Abstract
Objective:
Diabetes distress and depressive symptoms are common psychosocial concerns for people with diabetes. These are related yet distinct mood states, which have each been related to diabetes management and A1C among adolescents and adults with diabetes. However, they have not been examined concurrently in preadolescents with type 1 diabetes (T1D). Understanding the overlaps and distinctions between diabetes distress and depressive symptoms in youth would help guide decisions about psychosocial screening in diabetes clinical practice. This study aimed to categorize preadolescents based on clinical cut-offs of concurrently administered measures of depressive symptoms and diabetes distress and identify clinical and demographic characteristics of each group.
Method:
180 youth (aged 9–13 years, M age = 11.3 ± 1.3 years, 55% female, 56% Caucasian, M A1C = 8.4% (68 mmol/mol) ± 1.6%) completed measures of diabetes distress, depressive symptoms, and quality of life. Daily blood glucose monitoring frequency was calculated from meter download. A1C values were obtained from electronic medical records.
Results:
Depressive symptoms and diabetes distress each significantly correlated with A1C and quality of life. While most (69%) participants had no clinically significant elevations in either diabetes distress or depressive symptoms, 14% had elevated depressive symptoms only, and 17% had elevated distress without concurrent elevated depressive symptoms. Groups differed based on A1C, quality of life, and insurance status.
Conclusions:
Routine assessment of both depressive symptoms and diabetes distress may help to identify preadolescents with T1D who require psychosocial support.
Introduction
Depressive symptoms and diabetes distress are overlapping yet distinct constructs in people with type 1 diabetes [1–3]. Depressive symptoms are part of the diagnostic criteria for a depressive disorder (e.g., depressed mood, anhedonia, fatigue, feelings of worthlessness or guilt) [4]. Diabetes distress, on the other hand, is a normal emotional reaction to the burdensome self-management demands of diabetes, including frustration with treatment demands, worry about complications, feeling defeated or hopeless about one’s ability to manage diabetes, and low motivation for diabetes self-management [5]. Diabetes distress is related to “diabetes burnout,” a distinct construct that reflects a sense of detachment from diabetes care [6]. Diabetes distress may or may not precede diabetes burnout [7]. The 2018 Diabetes Canada Clinical Practice Guidelines highlight the need to assess for psychological disorders associated with diabetes in youth, including depression, and intervene early to minimize the negative impact throughout childhood development [8]. International practice guidelines for diabetes clinical care recommend regular assessment of both depressive symptoms and diabetes distress in youth [9, 10]. However, these recommendations are based on expert opinion and research with adolescents and adults with diabetes, as no study has examined both depressive symptoms and diabetes distress, concurrently, in preadolescents (i.e., 9–13 years old) with type 1 diabetes.
Routine screening for depressive symptoms and diabetes distress in diabetes clinical practice allows diabetes teams to identify patients who are struggling and refer them to appropriate mental/behavioral health professionals for evaluation and treatment. However, implementing psychological assessments requires time and resources, so diabetes clinics must prudently select which constructs to assess and how often [11]. Thus, information about the relevance of screening for each of these two constructs in preadolescents would have important clinical implications for routine psychological assessment in this population.
In adolescents, both depressive symptoms and diabetes distress are associated with lower quality of life, less engagement in self-management behaviors, and higher A1C [2, 12]. Moreover, in an Australian study with adolescents with type 1 diabetes, more than one-half of respondents endorsed moderate-to-high diabetes distress [13]. Participants who experienced higher diabetes distress were also more likely to report depressive symptoms. In another study, adolescents who reported depressive symptoms were four times more likely to endorse diabetes distress, highlighting some degree of co-occurrence of these concerns [12]. It is evident from these findings that both diabetes distress and depressive symptoms are present in adolescents with diabetes and are related to diabetes self-management behaviors and quality of life. However, preadolescence is a different stage of development with important distinctions in relation to mood concerns. The rate of depressive disorders is much lower in pre-adolescence (1–2%) vs. adolescence (8–9%) [14], and depressive disorders tend to have a later onset than anxiety and behavior disorders [15], making preadolescence a critically important period to study to inform prevention efforts in type 1 diabetes. Additionally, preadolescents, who are less cognitively and emotionally mature, may have less capacity to discern and report nuanced differences between depressive symptoms versus diabetes distress. The only studies to examine diabetes distress in preadolescent children specifically did not compare diabetes distress with depressive symptoms [16–18], leaving unanswered important questions about the relative rates of clinically significant diabetes distress and depressive symptoms in this unique developmental stage.
If there is significant overlap between these two constructs in preadolescents with type 1 diabetes, assessing one of them could adequately identify most youth who are in need of psychosocial support [19]. However, if measures of depressive symptoms and diabetes distress each identify a unique set of vulnerable youth, there would be a compelling rationale to screen for both. To understand the relative clinical importance of depressive symptoms and diabetes distress in preadolescent youth, this study assessed both constructs in youth age 9- to 13 with type 1 diabetes. We aimed to 1) examine whether associations among diabetes distress, depressive symptoms, and other clinical outcomes in this age group are consistent with documented associations in adolescents and adults; 2) determine whether scores on self-reported measures of depressive symptoms and diabetes distress could differentiate subgroups of preadolescents with type 1 diabetes; and 3) describe differences in clinical profiles between subgroup members.
We hypothesized that higher depressive symptoms and higher diabetes distress would individually be associated with higher A1C, less engagement in self-management behaviors, and lower quality of life. We also expected that some participants would fall into each of the following subgroups: A—no clinical elevation in diabetes distress or depressive symptoms, B—clinical elevation in only diabetes distress, C—clinical elevation in only depressive symptoms, and D—clinical elevation in both depressive symptoms and diabetes distress. We hypothesized these groups would exhibit unique clinical profiles, in that there would be significant differences between groups’ A1C, quality of life, blood glucose monitoring frequency, diabetes duration, insurance status, and insulin pump use. We hypothesized that participants with elevations in both depressive symptoms and diabetes distress (Group D) would have the highest A1C, least frequent blood glucose monitoring, and lowest quality of life compared with the other groups. We also hypothesized that group B (elevated diabetes distress only) would have higher A1C, longer diabetes duration, and more youth with private insurance than Group C (elevated depressive symptoms only).
