Abstract
Purpose
To assess the impact of the COVID-19 pandemic and associated mitigation measures on persons with sensory impairments (SI), including visual impairments (VI) and hearing impairments (HI).
Design
Cross-sectional survey.
Methods
Adults with VI (best-corrected visual acuity <20/60 in the better-seeing eye), HI (International Classification of Diseases, Tenth Revision, codes), and age- and sex-matched controls (n = 375) were recruited from the University of Michigan. The 34-item Coronavirus Disability Survey was administered. Both χ2 tests and logistic regression were used to compare survey responses between groups.
Results
All groups reported high levels of disruption of daily life, with 80% reporting “a fair amount” or “a lot” of disruption (VI: 76%, HI: 83%, CT: 82%, P = .33). Participants with VI had greater difficulty with day-to-day activities and were more likely to cite the following reasons: caregiver was worried about COVID-19 (odds ratio [OR]VI = 7.2, 95% CI = 3.5-14.4, P < .001) and decreased availability of public transportation (ORVI = 5.0, 95% CI = 1.5-15.6, P = .006). Participants with VI, but not HI, showed a trend toward increased difficulty accessing medical care (ORVI = 2.0, 95% CI = 0.99-4.0, P = .052) and began relying more on others for day-to-day assistance (ORVI = 3.1, 95% CI = 1.6-5.7, P < .001). Overall, 30% reported difficulty obtaining trusted information about the pandemic. Those with VI reported more difficulty seeing or hearing trusted information (ORVI = 6.1, 95% CI = 1.6-22.1, P = .006). Employed participants with HI were more likely to report a reduction in wages (ORHI = 2.5, 95% CI = 1.2-5.3, P = .02).
Conclusions
Individuals with VI have experienced increased disruption and challenges in daily activities related to the pandemic. People with SI may benefit from targeted policy approaches to the current pandemic and future stressors. Minimal differences in some survey measures may be due to the large impact of the pandemic on the population as a whole.
The SARS-CoV-2 (COVID-19) pandemic and public health mitigation measures have had an exceedingly large impact around the globe. As of the time of writing, more than 114 million global cases (28 million US) had been diagnosed, and there had been more than 2.5 million fatalities attributed to COVID-19 (517,000 US).1,2
The SARS-CoV-2 (COVID-19) pandemic, with accompanying emergency stay-at-home and lockdown orders, and public health advice to limit contact with individuals outside of one's own home, may worsen psychological, functional, financial, and transportation challenges for the entire population.3 Prior studies have revealed increased mental health challenges for the population as a whole due to COVID-19 and an outsized impact on loneliness and depression for certain subgroups of the population, including older adults and those with preexisting medical conditions.4, 5, 6 The impact of the COVID-19 pandemic on persons with sensory impairments (SI) has not been well described, and it is not known whether the pandemic has disproportionally affected this population.
We hypothesized that the COVID-19 pandemic and associated mitigation and containment responses may unequally exacerbate day-to-day challenges, social isolation, and poor health for adults with SI compared to those with normal sensory function. Those with hearing impairments (HI) struggle with communication as a result of the widespread use of personal protective equipment, including face masks.7 , 8 Similarly, those with visual impairments (VI) encounter barriers to typical modes of interaction, with reduced access to touch and tactile contact.9 These additional barriers may exacerbate disparities that affect individuals with SI even under normal circumstances, including challenges related to mental health, accessing healthcare, performing certain activities and instrumental activities of daily living, staying socially connected, and maintaining financial well-being.10, 11, 12, 13, 14, 15
To assess the impact of the pandemic on persons with various disabilities, we previously developed the Coronavirus Disability Survey (COV-DIS), which has been described elsewhere.16 We used this instrument to carry out a cross-sectional survey to assess the impact of the COVID-19 pandemic on adults with SI in southeastern Michigan, an area of the United States that was particularly hard hit during the early months of the pandemic in the spring of 2020.17
METHODS
A survey was conducted among adults with SI and controls with normal sensory function to determine the impact of the COVID-19 pandemic and accompanying mitigation measures on various domains of health and well-being. This study was approved by the University of Michigan Institutional Review Board and all participants provided informed consent.
PARTICIPANTS
All participants were recruited from Michigan Medicine, University of Michigan. Three groups of participants were recruited: (1) those with VI, (2) those with HI, and (3) controls without VI or HI. Inclusion criteria for all groups included: age ≥18 years, community dwelling, speaking and understanding English, and without dementia or a severe psychiatric condition. The VI and control groups were recruited using the Sight Outcomes Research Collaborative (SOURCE) Ophthalmology Electronic Health Record Data Repository.18 The HI group was recruited using DataDirect, a cohort identification tool that searches Michigan Medicine clinical encounters based on demographic criteria and administrative claims codes.
In the VI group, we recruited an approximately equal number of participants with moderate VI (<20/60-20/200 in the better-seeing eye) and severe VI/blindness (<20/200 in the better-seeing eye) from patients seen at Michigan Medicine from April 20, 2019, to April 20, 2020. Impairment was categorized based on best-corrected visual acuity, as all participants were receiving eye care. Participants with diagnosed HI were excluded by International Classification of Diseases, Tenth Revision (ICD-10) codes for HI or hearing loss. Inclusion and exclusion criteria for all groups are shown in Supplemental Table S1.
