Abstract
Background:
Statins are widely used to lower lipids and reduce cardiovascular events. In vitro studies and small studies in patients with hyperlipidemias show statins inhibit tissue factor (TF) and blood coagulation mechanisms. We assessed the effects of simvastatin on TF and coagulation biomarkers in patients entered in STATCOPE, a multicenter, randomized, placebo-controlled trial of simvastatin (40 mg daily) versus placebo on exacerbation rates in patients with chronic obstructive pulmonary disease (COPD).
Methods:
In 227 patients (114 simvastatin; 113 placebo; mean (±SEM) age 62 ± 0.53 years, 44.5 % women) we measured (baseline; 6 and 12 months): whole blood membrane TF-procoagulant activity (TF-PCA) and plasma factors VIIa, VII, VIII, fibrinogen, TF antigen, tissue factor pathway inhibitor (TFPI), thrombin-antithrombin complexes (TAT) and D-dimer. We excluded patients with diabetes, cardiovascular disease and those taking or requiring a statin.
Results:
In the statin group, there was a small increase in TF-PCA (from 25.18±1.08 to 30.36±1.10 U/ml; p=0.03) over 12 months; factors VIIa and VIII, fibrinogen, TAT and D-dimer did not change. Plasma TFPI (from 52.4±1.75 to 44.7±1.78 ng/mL; p< 0.0001) and FVIIC (1.23±0.04 to 1.15±0.03 U/mL; p=0.03) decreased and correlated with total cholesterol levels. No changes in biomarkers were observed with placebo.
Conclusions:
In contrast to previous studies on statins, in COPD patients without diabetes, cardiovascular disease or requiring a statin treatment, simvastatin (40 mg per day) did not decrease TF or factors VIIa and VIII, fibrinogen, TAT or D-dimer. The decreases in TFPI and factor VII reflect the decrease in serum lipids.
Keywords: Tissue factor pathway, Statin, Simvastatin, Blood coagulation, Chronic obstructive pulmonary disease
Introduction
Statins are widely used to lower lipids and reduce cardiac mortality and morbidity in the primary and secondary prevention of cardiovascular disease (CVD) (1, 2). In addition to lipid lowering, other effects attributed to statins, include anti-inflammatory effects and inhibition of the blood coagulation mechanisms (3, 4). Tissue factor (TF) is a membrane-bound protein expressed on monocytes and other cells and is the physiological initiating mechanism for blood coagulation (5). Circulating TF in blood is thrombogenic (5, 6) and levels are elevated in disorders associated with increased risk of thrombotic events, including diabetes mellitus (7, 8), cardiovascular disease (9), stroke (10), sickle cell disease (11) and chronic obstructive pulmonary disease (COPD) (12).
Several relatively small studies have reported that statins inhibit expression of TF and coagulation mechanisms, often with conflicting findings (4). This evidence comes from studies studying the effects of statins in vitro on monocytes from subjects with hypercholesterolemia or cardiovascular disease (4, 13, 14) and studies using monocytes stimulated with lipopolysaccharide (LPS) (13, 14), which acutely upregulates TF. In vivo evidence has come from animal models with hyperlipidemia (15, 16). In some studies statin effects were studied in human subjects administered LPS (17) or in the blood from skin bleeding time wounds of patients with cardiovascular disease and/or hypercholesterolemia (18–21) where simvastatin was shown to inhibit activation of prothrombin, factor V and factor XIII, and enhance factor Va inactivation (19, 21). Some authors have suggested that the effects of statins on the coagulation mechanisms are unrelated to their lipid lowering effects (4, 16). Statins have also been reported to decrease plasma coagulation factors, including factor VII, FVIII and fibrinogen, markers of thrombin generation, and tissue factor pathway inhibitor (TFPI) in hypercholesterolemic patients (4, 20, 22, 23). One study showed that fibrin clots in patients with COPD, including those with hyperlipidemia and diabetes mellitus, are denser and resistant to lysis than those from subjects matched for age, sex and cardiovascular risk factors and these aspects were improved by simvastatin (24). However, the effects of statins on blood coagulation mechanisms and tissue factor remain unclear, particularly in the absence of hyperlipidemia or LPS activation of monocytes.