Research Design and Methods
Participants and Procedures
As part of an observational study of resilience in preadolescents with type 1 diabetes, study staff recruited youth aged 9 to 13 years and their parents/primary caregivers (referred to as “parents”) from the diabetes clinic at Texas Children’s Hospital (Houston, TX, USA). The affiliated institutional review board approved the study. Inclusion criteria included age of 9 to 13 years old, diagnosed with type 1 diabetes per American Diabetes Association criteria for 6 months or longer, and parent and child fluency in English. Exclusion criteria included medical chart documentation or parent report of developmental delay or cognitive impairments in parent or child that would interfere with study participation and participation in any intervention research study within 3 months.
To recruit participants, research study staff previewed diabetes clinic schedules to identify potentially eligible youth with diabetes clinic appointments scheduled in upcoming weeks. Study staff mailed informational letters to these potentially eligible families that explained the purpose of the study and provided an option for how to opt out of any further communication. Study staff followed up with families by telephone to describe the study, assess interest, and schedule a meeting at the child’s upcoming diabetes clinic visit. At the diabetes clinic visit, staff confirmed eligibility, answered questions, and completed informed consent (parent) and assent (youth) procedures. Following enrollment, parents and youth separately completed a battery of questionnaires via a HIPAA-compliant secure web survey or on paper if preferred. Families completed surveys with the research staff member present, during a research appointment time, which typically took place immediately prior to their diabetes clinical visit. Participants were informed that the questionnaires were for research purposes only and would not be shared with their diabetes clinical team. Clinical staff did not have access to questionnaire data. Research staff also downloaded data from participants’ blood glucose meters. Participants were able to earn a maximum of $27 ($10 for completing the surveys and $12 for parking, and $5 for bringing in their meter.
Staff identified 260 potentially eligible families and sent letters. Six families opted out of being contacted about the study, citing lack of time and interest in participating in research as reasons for declining the invitation to participate. Staff were able to meet with 206 families to introduce the study, of whom 197 (96%) met eligibility criteria and 188 (95% of eligible) consented. Reasons for nonparticipation included time and disinterest in research. One participant did not provide complete baseline data, and seven did not complete the measure of depressive symptoms or diabetes distress, resulting in a final sample of n = 180.
Measures
Demographic and clinical characteristics
Parents reported demographic information, including youth race/ethnicity, number of parents in the household, and insurance coverage (used as a proxy for socioeconomic status). For the current study, we used demographic information from the parent questionnaires, and the self-report questionnaires were completed by youth. Participants’ electronic medical records provided diabetes duration and insulin regimen.
Diabetes distress
Youth 12 to 13 years of age completed the Problem Areas in Diabetes questionnaires for adolescents (PAID-T) [20], and youth 9 to 11 years of age completed the children’s version (PAID-C) [21]. Youth rated the degree to which they felt upset or bothered by diabetes-related situations over the past month on a Likert scale from 1 “not a problem” to 6 “big problem.” For this analysis, we used the items from the validated short-forms; 14 items for the PAID-T, 11 items for the PAID-C. Total scores ranged from 14 to 84 on the PAID-T and 11 to 66 on the PAID-C (higher indicating more diabetes distress). The reliability in this sample was excellent (α = 0.88 for both versions).
We used clinical cut-points for each measure to determine those who met the criteria for “elevated” diabetes distress. For the PAID-T, we used the clinical cut-point (total score ≥ 44) recommended by the authors, based on a ROC curve sensitivity analysis [22]. For the PAID-C, we used a clinical cut-point of 1 SD above the published mean for the PAID-C (total score ≥ 40.6), as recommended by the authors, since a ROC analysis has not yet been conducted for the PAID-C [21].
Depressive symptoms
Youth completed the second edition of the Children’s Depression Inventory-Short Form (CDI-SF), a 12-item screening measure of depressive symptoms [23]. For each symptom, participants chose one of three options representing the degree of severity over the past 2 weeks. The reliability in this sample was acceptable (α = 0.71). We converted the total score (range = 0–24) into a t-score (higher reflecting more frequent/intense depressive symptoms). We used a t-score of 60 or higher as a clinical cut-off for “elevated” depressive symptoms, as recommended by the authors [23]. For participants who scored above the clinical cut-off, a licensed psychologist contacted their caregivers to inform them and provide referrals as indicated.
Health-related quality of life (HRQOL)
Youth completed the Monitoring Individual Needs in Diabetes Youth Questionnaire (MY-Q) [24], adapted in collaboration with the original author for youth under age 12. Participants rated the degree to which they agreed with 24 items (ages 9–11, α = 0.69) or 32 items (ages 12–13, α = 0.59) on a 5-point Likert scale from 1 “all the time” to 5 “never,” related to the effect of diabetes on various domains of functioning (e.g., mood, social interactions, family activities). Scores were calculated on a 1–100 scale (higher indicating better HRQOL).
Diabetes self-management
For the current study, we measured diabetes self-management using objective time- and date-stamped data, downloaded from youths’ blood glucose meters by study staff (or extracted by hand for meters that were unable to be downloaded). Participants received an extra $5 for bringing all actively used meters to the study visit. When families reporting using other meters (e.g., at school), study staff made multiple efforts to collect data from those meters by telephone, email, or fax. Research staff compiled data from all available meters and calculated mean daily frequency of blood glucose monitoring over the previous 14 days.
