Graphical abstract
Key words: Respiratory tract infection/testing, Respiratory tract infection/diagnostic, Respiratory tract infection/self-collect, COVID-19, Emergency department
Abstract
Background
Nurses are the primary clinicians who collect specimens for respiratory tract infection testing. The specimen collection procedure is time and resource-consuming, but more importantly, it places nurses at risk for potential infection. The practice of allowing patients to self-collect their diagnostic specimens may provide an alternative testing model for the current COVID-19 outbreaks. The objective of this paper was to evaluate the accuracy and patient perception of self-collected specimens for respiratory tract infection diagnostics.
Methods
A concise clinical review of the recently published literature was conducted.
Results
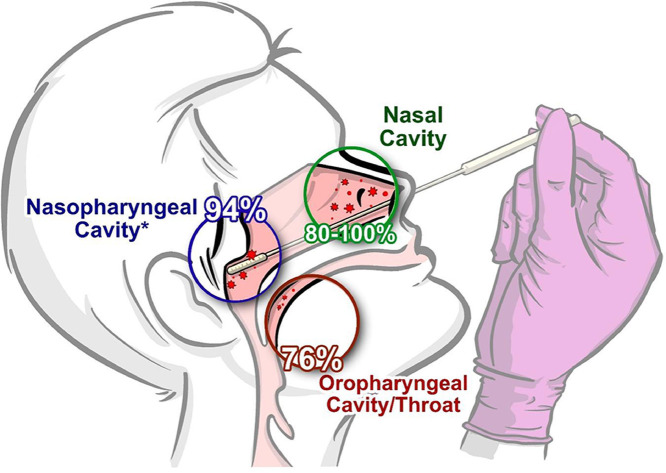
A total of 11 articles were included the review synthesis. The concept of self-collected specimens has a high patient acceptance rate of 83-99%. Self-collected nasal-swab specimens demonstrated strong diagnostic fidelity for respiratory tract infections with a sensitivity between 80-100%, this is higher than the 76% sensitivity observed with self-collected throat specimens. In a comparative study evaluating a professionally collected to a self-collected specimen for COVID-19 testing, a high degree of agreement (k = 0.89) was observed between the two methods.
Conclusion
As we continue to explore for testing models to combat the COVID-19 pandemic, self-collected specimens is a practical alternative to nurse specimen collection.
Introduction
Respiratory tract infections (RTIs) are prevalent communicable diseases and are the third leading cause of death worldwide.1 , 2 It is estimated that a new infectious disease emerges at a rate of one per year,3 making early disease detection critically important. As witnessed during the 2009 H1N1 outbreak and the 2020-2021 coronavirus disease 2019 (COVID-19) pandemic, emergency departments across the United States experienced surges in RTI presentations.4 Unanticipated swells in the patient census often result in downstream adverse effects on clinical operations, particularly to the nursing workforce.5 As the patient census increased so did the need for additional nursing coverage. Early diagnosis of RTIs is essential to the management of these patients as it can expedite decision points such as treatment, disposition, and containment. Furthermore, early diagnosis may aid patients with selecting the proper health care channel for their illness, potentially alleviating the problem of ED crowding. In this study, we explored the accuracy of self-collected specimens for RTI testing, the patient's perception of a self-collection model, and its potential role in the emergency department's clinical operations. For this article, the term COVID-19 was used to refer to both the virus (severe acute respiratory syndrome coronavirus 2 [SARS-CoV-2]) and the disease state (COVID-19).
Background
On March 11, 2020, just more than 2 months after the first confirmed case in China, the World Health Organization declared the COVID-19 outbreak a global pandemic. Delays in containment efforts, fueled by personnel and supply shortages, allowed millions to become infected with COVID-19.6, 7, 8, 9, 10 Nurses have been essential in the efforts to minimize the spread of COVID-19. In 2 studies evaluating nurse staffing models during the pandemic, facilities with higher staffing allocations for nurses experienced lower rates of COVID-19 infections and deaths.11 , 12 For this reason, it is important to develop strategies to safeguard the nursing workforce against the risk of infection, and one such strategy may be the implementation of a self-collection model for respiratory pathogens. In a self-collection model, patients swab themselves to procure the needed specimens for testing.