COPD patients have an increased incidence of cardiovascular disease (25) and venous thromboembolism (VTE) (26). We have previously shown that circulating TF procoagulant activity (TF-PCA) is elevated in patients with quiescent COPD (12). Exacerbations of COPD have been reported to increase lung and systemic inflammation which has been associated with an increased risk of acute myocardial infarction, congestive heart failure, pulmonary embolism, cardiac arrhythmias, stroke and venous thromboembolism (VTE) (27–29). Some of these events, such as VTE, pulmonary embolism, congestive heart failure, and acute myocardial infarction, share clinical presentations with COPD exacerbations, which are difficult for clinicians to discern (30). Based on retrospective and small studies reporting statins decrease exacerbations, hospitalizations, and mortality in patients with COPD (31), the efficacy of simvastatin to prevent COPD exacerbations was studied in a large multicenter, randomized, trial of simvastatin (40 mg daily) versus placebo (STATCOPE) (32). In STATCOPE, simvastatin did not affect exacerbation rates in moderate-to-severe COPD patients at high risk for exacerbations (32). However, we postulated that statins may have a beneficial effect on ameliorating the procoagulant blood biomarker profile of patients with moderate to severe COPD that were prone to exacerbations.
Herein we report the effects of simvastatin (40 mg daily) on TF and plasma coagulation biomarkers and their relationships to COPD exacerbation rates in patients studied in STATCOPE. In STATCOPE, patients with diabetes mellitus or cardiovascular disease and those who were taking statins or required statins were excluded, providing a unique group to study statin effects.
Methods
Study Design
We measured coagulation biomarkers in 227 patients (114 simvastatin; 113 placebo) enrolled in STATCOPE and from whom sequential blood samples were available at baseline, 6 and 12 months. Details regarding STATCOPE study design have been previously reported (32).
In STATCOPE, 885 moderate-to-severe COPD patients (mean (±SEM) age 62±0.53 years, 44% women) from 45 centers were randomized to treatment with simvastatin (40 mg daily) or placebo for 12–36 months. Patients were eligible if they were 40 to 80 years of age, had COPD (defined by a forced expiratory volume in 1 second [FEV1] of less than 80% and a ratio of FEV1 to forced vital capacity of less than 70%), smoking history of 10 or more pack-years, were receiving supplemental oxygen or treatment with glucocorticoids or antibiotic agents, or had an emergency department visit or hospitalization for COPD within the past year. Patients with diabetes or cardiovascular disease and those who were taking statins or who required statins on the basis of Adult Treatment Panel III criteria were excluded.
Biomarker Assays
Blood was collected into one-tenth volume of 3.2% sodium citrate from outpatients following fasting for 12 hours. Aliquots (1 ml) were frozen for whole blood TF-PCA assay. Blood samples were centrifuged (2000g, 30 min, room temperature) within 60 min of blood draw. Plasma was harvested immediately, frozen as multiple aliquots, and stored at −80°C. They were shipped on dry ice to the Sol Sherry Thrombosis Research Center, Temple University, Philadelphia. All biomarker assays were performed in blinded manner with respect to the treatment group.
Tissue factor procoagulant activity (TF-PCA) was measured in whole-blood cell lysates from blood collected into one-tenth volume of sodium citrate as previously described (33) (11)19 with a two-stage clotting assay using recombinant human FVIIa (Sekisui Diagnostics, LLC. Stamford, CT), human factor X (Haematologic Technologies Inc. Essex Junction, VT), and pooled normal human plasma (George King Bio-medical, Inc. Overland Park, KS) containing phospholipid vesicles. The TF-PCA assay measures cell-bound and microparticle-associated TF in lysed whole blood. HemosIL RecombiPlastin 2G (Instrumentation Laboratory, Bedford, MA) was used as a standard.