Glycemic control
As part of routine ambulatory diabetes care, trained medical assistants on the diabetes clinic staff obtained capillary blood samples from youth via finger stick, analyzed them using a point-of-care DCA 2000+ A1C Analyzer (Siemens-Bayer, Inc., Munich, Germany), and entered the result into each patient’s electronic medical record. Research staff then retrospectively reviewed electronic medical records to extract A1C values for research participants.
Data Analysis
For preliminary analyses, we conducted Pearson’s correlations to examine bivariate associations among depressive symptoms, diabetes distress, and clinical and demographic variables. We examined correlations separately for youth aged 9 to 11 and aged 12 to 13, as they completed slightly different versions of the PAID. To characterize youth based on elevated diabetes distress or depressive symptoms or both, we categorized participants into one of four groups using the CDI-SF clinical cut-off (t ≥ 60) and the clinical cut-offs described above for the PAID-C and the PAID-T. We combined all youth in this step of the analysis because PAID scores were categorized as above or below cut-off, according to the appropriate threshold for each measure. Group A participants had scores below cut-off for both depressive symptoms and diabetes distress; Group B had scores below cut-off for depressive symptoms and above cut-off (elevated) for diabetes distress; Group C had scores above cut-off (elevated) for depressive symptoms and below cut-off for diabetes distress; and Group D had scores above cut-offs (elevated) for both depressive symptoms and diabetes distress (Figure 1). We calculated descriptive statistics to characterize the demographic and disease-specific characteristics of each group. To identify whether the characteristics of youth differed across each group, we conducted a MANOVA comparing clinical (A1C, self-management, HRQOL, diabetes duration, insulin pump use) and socioeconomic (insurance coverage) variables among the groups overall. We conducted follow-up one-way ANOVAs to identify which variables were different between groups and then post-hoc analyses to probe significant between-group differences for each clinical and demographic variable. We used SPSS 25 for Windows (SPSS Inc., Chicago, IL, USA) for all analyses.
Figure 1.
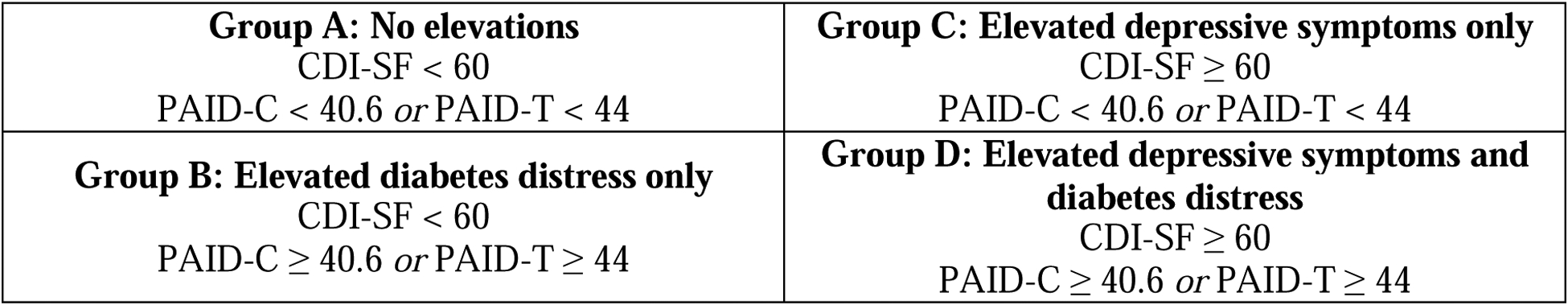
Group categorizations.
Results
The mean youth age was 11.3 ± 1.3 years and mean A1C was 8.4 ± 1.6%. Slightly more than one-half of participants (56%) were non-Hispanic white. Complete participant characteristics are detailed in Table 1.
Table 1.
Participant clinical and demographic characteristics (n = 180).
| Percent (n) | Mean ± SD | Range | |
|---|---|---|---|
| Youth age, years | 11.3 ± 1.3 | 9.1 – 13.9 | |
| Youth gender % female | 55.0 (99) | ||
| Highest parental education | |||
| < High school diploma | 3.3 (6) | ||
| High school diploma/GED | 13.9 (25) | ||
| Some college, no degree | 16.1 (29) | ||
| 2-year college degree/technical or associates | 13.3 (24) | ||
| 4-year college degree/bachelor’s | 30.6 (55) | ||
| Graduate degree | 22.8 (41) | ||
| Insurance, % private | 63.9 (115) | ||
| Youth race/ethnicity (parent report) | |||
| White, non-Hispanic | 56.1 (101) | ||
| Black, non-Hispanic | 19.4 (35) | ||
| Hispanic | 22.2 (40) | ||
| “Other” or more than 1 | 2.2 (4) | ||
| Diabetes duration, years | 4.1 ± 3.1 | 0.5 – 13.1 | |
| Insulin administration | |||
| Fixed dose insulin by injections | 12.2 (22) | ||
| Basal bolus injections | 43.9 (79) | ||
| Pump | 43.3 (78) | ||
| Glycemic control, % hemoglobin A1C (mmol/mol) | 8.4 (68) ± 1.6 | 5.1 (32) – 14.0 (130) | |
| Daily BGMF, from meter download | 4.7 ± 2.0 | 0.5 – 11.6 |
A1C = glycosylated hemoglobin A1C; BGMF = blood glucose monitoring frequency
Bivariate correlations for both younger and older youth in the sample revealed significant associations between each of the following: diabetes distress, depressive symptoms, A1C, and quality of life, in the expected directions (Table 2). Blood glucose monitoring frequency significantly correlated with A1C only.
Table 2.
