Abstract
In female reproduction, the oocyte number is limited after birth. To achieve a continuous ovulatory cycle, oocytes are stored in primordial follicles. Therefore, the regulation of primordial follicle dormancy and activation is important for reproductive sustainability, and its collapse leads to premature ovarian insufficiency. In this review, we summarize primordial follicle development and the molecular mechanisms underlying primordial follicle maintenance and activation in mice. We also overview the mechanisms discovered through in vitro culture of functional oocytes, including the establishment of primordial follicle induction by environmental factors, which revealed the importance of hypoxia and compression by the extra cellular matrix (ECM) for primordial follicle maintenance in vivo.
Keywords: Environmental factors, In vitro oogenesis, Primordial follicle
Development of Primordial Follicles
In mice, female germ cell development originates from primordial germ cells (PGCs) around embryonic day 7 (E7). PGCs are specified from the proximal region of the epiblast via extracellular signaling, especially bone morphogenetic protein 4 (BMP4) and wingless-type MMTV integration site family member 3 (Wnt3) signaling [1]. Once the PGCs are specified, they migrate to the embryonic gonads, where they undergo sex differentiation according to the gonadal environment [2]. Male PGCs proliferate and become arrested at the G1 phase of the mitotic cell cycle until birth. Then, they resume the cell cycle as spermatogonial stem cells and produce sperm throughout life. In contrast, female PGCs, hereinafter called oogonia, proliferate through incomplete cytokinesis to generate germ cell cysts. They then enter meiosis, which is initiated by retinoic acid signaling [3]. Around birth, germ cell cysts are broken down by a massive loss of germ cells known as fetal oocyte attrition (FOA) in mice [4]. The remaining germ cells form primordial follicles surrounded by flat granulosa cells. Distinct from the case of male spermatogonial stem cells, the number of oocytes is limited after birth, and the primordial follicles function to store the limited oocytes and remain in a quiescent state until activated to support a continuous ovulatory cycle. Therefore, the regulation of the activation and dormancy of oocytes in primordial follicles is critical. The collapse of this regulation leads to premature ovarian insufficiency (POI), which is characterized by the disappearance of the menstrual cycle associated with premature follicular depletion [5].
Regulatory Mechanisms of Primordial Follicle Formation, Maintenance and Activation
From the formation of primordial follicles to the regulation of their dormancy and activation, most of mechanistic insights come from gene manipulation models in mice (Table 1). These include both the interaction of oocytes with granulosa cells and intrinsic oocyte factors.
Table 1. The molecules which involved in primordial follicle formation and maintenance.
| Gene | Expression | Genetic manipulation | Phenotype | References |
|---|---|---|---|---|
| JNK | GC | inhibitor / knock down | More oocyte remained in the cyst | [6] |
| Wnt4 | Oocyte and GC | O/E (Lenti virus) | Cyst breakdown was delayed | [6] |
| E-cadherin | Oocyte | O/E (Lenti virus) | Cyst breakdown was obviously delayed | [6] |
| Notch2 | GC | flox (Amhr2-Cre) | Multi-oocyte follicles | [7] |
| Taf4b | GC | KO | Required for cyst breakdown and primordial follicle formation | [8] |
| Mael | Oocyte | KO | Massive elimination of fetal oocytes | [9] |
| KitL | GC | blocking Ab (ACK2) | Disruption of primordial follicle activation | [11] |
| Rptor | GC | flox (FOXL2-CreERT2) | Suppression of follicular activation | [12] |
| TSC1 | GC | flox (FOXL2-CreERT2) | Premature awaking of dormant oocyte | [12] |
| GDF9 | Oocyte | KO | Block in follicular development beyond primary follicle stage | [13] |
| BMP15 | Oocyte | KO | Sub fertile, BMP15-/-GDF9+/- show more severe defect than BMP15-/- | [14] |
| Nobox | Oocyte | KO | Abolish the transition from primordial to growing follicle | [15] |
| Figla | Oocyte | KO | Primordial follicles were not formed at birth | [17] |
| SOHLH1 | Oocyte | KO | Defect in primordial-to-primary follicle transition | [18] |
| LHX8 | Oocyte | KO | Similar to SOHLH1-KO (likely downstream of SOHLH1) | [18] |
| Foxo3a | Oocyte | KO | Global follicular activation | [19] |
| PTEN | Oocyte | flox (GDF9-Cre) | Entire primordial follicle pool becomes activated | [20] |
| KitY719F/Y719F | Oocyte | point mutation | Impaired follicle activation | [21] |
| Foxo3a | Oocyte | O/E (ΔNES) | Retarded oocyte growth and follicular development | [22] |
| p27 | Oocyte and GC | KO | Primordial follicle pool was prematurely activated | [23] |
| ELAVL2 | Oocyte | KO | Defective primordial follicle formation | [26] |
| DDX6 | Oocyte | flox (MVH-Cre) | Severely impairs the formation of primordial follicle | [26] |
GC, granulosa cell; O/E, over expression.
