Abstract
Background:
Decreased peripheral lymphocyte counts are associated with outcome after RT in several solid tumors, though appear late during or after the radiation course and often correlate with other clinical factors. Here we investigate if absolute lymphocyte counts (ALC) are independently associated with recurrence in pediatric medulloblastoma early during RT.
Methods:
We assessed 202 medulloblastoma patients treated between 2000 and 2016 and analyzed ALC throughout therapy, focusing on both early markers (ALC during week 1 – ALCwk1; grade 3+ Lymphopenia during week 2 – Lymphopeniawk2) and late markers (ALC nadir). Uni- and multivariable regressions were used to assess association of clinical and treatment variables with ALC and of ALC with recurrence.
Results:
Thirty-six recurrences were observed, with a median time to recurrence of 1.6 years (Range 0.2–10.3) and 7.1 years median follow-up. ALC during RT was associated with induction chemotherapy (p<0.001), concurrent carboplatin (p=0.009), age (p=0.01) and high-risk status (p=0.05). On univariable analysis, high-risk disease (HR 2.0[1.06–3.9],p=0.03) and M stage≥1 (HR 2.2[1.1–4.4]) were associated with recurrence risk, as was lower ALC early during RT (ALCwk1 HR 0.28[0.12–0.65],p=0.003; Lymphopeniawk2 HR 2.27[1.1–4.6],p=0.02). Neither baseline ALC nor nadir correlated with outcome. These associations persisted when excluding carboplatin and pre-RT chemotherapy patients, and in the multivariable analysis accounting for confounders lymphocyte counts remain significant (ALCwk1 HR 0.23[0.09–0.57],p=0.002; Lymphopeniawk2 HR 2.3[1.1–4.8],p=0.03).
Conclusion:
ALC during weeks 1 and 2 of RT was associated with recurrence and low ALC is an independent prognostic factor in medulloblastoma. Strategies to mitigate the risk of radiation-induced lymphopenia should be considered.
Keywords: medulloblastoma, lymphocyte counts, radiotherapy, recurrence risk, biomarker
Introduction
Medulloblastoma is the most common malignant pediatric brain tumor, occurring in patients from early age into adulthood with a peak in the range 3–6 years1,2. Current standard treatment entails surgery, typically followed by craniospinal irradiation (CSI) with a higher dose to the tumor bed and adjuvant chemotherapy. Five-year disease control for patients with standard risk (SR) disease is approximately 80% and 60–70% for patients with high risk (HR) disease3–5.
Patients over 3 years of age are stratified as SR or HR based on extent of postoperative disease, presence of disseminated disease (M stage), and histology. Management of SR patient includes 23.4 Gy CSI with a 54 Gy boost to the tumor bed, followed by adjuvant chemotherapy. For high-risk patients or those presenting with HR features, intensification of therapy can encompass CSI escalation to 36 Gy, boosts to bulky metastatic disease and use of concurrent carboplatin during RT. If medulloblastoma recurs after CSI the prognosis is very poor, with 5-year survival <10%, even for patients initially classified as SR6. This means even minor changes to the multi-modality approach can lead to large benefits to patients, either by increasing disease control or reducing impactful long-term toxicity. A successful example is the reduction of CSI dose for SR patients to 23.4Gy7,8 and the reduction of radiation boost volumes from whole posterior fossa (WPF) to involved field (IF) demonstrated on ACNS03319. However, ACNS0331 also proved that it is not feasible to further decrease the CSI dose from 23.4 to 18 Gy across the board in this patient group, i.e. for SR patients age 3–8 years, without increasing recurrence rates9.
CSI is also associated with considerable hematologic toxicity10,11 because a very high proportion of circulating immune cells in addition to a large volume of marrow are irradiated with each fraction. While the immune-stimulating effects of radiation have been a focus of research since the discovery of potential synergy between radiation and checkpoint inhibition12–14, the immune-suppressive effects of large field radiation, though recognized already in the 1970s15,16, have only lately garnered attention. Recently, lower absolute lymphocyte counts (ALC) during and after RT have been reported to correlate with survival in a range of cancers including adult glioma, liver, pancreas, lung and others17–22. A multitude of studies have shown the importance of lymphocytes, which are the main carriers of cell-mediated immunity, for tumor control after local therapy23–25. They recognize tumor antigens, play key roles in immune recruitment and activation, and most of our immune system’s mechanisms for identifying and removing tumor cells involve lymphocytes 26. However, the exact mechanisms behind the connection between outcomes and radiation-induced lymphopenia are still not well understood 27.
It is possible that immune status before, during, and after RT could be used as a prognostic biomarker in medulloblastoma, similar to recently identified molecular biomarkers that complement known clinical risk factors28 and can be used to group medulloblastoma into molecular subgroups with differing prognosis29. Several ongoing trials integrate these molecular classifications into stratification, offering reduced-dose CSI of 15–18Gy to patients with excellent prognosis (SJMB12 - NCT01878617, PNET5 - NCT02066220, ACNS1422 - NCT02724579). These study designs illustrate how rational biomarker-based risk stratification can be integrated into trial design with the goal of improving outcomes.
The primary objective of this study was to investigate the dynamics of peripheral lymphocyte counts in medulloblastoma patients during RT and determine if they are independently associated with increased recurrence risk. Furthermore, we examined the clinical and treatment-related factors that affect peripheral lymphocyte counts during radiotherapy.