Bivariate correlation matrix for depressive symptoms, diabetes distress, glycemic control, parent-reported adherence, blood glucose monitoring frequency, and quality of life. Youth age 9–11 below the diagonal (in yellow), age 12–13 above the diagonal (in blue).
| Age 9–11 (n = 116) | Age 12–13 (n = 59) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| PAID-C | CDI-SF | A1C | BGM Freq | MY-Q | Mean | SD | Mean | SD | |
| PAID-T | --- | 0.71** | 0.52** | −0.18 | −0.70** | 26.27 | 11.9 | 32.88 | 13.2 |
| CDI-SF | 0.60** | --- | 0.35** | −0.17 | −0.66** | 3.27 | 3.0 | 3.08 | 2.7 |
| A1C | 0.24** | 0.23** | --- | −0.37** | −0.47** | 8.43 | 1.5 | 8.26 | 1.7 |
| BGM Freq | −0.09 | −0.08 | −0.40** | --- | 0.07 | 4.92 | 2.1 | 4.55 | 2.1 |
| MY-Q | −0.63** | −0.55** | −0.26** | 0.09 | --- | 71.12 | 12.4 | 67.38 | 11.7 |
BGM Freq = blood glucose monitoring frequency
Classifying participants based on clinical cut-off scores resulted in the following: Group A (no elevations) included 124 participants, Group B (elevated PAID only) included 9 participants, Group C (elevated CDI-SF only) included 26 participants, and Group D (elevated PAID and CDI-SF) included 21 participants. To avoid over-interpretation of the relatively smaller subset of participants in Group B and given our research aim to evaluate the added value of screening for diabetes distress, we combined both groups with elevated diabetes distress (Groups B and D) into a single category. Thus, “Group B/D” included participants with elevated diabetes distress, with or without depressive symptoms. Table 3 provides descriptive data for each group.
Table 3.
Clinical and demographic characteristics of preadolescents with type 1 diabetes with elevated depressive symptoms and/or diabetes distress.
| DEPRESSIVE SYMPTOMS (CDI-SF) | |||
|---|---|---|---|
| BELOW CUT-OFF | ELEVATED | ||
| DIABETES DISTRESS (PAID) | BELOW CUT-OFF |
GROUP A: No Elevations n = 124 (68.9%) Age Range = 9–13 years; M±SD = 11.3±1.3 Gender (% Female) = 52.4% |
GROUP C: Elevated Depressive Symptoms Only n = 26 (14.4%) Age Range = 9–13; M±SD = 11.2±1.3 Gender (% Female) = 57.7% |
| ELEVATED |
GROUP B and D Combined: Elevated Diabetes Distress with or without Elevated Depressive Symptoms n = 30 (16.7%) Age Range = 9–13, M±SD = 11.4±1.3 Gender (% Female) = 63.3% |
||
There was a significant effect of group membership on participant characteristics, V = 0.44, F(12, 318) = 7.50, p < .01. One-way ANOVAs revealed significant differences among the groups on A1C, F(2,175) = 11.04, p < .01, MY-Q scores (HRQOL measure), F(2,172) = 38.63, p < .01, and insurance status, F(2,177) = 5.96, p < .01. There were no significant differences between groups for the other variables.
Bonferroni post-hoc probing of the ANOVAs revealed youth in Group B/D (M = 9.6 ± 1.6%) had significantly higher A1C than youth in Group A (M = 8.1 ± 1.5%, p < .01) and Group C (M = 8.4 ± 1.4%, p < .05). Participants in Group A and Group C did not differ significantly from each other in A1C. In terms of quality of life, youth in Group A (M = 74.1 ± 10.2) had significantly higher MY-Q scores than youth in Group B/D (M = 56.7 ± 9.9) and youth in Group C (M = 63.8 ± 10.9, all p < .01). Participants in Group C also had significantly higher MY-Q scores than participants in Group B/D (p< .05). In relation to socioeconomic status, Group C had a greater proportion of youth with public or no insurance (65.4%) compared with both Group A (30.6%, p < .01) and Group B/D (33.3%, p < .05). Groups A and B/D were not different from one another with regard to insurance status.
Discussion
In this first study to examine simultaneously diabetes distress and depressive symptoms in a relatively large and diverse sample of preadolescents with type 1 diabetes, findings supported the importance of screening for both clinically important constructs. Both were associated with A1C and diabetes-specific quality of life in the expected directions, which extends previous findings with adolescents [25] and adults [25, 26] to a younger age group (preadolescents). While most of this sample had no clinical elevations, almost one-third (groups B/D and C combined = 31%) reported experiencing elevated depressive symptoms and/or diabetes distress. This is a substantial portion of clinical patients who could benefit from consultation with a mental/behavioral health professional. Moreover, nine participants reported elevated diabetes distress without elevated depressive symptoms. Thus, a clinic that screens for depressive symptoms only would “miss” or overlook one in every 20 preadolescents they treat. These are children who could likely benefit from a behavioral health consult and who may be at risk for deteriorations in self-management as they transition into adolescence [27].
While these rates demonstrate the value of screening for both depressive symptoms and diabetes distress during the preadolescent period, it is possible that these rates underestimate the true need. In this sample, 30 participants (17%) scored above the cut-points for diabetes distress, which is somewhat lower than a national sample of Australian adolescents with type 1 diabetes, in which 36% reported “high” diabetes distress [13]. This discrepancy may be due to the specific measures or the cut-offs used or true differences in rates of distress between the United States and Australia. Alternatively, because the participants in the current study were younger than the Australian sample, it is also possible that, similarly as depressive symptoms, rates of high diabetes distress may increase in adolescence. More than one-quarter of youth (n = 47, 26%) scored above the cut-off for elevated depressive symptoms, which is slightly higher than other published rates of elevated depressive symptoms in youth (15–23%) [28, 29]. The reason for this difference is not known; it is possible that the higher representation of non-white youth and those with lower socioeconomic status in our sample may have contributed to these higher rates of elevated depressive symptoms, as demographic factors, such as minority race/ethnicity group membership and lower socioeconomic status, have been linked with depressive symptoms [30].