Coagulation biomarkers were measured in plasma harvested by centrifugation from blood collected into one-tenth volume of 3.2% sodium citrate (33). Coagulation factor VII (FVIIC), factor VIII (FVIIIC) and fibrinogen were measured by standard clotting assays (33). Plasma factor VIIa (the activated form of FVII) activity was measured by a commercially available assay (STACLOT VIIa-rTF, Diagnostica Stago Inc. Parsippany, NJ). Plasma thrombin-antithrombin complexes (Enzygnost TAT micro, Siemens Healthcare Diagnostics. Marburg, Germany), D-dimer (IMUCLONE D-dimer, Sekisui Diagnostics, LLC. Stamford, CT), tissue factor antigen (IMUBIND Tissue Factor, Sekisui Diagnostics, LLC. Stamford, CT), and TFPI (IMUBIND total TFPI, American Diagnostica GmbH. Greenwich, CT) were measured using ELISAs.
The methods for the measurements of serum lipids, C-reactive protein (CRP), blood sugar and HbA1c have been described (32).
Statistical Analysis
Descriptive statistics were generated and compared between groups, including demographics, baseline levels of coagulation biomarkers, lipid and CRP levels. Log transformations were employed for biomarkers (TF-PCA, FVIIa, TAT, D-dimer, TF antigen) with skewed distribution to achieve normality. Continuous data is expressed as mean and standard deviation (SD) and categorical data is expressed as frequency and percentage. Patient baseline characteristics and coagulation biomarkers were compared by Pearson Chi-Square test for categorical variables and two-sample t-test for continuous variables. A linear mixed-effects model was used to examine the effect of statin and placebo on the coagulation biomarker levels change over time, and the relationship to cancer and smoking status at baseline. Two-sample t-test was used to examine the relation between the coagulation marker at 12 months and the presence or absence of exacerbation at 12 months. Pearson’s correlation coefficients were calculated to examine the relationships among biomarkers, between biomarker levels and lipid levels at baseline, and relationship between biomarker levels at 12 months and the number of acute exacerbations. In addition, Pearson’s correlation coefficients were used to examine relationships between biomarkers using the average of the levels at three time points in each patient and separated by treatment groups. All statistical analyses were performed with the use of SAS software, version 9.4 (SAS Institute Inc., Cary, NC).
Results
Demographic and baseline clinical characteristics were similar in the statin and placebo groups (Table 1). These were also similar in patients in whom the coagulation biomarkers were measured, and in the patients in whom these measurements were not performed in the parent STATCOPE study.
Table 1.
Demographics of the Patients*.
| Placebo (N=113) |
||
|---|---|---|
| Age yr | 62.8±8.8 | 62.0±8.2 |
| Sex - n (%) Female | 47 (41.2%) | 54 (47.8%) |
| Black - n (%) | 24 (21.1%) | 21 (18.6%) |
| FEV1 - % of predicted value | 44.4±17.0 | 42.3±18.0 |
| Smoking history - pack year | 47.0±23.6 | 51.4±28.1 |
| Current Smoking Status - n (%) | 38 (33.3%) | 40 (35.4%) |
| Body Mass Index, BMI- (kg/m2) | 26.8±5.8 | 27.7±8.1 |
| High Blood Pressure - n (%) | 48 (42.1%) | 40 (35.4%) |
| History of Clots - n (%) | 4 (3.5%) | 2 (1.8%) |
| Cirrhosis - n (%) | 2 (1.8%) | 1.0 (0.9%) |
| Cancer - n (%) | 8 (7.0%) | 15 (13.3%) |
Shown are means ± SD. There were no significant differences between the simvastatin and the placebo groups. FEV1 denotes forced expiratory volume in 1 second.
Coagulation Biomarkers
Baseline levels of coagulation biomarkers were comparable between the placebo and statin groups. Over the 12-month follow-up mean TF-PCA levels minimally increased in the statin group (from 25.18±1.08 to 30.36±1.10 U/ml; p=0.03) but not in the placebo group (Figure 1). Plasma TFPI (p< 0.0001) and FVIIC (p=0.03) decreased in the statin group but were unchanged in the placebo group. Other markers (fibrinogen, FVIIIC, FVIIa, TAT, D-dimer) did not change over time in the statin group. In the placebo group, there was no change in any biomarker. TFPI levels were lower at 6 and 12 months in the statin compared to the placebo group (Figure 1); other biomarkers were similar between groups. There were 23 patients (15 placebo, 8 statin) with known cancer at baseline (Table 1). Among the coagulation biomarkers, only FVIIa was lower in the cancer group (3.96±0.83 mU/ml), n=23) compared to those without cancer (4.28+0.mU/ml, n=200) (p=0.023). A multivariable analyses performed taking into account the presence of cancer did not reveal a significant effect on the change of coagulation biomarkers between placebo and statin group.
