Graphical abstract
Keywords: COVID-19, Comorbidity, Meta-analysis
Abstract
Background
COVID-19 has infected over 35 million people worldwide and led to over 1 million deaths. Several risk factors that increase COVID-19 severity have emerged, including age and a history of cardiovascular disease, hypertension, or kidney disease. However, a number of outstanding questions persist, including whether the above comorbidities correlate with increased mortality from COVID-19 or whether age is a significant confounding variable that accounts for the observed relationship between COVID-19 severity and other comorbidities.
Methods and Findings
We conducted a systematic review and meta-analysis of studies documenting COVID-19 patients with hypertension, cardiovascular disease, cerebrovascular disease, or chronic kidney disease. We classified COVID-19 cases into severe/non-severe or deceased/surviving and calculated the odds ratio (OR) for each of the four comorbidities in these cohorts. 36 studies, comprising 22,573 patients, are included in our meta-analysis. We found that hypertension is the most prevalent comorbidity in deceased COVID-19 patients (55.4%; CI: 49.4–61.3%), followed by cardiovascular disease (30.7%; CI: 22.6–38.8%), cerebrovascular disease (13.4%; CI: 9.12–19.2%), then chronic kidney disease (9.05%; CI: 5.57–15.0%). The risk of death is also significantly higher for patients with these comorbidities, with the greatest risk factor being chronic kidney disease (OR: 8.86; CI: 5.27–14.89), followed by cardiovascular disease (OR: 6.87; CI: 5.56–8.50), hypertension (OR: 4.87; CI: 4.19–5.66), and cerebrovascular disease (OR: 4.28; CI: 2.86–6.41). These risks are significantly higher than previously reported, while correlations between comorbidities and COVID-19 severity are similar to previously reported figures. Using meta-regression analysis with age as a moderating variable, we observed that age contributes to the observed risks but does not explain them fully.
Conclusions
In this meta-analysis, we observed that cardiovascular, cerebrovascular, and kidney-related comorbidities in COVID-19 significantly contributes to greater risk of mortality and increased disease severity. We also demonstrated that age may not be a confounder to these associations.
1. Introduction
In December 2019, a novel Coronavirus Disease 2019 (COVID-19) caused by a new coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was first identified in Wuhan, China. Till date, the ongoing COVID-19 pandemic has had immense impact on healthcare institutions worldwide. Due to the high contagiousness of COVID-19, as well as its varied symptoms and differentiated clinical outcomes in patients [1], [2], [3], it is crucial to identify COVID-19 patients at risk of progressing to severe or critical illness to determine the most appropriate allocation of health system resources.
In a meta-analysis of 27 articles consisting of 22,753 patients globally, it was shown that the most common comorbidities in COVID-19 patients include hypertension (27.4%), cardiovascular disease (8.9%), and diabetes (17.4%) and that these comorbidities are associated with disease severity [4]. However, this study did not quantify study heterogeneity and did not examine correlations between different comorbidities or between comorbidities and potential confounding variables. In a separate study, chronic kidney disease (1.3%) and cerebrovascular disease (1.9%) were also found to be relevant underlying diseases that contribute to increased COVID-19 severity [5]. This study also did not include any multivariate analyses. Similar studies have also concluded that comorbidities, such as hypertension, cardiovascular disease, cerebrovascular disease, diabetes, and chronic kidney disease, significantly contribute to the progression to severe disease [6], [7], [8]. However, the lack of multivariate correction of results in most of these studies suggests that the existence of other variables that may be instead influencing COVID-19 severity cannot be precluded. A brief literature review conducted by Sisnieguez et. al. concluded that most studies, including both primary studies and meta-analyses, did not include multivariate analyses [9]. For two studies that evaluated the association between comorbidities and COVID-19 severity using a multivariate regression model, arterial hypertension was not the most significant predictor when other clinical variables are taken into account [10], [11]. Age has been shown to be the most likely moderating or confounding variable [12], [13].
Beyond the uncertainty over the presence of confounding variables, current research on whether comorbidities are associated with mortality is inconclusive. A number of primary studies and meta-analyses found no association between comorbidities and mortality, including a meta-analysis of 22,753 patients [4], [14], [15]. However, other large-scale have found significant association between comorbidities and mortality [16], [17], including an electronic medical record analysis of over 31,000 patients. In this study, we will attempt to address the inconsistencies and shortcomings of previous meta-analysis studies by examining the extent that age influences our correlation results and by analyzing patients with severe COVID-19 and patients who died from COVID-19 separately.
2. Materials and Methods
2.1. Search strategy and inclusion criteria for meta-analysis
A systematic search was conducted on studies published from the beginning of 2020 to May 25, 2020 in PubMed, medRxiv, and bioRxiv databases (Fig. S1). We used the search terms “covid19 comorbidity” AND “cardiovascular disease” OR “hypertension” OR “cerebrovascular disease” OR “chronic kidney disease” OR “stroke” OR “kidney damage”. Relevant articles were reviewed by two independent reviewers (LA and ACL). We included studies based on the following criteria: (1) Types of Studies: articles that reported the relationship between comorbidity and COVID-19; (2) Subjects: patients diagnosed with COVID-19; (3) Exposure intervention: COVID-19 patients with comorbidity including: hypertension, cardiovascular disease, cerebrovascular disease or chronic kidney disease. We excluded (1) editorials, reviews, meta-analyses; (2) studies with insufficient data, including case reports and letters; (3) studies where patients were not categorized based on severity of illness or survival; (4) studies not on the general population.
We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) standards for selection and inclusion of studies in our meta-analysis. From our search terms, we identified 899 potential articles for review. Out of these articles, 500 unique articles were identified. We excluded 437 articles based on Abstract review, and the remaining articles underwent full-text review. After full-text review, 14 reports were excluded because of insufficient data, 9 were excluded because patients were not sampled from the general population, and 3 were case series. Thus, a total of 36 articles were included for statistical analysis. Since it is not possible to conducted randomized case-control trials, all studies were primary case series consisting of a significant number of cases.
2.2. Data extraction
We extracted the following information from each article included in the study: (1). The year the article was published. (2). The population on whom the study was conducted. (3). The type of research. (4). What were the clinical variables included in each study and how were they measured? (5). How many individuals were included in the study? (6). If the clinical variables were measured in a continuous scale, what was the mean value of the variables among severe/deceased patients? (7). If the clinical variables were measured on a continuous scale, what was the mean of the clinical variables among non-severe/surviving patients? (8). If the clinical variables were measured on a continuous scale, what was the standard deviation of the clinical variables among severe/deceased patients? (9). If the clinical variables were measured on a continuous scale, what was the standard deviation of the clinical variables among non-severe/surviving patients? (10). If the clinical variables were measured on a binary scale, what were the number of people with the clinical variable in the severe/deceased cohort? (11). If the clinical variables were measured on a binary scale, what were the number of people with the clinical variable in the non-severe/surviving cohort?