Previous research with both adolescents and adults has found that diabetes distress correlates with A1C more strongly than do depressive symptoms [31, 13]. Our findings are largely congruent with these previous findings, in that the association between A1C and diabetes distress was stronger than the association between A1C and depressive symptoms in youth aged 12 to 13 years who completed the PAID-T. However, this pattern was not evident in the preadolescents aged 9 to 11 years in our sample, for whom correlations between diabetes distress scores and A1C were similar to correlations between depressive symptoms and A1C. It may be that in preadolescents distress is not as related to glycemic outcomes because parents are often more involved in diabetes management at that age, whereas older youth (12–13 years) often participate in diabetes management tasks more independently [32]. Additionally, the group with elevated diabetes distress had significantly higher A1C than the other groups, including the group with only elevated depressive symptoms. This finding further highlights that diabetes distress, rather than depressive symptoms, may be more clinically meaningful for understanding the connection between emotional concerns and glycemic outcomes among people with diabetes. This study extends this pattern, which has previously been reported in adolescents [25] and adults [26], down into preadolescence for the first time. Additional research is needed to understand the directionality of this association. For example, it is possible that those who are more distressed about their diabetes have difficulty maintaining self-management routines, have stress-induced higher glucose values, and ultimately have higher A1C. Alternatively, those with higher blood glucose levels may become more frustrated that they are not able to bring their blood glucose numbers down adequately, which could increase the distress they experience regarding their diabetes.
These data can help to understand the clinical characteristics of preadolescent youth with different levels of diabetes distress and depressive symptoms. Glycemic control was different between groups, such that members in Group B/D (elevated diabetes distress) had A1C values well over 1 percentage point higher than the other two groups (no elevations and elevated depressive symptoms only). This finding further supports the recommendation that clinics should screen for diabetes distress, especially when youth have high A1C results [10]. Members in each group also reported significantly different levels of quality of life, in descending order from highest quality of life in Group A (youth with no elevations), to lower quality of life in Group C (youth with elevated depressive symptoms only), to the lowest quality of life in Group B/D (youth with elevated diabetes distress with or without depressive symptoms). Thus, screening for depression alone may not identify youth at highest risk for poor quality of life, and adding a measure of diabetes distress may help to identify those youth who are struggling more globally. These findings can also shed light on the clinic patients who may be most vulnerable to mood symptoms. Specifically, members in Group C (elevated depression only) had around double the proportion of children with public or no insurance at 66.7% compared with 30.6% of children in Group A (no elevations) and 34.4% in Group B/D (elevated diabetes distress with or without depressive symptoms). In the United States, having no medical insurance or public insurance is a proxy for low socioeconomic status [33]. Thus, this finding is consistent with extensive literature that has identified low socioeconomic status as a risk factor for depression [34]. On the contrary, low socioeconomic status was not different based on clinical elevations in diabetes distress, indicating that high levels of diabetes distress may be less predictable based on sociodemographic factors than are high levels of depressive symptoms.
These findings suggest several clinical implications for teams working with preadolescents with type 1 diabetes. Given that most clinical and demographic variables were not significantly different between groups, providers are likely unable to rely on most clinical or demographic factors to accurately predict who may be at greatest risk for elevated depressive symptoms or diabetes distress without asking specifically about their patients’ experiences via psychosocial screeners. Preadolescents in this study were able to differentiate between depressive symptoms and diabetes distress using structured questionnaires designed to assess each construct, and many reported elevated diabetes distress, whether or not they also endorsed elevated depressive symptoms. Thus, screening with both measures would likely provide additive, not redundant, information about preadolescents’ emotional well-being and would be a worthwhile use of time and resources. Still, there are several considerations when choosing what screening tools meet a clinic’s needs, including clinical care guidelines and the clinic’s ability to respond to elevated scores (e.g., via referral to behavioral health specialists). Our team previously offered guidance about making these decisions and suggested specific measures that could be used to assess various aspects of emotional functioning [11]. In general, behavioral intervention outcomes are better when psychological symptoms and behavioral concerns are identified and treated early [35]. Thus, the findings of this study highlight the preventive importance of psychosocial screening during preadolescence.
These findings support international practice guidelines that encourage providers to consider assessing mental health factors, such as depression and diabetes distress, at periodic intervals [8, 9]. Our group previously provided practical recommendations for implementing psychological assessments/screening into clinical services for youth with type 1 diabetes [11]. Preadolescence is an important time to screen for these psychological concerns to provide support and try to prevent the well-documented deteriorations in A1C that occur during adolescence [36]. Although no clinical trials have identified efficacious interventions specifically for treating depression in youth with type 1 diabetes [19], there are successful interventions for treating depressive symptoms in adults with diabetes [37–41] and adolescents without diabetes [42–45], which clinicians may adapt for preadolescents with diabetes. In contrast with depressive symptoms, there are evidence-based recommendations for reducing diabetes distress in youth, including promoting parent-child collaboration and adaptive coping [19]. Additionally, Hood and colleagues demonstrated a reduction in adolescents’ diabetes distress following the resilience-building “Supporting Teens Problem Solving” intervention that targeted adaptive coping skills [44]. Integrating behavioral health providers into pediatric diabetes clinics is an optimal way to not only implement screeners of diabetes distress and depressive symptoms but also to ensure patients who are identified on the screeners can receive appropriate mental/behavioral health care [45].