Before the formation of primordial follicles, germ cell cysts are broken down by invading granulosa cells. It has been reported that c-Jun N-terminal kinase (JNK) inhibition or wingless-type MMTV integration site family, member 4 (Wnt4) overexpression delayed cyst breakdown according to the dysregulation of cadherin 1 (E-cadherin) at the junctions of germ cell cysts, leading to defective primordial follicle formation [6]. In addition, mice with Notch2 deficiency developed multi-oocyte follicles, indicating the importance of Notch2 in cyst breakdown, characterized as having a significantly reduced number of primordial follicles at postnatal day 18 (P18) [7]. Although the regulatory genes of Notch2 at cyst breakdown have not yet been elucidated, TATA-box binding protein associated factor 4b (Taf4b) is also known to regulate cyst breakdown. Taf4b is a gonadal-enriched coactivator subunit of the TATA box binding protein (Tbp, also known as TFIID). At birth, Taf4b-deficient ovaries exhibit defective primordial follicle formation and retain large cysts that have not been initiated or completely broken down [8]. Furthermore, during cyst breakdown, oocytes undergo meiosis, and meiosis is required for primordial follicle formation. During meiosis, it is important to suppress transposable elements (TEs), which are mainly regulated by maelstrom spermatogenic transposon silencers (Mael). Mael-/- ovaries exhibit defects in chromosome synapsis and dramatic loss of oocytes, leading to the formation of fewer primordial follicles [9]. In addition, primordial follicle formation is supported by a specific subpopulation of pregranulosa cells. There are two subpopulations of pregranulosa cells [10]. Bipotential pregranulosa (BPG) cells in the medulla express forkhead box L2 (FOXL2) earlier and support the first wave of oogenesis. Epithelial pregranulosa (EPG) cells express leucine-rich repeat-containing G protein coupled receptor 5 (Lgr5) during the embryonic stage and contributes to the formation of quiescent primordial follicles. An experiment using Lgr5-DTA-GFP mice, in which Lgr5+ cells were depleted during fetal follicle development, indicated that while primordial follicles were severely decreased, the primary follicles of the first wave of oogenesis were not. These results demonstrate the importance of EPG cells in primordial follicle formation.
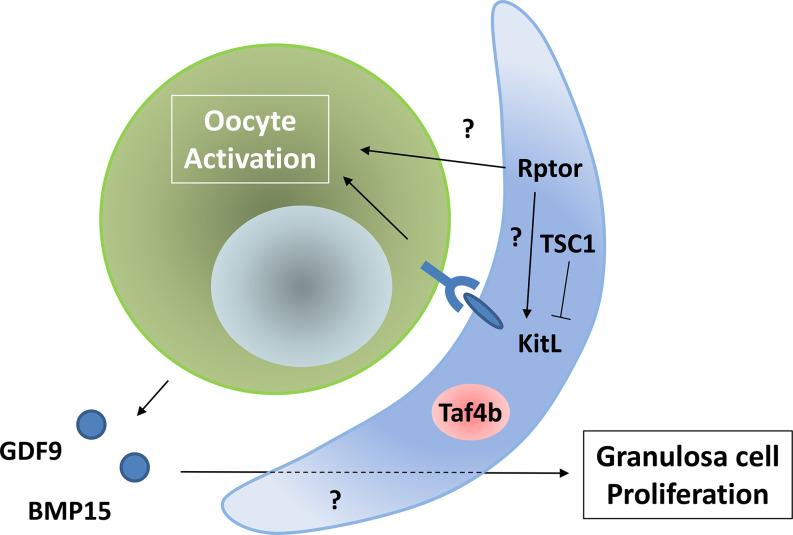
The interaction between oocytes and granulosa cells is also important for the regulation of primordial follicle activation. First, Kit ligand (KitL, also known as SCF) expressed on granulosa cells binds to its receptor, KIT proto-oncogene receptor tyrosine kinase (c-Kit), expressed in oocytes to initiate the activation of primordial follicles [11]. Moreover, in granulosa cells, the mechanistic target of the rapamycin kinase complex 1 (mTORC1) pathway is involved in oocyte activation. The regulatory associated protein of MTOR, complex 1 (Rptor), is a key component of the mTORC1 pathway, whose disruption in granulosa cells has been shown to suppress follicular activation and prevent the awakening of dormant oocytes [12]. In contrast, TSC complex subunit 1 (TSC1) is an inhibitor of mTORC1 signaling, and its deficiency in granulosa cells induces the premature awakening of all dormant oocytes [12]. Although TSC1-/- mice induced the upregulation of KitL on granulosa cells, the mechanism by which mTORC1 signaling regulates primordial follicle activation is not yet fully understood. In addition, secretion of growth differentiation factor 9 (GDF9) and bone morphogenetic protein 15 (BMP15) regulates the proliferation of granulosa cells. GDF9-deficient mice showed a block in follicular development beyond the primary one-layer follicle stage [13]. BMP15 was found to have a function similar to that of GDF9, but BMP15-/- mice showed a milder phenotype than GDF9-/- mice [14]. The interactions between the oocytes and granulosa cells are summarized in Fig. 1.
Fig. 1.
Interaction between oocytes and granulosa cells to activate primordial follicles.