2.3. Assessment of data quality
We sought to assign a quality score to each study we evaluated using a modification of the Newcastle/Ottawa scale. In this scale, one point is assigned for every “Yes” answered for the following questions: 1. Does the patient(s) represent(s) the whole experience of the investigator (center)? 2. Was the comorbidity adequately ascertained? 3. Was the outcome (COVID-19) adequately ascertained? 4. Were other alternative causes that may explain the observation ruled out? 5. Was there a challenge/rechallenge phenomenon? 6. Was there a dose–response effect? 7. Are the case described with sufficient details to allow other investigators to replicate the research or to allow practitioners make inferences related to their own practice? All points are summed to arrive at a quality score. A score of 5 or above is considered data for inclusion. All 36 studies at the final stage of article selection passed the quality score cutoff.
2.4. Statistical analyses
All data analysis was performed using R studio. Logit transformations were applied to proportional data where a majority of proportions were<0.2 or greater than 0.8. The Cochran’s Q test and the I2 index was used to test for and quantify study heterogeneity. Due to high heterogeneity (p < 0.05 for Q test, I2 greater than 50%), random effect models were used to estimate the summary proportions. We also performed leave-one-out sensitivity analysis to assess the effect of outliers on summary proportions. To account for some heterogeneity across all studies, we conducted moderator analysis using meta-regression. The odds ratios (ORs) describe the risk of different comorbidities in severe patients relative to non-severe patients, as well as the risk of the comorbidities in deceased relative to surviving patients. Publication bias was assessed using funnel plots and Egger test linear regression test (p < 0.05), and correlations between comorbidities were calculated using Spearman correlation test (p < 0.05).
3. Results
3.1. Prevalence of comorbidities in deceased COVID-19 patients
A total of 36 studies (Table 1), with a total of 22,573 patients, were included in this meta-analysis. 34 studies reported hypertension, 36 reported cardiovascular disease, 20 reported cerebrovascular disease and 27 reported chronic kidney disease. Each set of studies that report a certain comorbidity is analyzed separately. The studies used clinical symptoms and ICU admission to assess disease severity. Severe COVID-19 was defined as patients who had any of the following features at the time of, or after, admission: (1) respiratory distress (≥30 breaths per min); (2) oxygen saturation at rest ≤ 93%; (3) ratio of the partial pressure of arterial oxygen (PaO2) to a fractional concentration of oxygen inspired air (fiO2) ≤ 300 mmHg; or (4) critical complication (respiratory failure, septic shock, and or multiple organ dysfunction/failure) or admission into ICU.
Table 1.
Study characteristics of articles included in the meta-analysis.
| Source | Type of Study | Quality Score | Total No. of Patients | Geographical Region | Mortality Rate, % | Hypertension, % | Cardiovascular Disease, % | Stroke/Cerebrovascular Disease, % | Chronic Kidney Disease, % |
|---|---|---|---|---|---|---|---|---|---|
| Lijun Sun et al, 2020 | Observational Case Series | 6 | 55 | Beijing 302 Hospital | no deaths reported | 40 | 6.7 | N/A | 6.7 |
| Dominic Wichmann et al, 2020 | Prospective Cohort Study | 6 | 12 | University Medical Center Hamburg-Eppendorf | * | N/A | 25 | N/A | N/A |
| Fei Shao et al, 2020 | Case Series | 6 | 136 | Wuhan, China | 19.3 | 30.2 | 11 | 3.7 | N/A |
| Giacomo Grasselli et al, 2020 | Case Series | 6 | 1591 | Milan, Italy | 26 | 49 | 21 | N/A | 3 |
| Wei-Jie Guan et al, 2020 | Case Series | 6 | 1590 | 575 hospitals across mainland China | 3.1 | 16.9 | 3.7 | 1.9 | 1.3 |
| Chaolin Huang et al, 2020 | Case Series | 6 | 41 | Wuhan, China | 15 | 15 | 15 | N/A | 7 |
| Jin-jin Zhang et al, 2020 | Observational Case Series | 6 | 140 | Wuhan, China | no deaths reported | 32 | 5 | 2.1 | 1.4 |
| Char Leung et al, 2020 | Case Series | 6 | 46 | Wuhan, China | * | 40.5 | 18.6 | N/A | |
| Mohamad Nikpouraghdam et al, 2020 | Case Series | 6 | 2968 | Baqiyatallah Hospital - Tehran, Iran | 8.06 | 1.99 | 1.25 | N/A | 0.6 |
| QingChun Yao et al, 2020 | Case Series | 6 | 108 | Dabieshan Medical Center - Hubei, China | 48 | 14.8 | 3.7 | N/A | N/A |
| Xian Zhou et al, 2020 | Case Series | 6 | 110 | Wuhan Fourth Hospital | no deaths reported | 32.7 | 9.1 | 2.7 | 1.8 |
| Bicheng Zhang et al, 2020 | Case Series | 6 | 82 | Wuhan, China | * | 56.1 | 20.7 | 12.2 | 4.8 |
| Ying Huang et al, 2020 | Retrospective Case Series | 5 | 36 | Wuhan, China | * | 58.3 | 13.9 | 22.2 | 8.3 |
| Ao-Xiang Guo et al, 2020 | Retrospective Case Series | 6 | 159 | China | * | 41.5 | 28.3 | 10.7 | 5.7 |
| Gianpaolo Benelli et al, 2020 | Cohort Study | 6 | 411 | Crema, Italy | 17.5 | 47 | 22.6 | N/A | 5.3 |
| Fei Xiong et al, 2020 | Case Series | 6 | 131 | Wuhan, China | no deaths reported | N/A | 68.7 | N/A | N/A |