The emergency department has traditionally served as an access point for patients with acute RTIs, many of whom are likely to receive testing by means of a nasopharyngeal swab administered by a nurse. The Centers for Disease Control and Prevention endorsed in 2020 the nasopharyngeal swab as the preferred specimen collection method for COVID-19, with preliminary data suggesting higher viral concentration in the nasal and nasopharyngeal cavities.13 , 14 The nasopharyngeal swab procedure presents a considerable infection risk to the nurse owing to their proximity to the patient and the swab's propensity to induce sneezing or coughing.15 , 16
To further exacerbate the problem, mass testing initiatives for COVID-19 have been hampered by supply shortages such as the personal protective equipment needed to keep nurses safe.7 , 17 More importantly, an ease of community access to test sites has proven difficult16 as patients’ ability to use testing sites may be limited by a lack of transportation or the site's hours of operation. A potential solution to this problem is to offer an alternative testing option such as a self-collection model. In a community-based survey study by Hall et al,8 as many as 88% of participants reported a willingness to self-collect specimens. Self-collection diagnostic research has proven promising in the area of self-collected specimens for sexually transmitted infections. The implementation of a self-collection model for RTIs may alleviate unnecessary pressure on critical resource chains while improving community access to testing.18 In general, it is also felt that self-collection diagnostics have the potential for economic savings, with a self-collection model projected to be 5 times more cost-efficient than a professionally collected model.19
Before implementing a self-collection model for RTIs, it is important to determine the diagnostic accuracy of self-collected specimens. Misdiagnosis of COVID-19 could lead to the reintroduction of infected individuals back into the general population as seen in transmission cases in long-term care facilities.20 False-negative results could also lead to complacency when caring for patients with COVID-19 symptoms, and additional confirmatory testing such as chest computed tomography imaging21 can significantly increase the patient's ED length of stay and health care cost.
Although the self-collection research for respiratory viruses has been somewhat inconsistent,22 , 23 the results are promising, nonetheless. Studies evaluating alternative collection techniques such as the nasal or oropharyngeal swab methods demonstrated similar diagnostic outcomes to the nasopharyngeal swab but with stronger patient acceptance.7 , 19 Furthermore, in their recent update, the Centers for Disease Control and Prevention endorsed in 2020 both the nasal and oropharyngeal swab methods as acceptable sources for COVID-19 polymerase chain reaction (PCR) testing. Providing patients with alternative testing options should result in higher testing rates.
The objective of this article was to conduct a concise clinical review of the recently published literature to evaluate the accuracy and acceptance of self-collected specimens for RTI diagnosis. A meta-analysis of self-collected specimens for influenza diagnosis was published by Seaman et al19 as a comprehensive review of articles published between 2009 and 2017. Given the current COVID-19 pandemic, we reviewed more recent literature to explore the potential of a self-collection model for COVID-19 testing.
Methods
A literature search on the topic of self-collected specimens for RTI diagnostics was conducted, including articles from 2017 to September 1, 2020. Although our project was intended as a rapid, concise clinical review to inform practice and not meant to function as a full systematic review or meta-analysis of the literature, the Preferred Reporting Items for Systematic Reviews and Meta-Analyses 2009 guidelines24 were used to provide overall structure to the review process.
SEARCH PARAMETERS
The following search parameters were used to search PubMed/Medline, Scopus, and Embase databases: (influenza OR virus OR COVID-19 OR SARS-CoV-2) AND self-collect; (influenza OR virus OR COVID-19 OR SARS-CoV-2) AND self-collected; (influenza OR virus OR COVID-19 OR SARS-CoV-2) AND self-collection; (influenza OR virus OR COVID-19 OR SARS-CoV-2) AND patient-collected; and (influenza OR virus OR COVID-19 OR SARS-CoV-2) AND self-swab.
ELIGIBILITY CRITERIA
Search results were filtered to include only clinical trials, meta-analyses, randomized controlled trials, reviews, and systematic review–type articles. Results were also filtered to include only articles published between 2017 and September 1, 2020. All article titles were reviewed using a key word search to determine topic relevance. The key words included: respiratory tract infection, virus, influenza, COVID-19, SARS-CoV-2, self-collect, and self-swab. Articles with one or more of these words in their title progressed to a secondary screening in which the article titles and abstracts were reviewed for topic relevance.
SYNTHESIS
The level of evidence was assigned to each manuscript using the Johns Hopkins Nursing Evidence-Based Practice criteria.25 A concise summary of the findings of each study was synthesized in a table and a narrative.