Materials and Methods
Patient cohort
The study population is comprised of patients treated at the Massachusetts General Hospital between 2000 and 2016 with proton radiotherapy or a combination of photon and proton treatment. The majority of patients (153/202) were treated on three large prospective protocols at our institution (NCT01696721, NCT01063114, NCT00105560), and the rest were identified from an institutional database or enrolled on a prospective pediatric radiation registry, (the Pediatric Proton/Photon Consortium Registry, NCT01696721) and blood count data retrospectively collected. This study was approved by an institutional review board. All patients or their parents/guardians provided written informed consent. See Table 1 for a summary of patient characteristics and important clinical features.
Table 1.
Patient characteristics.
| all patients n= 202 | |
|---|---|
| Age, median [IQR] | 8.1 [5.8 – 12.4] |
| Histology | |
| Classic, Desmoplastic, NOS | 164 (81%) |
| Anaplastic, Large Cell | 38 (19%) |
| Risk Stratification | |
| Standard | 140 (69%) |
| High/Intermediate* | 62 (31%) |
| M Stage | |
| M0 | 149 (74%) |
| M1+ | 53 (26%) |
| CSI dose [Gy], median [range] | 23.4 [0 – 39.6] |
| Receiving high dose (≥36Gy) | 45 (22%) |
| RT partly delivered using photons | 34 (17%) |
| Pre-radiation chemotherapy | 36 (18%) |
| Concurrent carboplatin | 19 (9%) |
Intermediate risk was defined as patients with unfavorable histology (anaplastic/large cell) but otherwise standard-risk features. In accordance with the Children’s Oncology Group practice these patients were combined with high-risk patients for statistical analysis.
Treatment & Metrics for Blood Counts
The irradiation volume included CSI with a boost to the tumor bed or WPF per protocol or determined by physician discretion. The tumor bed volume was defined by contouring the resection cavity and any residual disease, expanding volumetrically by 0.8–1.5cm, anatomically confined to the WPF. CSI doses generally ranged from 18 to 36 GyRBE delivered in 1·8 GyRBE fractions, integrated with the boost fields.
For skeletally mature children as assessed by age, height, and bone age, the vertebral bodies were partly spared, i.e. the CSI target volume included the thecal sac, but not the whole vertebral body. A small fraction of patients received concurrent carboplatin (n=19, 9%), induction chemotherapy (n = 36, 18%), or both (n=2, 0.9%), and these variables were included in the multivariable analysis. Chemotherapy was most often given on or according to Children’s Oncology Group protocols30,31 (ACNS0331, ACNS0332 or ACNS0334, see www.childrensoncologygroup.org) or Headstart I, II, or III protocols 32,33. Chemotherapy given prior to radiation treatment was characterized as either high- or standard-dose, with high-dose defined as sufficiently intense to require peripheral stem cell rescue, given to young patients with at least one adverse prognostic factor. Standard-dose chemotherapy consisted of 1–16 weeks of well established agents, i.e. vincristine, cisplatin, and lomustine and/or cyclophosphamide, and was given due to delays in transfer of care to our center. Further detail regarding the treatment have been described previously34. Administration of dexamethasone is standard of practice peri-operatively in the neurosurgical setting. All patients were tapered off of dexamethasone for at least two weeks before radiation start.
Absolute lymphocyte counts were obtained via standard laboratory tests, measured as part of clinical care in counts per volume [109/L, i.e. 103/μL]. During radiotherapy blood draws were obtained at least weekly. The Common Toxicity Criteria for Adverse Events (CTCAE; Version 5.0) was used to grade hematologic toxicities. Grade 3 lymphopenia is defined as <0.5 ·109/L, and grade 4 as <0.2 ·109/L. If lymphocyte counts were measured multiple times in a specific week, the ALC for that week was the average of these measurements throughout the week.
We used three separate metrics to quantify peripheral lymphocyte counts throughout therapy, including two early and one late timepoint. Early timepoints included average lymphocyte counts during week 1 (ALC week 1) (dichotomized at the median) and the incidence of ≥grade 3 lymphopenia in week 2. The rationale behind this was to have one metric that is data driven and one that uses a clinically relevant threshold (grade 3+ lymphopenia). Week 2 was a logical timepoint for this metric, as prevalence of grade 3+ lymphopenia is very low in week 1, and >60% in week 3 (see results for details), so week 2 was the only timepoint where this metric could possibly have discriminatory power for our endpoint. The late endpoint was the ALC nadir, defined as minimum lymphocyte count measured during RT, which has been studied in a similar context in non-small cell lung20 and esophageal cancers21.
Statistical Analysis
Time to recurrence was measured from the start of RT, follow-ups were performed at least yearly and disease status assessed by imaging and clinical examination. We evaluated the association of clinical and treatment variables with our endpoints using multiple linear and logistic regression, applying variable transformations for variance stabilization where necessary. The relevance of prognostic factors for recurrence was evaluated using uni- and multi-variable Cox proportional hazards regression using the score test. Patients without documented recurrence were censored at the date of the last follow-up. One patient died of other causes after more than five years and was included in this analysis, omission of this patient does not change the analyses presented here.
We included all relevant factors possibly affecting outcome or lymphocyte counts: risk group, M stage, age, histology, gross total resection, age, baseline ALC, concurrent carboplatin, induction chemotherapy and CSI dose, see Table 2 and supplementary Table 3 for details. All statistical analyses were performed in R v3.6.135 using the survival package v2.3836., with p values based on a two-sided hypothesis test. CG, TIY, DS and BYY had access to the raw data.