The strengths of the current study include a relatively large, racially/ethnically and socioeconomically diverse sample, use of an objective measure of self-management, and an innovative approach to examining the intersection of depressive symptoms and diabetes distress during an important developmental period. Still, methodological limitations are also important to consider. This study was cross-sectional, and, thus, longitudinal follow-up is necessary to determine causal and temporal relations between the constructs (e.g., whether diabetes distress precedes or leads to depressive symptoms or vice versa) and stability or change in these groups over time. While we used validated screeners, we collected the data in the context of a research study during a diabetes clinic visit, not as part of routine clinical care. It is possible that youth may have responded differently if they knew their diabetes care team would see their answers. We determined the groups from this study using a published cut-point on the measure of depressive symptoms [23] and published clinical cut-point or mean on the measures of diabetes distress [20, 21]. Findings may differ if providers or researchers use other measures or other cut-points to establish the groups. There are slight differences between the two versions of the PAID (PAID-C and PAID-T), which might have biased findings between the two age groups. Still, the two versions performed similarly in our analyses and we used version-specific clinical cut-points rather than total scores to minimize this risk. The small cell size (n=9) of youth reporting elevated diabetes distress only precluded our ability to examine this group separately. It is possible that youth with elevated diabetes distress and elevated depressive symptoms have different characteristics than those without elevated depressive symptoms, so our results should be evaluated in a larger sample. While we describe our population as generally “preadolescent” based on age, we did not consider pubertal status. The children in our study were likely at various stages of pubertal development, which is also related to depressive symptoms [48] and glycemic outcomes [49]. Additionally, experiences may differ for youth receiving care in Canada versus the United States, given differences in health care delivery systems and access [50]. Finally, this study did not include a measure of anxiety. However, given then age of onset for anxiety disorders is in this age range group (11 years old) and that anxiety can often progress into subsequent depression in later years [15], future research including measures of general and diabetes-specific anxiety (e.g., fear of hypoglycemia) would be informative.
Conclusions
Overall, many preadolescents reported experiencing elevated depressive symptoms or diabetes distress or both that would merit referral to a mental/behavioral health professional for further assessment and treatment. Thus, routine psychological screening may be especially useful for children in this age range, and including both measures of depressive symptoms and diabetes distress is likely to identify more youth who are struggling and could benefit from professional support. Early recognition of emotional distress can help providers offer early intervention to prevent declines in A1C and quality of life that are common in the adolescent period. Indeed, future longitudinal research is needed to determine the predictive value of screening for these constructs over time. In the meantime, the findings of this study suggest that diabetes care providers should consider monitoring both depressive symptoms and diabetes distress through routine screening at diabetes clinic appointments.
Acknowledgements
The authors acknowledge the research staff and trainees who collected and managed study data: Viena Cao, Teresa Falk, Kelly Fegan-Bohm, Wendy Hammerman, Farida Khetani, Alani Mays, Karen Elizabeth Shults, and Courtney Titus.
Financial Support: This work was supported by the National Institutes of Diabetes and Digestive and Kidney Diseases (K12 DK 097696 PI: B Anderson).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: None
References
- [1].Fisher L, Gonzalez JS, Polonsky WH. The confusing tale of depression and distress in patients with diabetes: a call for greater clarity and precision. Diabet Med 2014;31:764–72. doi: 10.1111/dme.12428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Hagger V, Hendrieckx C, Sturt J, Skinner TC, Speight J. Diabetes distress among adolescents with type 1 diabetes: a systematic review. Curr Diab Rep 2016;16:9. doi: 10.1007/s11892-015-0694-2 [DOI] [PubMed] [Google Scholar]
- [3].Hessler DM, Fisher L, Polonsky WH, Masharani U, Strycker LA, Peters AL, et al. Diabetes distress is linked with worsening diabetes management over time in adults with type 1 diabetes. Diabet Med 2017;34:1228–34. doi: 10.1111/dme.13381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-5. 5th ed. Washington, DC: American Psychiatric Association; 2013. [Google Scholar]
- [5].Polonsky WH, Anderson BJ, Lohrer PA, Welch G, Jacobson AM, Aponte JE, et al. Assessment of diabetes-related distress. Diabetes Care 1995;18:754–60. doi: 10.2337/diacare.18.6.754 [DOI] [PubMed] [Google Scholar]
- [6].Abdoli S, Jones DH, Vora A, Stuckey H. Improving Diabetes Care: Should We Reconceptualize Diabetes Burnout? Diabetes Educ. 2019;45(2):214–224. doi: 10.1177/0145721719829066. [DOI] [PubMed] [Google Scholar]
- [7].Abdoli S, Hessler D, Smither B, Miller-Bains K, Burr EM, Stuckey HL. New insights into diabetes burnout and its distinction from diabetes distress and depressive symptoms: A qualitative study. Diabetes Research and Clinical Practice. 2020;169. doi: 10.1016/j.diabres.2020.108446. [DOI] [PubMed] [Google Scholar]