| Rong-Hui Du et.al, 2020 | Observational Case Series | 6 | 179 | Wuhan Pulmonary Hospital | 11.7 | 32.4 | 16.2 | 2.2 | |
| QingQing Chen et al, 2020 | Case Series | 6 | 145 | Taizhou, Zhejiang, China | no deaths reported | 15.2 | 0.7 | N/A | 2.1 |
| Yun Feng et al, 2020 | Case Series | 6 | 476 | Hospitals in Wuhan, Shanghai, and Anhui, China | no deaths reported | 23.7 | 8 | 3.6 | N/A |
| Wonjun Ji et al, 2020 | Retrospective Case Series | 6 | 5172 | South Korea | no deaths reported | 21.8 | 14.3 | 6.7 | 10.8 |
| Yunfei Liao et al, 2020 | Retrospective Case Series | 6 | 1848 | Wuhan, China | no deaths reported | 1.6 | 0.4 | 0.2 | 0.05 |
| Chen Yanover et al, 2020 | Cohort Study | 6 | 4353 | Israel | no deaths reported | 14.4 | 7.1 | 1.3 | 8.8 |
| Joseph E. Ebinger et al, 2020 | Observational Case Series | 6 | 442 | Los Angeles, USA | 2.5 | 36 | 11 | N/A | N/A |
| Valente-Acosta, B. et al, 2020 | Case Series | 6 | 33 | Mexico | no deaths reported | 36.4 | 9.1 | N/A | N/A |
| Kim, L. et al, 2020 | Cohort Study | 6 | 2490 | USA | 16.9 | 57.4 | 34.6 | N/A | 15.5 |
| Alberto M. Borobia et al, 2020 | Cohort Study | 6 | 2226 | Madrid, Spain | 20.7 | 41.3 | 19.3 | N/A | 7.8 |
| Tao Chen et al, 2020 | Case Series | 6 | 274 | Tongji Hospital - Wuhan, China | 41.2 | 34 | 8 | 1 | 1 |
| Fei Zhou et al, 2020 | Case Series | 6 | 191 | Wuhan, China | 28.3 | 30 | 8 | N/A | 1 |
| Lang Wang et al, 2020 | Case Series | 6 | 339 | Renmin Hospital, Wuhan University | 19.2 | 40.8 | 15.7 | 6.2 | 3.8 |
| Tian Gu et al, 2020 | Case-control Study | 6 | 275 | mainland China | 34.2 | 39.6 | 22.5 | 6.9 | 4.4 |
| Fan Zhang et al, 2020 | Retrospective Cohort Study | 6 | 48 | Wuhan, China | 35.4 | 66.7 | 27.1 | 22.9 | 10.4 |
| Shaobo Shi et al, 2020 | Case Series | 6 | 671 | Renmin Hospital, Wuhan University | 9.2 | 29.7 | 8.9 | 3.3 | 4.2 |
| Chen Chen et al, 2020 | Retrospective Case Series | 6 | 132 | Hubei Third People's Hospital, China | 9.8 | 68.2 | 23.5 | 5.3 | 4.5 |
| Guqin Zhang et al, 2020 | Retrospective Case Series | 6 | 221 | Wuhan, China | 20.5 | 24.4 | 10 | 6.8 | 2.7 |
| Michael G. Argenziano et al, 2020 | Retrospective Case Series | 6 | 1000 | New York City, USA | 21.1 | 60.1 | 23.3 | 7.9 | N/A |
| Shijiao Yan | Retrospective Case Series | 6 | 168 | Hainan, China | 3.6 | 14.3 | 7.1 | N/A | 0.6 |
| Christopher M. Petrilli et al, 2020 | Cross-sectional Study | 6 | 4103 | New York City, USA | 4.3 | 24 | 30.1 | N/A | 5.2 |
The results of this meta-analysis showed that the most prevalent comorbidity in deceased COVID-19 patients is hypertension (55.4%; CI: 49.4–61.3%), followed by cardiovascular disease (30.7%; CI: 22.6–38.8%), cerebrovascular disease (13.4%; CI: 9.12–19.2%), then chronic kidney disease (9.05%; CI: 5.57–15.0%) (Fig. S2). Few studies have quantified the prevalence of comorbidities in deceased COVID-19 patients. We found our prevalence percentages to be significantly higher than some current reports. In one meta-analysis of 38 studies, hypertension was only found in 32% of deceased patients, followed by cardiovascular disease at 13% [18]. The prevalence of chronic kidney disease was 9.6% in fatal cases in one review, which is similar to what we found [19]. We found that these four comorbidities were also associated with higher mortality rates.
Higher risk of hypertension (OR: 4.87; CI: 4.19–5.66), cardiovascular disease (OR: 6.87; CI: 5.56–8.50), cerebrovascular disease (OR: 4.28; CI: 2.86–6.41) and chronic kidney disease (OR: 8.86; CI: 5.27–14.89) were observed in deceased patients (Table 2). These ORs are also higher than previously reported. A meta-analysis of 25 studies (n = 484 patients) indicated an mortality OR of 2.25 for cardiovascular disease and 1.72 for hypertension [20].
Table 2.
Summary of odds ratios, Q statistics, I2 statistics for comorbidities.
| Variable Studied | No. of Studies | Odds Ratio | Q Statistic | I2 statistic, % |
|---|---|---|---|---|
| Proportion of deceased with HTN | 15/36 | 4.87 | 102.3 | 75.9 |
| Proportion of survivors with HTN | 9/36 | 82.1 | 93.8 | |
| Proportion of severe COVID-19 with HTN | 18/36 | 3.26 | 202.2 | 93.9 |
| Proportion of non-severe COVID-19 with HTN | 14/36 | 830.9 | 98.8 | |
| Proportion of deceased with CVD | 14/36 | 6.87 | 204.7 | 89.3 |
| Proportion of survivors with CVD | 8/36 | 53.2 | 93.6 | |
| Proportion of severe COVID-19 with CVD | 19/36 | 3.16 | 431.3 | 95.8 |
| Proportion of non-severe COVID-19 with CVD | 15/36 | 832.9 | 98.9 | |
| Proportion of deceased with CBVD | 8/36 | 4.28 | 17.9 | 64.9 |
| Proportion of survivors with CBVD | 5/36 | 17.6 | 82.8 | |
| Proportion of severe COVID-19 with CBVD | 9/36 | 1.90 | 43.7 | 81.5 |
| Proportion of non-severe COVID-19 with CBVD | 7/36 | 22.8 | 84.4 | |
| Proportion of deceased with CKD | 11/36 | 8.86 | 104.3 | 83.2 |
| Proportion of survivors with CKD | 5/36 | 4.9 | 0.0006 | |
| Proportion of severe COVID-19 with CKD | 11/36 | 1.92 | 237.2 | 95.2 |
| Proportion of non-severe COVID-19 with CKD | 9/36 | 39.5 | 98.3 |
*Abbreviations used: HTN - hypertension; CVD - cardiovascular disease; CBVD - cerebrovascular disease; CKD - chronic kidney disease.