With regard to the intrinsic effectors in oocytes, several transcription factors are known to be requisite for the development and maintenance of primordial follicles. NOBOX oogenesis homeobox (Nobox), an oocyte-specific homeobox gene, is expressed in germ cell cysts and primordial and growing oocytes. Nobox-deficient female mice have been reported to show no loss of germ cells before birth and normal initiation of primordial follicle formation. However, at P7, very few oocytes are apparent and most of those that are visible are in the process of being degraded. While Nobox-/- ovaries express the genes implicated in early folliculogenesis (Fgf2, BMP4, Wnt4, Gcna, and Foxo3a), a subset of genes that are preferentially expressed in postnatal oocytes are defective (Rfpl4, Fgf8, Zar1, Dnmt1o, Gdf9, BMP15, and H1oo) [15]. The germ cell-specific basic helix-loop-helix (bHLH) factor folliculogenesis-specific basic helix-loop-helix (Figla) is expressed specifically in postnatal oocytes, and was originally identified as a transcription factor that regulates the expression of zona pellucida (ZP) genes [16]. A loss-of-function mutant of Figla showed a failure of primordial follicle formation at birth. Although embryonic gonadogenesis appeared normal, no primordial follicles were present at P7 [17]. Although Figla-/- ovaries failed to express ZP transcripts, the mechanism by which it regulates primordial follicle formation is still elusive. Spermatogenesis- and oogenesis-specific basic helix-loop-helix 1 (Sohlh1) is expressed preferentially during early folliculogenesis. Sohlh1-deficient ovaries showed defects in the primordial-to-primary follicle transition, whereas they exhibited no significant differences in morphology or histology from the WT ovaries at birth [18]. In addition, LIM homeobox protein 8 (Lhx8) was drastically downregulated in Sohlh1-/- mice. Mice with Lhx8 mutations showed a phenotype similar to that of Sohlh1-/- mice, indicating that Lhx8 is likely downstream of Sohlh1 [18]. The Sohlh1 mutant mice showed an approximately 4-fold downregulation in Nobox and Figla expression, indicating that crosstalk occurs between these transcription factors [18].
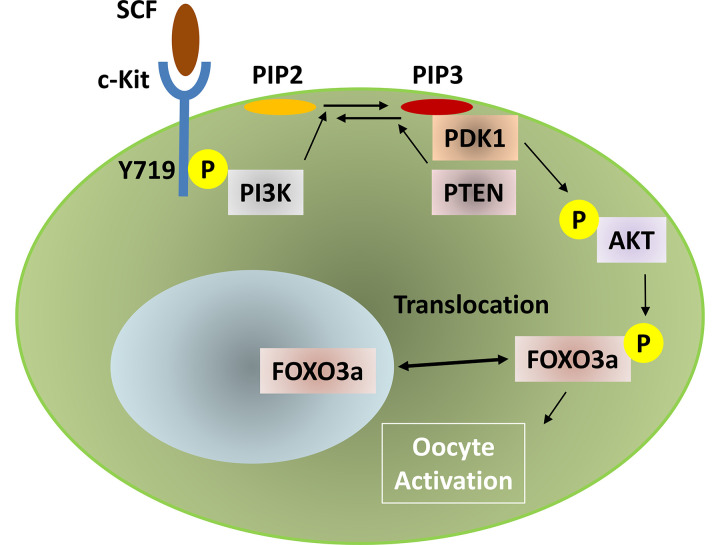
Among the oocyte-intrinsic factors, forkhead box O3a (FOXO3a) has been well characterized. Because FOXO3a functions at the earliest stage of follicular growth as a suppressor of primordial follicle activation, FOXO3a-/- mice show abnormal follicular activation [19]. Phosphorylation regulates FOXO3a localization from the nucleus to the cytoplasm, which suppresses its transcriptional function. This phosphorylation is mediated by the phosphoinositide-3-kinase (PI3K) thymoma viral proto-oncogene (AKT) signaling pathway, and phosphatase and tensin homolog (PTEN) regulate the pathway in the opposite direction. Therefore, in PTEN-mutant mice, primordial follicles are prevented from entering dormancy, resulting in enhanced oocyte activation [20]. Upstream of PI3K, SCF-mediated phosphorylation of c-Kit, especially in tyrosine at position 719 (Y719), functions in oocyte activation [21]. The signaling pathways for this are summarized in Fig. 2. ZP3 promoter-mediated overexpression of nuclear FOXO3a (through a mutation in the nuclear export signal [NES]) induced retarded oocyte growth and follicle development [22]. This nuclear FOXO3a overexpression resulted in the abnormal expression of cyclin-dependent kinase inhibitor 1 B (Cdkn1b, also known as p27), indicating that p27 is a downstream target of FOXO3a. In fact, p27-deficient mice exhibit prematurely activated primordial follicles [23]. However, the phenotypes were more severe in FOXO3a-/- than in p27-/- mice, and the double mutant for FOXO3a and p27 showed an additive effect for these phenotypes. These data indicate that FOXO3a has other target genes, and thus elucidating the molecular mechanisms by which FOXO3a regulates primordial follicle dormancy requires further investigation. PI3K-mediated FOXO3a phosphorylation is conserved during human primordial follicle activation. Treatment with the PTEN inhibitor bpV and the PI3K-activating peptide 740Y-P has been shown to induce human oocyte maturation, and thus has been clinically applied for patients with POI [24, 25].
Fig. 2.
Signaling pathway from SCF to the phosphorylation of FOXO3a for the activation of oocytes in primordial follicles.
In addition to transcription factors, it was recently reported that P-bodies (cytoplasmic RNP granules involved in the storage and degradation of RNA) regulate primordial follicle formation and dormancy. ELAV-like RNA binding protein 1 (ELAVL2) is an RNA-binding protein that directs the assembly of P-bodies by promoting the translation of DEAD (Asp-Glu-Ala-Asp) box polypeptide 6 (DDX6). Both ELAVL2 and DDX6 are indispensable for the formation of quiescent primordial follicles [26]. Notably, DDX6-deficient mice showed a dysregulation of PI3K-AKT signaling in primordial follicles. The assembly of P-bodies is induced by stress, which is mediated by the local environment [27]. As described in a later section, environmental factors are important for the regulation of primordial follicles. It would be interesting to study the mechanism underlying the induction of P-bodies during primordial follicle formation.