Results
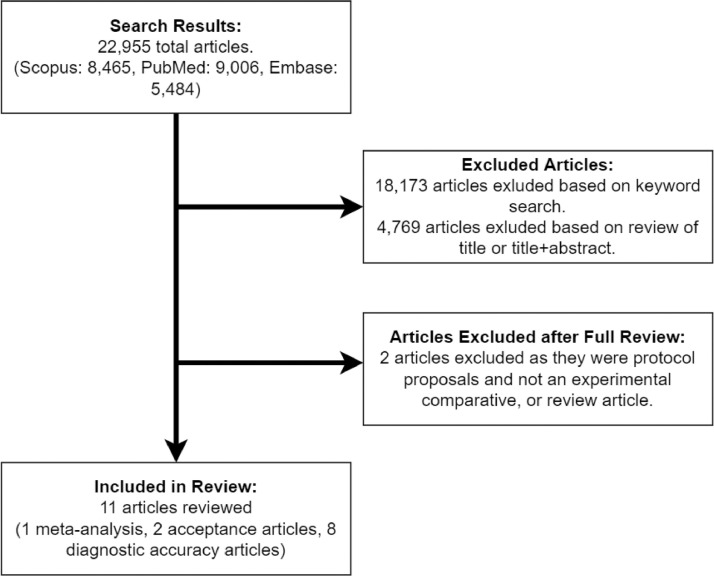
The literature search yielded a total of 22 955 articles across all databases (Scopus: 8465; PubMed: 9006; Embase: 5484). Using a key word search, it was determined 4782 had one or more key words related to the topic of interest. After reviewing each article's title alone or title and abstract, along with the removal of duplicated results, 13 articles were determined relevant to the topic of RTI self-collection research. Note that one of these articles was added during manuscript review and was originally missed using our methods owing to corrupted text in the title field in the downloaded file. Two additional articles were also removed after determining that they were protocol proposals and did not include any diagnostic or comparative data, resulting in 11 reviewable articles (Figure). Each article's level of evidence is presented in the evidence summary table (Table ).25 Of the articles included in this review, 1 was a meta-analysis, 2 studied the acceptance of self-collection by patients, and 8 articles evaluated the diagnostic accuracy of self-collected specimens either as a sole variable or in comparison with professionally collected specimens.
FIGURE.
PRISMA flowchart of literature search process and results. Search parameters yielded 22 995 articles, only 11 included in review synthesis based on topic relevance. PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
TABLE.
Level of evidence and results summary for the reviewed articles.*
| Authors | Level of Evidence | Results Summary | Study Location |
|---|---|---|---|
| Hall et al8 | IIIb | 1435 participants surveyed regarding self-collection of specimens for COVID-19 research. 88% reported high acceptance of saliva self-swab, while 83% reported high acceptance of a self-collected throat swab. Home self-collection was preferred over drive-through or clinic-based collection. | United States of America |
| Valentine-Graves et al9 | IIb | 148 participants surveyed regarding willingness to self-collect for COVID-19 testing, 84% reported high acceptance of a self-collection mail in testing model. 87% reported "confident" to "very confident" in their ability to collect an adequate specimen for testing. | United States of America |
| Adeniji17 | IIIc | Data from this literature review demonstrated self-collected specimens are equally as adequate as professionally collected specimens for respiratory tract infection testing. | South Africa |
| Tenover et al27 | IIIc | 135 self-collected specimens were mailed in for testing, 23% of these specimens had one or more packing or shipping errors. A comparative study evaluating the results of self-collected and professionally collected specimens demonstrated 95% agreement between the two collection methods with 53% of participants preferring the self-collection method. | United States of America |
| Wehrhahn et al18 | IIb | 236 participants, each with specimens collected by self-collection and professional collection. Both samples were evaluated for SARS-CoV-2 and other respiratory pathogens. The self-collected and professionally collected specimens demonstrated a high degree of agreement with a k = 0.89. | Australia |
| Seaman et al19 | IIa | A meta-analysis of 14 studies comparing self-collected with professionally collected specimens when testing for influenza. When compared to professionally collected specimens, self-collection had a pooled sensitivity of 87% and a specificity of 99%. | Australia |
| Altamirano et al23 | IIb | 30 participants, each providing 3 specimens (self-collected nasal swab, professionally collected nasal swab, and professionally collected oropharyngeal swab) for SARS-CoV-2 testing. The sensitivity and specificity of the self-collected specimens were 100% and 95%, respectively. | United States of America |
| Haussig et al26 | IIb | 102 participants provided 225 self-collected swabs. 100% of the swabs tested positive for c-myc DNA, suggesting specimen adequacy. 53% of the specimens tested positive for one or more viral pathogen(s). | Germany |