3.2. Prevalence of comorbidities in severely ill COVID-19 patients
The most prevalent comorbidity in COVID-19 patients with severe illness is hypertension (43.0%; CI: 34.0–51.9%), then cardiovascular disease (23.5%; CI: 15.0–32.0%), followed by cerebrovascular disease (8.21%; CI: 4.95–13.3%) and chronic kidney disease (8.46%; CI: 3.72–18.1%) (Fig. S3). These results are similar to current findings. A report by the COVID-19-Associated Hospitalization Surveillance Network showed that amongst hospitalized COVID-19 patients with data on underlying conditions, the most common comorbidities included hypertension (49.7%) and cardiovascular disease (27.8%) [21]. Higher risk of hypertension (OR: 3.26; CI: 2.96–3.59), cardiovascular disease (OR: 3.16; CI: 2.84–3.52), cerebrovascular disease (OR: 1.90; CI: 1.53–2.35) and chronic kidney disease (OR: 1.92; CI: 1.65–2.23) were observed in the severe group vs. non-severe group (Table 2). This is similar to other meta-analyses that show that higher risk of hypertension (OR 2.36, CI: 1.46–3.83) and cardiovascular disease (OR 3.42, CI: 1.88–6.22) were observed in severe COVID-19 [6].
3.3. Meta-regression with age as a moderating variable
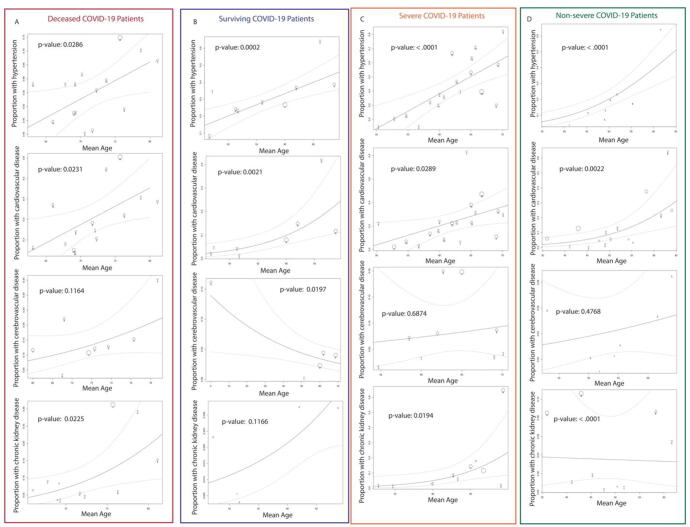
Age has been implicated to be a moderating or confounding variable to the results of correlations between comorbidities and COVID-19 severity/mortality [9]. To investigate the relationship between age and effect sizes, we used a meta-regression model. With a few exceptions, age was a significant moderator for most cohorts (Fig. 1) (p < 0.05).
Fig. 1.
Metaregression of the comorbidities in deceased (A), surviving (B), severe (C) and non-severe (D) COVID19 patients, with age as the moderating variable.
We found that in severe or non-severe patients with cerebrovascular disease, deceased patients with cerebrovascular disease and surviving patients with chronic kidney disease, age was not a significant moderator. In addition, we found that age had a slight negative association with the proportion of surviving patients with cerebrovascular disease. Interestingly, we noticed that age is a very strong moderating variable for the proportion of surviving patients with hypertension or cardiovascular diseases but a less strong moderating variable for the proportion of deceased patients with the same conditions (Fig. 1A,B), as shown by a p-value difference of more than 100 fold for hypertension and more than 10 fold for cardiovascular disease. In other words, age explains the presence of the above comorbidities very well in patients who survived COVID-19 but does not explain as well the presence of the same comorbidities in patients who did not survive COVID-19. The same trend was observed for the proportion of cardiovascular disease, cerebrovascular disease, and chronic kidney disease, but not hypertension, in severe vs. non-severe COVID-19 patients (Fig. 1C,D). This trend may indicate that age is not a significant confounding variable, although age does contribute to the correlation between comorbidities and COVID-19 severity or mortality.
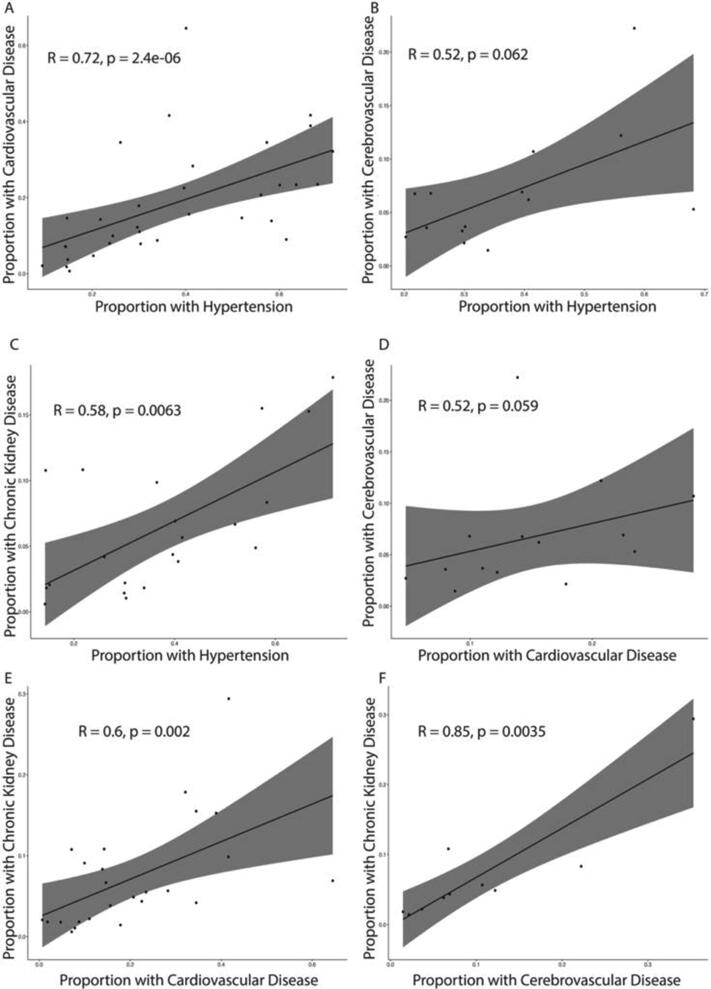
3.4. Correlation between the proportion of different comorbidities
We found a positive correlation between the proportions of all the comorbidities (Fig. 2). This is not unexpected as hypertension is a major risk factor for myocardial infarction, stroke, heart failure, and renal failure [22], [23]. Previous meta-analyses have not examined correlations between comorbidities in COVID-19 patients.
Fig. 2.
Scatterplots of significant Spearman correlations (p < 0.05) between comorbidities.