- [8].Diabetes Canada Clinical Practice Guidelines Expert Committee. Diabetes Canada 2018 clinical practice guidelines for the prevention and management of diabetes in Canada. Can J Diabetes 2018;42(Suppl 1):S1–S325. [DOI] [PubMed] [Google Scholar]
- [9].Delamater AM, de Wit M, McDarby V, Malik JA, Hilliard ME, Northam E, et al. ISPAD Clinical Practice Consensus Guidelines 2018: Psychological care of children and adolescents with type 1 diabetes. Pediatr Diabetes 2018;19:237–49. doi: 10.1111/pedi.12736 [DOI] [PubMed] [Google Scholar]
- [10].Young-Hyman D, de Groot M, Hill-Briggs F, Gonzalez JS, Hood K, Peyrot M. Psychosocial care for people with diabetes: a position statement of the American Diabetes Association. Diabetes Care 2016;39:2126–40. doi: 10.2337/dc16-2053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Hilliard ME, De Wit M, Wasserman RM, Butler AM, Evans M, Weissberg-Benchell J, et al. Screening and support for emotional burdens of youth with type 1 diabetes: strategies for diabetes care providers. Pediatr Diabetes 2018;19:534–43. doi: 10.1111/pedi.12575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Markowitz JT, Volkening LK, Butler DA, Laffel LM. Youth-perceived burden of type 1 diabetes: problem areas in diabetes survey-pediatric version (PAID-Peds). J Diabetes Sci Technol 2015;9:1080–5. doi: 10.1177/1932296815583506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Hagger V, Hendrieckx C, Cameron F, Pouwer F, Skinner TC, Speight J. Cut points for identifying clinically significant diabetes distress in adolescents with type 1 diabetes using the PAID-T: results from Diabetes MILES Youth–Australia. Diabetes Care 2017;40:1462–8. doi: 10.2337/dc17-0441 [DOI] [PubMed] [Google Scholar]
- [14].Horowitz JL, Garber J. The prevention of depressive symptoms in children and adolescents: A meta-analytic review. Journal of Consulting and Clinical Psychology. 2006;74(3):401–415. doi: 10.1037/0022-006X.74.3.401 [DOI] [PubMed] [Google Scholar]
- [15].Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- [16].Delamater AM, Patiño-Fernández AM, Smith KE, Bubb J. Measurement of diabetes stress in older children and adolescents with type 1 diabetes mellitus. Pediatric Diabetes. 2013;14(1):50–56. doi: 10.1111/j.1399-5448.2012.00894.x [DOI] [PubMed] [Google Scholar]
- [17].Fegan-Bohm K, Minard CG, Anderson BJ, et al. Diabetes distress and HbA1c in racially/ethnically and socioeconomically diverse youth with type 1 diabetes. Pediatric Diabetes. 2020;21(7):1362–1369. doi: 10.1111/pedi.13108 [DOI] [PubMed] [Google Scholar]
- [18].Evans MA, Weil LEG, Shapiro JB, et al. Psychometric Properties of the Parent and Child Problem Areas in Diabetes Measures. J Pediatr Psychol. 2019;44(6):703–713. doi: 10.1093/jpepsy/jsz018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Jaser SS. Psychological problems in adolescents with diabetes. Adolesc Med State Art Rev 2010;21:138–51. [PMC free article] [PubMed] [Google Scholar]
- [20].Weissberg-Benchell J, Antisdel-Lomaglio J. Diabetes-specific emotional distress among adolescents: feasibility, reliability, and validity of the problem areas in diabetes-teen version. Pediatr Diabetes 2011;12:341–4. doi: 10.1111/j.1399-5448.2010.00720.x [DOI] [PubMed] [Google Scholar]
- [21].Evans MA, Weil LEG, Shapiro JB, Anderson LM, Vesco AT, Rychlik K, et al. Psychometric properties of the Parent and Child Problem Areas in Diabetes measures. J Pediatr Psychol 2019;44:703–13. doi: 10.1093/jpepsy/jsz018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Shapiro JB, Vesco AT, Weil LEG, Evans MA, Hood KK, Weissberg-Benchell J. Psychometric properties of the Problem Areas in Diabetes: Teen and Parent of Teen versions. J Pediatr Psychol 2018;43:561–71. doi: 10.1093/jpepsy/jsx146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Kovacs M Children’s Depression Inventory 2nd Edition (CDI 2): Technical Manual North Tonawanda, NY: Multi-Health Systems; 2011. [Google Scholar]
- [24].de Wit M, Winterdijk P, Aanstoot HJ, Anderson B, Danne T, Deeb L, et al. Assessing diabetes-related quality of life of youth with type 1 diabetes in routine clinical care: the MIND Youth Questionnaire (MY-Q). Pediatr Diabetes 2012;13:638–46. doi: 10.1111/j.1399-5448.2012.00872.x [DOI] [PubMed] [Google Scholar]
- [25].Hagger V, Hendrieckx C, Cameron F, Pouwer F, Skinner TC, Speight J. Diabetes distress is more strongly associated with A1C than depressive symptoms in adolescents with type 1 diabetes: results from Diabetes MILES Youth-Australia. Pediatr Diabetes 2018;19:840–7. doi: 10.1111/pedi.12641 [DOI] [PubMed] [Google Scholar]
- [26].van Bastelaar KM, Pouwer F, Geelhoed-Duijvestijn PH, Tack CJ, Bazelmans E, Beekman AT, et al. Diabetes-specific emotional distress mediates the association between depressive symptoms and glycaemic control in type 1 and type 2 diabetes. Diabet Med 2010;27:798–803. doi: 10.1111/j.1464-5491.2010.03025.x [DOI] [PubMed] [Google Scholar]
- [27].Westen SC, Warnick J, Entessari M, Albanese-O’Neill A, Schatz D, Haller MJ, et al. Poor adherence in adolescents with type 1 diabetes associated with distress, fear of hypoglycemia, and executive functioning. Diabetes. 2018;67(Supplement 1). doi: 10.2337/db18-847-P [DOI] [Google Scholar]
- [28].Hood KK, Huestis S, Maher A, Butler D, Volkening L, Laffel LM. Depressive symptoms in children and adolescents with type 1 diabetes: association with diabetes-specific characteristics. Diabetes Care 2006;29:1389–91. doi: 10.2337/dc06-0087 [DOI] [PubMed] [Google Scholar]
- [29].Lawrence JM, Standiford DA, Loots B, Klingensmith GJ, Williams DE, Ruggiero A, et al. Prevalence and correlates of depressed mood among youth with diabetes: the SEARCH for Diabetes in Youth study. Pediatrics 2006;117:1348–58. doi: 10.1542/peds.2005-1398 [DOI] [PubMed] [Google Scholar]