| Goyal et al29 | IIb | 235 participants enrolled into a two-arm comparison study (community-108 or clinic-based-127). Self-collected nasal swabs had a sensitivity of 88% when compared with a professionally collected nasal swab. When compared with a professionally collected nasopharyngeal swab, self-collected nasal swabs had a sensitivity of 78%. The specificity was 100% for both methods. 99% of participants reported acceptance of the self-collected nasal swab method. | Thailand |
| Fisher et al28 | IIb | 63 participants provided 115 paired self-collected nasal and throat swabs. The sensitivity of the self-collected nasal swab was 96%, while the self-collected throat swab was 76%. Self-collected nasal swabs also had a lower median CT value when compared to self-collected throat swabs (25 vs 32). | United States of America |
| McCulloch et al30 | IIb | 185 participants each provided a self-collected nasal swab and professionally collected nasopharyngeal swab for SARS-CoV-2 testing. When compared with a professionally collected nasopharyngeal swab, the self-collected nasal swab had a sensitivity of 80% and a specificity of 98%. A high degree of agreement was observed with a k = 0.81. | United States of America |
Johns Hopkins Nursing Evidence-Based Practice criteria. CT, cycle threshold.
PATIENT PERCEPTION OF A SELF-COLLECTION MODEL
The acceptance of a self-collection model is important to pragmatic implementation in clinical practice. Research by teams Hall et al8 and Valentine-Graves et al9 provided some insight on patients’ perception of various self-collection methods for respiratory pathogens. According to data from Hall et al,8 1435 participants were surveyed with most (88%) rating in favor (agree or strongly agree) of a self-collected saliva specimen and an 83% acceptance rate for self-collected throat specimens. In a similar study conducted by Valentine-Graves et al,9 148 participants were surveyed regarding their perception of 3 mail-in self-collection methods (saliva, oropharyngeal swab, and dried blood spot card) with 84% of the participants reporting high acceptance of all 3 methods. Similar acceptance was seen in another study of adults and children with both cohorts, respectively, reporting 99% and 96% acceptance of a self-collection model.26 Valentine-Graves et al8 also asked the study participants to rate their confidence level regarding the integrity of their collected specimen with 87% reporting “confident” or “very confident.” Data from these studies provide a better understanding of the patient's willingness to not only self-collect for respiratory pathogens but also their acceptance of a distance testing model.
Critics of the self-collection model have cited collection errors by the patient as a potential barrier to a successful implementation. As reported in 1 study, approximately 24% of mail-in specimens had one or more errors related to packaging and shipping.27 In the same study, only 37 of 124 (30%) participants reported reviewing the instructional material before proceeding with the self-collection procedure. In a qualitative survey study assessing patients’ perception of a self-collection model, most of the dissatisfied comments pertained to unclear collection instructions or overly complicated collection kits.9 Despite the collection errors, the submitted specimens were still adequate for PCR testing. Nevertheless, these studies demonstrated the potential for patient errors that could translate to lower compliance rates or errors in the downstream diagnostic results.
SELF-COLLECTION DIAGNOSTIC ACCURACY
In the meta-analysis conducted by Seaman et al,19 13 articles on self-collected respiratory pathogens were reviewed to evaluate the diagnostic accuracy of self-collected specimens. When compared with a professionally collected nasal swab, self-collected nasal swabs had a pooled diagnostic sensitivity of 87% (95% CI, 80%-92%) and a specificity of 99% (95% CI, 98%-100%). Seaman et al19 also reported high acceptance of self-collected nasal swabs by patients.