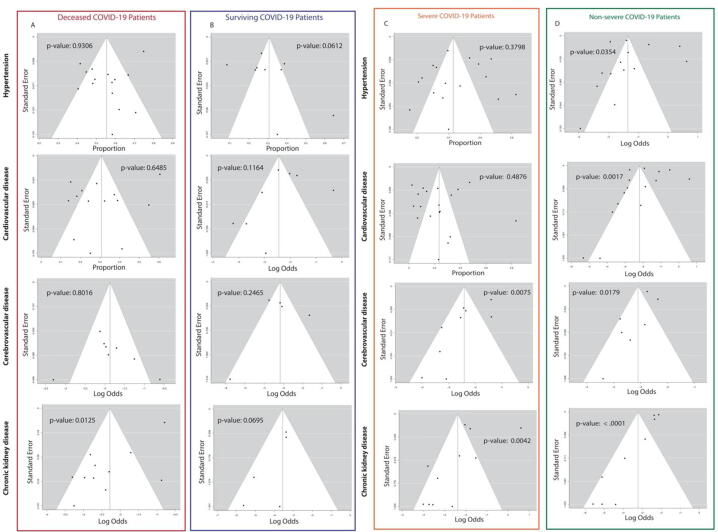
3.5. Examination of publication bias
The risk of publication bias was examined through funnel plots and Egger's tests (p greater than 0.05). For studies related to COVID-19 mortality, we found that all studies, except for those detailing the proportion of deceased COVID-19 patients with chronic kidney disease (Fig. 3A,B), showed no significant publication bias.
Fig. 3.
Funnel plots of the studies involving deceased (A), surviving (B), severe (C) and non-severe (D) COVID-19 patients.
For studies related to COVID-19 severity, we found that most studies showed significant publication bias, except for those detailing the proportion of severe COVID19 patients with hypertension and cardiovascular disease (Fig. 3C,D). This could be due to studies having different judgment criteria for severity of illness. To test the effect of study heterogeneity on summary effects, we performed leave-one-out sensitivity analysis to remove outlier studies and replotted forest plots after outlier removal. We discovered that summary effects were not significantly altered by the presence of outliers (Table S1).
4. Discussion
In our meta-analysis, we found that hypertension, cardiovascular disease, cerebrovascular disease, and chronic kidney disease are significant risk factors for greater COVID-19 severity, which is consistent with existing research. While we obtained similar OR as previous reports when comparing severe to non-severe COVID-19 patients for these comorbidities, we found OR of mortality to be much higher than previously reported for hypertension and cardiovascular disease [18], [20]. Compared to studies correlating comorbidities to COVID-19 severity, there are significantly fewer studies correlating comorbidities to COVID-19 mortality. Furthermore, studies do not agree on whether comorbidities are correlated with mortality [4], [14], [15]. In our study, we employed two classification systems for COVID-19 patients: dead vs. alive and severe vs. non-severe. We demonstrated that all four comorbidities are associated with mortality with a higher OR than comorbidities are associated with severity of disease, which suggests that underlying cardiovascular-related comorbidities are more significant contributors to death from COVID-19 than to greater COVID-19 severity. In other words, it can be inferred that while these comorbidities and other factors could all contribute to COVID-19 severity, these comorbidities contribute more to mortality than other risk factors that contribute to COVID-19 severity.
Previous reports have strongly implicated age as a significant risk factor for more severe COVID-19 and a risk factor for comorbidities. Therefore, it is highly likely that age is a confounding variable in any association between COVID-19 severity/mortality and comorbidities. We attempted to assess the effect of age on the proportion of COVID-19 patients with a specific comorbidity in the dead/alive classification and severe/non-severe classification. Age appeared to be a significant moderator for the proportion of COVID-19 patients with the comorbidities we analyzed. However, the moderating effect of age is generally significantly weaker in severe or deceased patients, compared to non-severe or surviving patients, for these comorbidities. If age is a confounding variable, age would be expected to correlate more with the proportion of patients with comorbidities in the severe/deceased cohort, since these cohorts are also composed of older patients. The fact that age does not correlate as well with comorbidities in older patients suggests that factors besides age are responsible for the presence of comorbidities in severe cases of COVID-19 and patients who died from COVID-19.
Several hypotheses could explain why cardiovascular, cerebrovascular, and kidney-related comorbidities are correlated with greater disease severity and mortality. The heart and kidneys were shown to be tissues with some of the highest angiotensin-converting enzyme 2 (ACE2) expression levels [24]. Moreover, patients with these existing comorbidities may be taking angiotensin-converting enzyme inhibitors (ACEI) and angiotensin receptor-blocking (ARB) drugs that further increase ACE2 expression in these organs [24], [25], [26]. The ACE2 receptor has been identified as the host receptor for SARS-CoV-2 [27], and research has shown that increased ACE2 expression may be associated with increased susceptibility to SARS-CoV-2 host cell entry [28], [29], leading to greater viral spread and more severe symptoms. However, there are also reports that higher ACE2 expression may play a protective role in COVID-19, as it has been shown to protect against H7N9-induced acute lung injury [30] and to reduce the inflammation in acute respiratory distress syndrome (ARDS) animal models [31]. Furthermore, it was observed that use of ACEI and ARB drugs by hypertensive COVID-19 patients is associated with lower risk of mortality and improved clinical outcomes [16], [32]. Instead of ACE2 expression, it could be possible that the immune dysregulations that accompanies these existing comorbidities make patients increasingly vulnerable to COVID-19. Further research must be done to explain the poorer prognosis of COVID-19 patients with these existing comorbidities.
Another point of interest is the possible causative relationship between cardiovascular and renal dysfunction, cardiorenal syndrome. While our results show that there is a significant positive correlation between the proportion of patients with cardiovascular disease and chronic kidney diseases, we cannot conclude if cardiovascular comorbidity increases the risk of renal dysfunction or vice versa. Current research shows that pre-existing cardiovascular comorbidities increase a patient’s risk of cardiovascular complications [33], such as including acute myocardial injury, myocarditis, arrhythmias, and venous thromboembolism, and that pre-existing renal comorbidities increase a patient’s risk of renal complications, such as acute kidney injury [34]. Therefore, whether cardiovascular comorbidities increases the risk of kidney dysfunction and vice versa, leading to cardiorenal syndrome, remains an important future research question.
We observed that elderly COVID-19 patients are more likely to progress to severe disease and have a higher mortality rate, which is consistent with existing literature [35], [36]. This could be due to older individuals having less robust innate and adaptive immune responses. For instance, the proportion of naive T cells decreases with age [37], making older adults more susceptible to novel pathogens. Naive T cells in older individuals also have functional defects, such as significantly shorter telomeres and a restricted TCR repertoire [38]. This is significant as the total number of T cells in COVID19 patients are greatly reduced, especially in patients requiring ICU care and older patients, limiting their antiviral response [39]. While we established that age may not be a confounding variable in our correlations through statistical analyses, there is no way to definitively rule out the possibility of confounders except through discovery of mechanistic relationships.