In Vitro Reconstitution of Primordial Dollicle Sevelopment
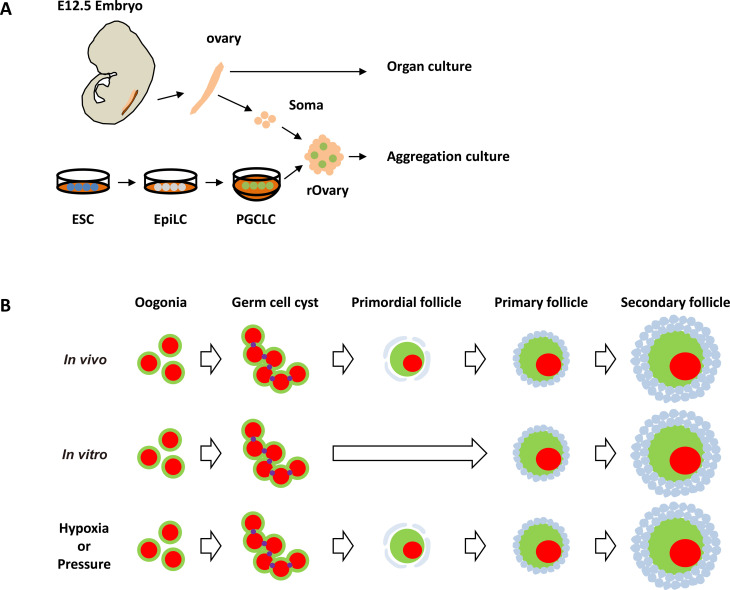
In vitro reconstitution of oocyte development has been established in mice [28, 29]. The procedure is divided into three steps: in vitro differentiation (IVDi), in vitro growth (IVG), and in vitro maturation (IVM) [30]. IVDi is a hormone-independent process that produces secondary follicles from PGCs. The two subsequent steps are follicle stimulating hormone (FSH)-dependent, and gonadotropin induces the maturation of MII oocytes. Finally, upon fertilization, in vitro-induced oocytes produce pups that can produce the next generation of mice. The method can be applied not only to fetal ovaries but also to reconstituted ovaries (rOvaries), which are composed of PGCLCs induced from pluripotent stem cells and fetal ovarian somatic cells (Fig. 3A). In vitro reconstitution culture expands the probability of oogenesis to be observed experimentally. The in vitro induction of oocytes from PGCs is a good model for in vivo female germline development, but there is a large difference in primordial follicle formation between oogenesis in vivo and in vitro. During in vitro oogenesis, primordial follicles were not observed and almost all of the oocytes were activated synchronously, even though it is not clear whether primordial follicles are temporarily formed (Fig. 3B). Because the genes involved in the in vitro generation of oocytes are identical to those involved in in vivo oogenesis, environmental effects are clearly important for the development and maintenance of primordial follicles.
Fig. 3.
A) Schematic diagram of in vitro culture from PGCs to oocytes. Both embryonic ovaries and rOvaries can be used in this method. B) Summary of oocyte development in vivo (upper panel), in vitro (middle panel) and in in vitro culture under hypoxia or pressure (lower panel).
Therefore, we focused on the environment in which primordial follicles were placed. It has been reported that the ovarian cortex, where the primordial follicles are located, contains only a negligible amount of platelet/endothelial cell adhesion molecule 1 (PECAM)-positive endothelial cells, as observed from 3D immunostaining analysis [31]. The sparse vascular network of the ovarian cortex suggests a hypoxic condition. In fact, during oocyte development, the expression of hypoxia inducible factor 1 (HIF1), which plays a central role in the hypoxic response of cells, indicated that oocytes in the primordial follicles are under hypoxia. In the female germline, HIF1 is expressed in all stages of fetal oogonia in newborn oocytes in mice. In particular, in the P5 ovary, high levels of HIF1 are expressed in the small oocytes located in the cortical region [32]. In addition, the ovarian cortex is surrounded by collagen embedded in the primordial follicles [33]. Collagen increases tissue stiffness [34], and fibrosis is mainly characterized by the accumulation of collagen; this indicates that the ovarian cortex was under compression. From this point of view, it is interesting to examine the granulosa cells of primordial follicles. The granulosa cells comprising the primordial follicles are squamous and cuboidal upon activation. It has been proposed that the adhesion-to-tension ratio defines the epithelial equilibrium state [35]. If the ratio increases, the tension decreases, and the epithelial morphology changes from squamous and cuboidal to columnar. These morphological features coincide with the shapes of the granulosa cells undergoing follicular activation. Therefore, the squamous shape of granulosa cells indicates a higher tension on primordial follicles.
In the in vitro induction of primordial follicles, the first clue towards the development of a system that recapitulates the induction of primordial follicles in vitro is derived from a comparison of the gene expression patterns between in vivo and in vitro oocyte development. We found that oocytes highly expressed FOXO3a in vivo, suggesting that oocytes of primordial follicles showed an upregulation of the genes involved in the reduction of reactive oxygen species (ROS) [36]. In contrast, when nuclear FOXO3a was expressed during in vitro oogenesis, the genes upregulated in the oocytes were involved in the response to hypoxia [36]. In addition, we have demonstrated that inhibition of mitochondrial activity affects growing oocytes, but not oocytes of primordial follicles, which indicates the independence of oxidative phosphorylation (OXPHOS) in primordial follicles [36]. These results clarified the relationship between hypoxia and primordial follicle development. Finally, we found that even in in vitro oogenesis, culture under hypoxic conditions induced primordial follicles [36].