In the HI group, participants were identified from among those seen at Michigan Medicine during the same timeframe (April 20, 2019, to April 20, 2020). We searched ICD-10 codes corresponding to a diagnosis of hearing loss or deafness to identify this cohort. Participants with a diagnosed major ocular disease (eg, glaucoma, diabetic retinopathy, macular degeneration, retinal detachment), low vision, or blindness were identified by ICD-10 codes and were excluded.
Control participants were recruited from among individuals who received an eye examination at Michigan Medicine from April 20, 2019, to April 20, 2020 and had best-corrected visual acuity ≥20/30 in both eyes, no visually impairing eye disease other than correctable refractive error, and no diagnosed HI. Five possible age- and sex-matched controls were identified for each respondent with HI and VI. All controls were sent an email, with a follow-up call per protocol until the desired sample size was reached, so the final distribution of participants was dependent on response rates and does not reflect exact case-control matching. Participants with diagnosed dementia, a severe psychiatric condition, or physical disability as well as non−community-dwelling status were excluded from all 3 groups using ICD-10 codes in the medical record.
THE COV-DIS SURVEY
The COV-DIS was used to collect survey response data for this study. The development of the COV-DIS has been described elsewhere,16 and the full survey is made freely available online (osf.io/p2w9j/). The COV-DIS was developed by expert consensus and has not undergone validation of psychometric properties or pilot testing among a representative sample at this time. The COV-DIS includes items related to general health, mental health, isolation, challenges performing daily activities, obtaining food, medicine and medical care, adaptations to the pandemic, financial strain, and access to information and transportation.
SURVEY ADMINISTRATION
Surveys were administered via phone or email. First, surveys were distributed by email with a link to complete the survey through Qualtrics. A follow-up email was sent to nonrespondents after 5 days. Two days later, those who still had not responded received a phone call from a study coordinator. Emails and phone calls were made from May 11, 2020, to July 2, 2020. During this time period, there were mask mandates throughout the state, and visitor restrictions were in place across the University of Michigan health system (no visitors for adult inpatients and 1 visitor allowed for outpatient visits, except as medical necessary).
CLINICAL AND DEMOGRAPHIC DATA
Participant age, race, ethnicity, sex, ZIP Code, visual acuity, Charlson Comorbidity Index (CCI), and marital status were collected from the medical record. The CCI is a general measure of multimorbidity and is strongly associated with mortality risk. The index is calculated based on diagnosed medical conditions; each condition contributes to the overall summary CCI score, for which a higher score indicates greater morbidity and mortality risk.19 The COV-DIS included a demographic question about household income. For participants with VI, data on visual acuity were extracted from the medical record. Nine-digit ZIP Codes were used to obtain an Area Deprivation Index (ADI) score for each participant based on their home address. Neighborhood disadvantage has been linked to many health outcomes, including cardiovascular disease, diabetes, visual impairment/blindness and mortality. This index accounts for factors including neighborhood-level income, education, employment, and housing quality. 20 , 21
SAMPLE SIZE
We calculated the necessary sample size to detect a 20% difference between controls and those with disability in the proportion answering, “unable” or “very difficult” vs “no” or “some difficulty” to the question “Since becoming aware of the coronavirus outbreak, how much difficulty have you had getting the routing medical care that you need?” Power was set at 80% with a 5% error rate under the assumption that the probability of answering “unable” or “very difficult” in the disability groups was 50%; if the probability was greater than or less than 50%, the required sample size would be smaller. The calculated sample size was 90 participants per group.
DATA ANALYSIS
Clinical and demographic data are reported as means (SD) for continuous data and as counts for categorical data. Exploratory factor analysis (EFA) was performed to determine the factor structure of the COV-DIS to permit summary scoring of items deemed to measure a single underlying factor. All items on an ordinal or continuous scale were included in the EFA. Factors with eigenvalues >1.0 were retained and item-to-factor loadings >|0.4| were considered significant. Differences between groups were tested using a 1-way analysis of variance (ANOVA) for continuous data and the Pearson χ2, Fisher exact test or logistic regression for categorical data. All response data were coded so that higher values represented worse outcomes. Survey response data for each item are reported stratified by SI status. For Likert-scaled survey items, the mean (SD) score for each SI group is reported and compared using a 1-way ANOVA. Summary survey response variables were constructed based on results of the EFA. In univariate analyses, marital status, CCI, and ADI were associated with SI status. Therefore, we modeled the association of SI status with all survey response data, adjusted for marital status, CCI, and ADI. These covariates were not significantly associated with survey responses and did not have an appreciable impact on the association of sSI status with survey responses. The single exception was in a model on difficulty accessing medical care, in which marital status appeared to play a significant role; thus, we report this key result.