Our study has several limitations. Firstly, we found high heterogeneity statistics, which could be due to the high variation in the sample sizes of studies. However, the elimination of outlier studies did not affect summary proportions significantly. Secondly, a number of publications were included that had incomplete or missing data on certain comorbidities, making it difficult to investigate their association with COVID-19 severity and mortality. In addition, Thirdly, most studies did not provide information on whether patients had multiple comorbidities. Thirdly, we have included observational studies, which may also be subject to interaction bias, and preprints that have not been peer reviewed. However, based on the Newcastle-Ottawa the scale, we have evaluated these preprints to be of sufficient quality to include in our meta-analysis. Lastly, we acknowledge that patients’ ongoing drug treatments [40], [41], [42] may affect our results as well. Metformin, typically taken by diabetic patients, is associated with reduced mortality in COVID-19 infection [40]. However, whether the patients in this meta-analysis had diabetes or what drugs they were taking were not available to us from the majority of the primary studies we sourced, due to the scope of our meta-analysis. In addition, the effect of drugs on COVID-19 severity and mortality in different patient populations appears to vary [43]. Thus, future meta-analyses can include examining the effect of drugs on COVID-19 severity and mortality in patients with different comorbidities.
5. Conclusion
We found that hypertension, cardiovascular disease, cerebrovascular disease, and chronic kidney disease are significant risk factors for COVID-19 severity and mortality. Our study reports some of the most conclusive correlations between comorbidities and COVID-19 mortality found in currently published literature. Through meta-regression analyses, we found that age does not explain as well the presence of hypertension and cardiovascular diseases in fatal cases of COVID-19 as it does the presence of these comorbidities in non-fatal cases, suggesting that it may not be a confounder. Given that it is still controversial whether there is an association between comorbidities and COVID-19 fatality and whether age is a confounder of these associations, our study provides critical evidence to address both questions.
Role of the funding source
This research was funded the University of California, Office of the President Emergency (Grant number: R00RG2369) to W.M.O.
CRediT authorship contribution statement
Abby C. Lee: Validation, Formal analysis, Investigation, Writing - original draft, Writing - review & editing, Visualization. Wei Tse Li: Validation, Formal analysis, Investigation, Writing - review & editing, Writing - original draft. Lauren Apostol: Validation, Formal analysis, Investigation. Jiayan Ma: Validation, Formal analysis, Investigation. Pam R. Taub: Writing - review & editing. Eric Y. Chang: Writing - review & editing. Mahadevan Rajasekaran: Writing - review & editing. Weg M. Ongkeko: Conceptualization, Methodology, Resources, Supervision, Writing - review & editing, Project administration, Funding acquisition.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.csbj.2021.06.038.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. Epub 2020/01/28 PubMed PMID: 31986264; PubMed Central PMCID: PMCPMC7159299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu K., Fang Y.Y., Deng Y., Liu W., Wang M.F., Ma J.P. Clinical characteristics of novel coronavirus cases in tertiary hospitals in Hubei Province. Chin Med J (Engl) 2020;133(9):1025–1031. doi: 10.1097/CM9.0000000000000744. Epub 2020/02/12. PubMed PMID: 32044814; PubMed Central PMCID: PMCPMC7147277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xu XW, Wu XX, Jiang XG, Xu KJ, Ying LJ, Ma CL, et al. Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS-Cov-2) outside of Wuhan, China: retrospective case series. BMJ. 2020;368:m606. Epub 2020/02/23. doi: 10.1136/bmj.m606. PubMed PMID: 32075786; PubMed Central PMCID: PMCPMC7224340 at www.icmje.org/coi_disclosure.pdf and declare: no support from any organisation for the submitted work; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work. [DOI] [PMC free article] [PubMed]
- 4.Bajgain K.T., Badal S., Bajgain B.B., Santana M.J. Prevalence of comorbidities among individuals with COVID-19: A rapid review of current literature. Am J Infect Control. 2020 doi: 10.1016/j.ajic.2020.06.213. Epub 2020/07/14. PubMed PMID: 32659414; PubMed Central PMCID: PMCPMC7351042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guan WJ, Liang WH, Zhao Y, Liang HR, Chen ZS, Li YM, et al. Comorbidity and its impact on 1590 patients with COVID-19 in China: a nationwide analysis. Eur Respir J. 2020;55(5). Epub 2020/03/29. doi: 10.1183/13993003.00547-2020. PubMed PMID: 32217650; PubMed Central PMCID: PMCPMC7098485 [DOI] [PMC free article] [PubMed]
- 6.Yang J, Zheng Y, Gou X, Pu K, Chen Z, Guo Q, et al. Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: a systematic review and meta-analysis. Int J Infect Dis. 2020;94:91-5. Epub 2020/03/17. doi: 10.1016/j.ijid.2020.03.017. PubMed PMID: 32173574; PubMed Central PMCID: PMCPMC7194638. [DOI] [PMC free article] [PubMed]
- 7.Pranata R, Huang I, Lim MA, Wahjoepramono EJ, July J. Impact of cerebrovascular and cardiovascular diseases on mortality and severity of COVID-19-systematic review, meta-analysis, and meta-regression. J Stroke Cerebrovasc Dis. 2020;29(8):104949. Epub 2020/09/16. doi: 10.1016/j.jstrokecerebrovasdis.2020.104949. PubMed PMID: 32927523. [DOI] [PubMed]
- 8.Tian W, Jiang W, Yao J, Nicholson CJ, Li RH, Sigurslid HH, et al. Predictors of mortality in hospitalized COVID-19 patients: A systematic review and meta-analysis. J Med Virol. 2020. Epub 2020/05/23. doi: 10.1002/jmv.26050. PubMed PMID: 32441789; PubMed Central PMCID: PMCPMC7280666. [DOI] [PMC free article] [PubMed]