Table 2.
Multivariable Cox regression associating baseline variables and lymphocyte counts with recurrence
| Multivariable Analysis ALC week 1 | Multivariable Analysis Lymphopenia Grade 3+ week 2 | |||
|---|---|---|---|---|
| Variable | HR [95% CI] | P | HR [95% CI] | P |
| Clinical & Baseline Factors | ||||
| High risk | - | - | - | - |
| M1+ | 1.7 [0.46 – 6.5] | 0.41 | 1.8 [0.75 – 4.3] | 0.19 |
| Anaplastic Histology | 1.7 [0.47 – 5.9] | 0.44 | 1.6 [0.72 – 3.5] | 0.25 |
| Gross Total Resection | - | - | - | - |
| Age at RT | - | - | - | - |
| Baseline (above/below median) | - | - | - | - |
| Lymphocyte counts during RT | ||||
| Early - ALC week 1 (above/below median) | 0.24 [0.1 – 0.59] | 0.002 | - | - |
| Early - Lymphopenia Grade 3+ in week 2 | - | - | 2.3 [1.1 – 4.8] | 0.03 |
| Late - ALC nadir (above/below median) | - | - | - | - |
| Treatment Factors | ||||
| Concurrent Carboplatin | 0.62 [0.19 – 2.0] | 0.43 | 0.68 [0.21 – 2.2] | 0.53 |
| Induction Chemotherapy | 0.43 [0.15 – 1.2] | 0.11 | 0.7 [0.26 – 1.9] | 0.47 |
| High CSI dose (≥36Gy) | - | - | - | - |
Results
Lymphocyte dynamics during radiotherapy & risk factors
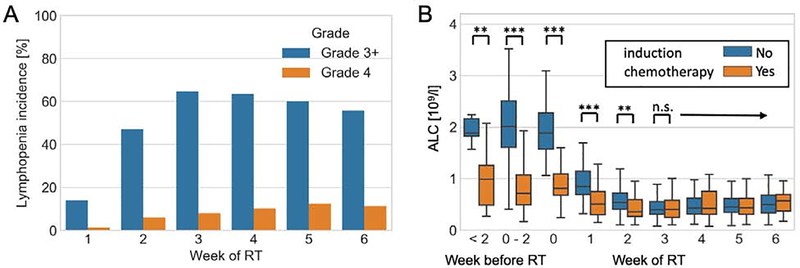
As expected, there was significant depletion in circulating lymphocytes shortly after commencing RT, with a very steep decline in ALC and increase in lymphopenia incidence in the first 3 weeks, leveling off thereafter. Figure 1A shows the incidence of grade 3+ and 4 lymphopenia in our entire patient cohort (n=202); 13% of patients had grade 3+ lymphopenia in the first week of treatment, rising to a peak of 63% at week 3. There were minor differences in dynamics between the patients receiving high vs low CSI doses (see Supplementary Figure 1).
Fig 1.
Lymphocytes counts and lymphopenia before and during RT (A) Lymphopenia incidence during RT (B) Boxplot of ALC stratified by induction chemotherapy, timepoints indicate more than 2 weeks before RT, within 2 weeks, exactly at RT start (week 0), and during RT. **=(p<0.01) ***=(p<0.001) according to two-sided Mann-Whitney U test.
Lymphocyte counts at baseline (defined as ≤2 weeks before RT start) were normally distributed, with median ALC of 1.8 ·109/L (IQR 1.3–2.3). After the start of RT, ALC declined significantly to a median of 1.2 ·109/L (IQR 0.86 – 1.58) during week 1, and then further to a nadir of median 0.32 ·109/L (IQR 0.23 – 0.47), see Supplementary Figure 2 for the ALC distributions throughout RT. The decline in ALC is accompanied by a skewing of the distribution, i.e. in some patients the lymphocyte counts were better maintained throughout RT, leading to a tail extending to the high end of the range (see Supplementary Figure 2).
As expected, induction chemotherapy has a strong effect on the circulating lymphocyte count, see Figure 1B. ALCs of patients who received pre-radiation chemotherapy are significantly different before the start of RT, but show similar declines during RT and at week 3 and thereafter they do not differ anymore from patients who did not receive pre-radiation chemotherapy.
To investigate the risk factors associated with lymphopenia, we performed a multivariable analysis to test association of clinical factors and interventions with our early (ALC during week 1 and lymphopenia grade 3+ in week 2) and late (ALC nadir) endpoints, as defined in the methods (Supplementary Table 1). Lymphocyte nadir in this patient population usually occurred in week 3–5, depending on the CSI dose level (see also Supplementary Figure 1). Induction chemotherapy (p<0.001) and use of concurrent carboplatin (p=0.009) were negatively associated with ALC during week 1; ≥grade 3 lymphopenia risk in week 2 was associated with concurrent carboplatin (p=0.01) and age at RT (p=0.01). Finally, ALC nadir was affected by HR status (p=0.05), but shows a positive correlation with age (p=0.01), for the detailed results see supplementary Table 1. In addition, we investigated the baseline lymphocyte count, see Supplementary Table 2. As expected, the baseline counts were negatively correlated with induction chemotherapy (p<0.001), but also with age (p=0.006).
