiratory and any other symptoms regardless of the purpose of the visit at the triage. Study staff then approached immediately those who reported at least one of the following symptoms of acute respiratory illness (ARI) for further screening: fever≥37.8°C, cough, sore throat, runny nose, headache, myalgia and phlegm. Individuals who reported ≥2 ARI symptoms, within 3 days of illness onset and ≥11 years of age were eligible to participate. After explaining the study to and obtaining informed consent from the participant, a rapid influenza diagnostic test, the Sofia Influenza A+B Fluorescent Immunoassay Analyzer (Cat #20218, Quidel, San Diego, CA), was used to identify influenza A or B virus infection as an incentive to participate. All participants provided a nasal swab for the rapid test, and an additional nasal swab and a separate throat swab for subsequent virologic confirmation at the laboratory. All participants also completed a questionnaire to record basic information including age, sex, symptom severity, medication, medical conditions and smoking history. In the first phase of the study from March 2013 to February 2014 (‘Influenza Study’), the result of the rapid test was used to determine eligibility for further participation in the study and exhaled breath collection; while in the second phase of the study from March 2014 to May 2016 (‘Respiratory Virus Study’), the rapid test did not affect eligibility. Eligible participants were then invited to provide an exhaled breath sample for 30 minutes in the same clinic visit.
Prior to the exhaled breath collection, each participant was randomly allocated in a 1:1 ratio to either wearing a surgical face mask (Cat #62356, Kimberly-Clark, Roswell, Georgia) or not during the collection. To mimic the real-life situation, under the observation by the study staff participants were asked to attach the surgical mask themselves, but instruction on how to wear the mask properly was given when the participant wore the mask incorrectly. Participants were instructed to breathe as normal during the collection, but (natural) coughing was allowed and the number of coughs was recorded by study staff. Participants were then invited to provide a second exhaled breath sample of the alternate type (e.g. if the participant was first assigned to wearing a mask he/she would then provide a second sample without a mask), but most participants did not agree to stay for a second measurement because of time constraints. Participants were compensated for each 30-minute exhaled breath collection with a supermarket coupon worth approximately US$30 and all participants were gifted a tympanic thermometer worth approximately US$20.
Ethical approval
Written informed consent was obtained from all participants ≥18 years of age, and written informed consent was obtained from parents or legal guardians of participants 11–17 years of age in addition to their own written informed consent. The study protocol was approved by the Institutional Review Board of The University of Hong Kong and the Clinical and Research Ethics Committee of Hong Kong Baptist Hospital.
Collection of swabs and exhaled breath particles
Nasal swabs and throat swabs were collected separately, placed in virus transport medium (VTM), stored and transported to the laboratory at 2–8°C, and the VTM were aliquoted and stored at −70°C until further analysis. Exhaled breath particles were captured and differentiated into two size fractions, the coarse fraction containing particles with aerodynamic diameter >5μm (referred to here as ‘respiratory droplets’) included droplets up to approximately 100 μm in diameter) and the fine fraction with particles ≤5μm (referred to here as ‘aerosols’) by the “G-II” bioaerosol collecting device12,15,19. In the G-II device, exhaled breath coarse particles >5μm were collected by a 5.0μm slit inertial Teflon impactor, and the remaining fine particles ≤5μm were condensed and collected into about 170ml of 0.1%BSA/PBS. Both the impactor and the condensate were stored and transported to the laboratory at 2–8°C. The virus on the impactor was recovered into 1ml, and the condensate was concentrated into 2ml of 0.1% BSA/PBS, aliquoted and stored at −70°C until further analysis. In a validation study, the G-II was able to recover over 85% of fine particles >0.05μm in size, and had comparable collection efficiency of influenza virus as the SKC BioSampler19.
Laboratory testing
Samples collected from the two Studies were tested at the same time. Nasal swab samples were first tested by a diagnostic-use viral panel, xTAG Respiratory Viral Panel (Abbott Molecular, Illinois, USA), to detect qualitatively twelve common respiratory viruses and subtypes including coronaviruses (NL63, OC43, 229E and HKU1), influenza A (non-specific, H1 and H3) and B viruses, respiratory syncytial virus (RSV), parainfluenza virus (types 1–4), adenovirus, human metapneumovirus, and enterovirus/rhinovirus. After one or more of the candidate respiratory viruses was detected by the Viral Panel from the nasal swab, all the samples from the same participant, i.e. the nasal swab, throat swab, the respiratory droplets and aerosols, were then tested with reverse transcriptase real-time polymerase chain reaction (RT-PCR) specific to the candidate virus(s) for determination of virus concentration in the samples. Infectious influenza virus was identified by viral culture using MDCK cells as described previously21, while viral culture was not done for coronavirus and rhinovirus.