RESULTS
A total of 375 individuals participated in this study: 112 participants with VI, 108 participants with HI, and 155 controls. Sociodemographic information is presented in Table 1 . Participants with VI were more likely to be aged >80 years compared to those with HI or controls (P < .001). There was a similar proportion of males and females in all 3 groups. All 3 groups were predominantly White and non-Hispanic. Compared to nonrespondents, those who participated in this study were slightly more likely to be non-Hispanic and White (respondents: 90%, vs nonrespondents: 82%, P < .001). Marital status differed between groups; participants with VI were less likely to be married than controls or those with HI (VI: 39%, HI: 81%, CT: 65%, P < .001). The groups also differed in ADI scores; participants with VI were more likely to have an ADI score of 50 to 100 (representing higher area deprivation) (VI: 53%, HI: 24%, CT: 24%, P < .001). The CCI scores were lower (e.g., fewer comorbidities) among those with HI compared to the other groups, and a larger proportion with VI received disability benefits (VI: 21%, HI: 1%, CT: 3%, P < .001). The mode of response was different between participant groups, with controls and participants with HI more likely to respond by email than those with VI (VI: 19%, HI: 87%, CT: 85%, P < .001) (Table 2 ). Email response rates were lower among participants with VI (VI: 15% vs HI: 27% vs CT: 27%, P < .001), and phone response rates were similar across groups (VI: 23% vs HI: 21% vs CT:20%, P = .84). These response rates include all potential participants with a valid phone number, including those who did not answer the phone. Overall response rates were similar between groups as well (VI: 20% vs HI: 26% vs CT: 25%, P = .13).
Table 1.
Sociodemographic Information for the Study Sample by Impairment Group
| Participant Group |
|||||
|---|---|---|---|---|---|
| Participant characteristic | Control | Visually Impaired | Hearing Impaired | Total | P |
| Age, y | .001 | ||||
| <50 | 17 (11%) | 17 (15%) | 13 (12%) | 47 | |
| 50-65 | 61 (39%) | 28 (25%) | 52 (48%) | 141 | |
| 65-80 | 61 (39%) | 41 (37%) | 41 (37%) | 143 | |
| >80 | 16 (10%) | 26 (23%) | 2 (2%) | 44 | |
| Sex | .81 | ||||
| Male | 82 (53%) | 56 (50%) | 53 (49%) | 191 | |
| Female | 73 (47%) | 56 (50%) | 55 (51%) | 184 | |
| Race | .17 | ||||
| White | 147 (95%) | 98 (88%) | 97 (90%) | 342 | |
| Other | 8 (5%) | 14 (12%) | 11 (10%) | 33 | |
| Ethnicity | .50 | ||||
| Hispanic | 1 (1%) | 4 (4%) | 2 (2%) | 7 | |
| Non-Hispanic/refused | 154 (99%) | 108 (96%) | 106 (98%) | 368 | |
| Marital status | .001 | ||||
| Married | 100 (65%) | 44 (39%) | 88 (81%) | 232 | |
| Unmarried | 55 (35%) | 68 (61%) | 20 (19%) | 143 | |
| Area Deprivation Indexa | .001 | ||||
| <25 | 54 (35%) | 16 (14%) | 46 (43%) | 116 | |
| 26-50 | 63 (41%) | 37 (33%) | 36 (32%) | 136 | |
| 51-75 | 22 (14%) | 36 (32%) | 19 (18%) | 77 | |
| 75-100 | 16 (10%) | 23 (21%) | 7 (6%) | 46 | |
| Missing/unavailable | 10 | ||||
| Charlson Comorbidity Indexb | .001 | ||||
| 0-1 | 28 (18%) | 24 (21%) | 61 (56%) | 113 | |
| 2-4 | 81 (52%) | 56 (50%) | 41 (38%) | 178 | |
| >5 | 46 (30%) | 32 (29%) | 6 (6%) | 84 | |
| Receiving disability benefits | 5 (3%) | 23 (21%) | 1 (1%) | .001 | |
Area Deprivation Index is a ranking of neighborhoods by socioeconomic status, including factors such as income, education, employment, and housing quality.
Charlson Comorbidity Index is a general measure of multimorbidity strongly associated with mortality risk.
Table 2.
Survey Response Format by Participant Group
| Group | Email Response | Phone Response | P |
|---|---|---|---|
| .0001 | |||
| Control | 131 (85%) | 24 (15%) | |
| Visual impairment | 21 (19%) | 91 (81%) | |
| Hearing impairment | 94 (87%) | 14 (13%) | |
| Total | 246 (66%) | 129 (34%) |
The EFA revealed 2 factors. Based on factor loadings, the first factor contained 4 COV-DIS items related to general disruption (question 8), and difficulty obtaining food (question 10), medicine (question 11), or medical care (question 12). The second factor contained the 2 items of the Patient Health Questionnaire (PHQ)−2 related to depressive symptoms (questions 3 and 4).22 Other items did not load onto a factor, suggesting the need to analyze results for these items separately (eg, without summary scoring).
Table 3 shows survey response data. Survey items measuring general health revealed worse scores for the VI group compared to the HI and control groups (P < .001), although a majority in all groups reported good, very good, or excellent overall health (VI: 39%, HI: 81%, CT: 65%, P < .001). A higher proportion of participants with VI reported getting help with their activities of daily living pre-pandemic compared with participants with HI or control participants (VI: 21% vs HI: 3% vs CT: 6% reporting someone else completing daily tasks for them, P < .001). No group endorsed appreciable change in their general health status since the start of the pandemic, with most participants in each group reporting that their health is “about the same” (VI: 75% vs HI: 85% vs CT: 83%, P = .28). Participants across groups reported equally high levels of disruption to their lives due to the pandemic, with most participants reporting “a fair amount” or “a lot” of disruption (VI: 76% vs HI: 82% vs CT: 83%, P = .33). The 3 groups reported similar levels of COVID-19 exposure (VI: 6% vs HI: 11% vs CT: 10%, P = .18) and infection (VI: 5% vs HI: 10% vs CT: 5%, P =. 41). Participants reported differing levels of change in isolation during the pandemic, with controls and those with HI reporting a significantly greater increase (VI: 67% vs HI: 88% vs CT: 76% reporting more isolation, P = .02). Composite scores on the PHQ-2 measure of depressive symptoms were similar among all 3 groups (VI: 1.55 vs HI:1.40 vs CT: 1.63, P = .28).