ALC early during RT correlates with outcome
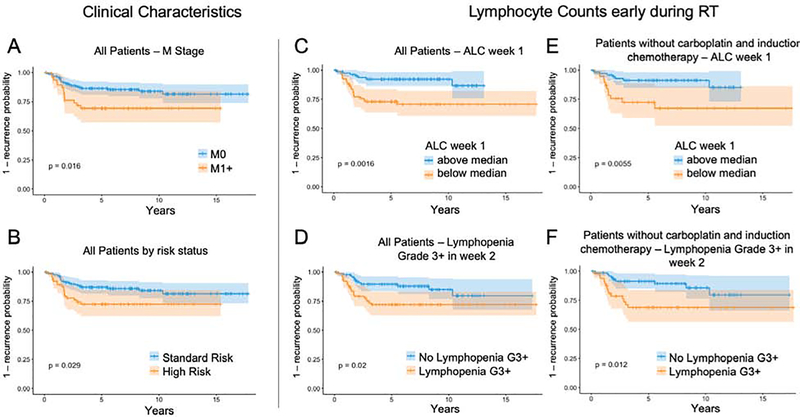
The median time to follow-up in disease-free patients was 7.1 years. Thirty-six patients recurred (18%), with a median time to recurrence of 1.6 years [IQR 1.1 – 2.4 years]. Supplementary Table 3 shows the results of the univariable Cox regression analyses of clinical characteristics, lymphocyte counts and treatment factors. Higher lymphocyte counts early during RT were strongly associated with decreased risk of recurrence, using either lymphopenia grade 3+ in week 2 (2.27 [1.11 – 4.6], p=0.02) or ALC during week 1 (HR 0.28 [0.12 – 0.65], p=0.003) as the metric. These two metrics used for early lymphocyte depletion naturally demonstrate a strong correlation, as shown in Supplementary Figure 3. The association of early lymphocyte depletion (ALC during week 1 / grade 3+ lymphopenia in week 2) with recurrence also persisted when restricting the analysis to patients not receiving induction chemotherapy (161 patients, p=0.002 / 0.02), not receiving concurrent carboplatin (178 patients, p=0.01 / 0.03) or neither (144 patients, p=0.006 / 0.02). Lymphocyte counts at baseline and the ALC nadir did not correlate with outcome. Among other factors only M stage (HR 2.2 [1.1 – 4.4], p=0.02) and high risk group (HR 2.0 [1.06 – 3.9, p=0.03) were associated with an increased risk of recurrence, with histology (HR 1.9 [0.94 – 3.9], p=0.08), age (HR 0.93 [0.86 – 1.00], p=0.06) and high dose CSI (1.86 [0.93 – 3.7], p=0.08) not being clearly associated. Note that the majority of these factors are strongly dependent on each other, i.e. HR being defined by M stage and histology, which in turn is associated with high dose CSI.
This strong association between recurrence and early lymphocyte depletion is confirmed in the multivariable analysis after controlling for a range of variables that could confound lymphocyte counts or outcome, shown in Table 2. Due to the limited number of events we did not include HR as a variable, since it is already represented by M stage and histology. However, including it instead of histology yields identical results. Similarly, including age instead or CSI dose also yields similar results. In agreement with the univariable analysis, baseline ALC or nadir did not correlate with recurrence risk in multivariable analyses either. The association of lymphocyte counts during the first week of treatment with recurrence also remain when restricting the analysis to high-risk patients for both the univariable (HR 0.19 [0.04 – 0.85], p=0.03) as well as the multivariable analysis (HR 0.14 [0.03 – 0.71], p=0.017). Furthermore, the results remain similar when using survival as endpoint instead of recurrence: univariable HR=0.18 [0.06 – 0.54] (p =0.0005), multivariable HR=0.14 [0.05 – 0.44] (p=0.0007).
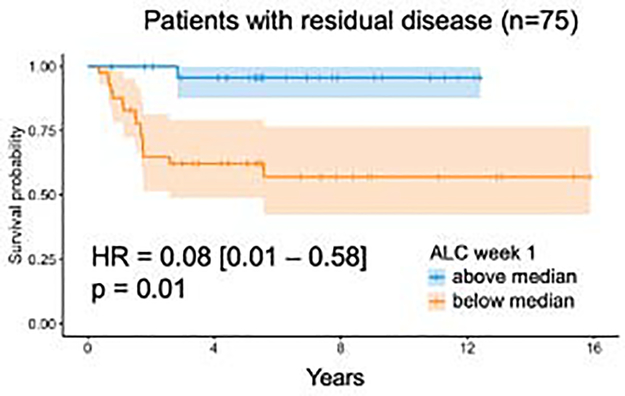
To illustrate the strong association of low early lymphocyte counts with recurrence, Figure 2 shows the time to recurrence curves split by M stage and HR status (2A/B), and early lymphocyte metrics in all patients (2C/D) and restricted to patients without induction chemotherapy or concurrent carboplatin (2E/F). To test the hypothesis that early lymphocyte counts matter more in patients with a larger burden of disease at the start of radiotherapy, we restricted our analysis only to patients with residual disease (n=75), shown in Figure 3. We also tested the hypothesis that the drop in ALC could be a proxy indicator of worsening overall patient status, and that this may be the underlying reason for the significance of ALC early during radiotherapy. However, the change in ALC was, similar to the baseline ALC as reported above, not associated with outcome.
Fig 2.