Statistical analyses
The primary outcome of the study was the virus generation rate in the tidal breathing of participants infected by different respiratory viruses, and the efficacy of face mask in preventing virus dissemination in exhaled breath, separately considering the respiratory droplets and aerosols. The secondary outcomes were the correlation between viral shedding in nose swabs, throat swabs, respiratory droplets and aerosols, and factors affecting viral shedding in respiratory droplets and aerosols.
We identified three groups of respiratory viruses with highest frequency of infection as identified by RT-PCR, namely coronavirus (including NL63, OC43, HKU1 and 229E), influenza virus, and rhinovirus, for further statistical analyses. We defined viral shedding as log10 virus copies per sample, and plotted viral shedding in each sample, i.e. the nasal swab, throat swab, respiratory droplets and aerosols, the latter two stratified by the mask intervention. As a proxy for the efficacy of face masks in preventing transmission of respiratory viruses via the respiratory droplet and aerosol routes, we compared the respiratory virus viral shedding in respiratory droplet and aerosol samples between participants wearing face mask or not, by comparing the frequency of detection with two-sided Fisher’s exact test, and by comparing viral load (defined as log10 virus copies per sample) by an unadjusted univariate Tobit regression model which allowed for censoring at the lower limit of detection of the RT-PCR assay. We also used the unadjusted univariate Tobit regression to investigate factors affecting viral shedding in respiratory droplets and aerosols without mask use, for example age, days since symptom onset, prior influenza vaccination, current medication and number of coughs during exhaled breath collection. We investigated the correlations between viral shedding in nasal swab, throat swab, respiratory droplets and aerosols with scatterplots and calculated the Spearman’s rank correlation coefficient between any two types of samples. We imputed 0.3 log10 virus copies/ml for the undetectable values before transformation to log10 virus copies per sample. All analyses were conducted with R version 3.6.022 and the VGAM package version 1.1.123.
DATA AVAILABILITY
Anonymized raw data and R syntax to reproduce all the analyses, figures, tables and supplementary tables in the published article are available at: https://doi.org/10.5061/dryad.w9ghx3fkt.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the General Research Fund (GRF) of the University Grants Committee [Grant No. 765811], the Health and Medical Research Fund (HMRF) [Grant No. 13120592] and a commissioned grant of the Food and Health Bureau, and the Theme-based Research Scheme [Project No. T11-705/14-N] of the Research Grants Council of the Hong Kong SAR Government. We wish to acknowledge colleagues including Rita Oi Pei Fung, Anita Kin Wa Li, Tiffany Waai Yan Ng, Teresa Hau Chi So, Peng Wu and Yanmy Xie for technical support in preparing and conducting this study and enrolling participants; Jo Kit Man Chan, Sin Ying Ho, Ying Zhi Liu and Amy Yu for laboratory support; Steve Ferguson, Wing Kam Leung, Jovan Pantelic, Jianjian Wei and Mike Wolfson for technical support in constructing and maintaining the G-II device; Vicky Jing Fang, Lai Ming Ho and Tom Tze Kit Lui for setting up the database; and Christina Woon Yee Cheung, Lawrence Fat Kwong Cheung, Patricia Tai Yin Ching, Ada Chu Hsu Lai, Deanna Wai Yee Lam, Suky Suk Yee Lo, Amy Siu Kuen Luk and other colleagues at the Out-Patient Centre and Infection Control Team of Hong Kong Baptist Hospital for facilitating this study.
Footnotes
COMPETING INTERESTS STATEMENT
BJC consults for Roche and Sanofi Pasteur. The authors report no other potential conflicts of interest.