In a study conducted by Fisher et al,28 self-collected nasal swabs and self-collected throat swabs by individuals with RTI symptoms showed a sensitivity of 96% (95% CI, 88%-99%) and 76% (95% CI, 65%-85%), respectively. These data are consistent with findings from a 3-arm (self-collected nasal swab vs professionally collected nasal swab and professionally collected oropharyngeal swab) study that evaluated self-collected nasal swab for COVID-19 testing, with a sensitivity of 100% (95% CI, 72%-100%) and specificity of 95% (95% CI, 74%-100%).23
In a comparative study conducted by Goyal et al,29 the acceptance rate and diagnostic accuracy of self-collected versus professionally collected specimens were evaluated in geriatric patients with RTI symptoms. Participants in the first cohort were asked to provide a self-collected nasal swab specimen at the onset of their symptoms, whereas the second cohort had 3 swabs (self-collected nasal swab, professionally collected nasal swab, and professionally collected nasopharyngeal swab) collected at the presentation to a geriatric clinic for their symptoms. All subjects were asked to rate their acceptance of the self-collected and professionally collected methods. Of the 235 participants, 99% reported that the self-collection method was acceptable and easy to perform. In the community cohort, 92% of the self-collected specimens tested positive for ribonuclease P, indicating it was an adequate specimen, whereas 99% of the clinic-based specimens were positive for ribonuclease P. The sensitivity of self-collected nasal swabs, when compared with professionally collected nasal swabs, was 88% (95% CI, 40%-100%), whereas self-collected nasal swabs versus professionally collected nasopharyngeal swabs had a sensitivity of 78% (95% CI, 40%-97%).29 Despite demonstrating a consistently higher sensitivity for respiratory pathogens, there were no significant differences between a nasopharyngeal swab (94%) and a nasal swab (89%).16 , 29 The sensitivity rate between a self-collected nasal swab and a professionally collected nasal swab was also not statistically significant.16 , 29 These data are consistent with another comparative study (self-collected vs professionally collected) by McCulloch et al30 in which the sensitivity and specificity of a self-collected nasal specimen were 80% (95% CI, 63%-91%) and 98% (95% CI, 94%-100%), respectively.
The 2 remaining comparative studies evaluated the diagnostic accuracy of self-collected specimens but implemented descriptive and Cohen's kappa statistics to report their findings. Haussig et al26 enrolled participants in a longitudinal study looking at self-collected respiratory specimens collected at the onset of symptoms. Participants were asked to self-collect nasal swab specimens and mail them in for testing. Of the 225 swabs received, 151 participants reported symptoms consistent with an RTI and had an overall 71% positive rate for 1 or more respiratory pathogen. By contrast, the asymptomatic cohort (58) only had a 14% positive rate for respiratory pathogens.26 In the Wehrhahn et al18 article, the diagnostic accuracy of self-collected specimens for COVID-19 testing was compared with professionally collected specimens. Using Cohen's kappa statistics, the authors found that self-collected specimens had a high agreement (κ = 0.89) with professionally collected specimens.18 In another study comparing self-collected with professionally collected specimens, there was also high agreement (95%) between the 2 collection methods when testing for influenza.27
To quantify specimen quality, cycle threshold (CT) values were collected in some of the reviewed studies. The CT value is the threshold in which the fluorescent signal used in PCR testing is able to detect the target gene of interest. In general, lower CT values (≤29) equate to higher concentrations of nucleic acid in the test specimen. The CT values from 2 studies showed consistent readings for self-collected specimens and professionally collected specimens,18 with a correlation coefficient of 0.81, P < .001.30 Another study showed the median CT values for self-collected nasal swabs (25) being consistently lower than self-collected throat swabs (32) when the data were aggregated from 8 different viral tests, suggesting a higher viral concentration with nasal swabs.28
Discussion
The diagnostic accuracy of self-collected respiratory specimens has received a lot of attention within the past decade of research, but the recent global pandemic has made it a priority to reevaluate self-collection as a viable alternative testing model. Self-collected specimens have shown similar diagnostic accuracy to professionally collected specimens while garnering higher patient acceptance.
The COVID-19 pandemic has become a world-changing event and has highlighted a grave need for a global reevaluation of our approach to managing epidemic or pandemic scale outbreaks. Delays in our testing initiatives allowed the disease to spread rapidly across borders, infecting millions, and resulting in global economic hardship.31 Despite efforts to contain the disease, infection and death rates continue to rise. Many health facilities are forced to operate at critical mass despite personnel and supply shortages.