1-recurrence curves split by M stage (A), high risk (B) and early lymphocyte metrics (C, D) in all patients. (E, F) show the results only in patients not receiving induction chemotherapy or concurrent carboplatin. All p-values according to log-rank method.
Fig 3.
Survival curve for patients with residual disease, i.e. without gross total resection or M2/3 disease (n=75, 17 events), split by median ALC during week 1. p-value according to log-rank method. Multivariable analysis including other factors yields identical results.
Discussion
The key finding from our investigation is that peripheral lymphocyte counts early during RT are independently associated with recurrence risk in pediatric medulloblastoma patients, even when accounting for clinical factors that could affect disease control or ALC itself. To our knowledge, this is the first report of an association between radiation-induced lymphopenia and outcomes in pediatric patients. It is also important to note that the associations between ALC loss and outcomes consistently grew stronger when controlling for other clinical variables in the multivariable analyses (Table 2 and Figure 2), and when restricting to more homogeneous subgroups such as high-risk patients. This is notable, as we also show that induction chemotherapy and concurrent carboplatin both associate with lower ALC early during RT themselves. Similarly, high-risk and M-stage associate with outcome in the univariable analysis (supplementary Table 3). The fact that, when including all of these factors in the multivariable analysis, we see an increase in significance of early ALC and a decrease in significance of other variables, indicates that while ALC early during treatment is confounded by these competing variables, it is itself more associated with outcome than they are.
Neither baseline ALC nor ALC nadir was associated with recurrence risk, although the latter has been frequently reported as an adverse prognostic marker in other disease sites20–22 and possible reasons for this are discussed below. While we used recurrence risk as the primary endpoint, overall survival yields very similar results as in this population death is almost exclusively caused by disease recurrence.
Our study has a number of strengths and weaknesses in the context of predictive biomarker studies and regarding ALC as predictor of disease outcome in particular. Among the strengths are the consistent treatment paradigm and radiation target, the long follow-up, the unbiased outcome metric, the strength of the effect compared to other known clinical biomarkers of outcome, and the novel biomarker of early ALC loss during treatment. The fact that CSI is essentially the same for all patients means that our analysis is not affected by variations in target volume location and size, which are major confounding factors complicating analyses in other disease sites, such as lung20, where larger fields increase lymphocyte depletion but are also associated with larger and more advanced tumors. The long follow-up (median 7.1 years with 90% of recurrences occurring within the first 5 years)5,34,37 and unbiased outcome metric (recurrence does not occur due to emergence of out-of-field lesions that could have been occult at baseline) strongly suggest that the results of this study are robust.
The early timepoint is another strength of a predictive biomarker that could possibly be used for treatment adaptation: while end-treatment ALC and ALC nadir have been shown to be associated with outcome in other disease sites including adult glioma, these markers can only be defined after RT has been completed and options to adapt therapy are limited. Early biomarkers that appear during the first 2 weeks of RT, on the other hand, may facilitate adaptive regimens that account for ALC loss dynamics. Finally, the relatively large size of our dataset allowed for possible confounding effects of systemic therapy to be analyzed with care. While the use of induction or concurrent chemotherapy may act as a surrogate for HR disease in addition to influencing circulating lymphocyte counts, we found that early ALC loss remained a robust marker for outcomes even when systemic therapy was thoroughly accounted for in the multivariable analysis.
The main weakness of this study is the unavailability of molecular subtypes for this patient population, essentially tied to the long follow-up for this cohort. While the results have yet to be verified in the context of molecular subtypes, there are two strong indications that the results will hold. First, the discovery of molecular subtypes did not diminish the importance of the other existing clinical biomarkers, such as residual disease, histology and M stage38,39, which is why current trials that investigate de-escalation based on molecular subtype exclude patients exhibiting these traditional HR features. Therefore, it is reasonable to assume that a marker as broad as ALC, which has shown associations in a range of other indications, could be valid also in the context of molecular biomarkers. Second, there is considerable heterogeneity within subtypes, and while WNT and SHH patients exhibit the best prognosis (the latter depending on age), they only represent a part of the population in which RT plays a major role (>3 years), and group 3/4 subtypes are more common and are diverse in terms of outcomes28,29. This indicates that the possible predictive value of ALC could be preserved within those subgroups and could be used to further refine patient prognostication, though this needs to be validated in a molecular-defined cohort. A recent study confirms that immune infiltration is low in all molecular subgroups of medulloblastoma 40. A minor weakness of our study is that we do not have data on administration of other non-chemotherapeutic drugs that could possible influence lymphocyte counts, such as Bactrim (only given to some of the patients receiving pre-RT chemotherapy) and Keppra (anti-epileptic). However, as these drugs are unlikely to influence our endpoint (recurrence), we assume the impact on our results is limited. Furthermore, all patients in our study have been treated mainly with proton therapy, and the results could differ for photon patients.
If validated in independent cohorts and in the context of molecular markers, early peripheral lymphocyte counts could be used as part of a composite biomarker (encompassing molecular subgroup, clinical features and ALC) either for improved patient stratification for therapeutic de-escalation, or to enrich trials testing intensified therapies with the goal of improving disease control in HR patients. While it is true that “on-the-fly” treatment adaptation and decision making are difficult in the current clinical RT work process41, it has been shown feasible in clinical trials41–43 and could be integrated if proven sufficiently impactful. There is a significant body of work44,45 modeling the risks of tumor recurrence and toxicity in medulloblastoma that could be adapted to include these new biomarkers. Our data also identify the prevention or mitigation of radiation-induced lymphopenia itself as a possible therapeutic target in pediatric medulloblastoma.