Table 3.
Survey Response Data by Participant Group
| Participant Group |
P |
|||
|---|---|---|---|---|
| Survey question | Control | Vision Impaired | Hearing Impaired | |
| General health before pandemic | <.001 | |||
| Excellent | 17 (11%) | 9 (8%) | 25 (23%) | |
| Very good | 71 (46%) | 30 (27%) | 53 (49%) | |
| Good | 53 (34%) | 44 (39%) | 24 (22%) | |
| Fair | 11 (7%) | 24 (21%) | 6 (6%) | |
| Poor | 3 (2%) | 5 (4%) | 0 (0%) | |
| Health change | .28 | |||
| Much better now | 1 (1%) | 2 (2%) | 3 (3%) | |
| Somewhat better now | 13 (8%) | 4 (4%) | 7 (7%) | |
| Same | 116 (75%) | 95 (85%) | 89 (83%) | |
| Somewhat worse now | 23 (15%) | 10 (9%) | 8 (7%) | |
| Much worse now | 1 (1%) | 1 (1%) | 0 (0%) | |
| Help with activities before pandemic | <.001 | |||
| No help needed | 121 (78%) | 26 (23%) | 90 (83%) | |
| Some help needed | 27 (17%) | 63 (56%) | 15 (14%) | |
| Someone else completes tasks | 7 (6%) | 23 (21%) | 3 (3%) | |
| Disruption of daily life because of COVID-19 | .33 | |||
| A lot | 48 (31%) | 31 (28%) | 36 (33%) | |
| A fair amount | 79 (51%) | 54 (48%) | 51 (50%) | |
| Just a little | 25 (16%) | 21 (19%) | 20 (19%) | |
| Not at all | 2 (1%) | 6 (5%) | 1 (1%) | |
| COVID-19 infection | .41 | |||
| Yes | 7 (5%) | 6 (5%) | 11 (10%) | |
| No | 125 (81%) | 91 (82%) | 82 (76%) | |
| COVID-19 exposure (of those not infected) | .21 | |||
| Yes | 15 (10%) | 6 (6%) | 11 (11%) | |
| No | 74 (50%) | 68 (64%) | 51 (53%) | |
| Isolation change | .02 | |||
| Much less now | 0 (0%) | 3 (3%) | 2 (2%) | |
| Less now | 12 (8%) | 3 (3%) | 7 (7%) | |
| Same | 24 (16%) | 31 (28%) | 14 (13%) | |
| More now | 66 (43%) | 39 (35%) | 54 (51%) | |
| Much more now | 51 (33%) | 36 (32%) | 28 (27%) | |
| Difficulty obtaining food | .30 | |||
| No or some difficulty | 152 (99%) | 105 (94%) | 105 (97%) | |
| A lot of difficulty or unable | 2 (1%) | 7 (6%) | 3 (3%) | |
| Difficulty obtaining medicine | .51 | |||
| No or some difficulty | 154 (99%) | 99 (88%) | 93 (86%) | |
| A lot of difficulty or unable | 1 (1%) | 13 (12%) | 15 (14%) | |
| Difficulty obtaining medical care | .052 | |||
| No or some difficulty | 139 (90%) | 91 (81%) | 94 (89%) | |
| A lot of difficulty or unable | 16 (10%) | 21 (19%) | 13 (11%) | |
| Job loss | .15 | |||
| Yes | 5 (3%) | 9 (9%) | 5 (5%) | |
| No | 140 (97%) | 89 (91%) | 97 (95%) | |
| Pay cut (of employed) | .049 | |||
| Yes | 13 (10%) | 6 (8%) | 21 (21%) | |
| No | 122 (90%) | 68 (92%) | 78 (78%) | |
| General transportation | <.001 | |||
| Drove themselves | 138 (89%) | 23 (21%) | 104 (96%) | |
| Driven by family/friends | 22 (14%) | 81 (72%) | 5 (5%) | |
| Public transportation | 7 (5%) | 16 (14%) | 5 (5%) | |
| Chance of running out of money | 8.9% | 7.06% | 4.26% | .60 |
| PHQ-2 score, mean (SD) | 1.63 (1.79) | 1.55 (1.76) | 1.40 (1.66) | .28 |
| Leaving home less often | 134 (87%) | 95 (85%) | 99 (92%) | .24 |
| Not interacting with friends as often | 126 (81%) | 86 (77%) | 96 (89%) | .07 |
| Connecting more via phone, tablet, computer | 97 (63%) | 76 (63%) | 78 (72%) | .23 |
PHQ = Patient Health Questionnaire (higher scores correlate with depression).
The majority of the sample had no difficulty obtaining medicines (P = .51) or food (P = .30), and there were no significant differences between groups. Those with VI, but not HI, showed a trend toward increased odds of reporting difficulty accessing medical care (odds ratio [OR]VI = 2.0, 95% CI = 0.99-4.0, P =.05) compared to controls, an association that was somewhat attenuated after adjusting for marital status (ORVI = 1.8, 95% CI = 0.9-3.9, P = .08), a potential confounder of this association.