- [30].Jackson B, Goodman E. Low social status markers: do they predict depressive symptoms in adolescence? Race Soc Probl 2011;3:119–28. doi: 10.1007/s12552-011-9047-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Strandberg RB, Graue M, Wentzel-Larsen T, Peyrot M, Rokne B. Relationships of diabetes-specific emotional distress, depression, anxiety, and overall well-being with A1C in adult persons with type 1 diabetes. J Psychosom Res 2014;77:174–9. doi: 10.1016/j.jpsychores.2014.06.015 [DOI] [PubMed] [Google Scholar]
- [32].Comeaux SJ, Jaser SS. Autonomy and insulin in adolescents with type 1 diabetes. Pediatr Diabetes 2010;11:498–504. doi: 10.1111/j.1399-5448.2009.00625.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].McGrail KM, van Doorslaer E, Ross NA, Sanmartin C. Income-related health inequalities in Canada and the United States: a decomposition analysis. Am J Public Health 2009;99:1856–63. doi: 10.2105/AJPH.2007.129361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Lorant V, Deliège D, Eaton W, Robert A, Philippot P, Ansseau M. Socioeconomic inequalities in depression: a meta-analysis. Am J Epidemiol 2003;157:98–112. doi: 10.1093/aje/kwf182 [DOI] [PubMed] [Google Scholar]
- [35].Graber JA, Hill JC, Saczawa ME. Childhood and the Entry into Adolescence: A Pivotal Period in Health-Related Behaviors and Prevention. In: Sloboda Z, Petras H, eds. Defining Prevention Science. Advances in Prevention Science. Springer US; 2014:59–86. doi: 10.1007/978-1-4899-7424-2 [DOI] [Google Scholar]
- [36].Foster NC, Beck RW, Miller KM, Clements MA, Rickels MR, DiMeglio LA, et al. State of type 1 diabetes management and outcomes from the T1D Exchange in 2016–2018. Diabetes Technol Ther 2019;21:66–72. doi: 10.1089/dia.2018.0384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Quinn CC, Swasey KK, Crabbe JCF, Shardell MD, Terrin ML, Barr EA, et al. The impact of a mobile diabetes health intervention on diabetes distress and depression among adults: secondary analysis of a cluster randomized controlled trial. JMIR Mhealth Uhealth 2017;5:e183. doi: 10.2196/mhealth.8910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Zagarins SE, Allen NA, Garb JL, Welch G. Improvement in glycemic control following a diabetes education intervention is associated with change in diabetes distress but not change in depressive symptoms. J Behav Med 2012;35:299–304. doi: 10.1007/s10865-011-9359-z [DOI] [PubMed] [Google Scholar]
- [39].Lutes LD, Cummings DM, Littlewood K, Solar C, Carraway M, Kirian K, et al. COMRADE: a randomized trial of an individually tailored integrated care intervention for uncontrolled type 2 diabetes with depression and/or distress in the rural southeastern US. Contemp Clin Trials 2018;70:8–14. doi: 10.1016/j.cct.2018.04.007 [DOI] [PubMed] [Google Scholar]
- [40].Friis AM, Johnson MH, Cutfield RG, Consedine NS. Kindness matters: a randomized controlled trial of a mindful self-compassion intervention improves depression, distress, and A1C among patients with diabetes. Diabetes Care 2016;39:1963–71. doi: 10.2337/dc16-0416 [DOI] [PubMed] [Google Scholar]
- [41].Mathiesen AS, Egerod I, Jensen T, Kaldan G, Langberg H, Thomsen T. Psychosocial interventions for reducing diabetes distress in vulnerable people with type 2 diabetes mellitus: a systematic review and meta-analysis. Diabetes Metab Syndr Obes 2019;12:19–33. doi: 10.2147/DMSO.S179301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Wood A, Harrington R, Moore A. Controlled trial of a brief cognitive–behavioural intervention in adolescent patients with depressive disorders. J Child Psychol Psychiatry 1996;37:737–46. doi: 10.1111/j.1469-7610.1996.tb01466.x [DOI] [PubMed] [Google Scholar]
- [43].Garber J, Clarke GN, Weersing VR, Beardslee WR, Brent DA, Gladstone TRG, et al. Prevention of depression in at-risk adolescents: a randomized controlled trial. JAMA 2009;301:2215–24. doi: 10.1001/jama.2009.788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Young JF, Mufson L, Gallop R. Preventing depression: a randomized trial of interpersonal psychotherapy-adolescent skills training. Depress Anxiety 2010;27:426–33. doi: 10.1002/da.20664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Stice E, Shaw H, Bohon C, Marti CN, Rohde P. A meta-analytic review of depression prevention programs for children and adolescents: factors that predict magnitude of intervention effects. J Consult Clin Psychol 2009;77:486–503. doi: 10.1037/a0015168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Hood KK, Iturralde E, Rausch J, Weissberg-Benchell J. Preventing diabetes distress in adolescents with type 1 diabetes: results 1 year after participation in the STePS program. Diabetes Care 2018;41:1623–30. doi: 10.2337/dc17-2556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Kichler JC, Harris MA, Weissberg-Benchell J. Contemporary roles of the pediatric psychologist in diabetes care. Curr Diabetes Rev 2015;11:210–21. doi: 10.2174/1573399811666150421104449 [DOI] [PubMed] [Google Scholar]
- [48].Thapar A, Collishaw S, Pine DS, Thapar AK. Depression in adolescence. Lancet 2012;379:1056–67. doi: 10.1016/S0140-6736(11)60871-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Moreland EC, Tovar A, Zuehlke JB, Butler DA, Milaszewski K, Laffel LM. The impact of physiological, therapeutic and psychosocial variables on glycemic control in youth with type 1 diabetes mellitus. J Pediatr Endocrinol Metab 2004;17:1533–44. doi: 10.1515/jpem.2004.17.11.1533 [DOI] [PubMed] [Google Scholar]
- [50].Kaiser SV, Sundaram V, Cohen E, Shulman R, Guan J, Sanders L, et al. Health care for children with diabetes mellitus from low-income families in Ontario and California: a population-based cohort study. CMAJ Open 2016;4:E729–E36. doi: 10.9778/cmajo.20160075 [DOI] [PMC free article] [PubMed] [Google Scholar]