We have also performed research focused on the extracellular matrix (ECM) and compression. We demonstrated the importance of the ECM in the dormant state of oocytes in primordial follicles [37]. When we treated newborn mouse ovaries with CTK (collagenase, trypsin, KSR) solution, the ECM was cleaved, which caused the translocation of FOXO3a from the nucleus to the cytoplasm in oocytes of the primordial follicles, indicating follicular activation. However, this activation of primordial follicles by CTK was prevented when the treatment was performed under exogenous pressure [37]. These results indicate the importance of ECM-mediated pressure in primordial follicle dormancy. Interestingly, when the culture was subjected to exogenous pressure, primordial follicles were induced from PGCs [37]. Taken together, these results show that we succeeded in inducing primordial follicles in the context of in vitro oocyte development by modulating the culture conditions. These findings underscore the importance of hypoxia and physical pressure in the development and maintenance of primordial follicles.
The mechanism by which hypoxia and physical pressure induce primordial follicles in vitro is under investigation. However, when FOXO3a is expressed under normoxia, almost all FOXO3a is localized in the cytoplasm. Thus, hypoxia may regulate the nuclear localization of FOXO3a. In addition, hypoxia has been reported to activate FOXO3a expression [38]. On the other hand, with respect to pressure, the actual amount of pressure on the primordial follicle in the ovarian cortex is not known; neither is it known how much this pressure differs from the pressure in the medullary region. In other words, the measurement of pressure in the ovary has not yet been achieved. To date, the pressure in cells is generally measured using an atomic force microscope [46]. However, such measurements require direct contact to obtain sufficient resolution, and the values are averaged along the contact direction. These features make it difficult to measure the inside of a layered structure, such as tissue. In recent years, there have been reports of new technologies, such as Brillouin microscopy, which might be applicable for taking measurements within tissues [39] . Brillouin microscopy has been developed and used in the field of materials science, but by combining this technology with a confocal microscope, living cells can be analyzed in three-dimensional space with high resolution without contact. In addition, the molecular mechanisms by which pressure regulates cell fate are largely unknown, although it is considered that the main mechanism likely involves changes in signaling caused by pressure-induced structural changes. The most studied molecule with regard to the differences in cell fate in relation to pressure is the large-conductance ion mechanosensitive channel (MscL) in bacteria. MscLs consist of transmembrane helices (TM) 1 and TM2, which are the inner and outer helices, respectively. TM1 constitutes an ion transmission path (pore) while TM2 interacts with the lipid membrane [40]. The opening and closing of the channel are controlled by physical stress. It has also been reported that the reactivity of the Crk-associated substrate (Cas) differed, owing to the structural changes caused by extension of experimental pulling [41]. In oocytes, changes in cell volume were observed in response to CTK treatment and pressure [37]. This makes it easy to imagine changes such as expansion and contraction of cell membranes, suggesting that structural changes in some molecules may be the mechanistic key to the regulation of gene expression by pressure. In addition, it has been reported that phosphatidylinositol (PI) undergoes structural changes under hydrostatic pressure [42]. Therefore, it is possible that pressure regulates PI3K signaling through the modulation of PI.
In addition to clarifying how the individual mechanisms of hypoxia and pressure affect primordial follicle formation in vitro, it is important to analyze the combined effects of hypoxia and pressure. It has been predicted that there is crosstalk between hypoxia and pressure. The ovarian stroma is enriched in hyaluronic acid (HA), which makes the tissue soft [34]. While HA is catalyzed by hyaluronidase to produce low-molecular-weight HA, the activity of hyaluronidase is suppressed by ROS [43]. In hypoxia, it is predicted that the levels of ROS will be lower, indicating the weaker limiting effect of hyaluronidase, making the tissue stiffer. In addition, HA affects cellular metabolism. The reduction of HA has been shown to increase glucose uptake [44]. Increased glucose uptake is suitable for primordial follicles that depend on glycolysis. However, the effect of HA on primordial follicle maintenance has not been investigated directly. Therefore, it is possible that hypoxia and pressure work independently for the maintenance of primordial follicles.
Nuclear Rotation of Oocytes in Primordial Follicles
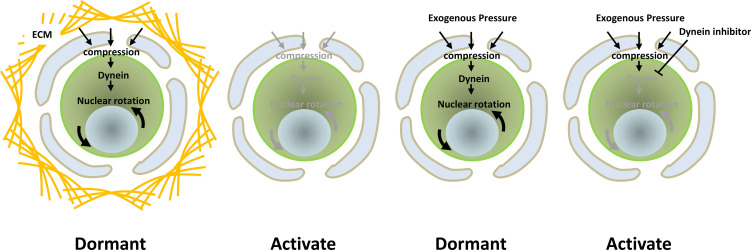
In addition to maintaining dormancy, we found that pressure induces nuclear rotation in the oocytes of primordial follicles in mice [37]. While such rotation is stopped by CTK treatment, it continues under pressure [37], indicating that the ECM-generated compression to oocytes in the primordial follicles induces nuclear rotation. In fact, there are several reports showing that physical stress induces nuclear rotation. One of these reports proposed that nuclear rotation occurs in a dynein-dependent manner [45, 46]. Another study showed that a dynein inhibitor, Ciliobrevin D (CD), ceased the nuclear rotation of oocytes in primordial follicles. Finally, CD treatment was shown to enhance the activation of primordial follicles, indicating the importance of nuclear rotation for the maintenance of primordial follicle dormancy (Fig. 4).