- 9.Leiva Sisnieguez CE, Espeche WG, Salazar MR. Arterial hypertension and the risk of severity and mortality of COVID-19. Eur Respir J. 2020;55(6). Epub 2020/05/14. doi: 10.1183/13993003.01148-2020. PubMed PMID: 32398296; PubMed Central PMCID: PMCPMC7236824. [DOI] [PMC free article] [PubMed]
- 10.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054-62. Epub 2020/03/15. doi: 10.1016/S0140-6736(20)30566-3. PubMed PMID: 32171076; PubMed Central PMCID: PMCPMC7270627. [DOI] [PMC free article] [PubMed]
- 11.Chen C, Chen C, Yan JT, Zhou N, Zhao JP, Wang DW. [Analysis of myocardial injury in patients with COVID-19 and association between concomitant cardiovascular diseases and severity of COVID-19]. Zhonghua Xin Xue Guan Bing Za Zhi. 2020;48(7):567-71. Epub 2020/03/07. doi: 10.3760/cma.j.cn112148-20200225-00123. PubMed PMID: 32141280. [DOI] [PubMed]
- 12.Wang B, Li R, Lu Z, Huang Y. Does comorbidity increase the risk of patients with COVID-19: evidence from meta-analysis. Aging (Albany NY). 2020;12(7):6049-57. Epub 2020/04/09. doi: 10.18632/aging.103000. PubMed PMID: 32267833; PubMed Central PMCID: PMCPMC7185114. [DOI] [PMC free article] [PubMed]
- 13.Liu K, Chen Y, Lin R, Han K. Clinical features of COVID-19 in elderly patients: A comparison with young and middle-aged patients. J Infect. 2020;80(6):e14-e8. Epub 2020/03/17. doi: 10.1016/j.jinf.2020.03.005. PubMed PMID: 32171866; PubMed Central PMCID: PMCPMC7102640. [DOI] [PMC free article] [PubMed]
- 14.Liu H, Chen S, Liu M, Nie H, Lu H. Comorbid Chronic Diseases are Strongly Correlated with Disease Severity among COVID-19 Patients: A Systematic Review and Meta-Analysis. Aging Dis. 2020;11(3):668-78. Epub 2020/06/04. doi: 10.14336/AD.2020.0502. PubMed PMID: 32489711; PubMed Central PMCID: PMCPMC7220287. [DOI] [PMC free article] [PubMed]
- 15.Huang S, Wang J, Liu F, Liu J, Cao G, Yang C, et al. COVID-19 patients with hypertension have more severe disease: a multicenter retrospective observational study. Hypertens Res. 2020;43(8):824-31. Epub 2020/06/03. doi: 10.1038/s41440-020-0485-2. PubMed PMID: 32483311; PubMed Central PMCID: PMCPMC7261650. [DOI] [PMC free article] [PubMed]
- 16.Zhang P, Zhu L, Cai J, Lei F, Qin JJ, Xie J, et al. Association of Inpatient Use of Angiotensin-Converting Enzyme Inhibitors and Angiotensin II Receptor Blockers With Mortality Among Patients With Hypertension Hospitalized With COVID-19. Circ Res. 2020;126(12):1671-81. Epub 2020/04/18. doi: 10.1161/CIRCRESAHA.120.317134. PubMed PMID: 32302265; PubMed Central PMCID: PMCPMC7265882. [DOI] [PMC free article] [PubMed]
- 17.Harrison SL, Fazio-Eynullayeva E, Lane DA, Underhill P, Lip GYH. Comorbidities associated with mortality in 31,461 adults with COVID-19 in the United States: A federated electronic medical record analysis. PLoS Med. 2020;17(9):e1003321. Epub 2020/09/11. doi: 10.1371/journal.pmed.1003321. PubMed PMID: 32911500; PubMed Central PMCID: PMCPMC7482833 following competing interests. GYHL is a Consultant for Bayer/Janssen, BMS/Pfizer, Medtronic, Boehringer Ingelheim, Novartis, Verseon, and Daiichi-Sankyo and a Speaker for Bayer, BMS/Pfizer, Medtronic, Boehringer Ingelheim, and Daiichi-Sankyo. No fees are directly received personally. DAL has received investigator-initiated educational grants from Bristol-Myers Squibb (BMS) and Boehringer Ingelheim; has been a speaker for Boehringer Ingelheim and BMS/Pfizer; and has consulted for BMS, Boehringer Ingelheim, and Daiichi-Sankyo. EF-E and PU are employees of TriNetX. SLH has declared no competing interests. [DOI] [PMC free article] [PubMed]
- 18.Espinosa OA, Zanetti ADS, Antunes EF, Longhi FG, Matos TA, Battaglini PF. Prevalence of comorbidities in patients and mortality cases affected by SARS-CoV2: a systematic review and meta-analysis. Rev Inst Med Trop Sao Paulo. 2020;62:e43. Epub 2020/06/25. doi: 10.1590/S1678-9946202062043. PubMed PMID: 32578683; PubMed Central PMCID: PMCPMC7310609. [DOI] [PMC free article] [PubMed]
- 19.Bajgain K.T., Badal S., Bajgain B.B., Santana M.J. Prevalence of comorbidities among individuals with COVID-19: A rapid review of current literature. Am J Infect Control. 2021;49(2):238–246. doi: 10.1016/j.ajic.2020.06.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ssentongo P., Ssentongo A.E., Heilbrunn E.S., Ba D.M., Chinchilli V.M., Hirst J.A. Association of cardiovascular disease and 10 other pre-existing comorbidities with COVID-19 mortality: A systematic review and meta-analysis. PLoS ONE. 2020;15(8):e0238215. doi: 10.1371/journal.pone.0238215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garg S, Kim L, Whitaker M, O'Halloran A, Cummings C, Holstein R, et al. Hospitalization Rates and Characteristics of Patients Hospitalized with Laboratory-Confirmed Coronavirus Disease 2019 - COVID-NET, 14 States, March 1-30, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(15):458-64. Epub 2020/04/17. doi: 10.15585/mmwr.mm6915e3. PubMed PMID: 32298251. [DOI] [PMC free article] [PubMed]
- 22.Lee D.S., Massaro J.M., Wang T.J., Kannel W.B., Benjamin E.J., Kenchaiah S. Antecedent blood pressure, body mass index, and the risk of incident heart failure in later life. Hypertension. 2007;50(5):869–876. doi: 10.1161/HYPERTENSIONAHA.107.095380. Epub 2007/09/26. PubMed PMID: 17893376. [DOI] [PubMed] [Google Scholar]
- 23.Ong KL, Cheung BM, Man YB, Lau CP, Lam KS. Prevalence, awareness, treatment, and control of hypertension among United States adults 1999-2004. Hypertension. 2007;49(1):69-75. Epub 2006/12/13. doi: 10.1161/01.HYP.0000252676.46043.18. PubMed PMID: 17159087. [DOI] [PubMed]
- 24.Gallagher PE, Ferrario CM, Tallant EA. MAP kinase/phosphatase pathway mediates the regulation of ACE2 by angiotensin peptides. Am J Physiol Cell Physiol. 2008;295(5):C1169-74. Epub 2008/09/05. doi: 10.1152/ajpcell.00145.2008. PubMed PMID: 18768926; PubMed Central PMCID: PMCPMC2585001. [DOI] [PMC free article] [PubMed]