Figure 1.
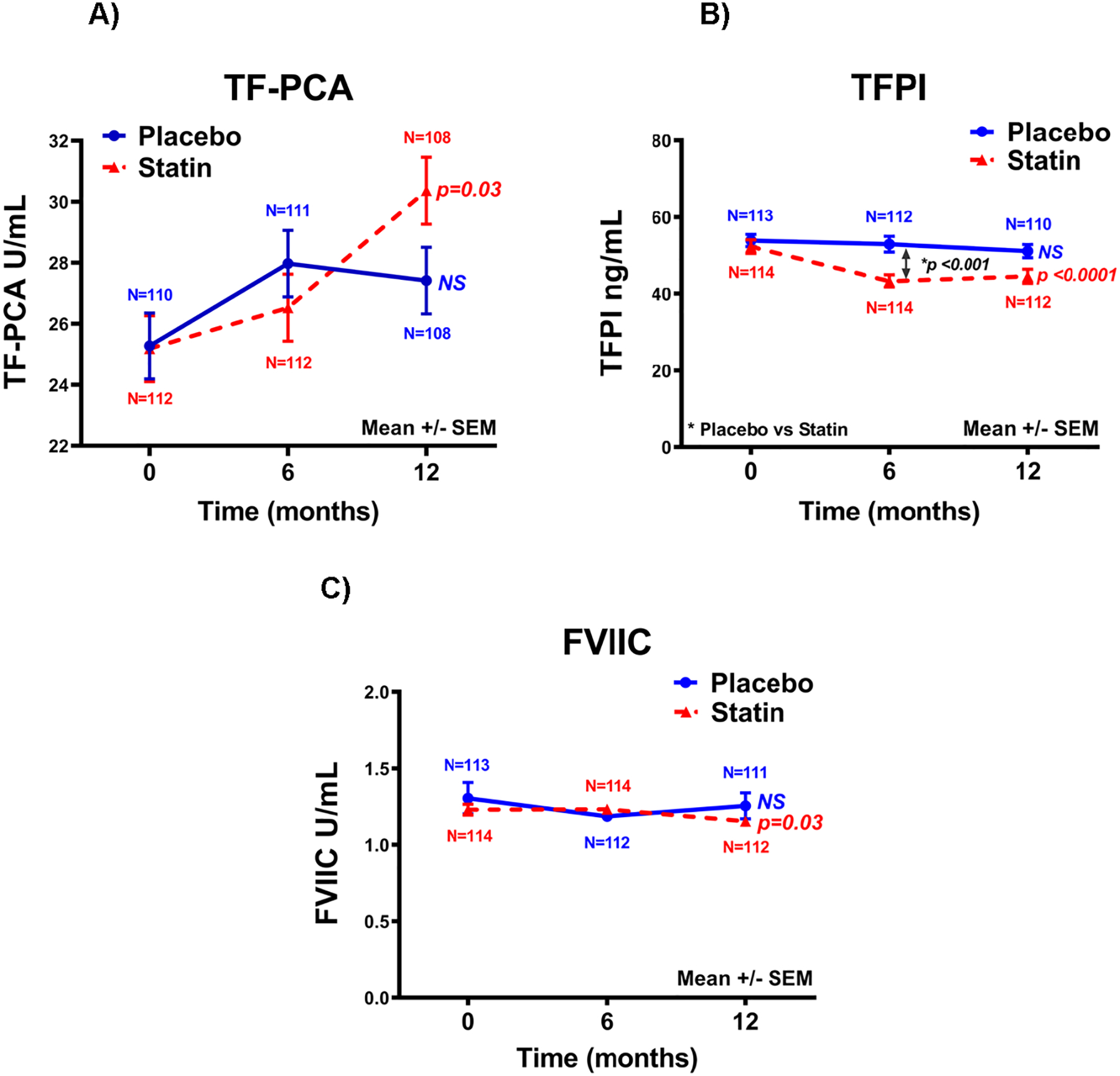
Effect of simvastatin and placebo on (A) tissue factor procoagulant activity (TF-PCA), (B) tissue factor pathway inhibitor (TFPI) and (C) factor VII coagulant activity (FVIIC). Shown are mean ± SEM for patients (n=108–113) in the placebo and the simvastatin (n=108–114) groups. The p-values shown are for change over time in the placebo or statin group. TFPI levels were different between placebo and statin groups (p<0.0001) and this is shown. TF-PCA and FVIIC levels were not different between the two groups.