Fig. 4.
Summary of the effects of pressure for nuclear rotation and oocyte activation.
Although nuclear rotation has been investigated for 50 years, its molecular mechanisms and biological significance are largely unknown. To date, nuclear rotation has been observed in migrating cells that are pulled in the same direction [45]. However, in recent years, it has been suggested that a relationship exists between nuclear rotation and certain cell functions such as cell differentiation and virus replication [47, 48]. As it has been reported that a deficiency in Vimentin or Lamin B1 in fibroblasts induced nuclear rotation, it is possible that nucleus rotation is a result of cytoskeletal remodeling under physical stress [49, 50]. However, it would be intriguing to study why dormant oocytes in primordial follicles actively rotate their nuclei.
Conclusion
Primordial follicles sustain the ovulatory cycle through the regulation of dormancy and activation. The development of primordial follicles has been analyzed mainly by genetic manipulation in mice. The regulation of FOXO3a in the activation of primordial follicles has been well characterized. SCF from granulosa cells binds to c-Kit on oocytes, activating PI3K-AKT signals that phosphorylate FOXO3a and induce their translocation from the nucleus to the cytoplasm, inactivating the transcriptional activity of FOXO3a. However, the precise mechanisms, and especially the target genes of FOXO3a in primordial follicles, are not yet fully understood. Although in vitro oocytes mature and have the potential to produce pups after IVF, primordial follicles were not detected. This raised new insights into the role of environmental factors in primordial follicle formation. For example, primordial follicles are under hypoxia and are compressed by the ECM. Thus, it appears that culture under hypoxia and pressure could induce primordial follicles in a dormant state in vitro. This, in turn, opens the possibility of not only a mechanistic analysis but also the reconstitution of sustainable oogenesis in vitro, which could lead to clinical applications regarding reconstituted ovaries.
Conflict of interests
The author declares that there is no conflict of interest
Acknowledgments
I thank all lab members for their critical discussions. G. Nagamatsu was supported in part by KAKENHI Grants-in-Aid from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (no. 18K06261).
References
- 1.Cantú AV, Laird DJ. Wnt and Bmp fit germ cells to a T. Dev Cell 2013; 27: 485–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harikae K, Miura K, Kanai Y. Early gonadogenesis in mammals: significance of long and narrow gonadal structure. Dev Dyn 2013; 242: 330–338. [DOI] [PubMed] [Google Scholar]
- 3.Bowles J, Knight D, Smith C, Wilhelm D, Richman J, Mamiya S, Yashiro K, Chawengsaksophak K, Wilson MJ, Rossant J, Hamada H, Koopman P. Retinoid signaling determines germ cell fate in mice. Science 2006; 312: 596–600. [DOI] [PubMed] [Google Scholar]
- 4.Malki S, Tharp ME, Bortvin A. A whole-mount approach for accurate quantitative and spatial assessment of fetal oocyte dynamics in mice. Biol Reprod 2015; 93: 113. [DOI] [PubMed] [Google Scholar]
- 5.Barros F, Carvalho F, Barros A, Dória S. Premature ovarian insufficiency: clinical orientations for genetic testing and genetic counseling. Porto Biomed J 2020; 5: e62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Niu W, Wang Y, Wang Z, Xin Q, Wang Y, Feng L, Zhao L, Wen J, Zhang H, Wang C, Xia G. JNK signaling regulates E-cadherin junctions in germline cysts and determines primordial follicle formation in mice. Development 2016; 143: 1778–1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu J, Gridley T. Notch2 is required in somatic cells for breakdown of ovarian germ-cell nests and formation of primordial follicles. BMC Biol 2013; 11: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grive KJ, Seymour KA, Mehta R, Freiman RN. TAF4b promotes mouse primordial follicle assembly and oocyte survival. Dev Biol 2014; 392: 42–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Malki S, van der Heijden GW, O’Donnell KA, Martin SL, Bortvin A. A role for retrotransposon LINE-1 in fetal oocyte attrition in mice. Dev Cell 2014; 29: 521–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Niu W, Spradling AC. Two distinct pathways of pregranulosa cell differentiation support follicle formation in the mouse ovary. Proc Natl Acad Sci USA 2020; 117: 20015–20026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jones RL, Pepling ME. KIT signaling regulates primordial follicle formation in the neonatal mouse ovary. Dev Biol 2013; 382: 186–197. [DOI] [PubMed] [Google Scholar]