On the other hand, the ability to enrich adjuvant drug trials with HR patients can be extremely useful for current trials studying various immunotherapeutic approaches in medulloblastoma (NCT02359565 – Pembrolizumab, NCT03130959 - ‘Checkmate 908’ – Nivolumab+-Ipilimumab, NCT02502708 – Indoximod + temozolomide, NCT02962167 – viral cancer vaccine, all for recurrent medulloblastoma). The high success rate of the current standard of care necessitates large trial cohorts to show an effect of treatment intensification, which results in additional drug exposure and potential toxicity for patients that would not need additional therapy. Therefore, better stratification of low- as well as high-risk groups could be impactful in two ways, either as criterion for de-escalation or to improve statistical power in adjuvant drug trials.
The connection of peripheral lymphocyte counts during or after RT and outcome has been shown retrospectively and prospectively in a range of disease sites, and the published literature has been reviewed by Grassberger et al22. While similar associations have been observed in the setting of immune checkpoint inhibition46,47, also in combination with RT48, the exact mechanisms are unclear. Multiple ways in which depletion in lymphocytes could suppress a nascent immune response have been proposed, from lymphopenia marking a state of T cell exhaustion46 to the fact that fewer lymphocytes means lower probability for new antigen presentation to T cells that could initiate a response, especially as RT has been shown to preferentially deplete naive and early memory T cells49. It has recently been shown that specifically medulloblastoma seems to reside in a non-inflammatory microenvironment which attracts few immune cells 40, which could explain the strong association of outcome with lymphocyte counts. The same study explored variations in lymphocyte subsets, and showed that more CD4+ T-cells were observed in the group 4 subtype. This further underscores the importance of studying these associations prospectively and including molecular sub-types and lymphocyte sub-populations.
Our observation that early lymphocyte counts are associated with outcome, but not the lymphocyte nadir, is a notable difference compared to studies in other indications20,21. In the currently published literature demonstrating that the nadir is important, concurrent chemo-radiation is the main cytotoxic therapeutic mechanism. Recurrence is caused by certain populations surviving both chemotherapy and RT, i.e. present towards the end of the treatment regimen, and it is essential for these populations to be recognized by antigen presenting cells, which is why the late timepoint (nadir) is important. In medulloblastoma on the other hand, the majority of tumor burden has been removed via surgery and possibly pre-RT chemotherapy. Therefore, the disease load is very small, and it is conceivable that additional antigen presentation matters more at the beginning of RT (during CSI). Furthermore, most patients experience severe, grade 3+ lymphopenia towards the end of RT (see Figure 1), possibly impeding antigen recognition at this stage. This rationale would indicate that lymphocyte counts should matter more for patients with residual disease after surgery and indeed, as shown in Figure 3, restriction of the analysis to these patients increases the importance of ALC early during RT further. However, the number of patients in this subset is low (n=75) and additional prospective validation in larger datasets is required.
Conclusion
This is the first study to show that circulating lymphocyte counts are independently associated with recurrence risk in a pediatric population, taking into account factors that might interact with ALC and outcome. Patients with peripheral lymphocyte counts below median during the first week of RT or with grade 3+ lymphopenia in week 2 had a 4-fold and 2.3-fold risk of recurrence in multivariable Cox analysis, respectively. These data now merit study in the different molecular sup-types of medulloblastoma, and if validated in other cohorts will provide an early prognostic indicator that can be used for treatment intensification or de-escalation.
Supplementary Material
Acknowledgements:
The authors thank H. Paganetti and M. Cobbold for their insights and helpful discussions. This work has been previously presented at the 61st Annual Meeting of the American Society for Radiation Oncology in Chicago, Illinois.
Funding: The present research was supported in part by National Cancer Institute U19 CA21239 (C.G., T.Y.) and C06 CA059267 (B.Y.Y.)
Footnotes
Conflict of Interest Disclosures: The authors declare no potential conflicts of interest.