Relationship of Coagulation Biomarkers and COPD Exacerbations
In the statin group, fibrinogen (p=0.003) and FVIIIC (p=0.0004) were higher in those who reported exacerbations over the 1-year post randomization in STATCOPE compared to those without exacerbation; this was not observed in the placebo group (Figure 2). In the placebo group there were weak correlations between TF-PCA (r= 0.19; p=0.05), TAT (r= −0.20, p=0.04) and D-dimer (r= −0.20, p=0.03) levels and the number of acute exacerbations. In the statin group, there was no relationship between the number of exacerbations and biomarker levels.
Figure 2.
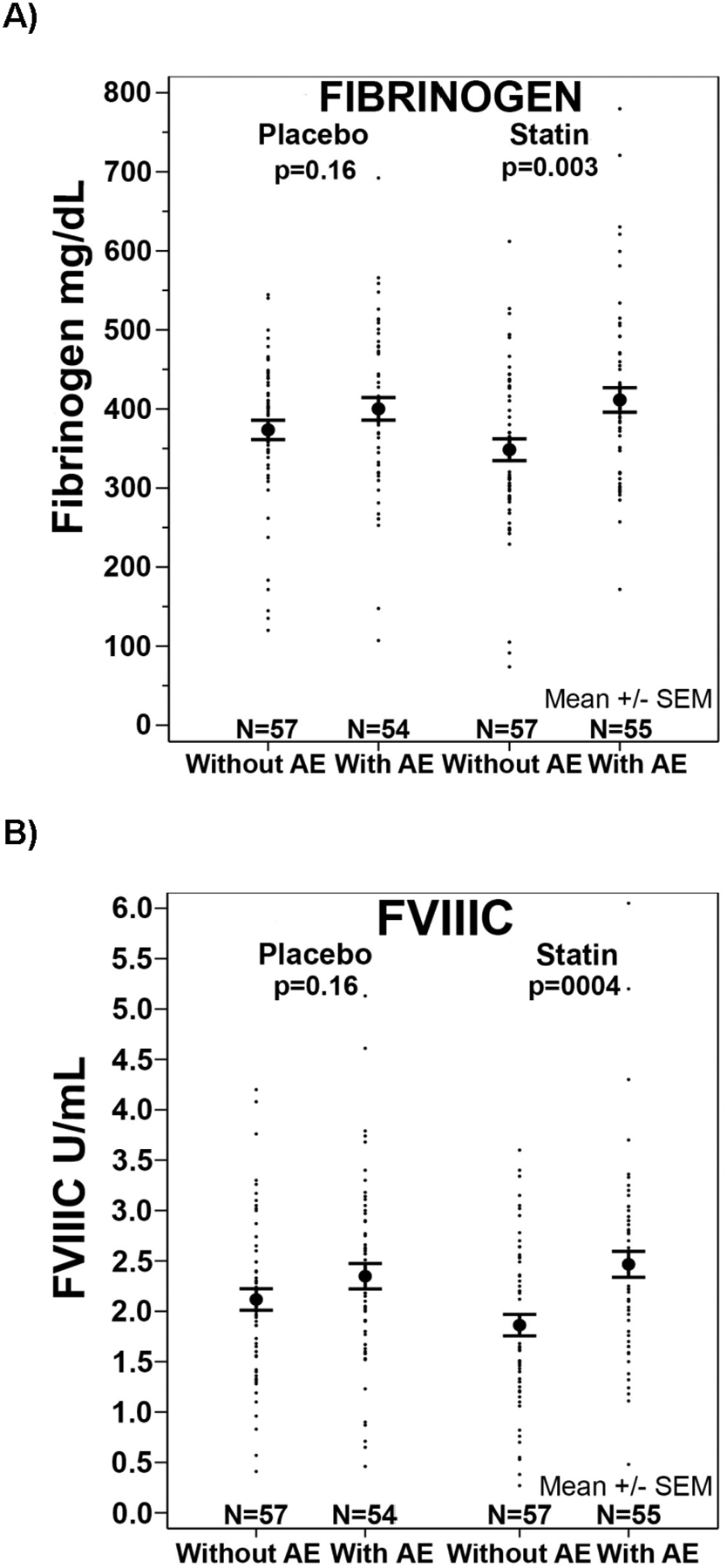
Plasma fibrinogen (A) and factor VIII (B) (mean ± SEM) at 12 months in patients with and without acute exacerbation (AE) at 1 year in the placebo or statin groups.