- 12.Zhang H, Risal S, Gorre N, Busayavalasa K, Li X, Shen Y, Bosbach B, Brännström M, Liu K. Somatic cells initiate primordial follicle activation and govern the development of dormant oocytes in mice. Curr Biol 2014; 24: 2501–2508. [DOI] [PubMed] [Google Scholar]
- 13.Dong J, Albertini DF, Nishimori K, Kumar TR, Lu N, Matzuk MM. Growth differentiation factor-9 is required during early ovarian folliculogenesis. Nature 1996; 383: 531–535. [DOI] [PubMed] [Google Scholar]
- 14.Yan C, Wang P, DeMayo J, DeMayo FJ, Elvin JA, Carino C, Prasad SV, Skinner SS, Dunbar BS, Dube JL, Celeste AJ, Matzuk MM. Synergistic roles of bone morphogenetic protein 15 and growth differentiation factor 9 in ovarian function. Mol Endocrinol 2001; 15: 854–866. [DOI] [PubMed] [Google Scholar]
- 15.Rajkovic A, Pangas SA, Ballow D, Suzumori N, Matzuk MM. NOBOX deficiency disrupts early folliculogenesis and oocyte-specific gene expression. Science 2004; 305: 1157–1159. [DOI] [PubMed] [Google Scholar]
- 16.Liang L, Soyal SM, Dean J. FIGalpha, a germ cell specific transcription factor involved in the coordinate expression of the zona pellucida genes. Development 1997; 124: 4939–4947. [DOI] [PubMed] [Google Scholar]
- 17.Soyal SM, Amleh A, Dean J. FIGalpha, a germ cell-specific transcription factor required for ovarian follicle formation. Development 2000; 127: 4645–4654. [DOI] [PubMed] [Google Scholar]
- 18.Pangas SA, Choi Y, Ballow DJ, Zhao Y, Westphal H, Matzuk MM, Rajkovic A. Oogenesis requires germ cell-specific transcriptional regulators Sohlh1 and Lhx8. Proc Natl Acad Sci USA 2006; 103: 8090–8095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Castrillon DH, Miao L, Kollipara R, Horner JW, DePinho RA. Suppression of ovarian follicle activation in mice by the transcription factor Foxo3a. Science 2003; 301: 215–218. [DOI] [PubMed] [Google Scholar]
- 20.Reddy P, Liu L, Adhikari D, Jagarlamudi K, Rajareddy S, Shen Y, Du C, Tang W, Hämäläinen T, Peng SL, Lan ZJ, Cooney AJ, Huhtaniemi I, Liu K. Oocyte-specific deletion of Pten causes premature activation of the primordial follicle pool. Science 2008; 319: 611–613. [DOI] [PubMed] [Google Scholar]
- 21.Kissel H, Timokhina I, Hardy MP, Rothschild G, Tajima Y, Soares V, Angeles M, Whitlow SR, Manova K, Besmer P. Point mutation in kit receptor tyrosine kinase reveals essential roles for kit signaling in spermatogenesis and oogenesis without affecting other kit responses. EMBO J 2000; 19: 1312–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu L, Rajareddy S, Reddy P, Du C, Jagarlamudi K, Shen Y, Gunnarsson D, Selstam G, Boman K, Liu K. Infertility caused by retardation of follicular development in mice with oocyte-specific expression of Foxo3a. Development 2007; 134: 199–209. [DOI] [PubMed] [Google Scholar]
- 23.Rajareddy S, Reddy P, Du C, Liu L, Jagarlamudi K, Tang W, Shen Y, Berthet C, Peng SL, Kaldis P, Liu K. p27kip1 (cyclin-dependent kinase inhibitor 1B) controls ovarian development by suppressing follicle endowment and activation and promoting follicle atresia in mice. Mol Endocrinol 2007; 21: 2189–2202. [DOI] [PubMed] [Google Scholar]
- 24.Li J, Kawamura K, Cheng Y, Liu S, Klein C, Liu S, Duan EK, Hsueh AJ. Activation of dormant ovarian follicles to generate mature eggs. Proc Natl Acad Sci USA 2010; 107: 10280–10284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kawamura K, Cheng Y, Suzuki N, Deguchi M, Sato Y, Takae S, Ho CH, Kawamura N, Tamura M, Hashimoto S, Sugishita Y, Morimoto Y, Hosoi Y, Yoshioka N, Ishizuka B, Hsueh AJ. Hippo signaling disruption and Akt stimulation of ovarian follicles for infertility treatment. Proc Natl Acad Sci USA 2013; 110: 17474–17479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kato Y, Iwamori T, Ninomiya Y, Kohda T, Miyashita J, Sato M, Saga Y. ELAVL2-directed RNA regulatory network drives the formation of quiescent primordial follicles. EMBO Rep 2019; 20: e48251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tauber D, Tauber G, Parker R. Mechanisms and Regulation of RNA Condensation in RNP Granule Formation. Trends Biochem Sci 2020; 45: 764–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morohaku K, Tanimoto R, Sasaki K, Kawahara-Miki R, Kono T, Hayashi K, Hirao Y, Obata Y. Complete in vitro generation of fertile oocytes from mouse primordial germ cells. Proc Natl Acad Sci USA 2016; 113: 9021–9026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hikabe O, Hamazaki N, Nagamatsu G, Obata Y, Hirao Y, Hamada N, Shimamoto S, Imamura T, Nakashima K, Saitou M, Hayashi K. Reconstitution in vitro of the entire cycle of the mouse female germ line. Nature 2016; 539: 299–303. [DOI] [PubMed] [Google Scholar]
- 30.Hayashi K, Hikabe O, Obata Y, Hirao Y. Reconstitution of mouse oogenesis in a dish from pluripotent stem cells. Nat Protoc 2017; 12: 1733–1744. [DOI] [PubMed] [Google Scholar]