Data Sharing Statement: Research data are not available at this time, please contact the corresponding author for specific requests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Millard NE & De Braganca KC Medulloblastoma. J. Child Neurol. 31, 1341–1353 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McGovern SL, Grosshans D & Mahajan A Embryonal brain tumors. Cancer J 20, 397–402 (2014). [DOI] [PubMed] [Google Scholar]
- 3.Salloum R et al. Late Morbidity and Mortality Among Medulloblastoma Survivors Diagnosed Across Three Decades: A Report From the Childhood Cancer Survivor Study. J. Clin. Oncol 37, 731–740 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Packer RJ et al. Phase III Study of Craniospinal Radiation Therapy Followed by Adjuvant Chemotherapy for Newly Diagnosed Average-Risk Medulloblastoma. JCO 24, 4202–4208 (2006). [DOI] [PubMed] [Google Scholar]
- 5.Jakacki RI et al. Outcome of children with metastatic medulloblastoma treated with carboplatin during craniospinal radiotherapy: a Children’s Oncology Group Phase I/II study. J. Clin. Oncol 30, 2648–2653 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johnston DL et al. Survival Following Tumor Recurrence in Children With Medulloblastoma. J. Pediatr. Hematol. Oncol 40, e159–e163 (2018). [DOI] [PubMed] [Google Scholar]
- 7.Merchant TE et al. Multi-institution prospective trial of reduced-dose craniospinal irradiation (23.4 Gy) followed by conformal posterior fossa (36 Gy) and primary site irradiation (55.8 Gy) and dose-intensive chemotherapy for average-risk medulloblastoma. Radiation Oncology Biology 70, 782–787 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thomas PR et al. Low-stage medulloblastoma: final analysis of trial comparing standard-dose with reduced-dose neuraxis irradiation. JCO 18, 3004–3011 (2000). [DOI] [PubMed] [Google Scholar]
- 9.Michalski JM et al. Results of COG ACNS0331: A Phase III Trial of Involved-Field Radiotherapy (IFRT) and Low Dose Craniospinal Irradiation (LD-CSI) with Chemotherapy in Average-Risk Medulloblastoma: A Report from the Children’s Oncology Group. Radiation Oncology Biology 96, 937–938 (2016). [Google Scholar]
- 10.Miljkovic MD, Grossman SA, Ye X, Ellsworth S & Terezakis S Patterns of Radiation-Associated Lymphopenia in Children with Cancer. Cancer Investigation 34, 32–38 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wong KK et al. Acute toxicity of craniospinal irradiation with volumetric-modulated arc therapy in children with solid tumors. Pediatr Blood Cancer 65, e27050 (2018). [DOI] [PubMed] [Google Scholar]
- 12.Formenti SC & Demaria S Systemic effects of local radiotherapy. The Lancet Oncology 10, 718–726 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Demaria S et al. Immune-mediated inhibition of metastases after treatment with local radiation and CTLA-4 blockade in a mouse model of breast cancer. Clin Cancer Res 11, 728–734 (2005). [PubMed] [Google Scholar]
- 14.Dewan MZ et al. Fractionated but not single-dose radiotherapy induces an immune-mediated abscopal effect when combined with anti-CTLA-4 antibody. Clin Cancer Res 15, 5379–5388 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.MacLennan IC & Kay HE Analysis of treatment in childhood leukemia. IV. The critical association between dose fractionation and immunosuppression induced by cranial irradiation. Cancer 41, 108–111 (1978). [DOI] [PubMed] [Google Scholar]
- 16.Harisiadis L, Kopelson G & Chang CH Lymphopenia caused by cranial irradiation in children receiving craniospinal radiotherapy. Cancer 40, 1102–1108 (1977). [DOI] [PubMed] [Google Scholar]
- 17.Grassberger C et al. Differential Association Between Circulating Lymphocyte Populations With Outcome After Radiation Therapy in Subtypes of Liver Cancer. Int. J. Radiat. Oncol. Biol. Phys 101, 1222–1225 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wild AT et al. The Association Between Chemoradiation-related Lymphopenia and Clinical Outcomes in Patients With Locally Advanced Pancreatic Adenocarcinoma. Am. J. Clin. Oncol 38, 259–265 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grossman SA et al. Survival in Patients With Severe Lymphopenia Following Treatment With Radiation and Chemotherapy for Newly Diagnosed Solid Tumors. J Natl Compr Canc Netw 13, 1225–1231 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tang C et al. Lymphopenia association with gross tumor volume and lung V5 and its effects on non-small cell lung cancer patient outcomes. Int. J. Radiat. Oncol. Biol. Phys 89, 1084–1091 (2014). [DOI] [PubMed] [Google Scholar]
- 21.Davuluri R et al. Lymphocyte Nadir and Esophageal Cancer Survival Outcomes After Chemoradiation Therapy. Int. J. Radiat. Oncol. Biol. Phys 99, 128–135 (2017). [DOI] [PubMed] [Google Scholar]
- 22.Grassberger C, Ellsworth SG, Wilks MQ, Keane FK & Loeffler JS Assessing the interactions between radiotherapy and antitumour immunity. Nat Rev Clin Oncol 16, 729–745 (2019). [DOI] [PubMed] [Google Scholar]
- 23.Marciscano AE et al. Elective nodal irradiation attenuates the combinatorial efficacy of stereotactic radiation therapy and immunotherapy. Clin Cancer Res clincanres.3427.2017 (2018). doi: 10.1158/1078-0432.CCR-17-3427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hegde S et al. Dendritic Cell Paucity Leads to Dysfunctional Immune Surveillance in Pancreatic Cancer. Cancer Cell 37, 289–307.e9 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tseng YD et al. Effect of Patient Immune Status on the Efficacy of Radiation Therapy and Recurrence-Free Survival Among 805 Patients With Merkel Cell Carcinoma. Int. J. Radiat. Oncol. Biol. Phys 102, 330–339 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yousefi H, Yuan J, Keshavarz-Fathi M, Murphy JF & Rezaei N Immunotherapy of cancers comes of age. Expert Rev Clin Immunol 13, 1001–1015 (2017). [DOI] [PubMed] [Google Scholar]