- 25.Jessup JA, Gallagher PE, Averill DB, Brosnihan KB, Tallant EA, Chappell MC, et al. Effect of angiotensin II blockade on a new congenic model of hypertension derived from transgenic Ren-2 rats. American journal of physiology Heart and circulatory physiology. 2006;291(5):H2166-72. Epub 2006/06/13. doi: 10.1152/ajpheart.00061.2006. PubMed PMID: 16766648. [DOI] [PubMed]
- 26.Ferrario C.M., Jessup J., Chappell M.C., Averill D.B., Brosnihan K.B., Tallant E.A. Effect of angiotensin-converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin-converting enzyme 2. Circulation. 2005;111(20):2605–2610. doi: 10.1161/CIRCULATIONAHA.104.510461. Epub 2005/05/18. PubMed PMID: 15897343. [DOI] [PubMed] [Google Scholar]
- 27.Bourgonje AR, Abdulle AE, Timens W, Hillebrands JL, Navis GJ, Gordijn SJ, et al. Angiotensin-converting enzyme 2 (ACE2), SARS-CoV-2 and the pathophysiology of coronavirus disease 2019 (COVID-19). J Pathol. 2020;251(3):228-48. Epub 2020/05/18. doi: 10.1002/path.5471. PubMed PMID: 32418199; PubMed Central PMCID: PMCPMC7276767. [DOI] [PMC free article] [PubMed]
- 28.Hoffmann M, Kleine-Weber H, Schroeder S, Kruger N, Herrler T, Erichsen S, et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. 2020;181(2):271-80 e8. Epub 2020/03/07. doi: 10.1016/j.cell.2020.02.052. PubMed PMID: 32142651; PubMed Central PMCID: PMCPMC7102627. [DOI] [PMC free article] [PubMed]
- 29.Wrapp D., Wang N., Corbett K.S., Goldsmith J.A., Hsieh C.L., Abiona O. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367(6483):1260–1263. doi: 10.1126/science.abb2507. Epub 2020/02/23. PubMed PMID: 32075877; PubMed Central PMCID: PMCPMC7164637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ciaglia E, Vecchione C, Puca AA. COVID-19 Infection and Circulating ACE2 Levels: Protective Role in Women and Children. Front Pediatr. 2020;8:206. Epub 2020/05/12. doi: 10.3389/fped.2020.00206. PubMed PMID: 32391299; PubMed Central PMCID: PMCPMC7192005. [DOI] [PMC free article] [PubMed]
- 31.Zhang H., Baker A. Recombinant human ACE2: acing out angiotensin II in ARDS therapy. Crit Care. 2017;21(1):305. doi: 10.1186/s13054-017-1882-z. Epub 2017/12/15. PubMed PMID: 29237475; PubMed Central PMCID: PMCPMC5729230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meng J, Xiao G, Zhang J, He X, Ou M, Bi J, et al. Renin-angiotensin system inhibitors improve the clinical outcomes of COVID-19 patients with hypertension. Emerg Microbes Infect. 2020;9(1):757-60. Epub 2020/04/02. doi: 10.1080/22221751.2020.1746200. PubMed PMID: 32228222; PubMed Central PMCID: PMCPMC7170368. [DOI] [PMC free article] [PubMed]
- 33.Driggin, Elissa et al. “Cardiovascular Considerations for Patients, Health Care Workers, and Health Systems During the COVID-19 Pandemic.” Journal of the American College of Cardiology vol. 75,18 (2020): 2352-2371. doi:10.1016/j.jacc.2020.03.031 [DOI] [PMC free article] [PubMed]
- 34.Kunutsor S.K., Laukkanen J.A. Renal complications in COVID-19: a systematic review and meta-analysis. Ann Med. 2020;52(7):345–353. doi: 10.1080/07853890.2020.1790643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen Y., Li L. SARS-CoV-2: virus dynamics and host response. Lancet Infect Dis. 2020;20(5):515–516. doi: 10.1016/S1473-3099(20)30235-8. Epub 2020/03/28. PubMed PMID: 32213336; PubMed Central PMCID: PMCPMC7156233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shahid Z., Kalayanamitra R., McClafferty B., Kepko D., Ramgobin D., Patel R. COVID-19 and older adults: What we know. J Am Geriatr Soc. 2020;68(5):926–929. doi: 10.1111/jgs.16472. Epub 2020/04/08. PubMed PMID: 32255507; PubMed Central PMCID: PMCPMC7262251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weiskopf D., Weinberger B., Grubeck-Loebenstein B. The aging of the immune system. Transpl Int. 2009;22(11):1041–1050. doi: 10.1111/j.1432-2277.2009.00927.x. Epub 2009/07/25. PubMed PMID: 19624493. [DOI] [PubMed] [Google Scholar]
- 38.Pfister G., Weiskopf D., Lazuardi L., Kovaiou R.D., Cioca D.P., Keller M. Naive T cells in the elderly: are they still there? Ann N Y Acad Sci. 2006;1067:152–157. doi: 10.1196/annals.1354.018. Epub 2006/06/29. PubMed PMID: 16803980. [DOI] [PubMed] [Google Scholar]
- 39.Diao B, Wang C, Tan Y, Chen X, Liu Y, Ning L, et al. Reduction and Functional Exhaustion of T Cells in Patients With Coronavirus Disease 2019 (COVID-19). Front Immunol. 2020;11:827. Epub 2020/05/20. doi: 10.3389/fimmu.2020.00827. PubMed PMID: 32425950; PubMed Central PMCID: PMCPMC7205903. [DOI] [PMC free article] [PubMed]
- 40.Hariyanto T.I., Kurniawan A. Dipeptidyl peptidase 4 (DPP4) inhibitor and outcome from coronavirus disease 2019 (COVID-19) in diabetic patients: a systematic review, meta-analysis, and meta-regression. J Diabetes Metab Disord. 2021;20(1):543–550. doi: 10.1007/s40200-021-00777-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hariyanto, Ivan Timotius, Kurniawan Andree. Metformin use is associated with reduced mortality rate from coronavirus disease 2019 (COVID-19) infection. Obes Med. 2019;19(2020) doi: 10.1016/j.obmed.2020.100290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hariyanto, Timotius I., and Andree Kurniawan. “Statin And Outcomes Of Coronavirus Disease 2019 (COVID-19): A Systematic Review, Meta-Analysis, And Meta-Regression”. Nutrition, Metabolism And Cardiovascular Diseases, vol 31, no. 6, 2021, pp. 1662-1670. Elsevier BV, doi:10.1016/j.numecd.2021.02.020. Accessed 21 June 2021. [DOI] [PMC free article] [PubMed]
- 43.Cariou Bertrand, Goronflot Thomas, Rimbert Antoine, Boullu Sandrine, Le May Cédric, Moulin Philippe. Routine use of statins and increased COVID-19 related mortality in inpatients with type 2 diabetes: Results from the CORONADO study. Diab Metab. 2021;47(2):101202. doi: 10.1016/j.diabet.2020.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.