- 31.Feng Y, Cui P, Lu X, Hsueh B, Möller Billig F, Zarnescu Yanez L, Tomer R, Boerboom D, Carmeliet P, Deisseroth K, Hsueh AJW. CLARITY reveals dynamics of ovarian follicular architecture and vasculature in three-dimensions. Sci Rep 2017; 7: 44810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Takahashi N, Davy PM, Gardner LH, Mathews J, Yamazaki Y, Allsopp RC. Hypoxia inducible factor 1 alpha is expressed in germ cells throughout the murine life cycle. PLoS One 2016; 11: e0154309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bochner F, Fellus-Alyagor L, Kalchenko V, Shinar S, Neeman M. A novel intravital imaging window for longitudinal microscopy of the mouse ovary. Sci Rep 2015; 5: 12446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Amargant F, Manuel SL, Tu Q, Parkes WS, Rivas F, Zhou LT, Rowley JE, Villanueva CE, Hornick JE, Shekhawat GS, Wei JJ, Pavone ME, Hall AR, Pritchard MT, Duncan FE. Ovarian stiffness increases with age in the mammalian ovary and depends on collagen and hyaluronan matrices. Aging Cell 2020; 19: e13259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Derganc J, Svetina S, Žekš B. Equilibrium mechanics of monolayered epithelium. J Theor Biol 2009; 260: 333–339. [DOI] [PubMed] [Google Scholar]
- 36.Shimamoto S, Nishimura Y, Nagamatsu G, Hamada N, Kita H, Hikabe O, Hamazaki N, Hayashi K. Hypoxia induces the dormant state in oocytes through expression of Foxo3. Proc Natl Acad Sci USA 2019; 116: 12321–12326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nagamatsu G, Shimamoto S, Hamazaki N, Nishimura Y, Hayashi K. Mechanical stress accompanied with nuclear rotation is involved in the dormant state of mouse oocytes. Sci Adv 2019; 5: v9960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bakker WJ, Harris IS, Mak TW. FOXO3a is activated in response to hypoxic stress and inhibits HIF1-induced apoptosis via regulation of CITED2. Mol Cell 2007; 28: 941–953. [DOI] [PubMed] [Google Scholar]
- 39.Prevedel R, Diz-Muñoz A, Ruocco G, Antonacci G. Brillouin microscopy: an emerging tool for mechanobiology. Nat Methods 2019; 16: 969–977. [DOI] [PubMed] [Google Scholar]
- 40.Chang G, Spencer RH, Lee AT, Barclay MT, Rees DC. Structure of the MscL homolog from Mycobacterium tuberculosis: a gated mechanosensitive ion channel. Science 1998; 282: 2220–2226. [DOI] [PubMed] [Google Scholar]
- 41.Sawada Y, Tamada M, Dubin-Thaler BJ, Cherniavskaya O, Sakai R, Tanaka S, Sheetz MP. Force sensing by mechanical extension of the Src family kinase substrate p130Cas. Cell 2006; 127: 1015–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Carrier D, Wong PTT. Effect of dehydration and hydrostatic pressure on phosphatidylinositol bilayers: an infrared spectroscopic study. Chem Phys Lipids 1996; 83: 12. [Google Scholar]
- 43.Monslow J, Govindaraju P, Puré E. Hyaluronan - a functional and structural sweet spot in the tissue microenvironment. Front Immunol 2015; 6: 231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sullivan WJ, Mullen PJ, Schmid EW, Flores A, Momcilovic M, Sharpley MS, Jelinek D, Whiteley AE, Maxwell MB, Wilde BR, Banerjee U, Coller HA, Shackelford DB, Braas D, Ayer DE, de Aguiar Vallim TQ, Lowry WE, Christofk HR. Extracellular matrix remodeling regulates glucose metabolism through TXNIP destabilization. Cell 2018; 175: 117–132.e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Levy JR, Holzbaur ELF. Dynein drives nuclear rotation during forward progression of motile fibroblasts. J Cell Sci 2008; 121: 3187–3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Christophorou N, Rubin T, Bonnet I, Piolot T, Arnaud M, Huynh JR. Microtubule-driven nuclear rotations promote meiotic chromosome dynamics. Nat Cell Biol 2015; 17: 1388–1400. [DOI] [PubMed] [Google Scholar]
- 47.Brosig M, Ferralli J, Gelman L, Chiquet M, Chiquet-Ehrismann R. Interfering with the connection between the nucleus and the cytoskeleton affects nuclear rotation, mechanotransduction and myogenesis. Int J Biochem Cell Biol 2010; 42: 1717–1728. [DOI] [PubMed] [Google Scholar]
- 48.Procter DJ, Banerjee A, Nukui M, Kruse K, Gaponenko V, Murphy EA, Komarova Y, Walsh D. The HCMV assembly compartment is a dynamic golgi-derived MTOC that controls nuclear rotation and virus spread. Dev Cell 2018; 45: 83–100.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gerashchenko MV, Chernoivanenko IS, Moldaver MV, Minin AA. Dynein is a motor for nuclear rotation while vimentin IFs is a “brake”. Cell Biol Int 2009; 33: 1057–1064. [DOI] [PubMed] [Google Scholar]
- 50.Ji JY, Lee RT, Vergnes L, Fong LG, Stewart CL, Reue K, Young SG, Zhang Q, Shanahan CM, Lammerding J. Cell nuclei spin in the absence of lamin b1. J Biol Chem 2007; 282: 20015–20026. [DOI] [PubMed] [Google Scholar]