A self-collection model is a logical shift in the testing paradigm. As demonstrated, patients are very accepting of the self-collection concept8 , 9 , 19 , 32 and have shown that they can collect reliable specimens.18 , 28 The diagnostic sensitivity and specificity for self-collected specimens have been largely consistent with professionally collected specimens when testing for RTIs,16 , 19 , 23 , 28 , 29 with similar results observed for COVID-19 testing.18 , 23
Limitations
We must acknowledge the limitations in our review findings and the potential barriers to a successful implementation of a self-collection model. Patients have openly admitted to not reviewing the instructional material included in the self-collection kits, potentially resulting in collection or packaging errors.27 In addition, reliance on a courier service to collect specimens may not be a cost-effective means of gathering specimens, particularly if an ad hoc approach is implemented.
The research team followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines when developing our search parameters. However, our search parameters were not registered on a prospective register such as Prospero, limiting the repeatability of our study design. We recognize the potential for missed literature as our search yielded a small collection of articles. Most of the studies in this literature review were pilots or feasibility studies with small sample sizes, resulting in generally wider CIs. An additional limitation of findings reported in this review is the lack of a gold standard when comparing the sensitivities of self- and professionally collected specimens. Therefore, this could result in a compounding effect leading to an overestimate of the true sensitivity of the test for the disease. Each study implemented varied collection methods, specimen sites, and onset of symptom windows—all critical factors in determining specimen quality and diagnostic outcomes.29 The articles reviewed included a wide distribution of studies across multiple nations with differing cultural preferences and resource systems. It is important to consider these variables when trying to generalize the findings.
Implications for Emergency Nursing Practice
Emergency departments have experienced significant surges in their patient census since the COVID-19 pandemic began, and these fluctuations have proven taxing to the nursing discipline, including nurses who are working additional and longer shifts.33 , 34 The implementation of a self-collection model for RTIs can offset the burden of specimen procurement from the nursing staff while mitigating their infection risk. By allowing patients to collect their own specimens, whether for home testing or in an emergency department, nurses are freed to prioritize their efforts to other tasks, such as caring for the critically ill. Furthermore, providing patients with the means to confirm their diagnosis before engaging with the health care system could significantly improve their length of stay in the emergency department. Alternatively, a prehospital diagnosis could prove valuable for emergency departments with an established telemedicine infrastructure to care for patients with lower acuity symptoms. More importantly, patients who were previously unable or unwilling to access conventional testing sites now have an alternative testing option. Information from a home test kit could also aid patients in making better-informed decisions regarding the proper use of health care channels. The benefits of a self-collection model also include potential economic savings as it reduces our reliance on costly personal protective equipment and the personnel needed to staff testing sites. These are all important variables for future pandemic planning.
Conclusion
Nurses are the primary clinicians who collect respiratory specimens, potentially placing nurses at risk for infection. Nurses have also been extracted from their home departments to staff testing facilities during the pandemic, further exacerbating the nursing shortage. As we continue to explore for alternative testing models to combat the COVID-19 pandemic, a self-collection model is a practical option. The reallocation of this task to the patient has the potential for cost savings but more importantly, improved patient and nursing satisfaction.
Author Disclosures
Conflicts of interest: none to report.
This project was funded by the University of Nebraska Medical Center – COVID Rapid Response Grant.
Biographies
Thang T. Nguyen is Instructor, University of Nebraska Medical Center - Department of Emergency Medicine, Omaha, NE. ORCID identifier: https://orcid.org/0000-0002-9071-7771
Wesley G. Zeger is Associate Professor, University of Nebraska Medical Center - Department of Emergency Medicine, Omaha, NE.
Michael C. Wadman is Professor, University of Nebraska Medical Center - Department of Emergency Medicine, Omaha, NE.
Aaron N. Barksdale is Associate Professor, University of Nebraska Medical Center - Department of Emergency Medicine, Omaha, NE.
Footnotes
Section Editor: Amber Adams, DNP, RN, CEN
For presubmission guidance, please contact Amber Adams, DNP, RN, CEN at: bcamber19@gmail.com. Submit a manuscript directly to JEN.