There were no significant differences in the impact of the pandemic on job loss between groups (P = .15). In addition, there was a low level of worry about running out of money; on average, participants estimated that there was a 7% chance that they would run out of money in the following 3 months, and this finding was similar across groups (P = .6). Those with HI were most likely to report pay reductions compared to those in the control and VI groups (VI: 6%, HI: 21%, CT: 10% among employed participants, ORHI = 2.5, 95% CI = 1.2-5.3, P = .02).
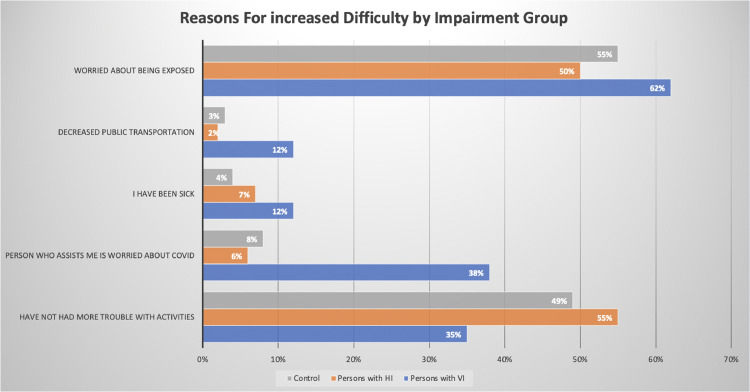
Since the start of the pandemic, participants in all groups have had more trouble taking care of day-to-day activities. However, the reasons for this varied across groups (Figure 1). Those with VI cited greater difficulty because of caregivers’ worry about COVID-19 (VI: 38%, HI: 6%, CT: 5%, ORVI = 7.2, 95% CI = 3.5-14.5, P < .001) and decreased availability of public transportation (VI: 12%, HI: 2%, CT: 3%, ORVI = 5.0, 95% CI = 1.6-15.6, P < .001).
Participants with VI were more likely to have begun relying more on family or friends for assistance (VI: 31%, HI: 7%, CT: 13%, ORVI = 3.1, 95% CI = 1.7-5.7, P < .001). Not surprisingly, a majority in all groups were leaving their house less than pre-pandemic, interacting with friends less, and using phone, tablets, or computers to connect with others. These findings were similar across all groups. It is notable that this increased difficulty with daily activities was present despite the finding that participants with VI were more likely to rely on others for daily tasks.
Among all participants, 30% reported difficulty obtaining trusted information about the pandemic. Compared to controls, those with VI were more likely to note that trusted information was difficult to see or hear (VI: 11%, HI: 2%, CT: 1%, ORVI = 3.1, 95% CI = 1.7-22.1, P < .001). Unadjusted odds ratios describing significant differences between study groups are shown in Table 4 ; there was a trend that did not reach statistical significance (P = .052) toward greater difficulty accessing medical care for those with VI.
Table 4.
Unadjusted Odds Ratios Comparing Sensory Impairment and Control Groups
| Survey question | OR, VI vs Control (95% CI) | OR, HI vs Control (95% CI) |
|---|---|---|
| Increased difficulty with daily activities for the following reasons: | ||
| Person assisting worried about exposure | 7.2 (3.5-14.4) | 0.7 (0.2-1.9) |
| Decreased public transit availability | 5.0 (1.6-15.6) | 0.7 (0.1-4.0) |
| Relying more on family and friends since the start of the pandemic | 3.1 (1.7-5.7) | 0.5 (0.2-1.3) |
| Difficulty seeing or hearing trusted information about the pandemic | 6.1 (1.7-22.1) | 0.5 (0.05-4.6) |
| Difficulty accessing routine medical care | 2.0 (0.99-4.0) | 1.2 (0.5-2.6) |
HI = hearing impairment; OR = odds ratio; VI = visual impairment.
Being married was associated with lower depressive symptoms (married: 32%, not married: 24% endorsing depressive symptoms, P < .001). When we adjusted for marital status, the association of VI with difficulty accessing routine medical care was attenuated (ORVI = 1.9, 95% CI = 0.9-3.9, P = .08) compared to the unadjusted model (ORVI = 2.0, 95% CI = 0.99-4.0, P = .052). The remaining survey responses were not significantly associated with CCI, ADI, disability benefits, or mode of survey response for any participant group.
DISCUSSION
Over 295 million persons worldwide have distance VI and 43.3 million are blind,23 and 466 million persons worldwide have HI.24 Although there is reason to suspect that the COVID-19 pandemic and associated mitigation measures may have a disproportionate impact on adults with SI, little work has been undertaken specifically to address the needs of this population during the pandemic.9 , 25 , 26 This study was designed to provide needed insight into the impact of the pandemic on adults living with VI and HI.
Not surprisingly, our study confirms the large impact of the pandemic across the population, including individuals without an SI. In fact, 80% of our sample reported at least a fair amount of disruption to their daily life. Despite reporting similar levels of COVID-19 infection and exposure, several key findings from this study highlight the outsized impact of the pandemic on individuals with SI, and the need for increased attention and planning to address the effects of a high-impact social stressor such as the COVID-19 pandemic on persons with VI.