- 27.Gutiontov SI, Pitroda SP, Chmura SJ, Arina A & Weichselbaum RR Cytoreduction and the Optimization Of Immune Checkpoint Inhibition with Radiation Therapy. Int. J. Radiat. Oncol. Biol. Phys 108, 17–26 (2020). [DOI] [PubMed] [Google Scholar]
- 28.Taylor MD et al. Molecular subgroups of medulloblastoma: the current consensus. Acta Neuropathol. 123, 465–472 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Louis DN et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol. 131, 803–820 (2016). [DOI] [PubMed] [Google Scholar]
- 30.Olson J Efficacy of Carboplatin Administered Concomitantly With Radiation and Isotretinoin as a Pro-Apoptotic Agent in Other Than Average Risk Medulloblastoma/PNET Patients. Children’s Oncology Group Protocol ACNS 0332. Version 6/30/17. Available from: https://cogmembersorg/Prot/acns0332/acns0332docpdf [Google Scholar]
- 31.Michalski JM A Study Evaluating Limited Target Volume Boost Irradiation and Reduced Dose Craniospinal Radiotherapy (18.00 Gy) and Chemotherapy in Children with Newly Diagnosed Standard Risk Medulloblastoma: A Phase 3 Double Randomized Trial. Children’s Oncology Group Protocol ACNS 0331. Version 6/37/16. 2004; Available from: https://cogmembers.org/Prot/ACNS0331/ACNS0331DOC.pdf. [Google Scholar]
- 32.Dhall G et al. Outcome of children less than three years old at diagnosis with non-metastatic medulloblastoma treated with chemotherapy on the ‘Head Start’ I and II protocols. Pediatr Blood Cancer 50, 1169–1175 (2008). [DOI] [PubMed] [Google Scholar]
- 33.Zaky W et al. Intensive induction chemotherapy followed by myeloablative chemotherapy with autologous hematopoietic progenitor cell rescue for young children newly-diagnosed with central nervous system atypical teratoid/rhabdoid tumors: the Head Start III experience. Pediatr Blood Cancer 61, 95–101 (2014). [DOI] [PubMed] [Google Scholar]
- 34.Yock TI, Yeap BY, Ebb DH, et al. Long-term toxic effects of proton radiotherapy for paediatric medulloblastoma: a phase 2 single-arm study. Lancet Oncol 2016;17:287–298 [DOI] [PubMed] [Google Scholar]
- 35.Team, R. C. R: A language and environment for statistical computing. (2017).
- 36.Therneau TM & Grambsch PM Modeling survival data: extending the Cox model. (2013). [Google Scholar]
- 37.Hoff, von K et al. Long-term outcome and clinical prognostic factors in children with medulloblastoma treated in the prospective randomised multicentre trial HIT’91. Eur. J. Cancer 45, 1209–1217 (2009). [DOI] [PubMed] [Google Scholar]
- 38.Ryan SL et al. MYC family amplification and clinical risk-factors interact to predict an extremely poor prognosis in childhood medulloblastoma. Acta Neuropathol. 123, 501–513 (2012). [DOI] [PubMed] [Google Scholar]
- 39.Ellison DW et al. Medulloblastoma: clinicopathological correlates of SHH, WNT, and non-SHH/WNT molecular subgroups. Acta Neuropathol. 121, 381–396 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Diao S, Gu C, Zhang H & Yu C Immune cell infiltration and cytokine secretion analysis reveal a non-inflammatory microenvironment of medulloblastoma. Oncology Letters 20, 1–1 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Matuszak MM et al. Functional Adaptation in Radiation Therapy. Semin Radiat Oncol 29, 236–244 (2019). [DOI] [PubMed] [Google Scholar]
- 42.Kong F-M et al. Effect of Midtreatment PET/CT-Adapted Radiation Therapy With Concurrent Chemotherapy in Patients With Locally Advanced Non-Small-Cell Lung Cancer: A Phase 2 Clinical Trial. JAMA Oncol 3, 1358–1365 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bussink J, van Herpen CML, Kaanders JHAM & Oyen WJG PET-CT for response assessment and treatment adaptation in head and neck cancer. The Lancet Oncology 11, 661–669 (2010). [DOI] [PubMed] [Google Scholar]
- 44.Brodin NP et al. Optimizing the radiation therapy dose prescription for pediatric medulloblastoma: Minimizing the life years lost attributable to failure to control the disease and late complication risk. Acta Oncologica 53, 462–470 (2014). [DOI] [PubMed] [Google Scholar]
- 45.Brodin NP, Vogelius IR, Bjork-Eriksson T, Rosenschöld PMA & Bentzen SM Modeling Freedom From Progression for Standard-Risk Medulloblastoma: A Mathematical Tumor Control Model With Multiple Modes of Failure. International Journal of Radiation Oncology*Biology*Physics 87, 422–429 (2013). [DOI] [PubMed] [Google Scholar]
- 46.Diehl A, Yarchoan M, Hopkins A, Jaffee E & Grossman SA Relationships between lymphocyte counts and treatment-related toxicities and clinical responses in patients with solid tumors treated with PD-1 checkpoint inhibitors. Oncotarget 8, 114268–114280 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ku GY et al. Single-institution experience with ipilimumab in advanced melanoma patients in the compassionate use setting. Cancer 116, 1767–1775 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pike LRG et al. The Impact of Radiation Therapy on Lymphocyte Count and Survival in Metastatic Cancer Patients Receiving PD-1 Immune Checkpoint Inhibitors. Int. J. Radiat. Oncol. Biol. Phys. 103, 142–151 (2019). [DOI] [PubMed] [Google Scholar]
- 49.Tabi Z et al. Resistance of CD45RA- T cells to apoptosis and functional impairment, and activation of tumor-antigen specific T cells during radiation therapy of prostate cancer. J. Immunol. 185, 1330–1339 (2010). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.