REFERENCES
- 1.Brendish JN, Malachira KA, Armstrong L. Routine molecular point-of-care testing for respiratory viruses in adults presenting to hospital with acute respiratory illness (ResPOC): a pragmatic, open-label, randomised controlled trial. Lancet Respir Med. 2017;5(5):401–411. doi: 10.1016/S2213-2600(17)30120-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zumla A, Al-Tawfiq A, Enne VI. Rapid point of care diagnostic tests for viral and bacterial respiratory tract infections—needs, advances, and future prospects. Lancet Infect Dis. 2014;14(11):1123–1135. doi: 10.1016/S1473-3099(14)70827-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abad X. Biocontainment in low income countries: a short discussion. Med Saf Glob Health. 2018;7(1):1–3. doi: 10.4172/2574-0407.1000139. [DOI] [Google Scholar]
- 4.Sugerman D, Nadeau HK, Lafond K. A survey of emergency department 2009 pandemic influenza A (H1N1) surge preparedness–Alanta, Georgia, July–October 2009. Clin Infect Dis. 2011;11(suppl 1):177–182. doi: 10.1093/cid/ciq035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wong EL, Wong SY, Lee N, Cheung A, Griffiths S. Healthcare worker's duty concerns of working in the isolation ward during the novel H1N1 pandemic. J Clin Nurs. 2011;21(9-10):1466–1475. doi: 10.1111/j.1365-2702.2011.03783.x. [DOI] [PubMed] [Google Scholar]
- 6.Cucinotta D, Vanelli M. WHO declares COVID-19 a pandemic. Acta Biomed. 2020;91(1):157–160. doi: 10.23750/abm.v91i1.9397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Siegler JA, Sullivan SP, Sanchez T. Protocol for a national probability survey using home specimen collection methods to assess prevalence and incidence of SARS-CoV-2 infection and antibody responses. Ann Epidemiol. 2020;49:50–60. doi: 10.1016/j.annepidem.2020.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hall WE, Luisi N, Zlotorzynska M. Willingness to use home collection methods to provide specimens for SARS-CoV-2/COVID-19 research: survey study. J Med Internet Res. 2020;22(9):1–13. doi: 10.2196/19471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Valentine-Graves M, Hall E, Guest J. At-home self-collection of saliva, oropharyngeal swabs and dried blood spots for SARS-CoV-2 diagnosis and serology: post-collection acceptability of specimen collection process ad patient confidence in specimens. PLoS One. 2020;15 doi: 10.1371/journal.pone.0236775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Livingston E, Desai A, Berkwits M. Sourcing personal protective equipment during the COVID-19 pandemic. J Am Med Assoc. 2020;323(19):1912–1914. doi: 10.1001/jama.2020.5317. [DOI] [PubMed] [Google Scholar]
- 11.Gorges RJ, Konetzka RT. Staffing levels and COVID-19 cases and outbreaks in the U.S. nursing homes. J Am Geriatr Soc. 2020;68(11):2462–2466. doi: 10.1111/jgs.16787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Szczerbinska K. Could we have done better with COVID-19 in nursing homes? Eur Geriatr Med. 2020;11(4):639–643. doi: 10.1007/s41999-020-00362-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Centers for Disease Control and Prevention. Interim guidelines for collecting, handling of clinical specimens for COVID-19 testing. Published 2020. Accessed August 16, 2020. https://www.cdc.gov/coronavirus/2019-ncov/lab/guidelines-clinical-specimens.html
- 14.Zou L, Ruan F, Huang M. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N Engl J Med. 2020;382(12):1177–1179. doi: 10.1056/NEJMc2001737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.To KK, Tsang OT, Leung WS. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study. Lancet Infect Dis. 2020;20(5):565–574. doi: 10.1016/S1473-3099(20)30196-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Black JR, Bailey C, Przewrocka J, Dijkstra KK, Swanton C. COVID-19: the case for health-care worker screening to prevent hospital transmission. Lancet. 2020;395(10234):1418–1420. doi: 10.1016/S0140-6736(20)30917-X. Published correction appears in Lancet. 2020;395(10234):1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Adeniji AA. Self-collected upper respiratory tract swabs for COVID-19 test: a feasible way to increase overall testing rate and conserve resources in South Africa. Afr J Prim Health Care Fam Med. 2020;12(1):e1–e4. doi: 10.4102/phcfm.v12i1.2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wehrhahn CM, Robson J, Brown S. Self-collection: an appropriate alternative during the SARS-CoV-2 pandemic. J Clin Virol. 2020;128:1–5. doi: 10.1016/j.jcv.2020.104417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seaman CP, Tran LLT, Cowling BJ, Sullivan SG. Self-collected compared with professional-collected swabbing in the diagnosis of influenza in symptomatic individuals: a meta-analysis and assessment of validity. J Clin Virol. 2019;118:28–35. doi: 10.1016/j.jcv.2019.07.010. [DOI] [PubMed] [Google Scholar]