Approximately 1 in 5 individuals with VI who were surveyed reported difficulty obtaining routine medical care, which was about twice the rate observed in the HI and control groups and trended toward a statistically significant difference between groups (P = .052). This finding may be partially confounded by marital status, as those with VI were less likely to be married, and unmarried participants were more likely to report difficulty accessing medical care. Notwithstanding, this finding may have important implications for targeting interventions and outreach to ensure healthcare access for vulnerable populations, including those with VI.
Persons with VI were also more likely to experience difficulty taking care of their daily activities because of the dual burden of being worried about COVID-19 exposure themselves and having caretakers who were also concerned about exposure. Concomitantly, their reliance on others to attend to day-to-day needs also increased, a trend that was not observed among the other groups. These findings confirm and build on findings from the recently published Flatten Inaccessibility survey that individuals with VI expressed pandemic-related concerns about their healthcare and access to transportation.27 , 28
Participants with VI also relied more heavily on public transportation and cited increased difficulty because of its decreased availability at rates 5 times higher than that of controls. Lack of access to reliable transportation is 1 of the key factors affecting the ability of people with VI to find and/or to maintain steady employment.29 , 30 Public transportation is essential for the many people with disabilities who cannot drive or do not have access to an accessible vehicle, and persons with VI appear to face an increased burden from this disruption.
The impact on public transportation of the COVID-19 pandemic and related containment policies, such as physical distancing and alternate seating (e.g., capacity limits), have led to a sharp decline in public transit ridership.31 The resulting decrease in revenue has caused many transit agencies to adopt drastic cost-cutting measures such as reducing services (eg, by eliminating and/or combining routes, lowering peak time capacity) and/or reducing their workforce (eg, laying off bus and paratransit drivers, discontinuing paratransit contracts). In 2020, 65% of US transit agencies resorted to cutting services because of the funding shortfall; 30% of agencies eliminated some routes, and 22% had to lay off employees.31 Persons with sensory and mobility impairments may experience a disproportionately greater effect. For instance, new and/or unfamiliar transit routes are a known source of transportation stress among persons with VI using public transit.32 Taken together, this suggests the need for targeted support for transportation needs for those with VI during the current pandemic.
In addition, a recent survey of individuals with low vision in Hungary reported that people with VI have faced difficulty with shopping for essential products and have relied on additional support.33 Notably, however, all participants in that study had VI, so there was no control group for comparison. Our data support and extend this finding by offering a comparison with controls and participants with HI.
The current study also has important implications for public health messaging and information access, with more than 30% of all participants reporting that they had trouble obtaining trustworthy information about the pandemic. Those with VI were more than 6 times as likely as controls to have difficulty finding trusted information that they could see and hear well. This finding highlights the need for targeted information campaigns that use auditory and visual information, large print, high contrast, and other adaptations to meet the needs of those with low vision. The survey of individuals with VI in Hungary also reported a large increase in the need to learn new applications and software for online work or study, with a majority of persons with VI needing support to learn to use these platforms, suggesting the need for targeted interventions in multiple domains of information sharing.33
One counterintuitive finding was that participants with VI reported a smaller change in isolation since the start of the pandemic compared to those with HI and controls. This finding contradicts a recent study of people with eye diseases in the United Kingdom that found that severity of VI was associated with greater loneliness, although this study recruited only from eye support charities and support groups, so it may have lacked an appropriate control group for comparison.34 We suspect that participants with VI in our study may have been more socially isolated before the pandemic, so they experienced less of a change than others.35 Future qualitative studies may be helpful to better understand this finding and to characterize the impact of the pandemic on social functioning in adults with VI.
Persons with HI appeared to experience fewer adverse outcomes than did those with VI. This may be due to socioeconomic and demographic differences between groups. The HI group had higher lower ADI scores and a lower burden of multimorbidity compared to others. These factors may have served to buffer individuals from some of the effects of the pandemic despite their prevalent HI. Ascertaining HI solely based on ICD-10 codes precluded us from determining the severity of the hearing loss. It is possible that those with more severe levels of hearing loss may experience the pandemic differently compared to those with milder levels.
Several survey items did not reveal significantly different results between groups. Participants reported similar levels of overall health change, with most reporting no change. The overwhelming majority of all groups reported no difficulty obtaining food or medicines. A similarly low proportion reported depressive symptoms, fear of running out of money, or loss of a job. The limited differences with some COV-DIS measures may have been due to a strong floor/ceiling effect. Future research may be useful to evaluate these differences further.
There are currently no consistent, specific, evidence-based recommendations for communities to work toward mitigating the outsized impact of the pandemic on persons with SI. There have been calls to ensure that the COVID-19 response is disability inclusive, with discussion of the potential for a larger impact among disadvantaged groups.25 , 26 Recent editorials provide guidelines for healthcare access for patients with hearing loss.7 , 8 , 36 Our study builds on these efforts and calls to action to inform future policies and recommendations.
Our study has several important limitations. Our sample was largely racially and ethnically homogeneous, which may limit the generalizability of our findings. In addition, all participants in this study had been under medical care at the University of Michigan, which suggests that they may have had better baseline access to healthcare services than other, more vulnerable, groups. Survey respondents also differed somewhat from nonrespondents; individuals from minority groups may have been underrepresented, which may limit the generalizability of our findings. Although marital status may provide an indication of possible support at home, our survey did not specifically include an item on co-habitation and was limited in the data that it provided on formal or informal help available in the home. These factors may be associated with more favorable outcomes and should be assessed in future research. The ascertainment of HI based on ICD-10 codes precluded us from determining the severity of hearing loss with the same level of granularity that we applied to VI. In addition, our survey has not yet undergone psychometric validation.