Because of the potential effects of smoking on disease progression and coagulation parameters, we examined the relationship between the coagulation factor levels and smoking status at enrollment; 33.3 and 35.45% of patients were current smokers in the statin and placebo groups, respectively. The data was analyzed combining patients in the two treatment groups by repeated measures analyses; only plasma D-dimer (p=0.007) and TAT (p=0.037) were different between current smokers versus non-smokers (Figure 3). The change in these biomarkers over time was not statistically significant between smokers versus non-smokers. Plasma D-dimer was lower at all three time points in current smokers compared to non-smokers. TAT levels were higher in current-smokers at baseline and no different at 6 and 12 months compared to non-smokers (Figure 3). These findings indicate the effect of smoking status on these parameters and suggest there may be differential effects of smoking on TAT and D-dimer levels.
Figure 3.
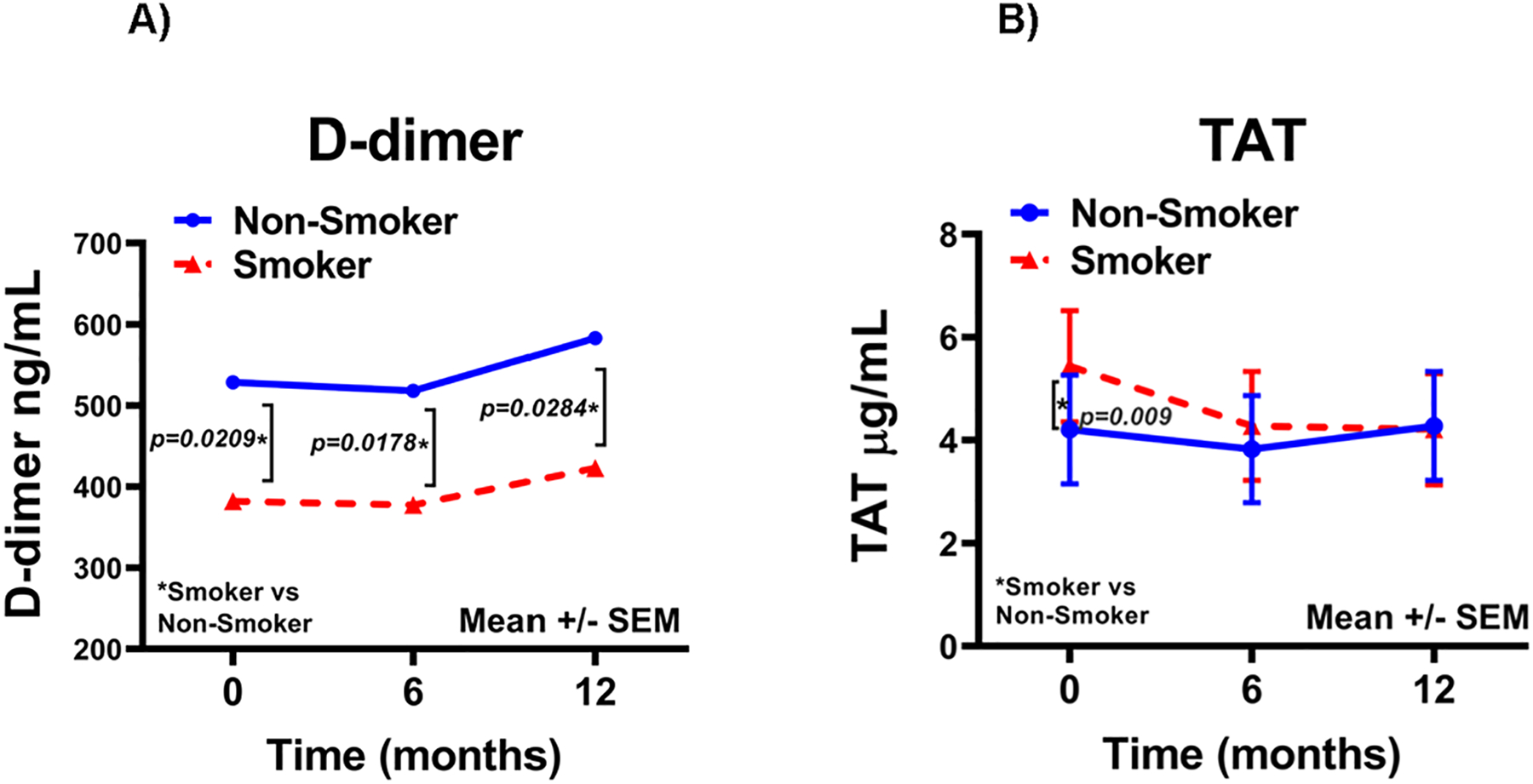
Plasma D-dimer (A) and TAT (B) (mean ± SEM) in current smokers (n=78) and non-smokers (n=149) at baseline. The data was analyzed combining patients in the statin and placebo groups.
Relationships between blood coagulation biomarker levels
We explored the relationships between levels of blood coagulation biomarkers, TF-PCA, plasma fibrinogen and FVIIIC, all of which are acute phase reactants, at baseline (Table 2). Combining both the statin and placebo groups, there was a positive relationship between membrane-bound TF-PCA with plasma fibrinogen (r=0.41, p=0.0001); and between fibrinogen and FVIIIC (r=0.27, p<0.0001). TF-PCA (r=0.19, p=0.004) and fibrinogen (r=0.39, p=0.0001) levels correlated with CRP levels. TF is a membrane protein and in this study we measured it in membranes (TF-PCA) and in plasma (TF antigen). There was no correlation between plasma TF antigen with TF-PCA, plasma FVIIIC or plasma fibrinogen. Plasma TF correlated with FVIIa (r=0.19, p=0.002). Plasma FVIIC correlated with FVIIa (r=0.24, p=0.0003), and TAT correlated with D-dimer (r=0.21, p=0.001). FVIIC (r=0.19, p=0.004) and FVIIa (r=0.21, p=0.001) correlated with TAT levels. In addition, we examined the above relationships using the average of the levels at three time points in each patient, separated by treatment groups, and found the relationships to be the similar (Supplemental Table 1).
Table 2.
Correlations between baseline levels of coagulation markers.
| TFPI | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| TFPCA |
r p |
−0.05 0.48 |
0.08 0.23 |
−0.003 0.97 |
0.27 <.0001 |
0.41 <.0001 |
−0.05 0.50 |
0.05 0.47 |
0.04 0.56 |
| TF-antigen |
r p |
0.09 0.19 |
0.19 0.002 |
0.008 0.91 |
−0.03 0.61 |
0.07 0.30 |
0.15 0.02 |
0.03 0.63 |
|
| FVIIC |
r p |
0.24 <.0003 |
−0.06 0.34 |
−0.11 0.09 |
0.19 0.004 |
−0.07 0.33 |
0.08 0.24 |
||
| FVIIa |
r p |
−0.01 0.89 |
−0.12 0.06 |
0.21 0.001 |
−0.04 0.60 |
0.04 0.58 |
|||
| FVIIIC |
r p |
0.30 <.0001 |
0.02 0.74 |
0.10 0.12 |
0.11 0.10 |
||||
| Fibrinogen |
r p |
-0.16 0.02 |
−0.05 0.44 |
0.09 0.2 |
|||||
| TAT |
r p |
0.21 0.001 |
0.07 0.32 |
||||||
| D-dimer |
r p |
0.13 0.05 |
Shown are Pearson’s correlations coefficients and p-values, and includes patients from placebo and statin group (N=222–227).