- 20.Kimball A, Hatfield MK, Arons M. Asymptomatic and presymptomatic SARS-CoV-2 infections in residents of a long-term care skilled nursing facility – King County, Washington. MMWR Morb Mortal Wkly Rep. 2020;69(13):337–381. doi: 10.15585/mmwr.mm6913e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Winichakoon P, Chaiwarith R, Liwsrisakun C. Negative nasopharyngeal and oropharyngeal swabs do not rule out COVID-19. J Clin Microbiol. 2020;58(5):e00297–20. doi: 10.1128/JCM.00297-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dhiman N, Miller RM, Finley JL. Effectiveness of patient-collected swabs for influenza testing. Mayo Clin Proc. 2012;87(6):548–554. doi: 10.1016/j.mayocp.2012.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Altamirano J, Govindarajan P, Blomkalns AL. Assessment of sensitivity and specificity of patient-collected lower nasal specimens for severe acute respiratory syndrome coronavirus 2 testing. JAMA Netw Open. 2020;3(6) doi: 10.1001/jamanetworkopen.2020.12005. Published correction appears in JAMA Netw Open. 2020;3(7):e2014910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moher D, Liberati A, Tetzlaf J, Altman DG, PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009;6(7) doi: 10.1371/journal.pmed1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dang D, Dearholt SL. 3rd ed. Sigma Theta Tau International; 2018. Johns Hopkins Nursing Evidence-Based Practice: Model & Guidelines.https://libguides.ohsu.edu/ebptoolkit/levelsofevidence Accessed January 30, 2021. [Google Scholar]
- 26.Haussig JM, Targosz A, Engelhart S. Feasibility study for the use of self-collected nasal swabs to identify pathogens among participants of a population-based surveillance system for acute respiratory infections (GrippWeb-Plus) – Germany, 2016. Influ Other Respir Viruses. 2018;13(4):319–330. doi: 10.1111/irv.12644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tenover FC, Jo Baron E, Gaydos CA. Self-collected specimens for infectious diseases testing. Clin Microbiol. 2017;7(39):51–56. doi: 10.1016/j.clinmicnews.2017.03.004. [DOI] [Google Scholar]
- 28.Fisher EC, Boeckh M, Jerome RK, Englund J, Kuypers J. Evaluating addition of self-collected throat swabs to nasal swabs for respiratory virus detection. J Clin Virol. 2019;115:43–46. doi: 10.1016/j.jcv.2019.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goyal S, Prasert K, Praphasiri P. The acceptability and validity of self-collected nasal swabs for detection of influenza virus infection among older adults in Thailand. Influenza Other Respir Viruses. 2017;11(5):412–417. doi: 10.1111/irv.12471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McCulloch DJ, Kim AE, Wilcox NC. Comparison of unsupervised home self-collected midnasal swabs with clinician-collected nasopharyngeal swabs for detection of SARS-CoV-2 infection. JAMA Netw Open. 2020;3(7) doi: 10.1001/jamanetworkopen.2020.16382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baldwin R, Weder di Mauro B. Centre for Economic Policy Research; 2020. Economics in the Time of COVID-19. [Google Scholar]
- 32.Valentine-Graves M, Hall E, Guest J, Adam E, Valencia R, Hardee I. At-home self-collection of saliva, oropharyngeal swabs and dried blood spots for SARS-CoV-2 diagnosis and serology: post-collection acceptability of specimen collection process ad patient confidence in specimens. Plos One. Published online August 5, 2020 doi: 10.1371/journal.pone.0236775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.García-Martín M, Roman P, Rodriguez-Arrastia M, Diaz-Cortes MDM, Soriano-Martin PJ, Ropero-Padilla C. Novice nurse's transitioning to emergency nurse during COVID-19 pandemic: a qualitative study. J Nurs Manag. 2021;29(2):258–267. doi: 10.1111/jonm.13148. [DOI] [PubMed] [Google Scholar]
- 34.Bernstein L. Some places were short on nurses before the virus. The pandemic is making it much worse. The Washington Post. Published 2020. Accessed February 3, 2021. https://www.washingtonpost.com/health/some-places-were-short-on-nurses-before-the-virus-the-pandemic-is-making-it-much-worse/2020/11/16/8d3755a0-25c4-11eb-a688-5298ad5d580a_story.html