REFERENCES
- 1.Nichols WG, Peck Campbell AJ & Boeckh M Respiratory viruses other than influenza virus: impact and therapeutic advances. Clin. Microbiol. Rev. 21, 274–290, table of contents, doi: 10.1128/CMR.00045-07 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shiu EYC, Leung NHL & Cowling BJ Controversy around airborne versus droplet transmission of respiratory viruses: implication for infection prevention. Curr. Opin. Infect. Dis. 32, 372–379, doi: 10.1097/QCO.0000000000000563 (2019). [DOI] [PubMed] [Google Scholar]
- 3.Tellier R, Li Y, Cowling BJ & Tang JW Recognition of aerosol transmission of infectious agents: a commentary. BMC Infect. Dis. 19, 101, doi: 10.1186/s12879-019-3707-y (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xiao J et al. Nonpharmaceutical Measures for Pandemic Influenza in Nonhealthcare Settings-Personal Protective and Environmental Measures. Emerg. Infect. Dis. 26, doi: 10.3201/eid2605.190994 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kutter JS, Spronken MI, Fraaij PL, Fouchier RAM & Herfst S Transmission routes of respiratory viruses among humans. Curr. Opin. Virol. 28, 142–151, doi: 10.1016/j.coviro.2018.01.001 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cowling BJ & Leung GM Epidemiological research priorities for public health control of the ongoing global novel coronavirus (2019-nCoV) outbreak. Euro Surveill. 25, doi: 10.2807/1560-7917.ES.2020.25.6.2000110 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Han Q, Lin Q, Ni Z & You L Uncertainties about the transmission routes of 2019 novel coronavirus. Influenza Other Respi. Viruses, doi: 10.1111/irv.12735 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.MacIntyre CR & Chughtai AA Facemasks for the prevention of infection in healthcare and community settings. BMJ 350, h694, doi: 10.1136/bmj.h694 (2015). [DOI] [PubMed] [Google Scholar]
- 9.Ha’eri GB & Wiley AM The efficacy of standard surgical face masks: an investigation using “tracer particles”. Clin. Orthop. Relat. Res., 160–162 (1980). [PubMed] [Google Scholar]
- 10.Patel RB, Skaria SD, Mansour MM & Smaldone GC Respiratory source control using a surgical mask: An in vitro study. J. Occup. Environ. Hyg. 13, 569–576, doi: 10.1080/15459624.2015.1043050 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johnson DF, Druce JD, Birch C & Grayson ML A quantitative assessment of the efficacy of surgical and N95 masks to filter influenza virus in patients with acute influenza infection. Clin. Infect. Dis. 49, 275–277, doi: 10.1086/600041 (2009). [DOI] [PubMed] [Google Scholar]
- 12.Milton DK, Fabian MP, Cowling BJ, Grantham ML & McDevitt JJ Influenza virus aerosols in human exhaled breath: particle size, culturability, and effect of surgical masks. PLoS Pathog. 9, e1003205, doi: 10.1371/journal.ppat.1003205 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huynh KN, Oliver BG, Stelzer S, Rawlinson WD & Tovey ER A new method for sampling and detection of exhaled respiratory virus aerosols. Clin. Infect. Dis. 46, 93–95, doi: 10.1086/523000 (2008). [DOI] [PubMed] [Google Scholar]
- 14.Stelzer-Braid S et al. Exhalation of Respiratory Viruses by Breathing, Coughing, and Talking. J. Med. Virol. 81, 1674–1679, doi:Doi 10.1002/Jmv.21556 (2009). [DOI] [PubMed] [Google Scholar]
- 15.Yan J et al. Infectious virus in exhaled breath of symptomatic seasonal influenza cases from a college community. Proc. Natl. Acad. Sci. U. S. A. 115, 1081–1086, doi: 10.1073/pnas.1716561115 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tovey ER et al. Rhinoviruses significantly affect day-to-day respiratory symptoms of children with asthma. J. Allergy Clin. Immunol. 135, 663–669.e612, doi: 10.1016/j.jaci.2014.10.020 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Booth TF et al. Detection of airborne severe acute respiratory syndrome (SARS) coronavirus and environmental contamination in SARS outbreak units. J. Infect. Dis. 191, 1472–1477, doi: 10.1086/429634 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim SH et al. Extensive Viable Middle East Respiratory Syndrome (MERS) Coronavirus Contamination in Air and Surrounding Environment in MERS Isolation Wards. Clin. Infect. Dis. 63, 363–369, doi: 10.1093/cid/ciw239 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McDevitt JJ et al. Development and Performance Evaluation of an Exhaled-Breath Bioaerosol Collector for Influenza Virus. Aerosol Sci Technol 47, 444–451, doi: 10.1080/02786826.2012.762973 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jennings LC & Dick EC Transmission and control of rhinovirus colds. Eur. J. Epidemiol. 3, 327–335, doi: 10.1007/bf00145641 (1987). [DOI] [PubMed] [Google Scholar]
- 21.Chan KH, Peiris JS, Lim W, Nicholls JM & Chiu SS Comparison of nasopharyngeal flocked swabs and aspirates for rapid diagnosis of respiratory viruses in children. J. Clin. Virol. 42, 65–69, doi: 10.1016/j.jcv.2007.12.003 (2008). [DOI] [PubMed] [Google Scholar]
- 22.R: A language and environment for statistical computing (R Foundation for Statistical Computing, Vienna, Austria, 2019). [Google Scholar]
- 23.Yee TW Vector Generalized Linear and Additive Models: With an Implementation in R. (Springer; New York, 2016). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Anonymized raw data and R syntax to reproduce all the analyses, figures, tables and supplementary tables in the published article are available at: https://doi.org/10.5061/dryad.w9ghx3fkt.