This study also had notable strengths. Participants were surveyed with an instrument expressly designed to understand relevant metrics surrounding pandemic-specific disruption for persons with disabilities. The survey took place in a location particularly hard-hit by the pandemic at the time of administration and was rapidly disseminated to groups who were likely to face additional disruption as a result of their sensory disability. However, in the months following the survey administration, southeastern Michigan experienced additional surges of COVID-19 that may have resulted in further impact on these populations. In addition, the survey was available either by telephone or by email. There may have been response bias and a mode effect if phone respondents were less likely to provide accurate answers to sensitive questions, such as income and employment status. However, this limitation is balanced by the benefit of allowing each participant the flexibility to determine which survey mode was most accessible.
In conclusion, the COVID-19 pandemic and related mitigation measures appear to have a disproportionate impact on adults with VI. Findings from this study have the potential to inform data-driven policy and public health decisions during the current pandemic and with future high-impact social stressors. Figure 1
Figure 1.
Reasons for increased difficulty endorsed by study participants with visual impairment, hearing impairment, or no sensory impairment.
Acknowledgments
Funding/Support: Funding was provided by an unrestricted grant from the Lighthouse Guild to the University of Michigan Department of Ophthalmology and Visual Sciences. J.R.E. is supported by a grant from the National Institutes of Health (K23EY027848). This work was supported by a grant to A.B. from the Michigan Institute for Clinical and Health Research with funding provided by the National Institutes of Health (TL1TR002242).
Financial Disclosures: The authors indicate no financial support or conflicts of interest. All authors attest that they meet the current ICMJE criteria for authorship.
Footnotes
Supplemental Material available at AJO.com.
Abstract accepted for presentation at the Association for Research in Vision and Ophthalmology 2021 Annual Meeting Virtual, May 1-7, 2021.
Appendix. Supplementary materials
REFERENCES
- 1.CDC. COVID Data Tracker. Centers for Disease Control and Prevention. Published March 28, 2020. Accessed February 7, 2021. https://covid.cdc.gov/covid-data-tracker
- 2.WHO Coronavirus Disease (COVID-19) Dashboard. Accessed February 7, 2021. https://covid19.who.int
- 3.Gershman J. A guide to state coronavirus reopenings and lockdowns. The Wall Street Journal, Dow Jones& Company. 2020;8 www.wsj.com/articles/a-state-by-state-guide-to-coronavirus-lockdowns-11584749351. Published May. [Google Scholar]
- 4.Xiong J, Lipsitz O, Nasri F, et al. Impact of COVID-19 pandemic on mental health in the general population: a systematic review. J Affect Disord. 2020;277:55–64. doi: 10.1016/j.jad.2020.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Palgi Y, Shrira A, Ring L, et al. The loneliness pandemic: loneliness and other concomitants of depression, anxiety and their comorbidity during the COVID-19 outbreak. J Affect Disord. 2020;275:109–111. doi: 10.1016/j.jad.2020.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pfefferbaum B, North CS. Mental health and the Covid-19 pandemic. N Engl J Med. 2020;383(6):510–512. doi: 10.1056/NEJMp2008017. [DOI] [PubMed] [Google Scholar]
- 7.Moreland CJ, Ruffin CV, Morris MA, McKee M. Unmasked: how the COVID-19 pandemic exacerbates disparities for people with communication-based disabilities. J Hosp Med. 2021;16(3):185–188. doi: 10.12788/jhm.3562. [DOI] [PubMed] [Google Scholar]
- 8.McKee M, Moran C, Zazove P. Overcoming additional barriers to care for deaf and hard of hearing patients during COVID-19. JAMA Otolaryngol Neck Surg. 2020;146(9):781. doi: 10.1001/jamaoto.2020.1705. [DOI] [PubMed] [Google Scholar]
- 9.Senjam SS. Impact of COVID-19 pandemic on people living with visual disability. Indian J Ophthalmol. 2020;68(7):1367–1370. doi: 10.4103/ijo.IJO_1513_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gopinath B, Schneider J, McMahon CM, Teber E, Leeder SR, Mitchell P. Severity of age-related hearing loss is associated with impaired activities of daily living. Age Ageing. 2012;41(2):195–200. doi: 10.1093/ageing/afr155. [DOI] [PubMed] [Google Scholar]
- 11.Patel N, Stagg BC, Swenor BK, Zhou Y, Talwar N, Ehrlich JR. Association of co-occurring dementia and self-reported visual impairment with activity limitations in older adults. JAMA Ophthalmol. 2020;138(7):756. doi: 10.1001/jamaophthalmol.2020.1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Assi L, Varadaraj V, Shakarchi AF, et al. Association of vision impairment with preventive care use among older adults in the United States. JAMA Ophthalmol. 2020;138(12):1298. doi: 10.1001/jamaophthalmol.2020.4524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shah K, Frank CR, Ehrlich JR. The association between vision impairment and social participation in community-dwelling adults: a systematic review. Eye Lond Engl. 2020;34(2):290–298. doi: 10.1038/s41433-019-0712-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shukla A, Harper M, Pedersen E, et al. Hearing loss, loneliness, and social isolation: a systematic review. Otolaryngol Neck Surg. 2020;162(5):622–633. doi: 10.1177/0194599820910377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reed NS, Altan A, Deal JA, et al. Trends in health care costs and utilization associated with untreated hearing loss over 10 years. JAMA Otolaryngol Neck Surg. 2019;145(1):27. doi: 10.1001/jamaoto.2018.2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bernard A, Weiss S, Stein JD, et al. Assessing the impact of COVID-19 on persons with disabilities: development of a novel survey. Int J Public Health. 2020;65(6):755–757. doi: 10.1007/s00038-020-01433-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Michigan Coronavirus Map and Case Count . 2020. The New York Times.https://www.nytimes.com/interactive/2020/us/michigan-coronavirus-cases.html Published April 1. [Google Scholar]
- 18.Stein JD, Rahman M, Andrews C, et al. Evaluation of an algorithm for identifying ocular conditions in electronic health record data. JAMA Ophthalmol. 2019;137(5):491. doi: 10.1001/jamaophthalmol.2018.7051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sottile PD, Albers D, DeWitt PE, et al. Real-time electronic health record mortality prediction during the COVID-19 pandemic: a prospective cohort study. Health Informatics. 2021 doi: 10.1101/2021.01.14.21249793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Messer LC, Laraia BA, Kaufman JS, et al. The development of a standardized neighborhood deprivation index. J Urban Health. 2006;83(6):1041–1062. doi: 10.1007/s11524-006-9094-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yip JLY, Luben R, Hayat S, et al. Area deprivation, individual socioeconomic status and low vision in the EPIC-Norfolk Eye Study. J Epidemiol Community Health. 2014;68(3):204–210. doi: 10.1136/jech-2013-203265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kroenke K, Spitzer RL, Williams JBW. The Patient Health Questionnaire-2: validity of a two-item depression screener. Med Care. 2003;41(11):1284–1292. doi: 10.1097/01.MLR.0000093487.78664.3C. [DOI] [PubMed] [Google Scholar]
- 23.Bourne R, Steinmetz JD, Flaxman S, et al. Trends in prevalence of blindness and distance and near vision impairment over 30 years: an analysis for the Global Burden of Disease Study. Lancet Glob Health. 2021;9(2):e130–e143. doi: 10.1016/S2214-109X(20)30425-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Davis AC, Hoffman HJ. Hearing loss: rising prevalence and impact. Bull World Health Organ. 2019;97(10):646. doi: 10.2471/BLT.19.224683. –646A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Armitage R, Nellums LB. The COVID-19 response must be disability inclusive. Lancet Public Health. 2020;5(5):e257. doi: 10.1016/S2468-2667(20)30076-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.The Lancet Public Health. COVID-19 puts societies to the test. Lancet Public Health. 2020;5(5):e235. doi: 10.1016/S2468-2667(20)30097-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rosenblum LP. Unprecedented times call for unprecedented collaboration: how two COVID-19 surveys were created with input from across the field of visual impairment to analyze the needs of adults, students, teachers, and orientation and mobility practitioners. J Vis Impair Blind. 2020;114(3):237–239. doi: 10.1177/0145482X20927129. [DOI] [Google Scholar]
- 28.American Foundation for the Blind. Executive summary. Accessed February 17, 2021. https://www-afb-org.proxy.lib.umich.edu/research-and-initiatives/flatten-inaccessibility-survey/executive-summary
- 29.Crudden A, Sansing W, Butler S. Overcoming barriers to employment: strategies of rehabilitation providers. J Vis Impair Blind. 2005;99(6):325–335. doi: 10.1177/0145482X0509900602. [DOI] [Google Scholar]
- 30.Crudden A, McBroom LW. Barriers to employment: a survey of employed persons who are visually impaired. J Vis Impair Blind. 1999;93(6):341–350. doi: 10.1177/0145482X9909300602. [DOI] [Google Scholar]
- 31.APTA. Policy brief: COVID-19 pandemic threatens public transit jobs and service. Accessed March 5, 2021. https://www.apta.com/wp-content/uploads/APTA-Survey-Brief-Agency-Jan-2021.pdf
- 32.Crudden A, Cmar JL, McDonnall MC. Stress associated with transportation: a survey of persons with visual impairments. J Vis Impair Blind. 2017;111(3):219–230. doi: 10.1177/0145482X1711100303. [DOI] [Google Scholar]
- 33.Gombas J, Csakvari J. Experiences of individuals with blindness or visual impairment during the COVID-19 pandemic lockdown in Hungary. Br J Vis Impair. 2021 doi: 10.1177/0264619621990695. Published online February 140264619621990695. [DOI] [Google Scholar]
- 34.Ting DSJ, Krause S, Said DG, Dua HS. Psychosocial impact of COVID-19 pandemic lockdown on people living with eye diseases in the UK. Eye. 2020:1–3. doi: 10.1038/s41433-020-01130-4. Published online August 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brunes A, Hansen MB, Heir T. Loneliness among adults with visual impairment: prevalence, associated factors, and relationship to life satisfaction. Health Qual Life Outcomes. 2019;17(1):24. doi: 10.1186/s12955-019-1096-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.West JS, Franck KH, Welling DB. Providing health care to patients with hearing loss during COVID-19 and physical distancing. Laryngoscope Investig Otolaryngol. 2020;5(3):396–398. doi: 10.1002/lio2.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.