Relationship between coagulation biomarkers and serum lipids, HbA1c and CRP
Baseline levels of total cholesterol (Mean ± SD: 193.4±37.0 vs. 195.4±36.7 mg per deciliter, p=0.44), LDL cholesterol (Mean: 112.0±29.0 vs. 114.0±29.0 mg per deciliter, p=0.62) and HDL cholesterol (Mean: 61.5±22.4 vs. 63.0±26.3 mg per deciliter, p=0.99), and triglycerides (Mean: 98.8+57.5 vs. 92.2+49.8 mg per deciliter, p=0.32) were similar in the statin and placebo groups. At 1 year, LDL cholesterol (79.8±31.7 vs 105.4±28.6 mg per deciliter; p<0.0001) and total cholesterol (164.2±39.4 vs 189.1±39.4 mg per deciliter; p<0.0001) were lower in the statin group compared to the placebo group; HDL cholesterol levels were similar (65.4±27.6 vs 62.9±24.5 mg per deciliter (p=0.57). APOA1, triglycerides and glucose levels at baseline and 12 months, and HbA1c measured at baseline were not different between the groups (data not shown) (Supplemental Table 2). Baseline CRP levels were similar in both treatment groups (Supplemental Table 2) and did not change over time in either group.
We explored the relationship between baseline coagulation biomarker and serum lipid levels, combining both groups (Table 3). There was a weak relationship of plasma FVIIC with total cholesterol (r=0.18, p=0.007) and APOA1 (r=0.14; p=0.03); and of plasma FVIIa with HDL (r=0.18, p=0.009) and APOA1 (r=0.15, p=0.02) levels. A stronger relationship was noted between plasma TFPI and total cholesterol (r=0.47, p<0.0001), LDL (r=0.44, p<0.0001), HDL (r=0.18, p=0.006), and APOA1 (r=0.22, p=0.001). There was a weak relationship between FVIII and HDL (r=0.17; p=0.009) and APOA1 (r= 0.15; p=0.02). No relationships were observed between TF-PCA and serum lipid levels. TF-PCA (r=0.16; p=0.02) and FVIIIC (r=0.21, p=0.002) correlated with HbA1c at baseline. CRP levels correlated with TF-PCA (r=0.19, p=0.004), fibrinogen (0.39, p<0.0001) and D-dimer (r=0.20, p=0.002). In addition, we examined the above relationships using an average of the three timed measurements in each patient and separated by treatment groups and found the same relationships (Supplemental Table 3).
Table 3.
Correlation between baseline levels of coagulation biomarkers and lipids, HbA1c, blood glucose and CRP.
| TFPI | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total Cholesterol |
r p |
0.007 0.91 |
0.06 0.37 |
0.18 0.007 |
0.07 0.33 |
0.05 0.47 |
0.05 0.45 |
−0.01 0.88 |
0.01 0.86 |
0.47 <.0001 |
| LDL |
r p |
0.04 0.59 |
−0.03 0.70 |
0.12 0.07 |
−0.05 0.46 |
−0.08 0.21 |
0.11 0.12 |
0.03 0.66 |
0.08 0.23 |
0.44 <.0001 |
| HDL |
r p |
−0.06 0.42 |
0.11 0.11 |
0.07 0.29 |
0.17 0.008 |
0.17 0.009 |
−0.05 0.47 |
−0.04 0.51 |
−0.08 0.24 |
0.18 0.006 |
| Triglycerides |
r p |
0.05 0.50 |
0.03 0.63 |
0.12 0.07 |
−0.04 0.55 |
−0.004 0.95 |
0.001 0.99 |
−0.02 0.81 |
−0.003 0.96 |
0.02 0.77 |
| APOA1 |
r p |
−0.09 0.17 |
0.15 0.03 |
0.14 0.03 |
0.15 0.02 |
0.15 0.02 |
−0.07 0.31 |
−0.02 0.72 |
−0.05 0.45 |
0.22 0.001 |
| HbA1c |
r p |
0.16 0.02 |
0.09 0.18 |
0.08 0.28 |
0.02 0.80 |
0.21 0.001 |
0.08 0.23 |
0.11 0.08 |
0.10 0.12 |
−0.01 0.81 |
| Blood glucose |
r p |
−0.06 0.39 |
−0.08 0.25 |
−0.03 0.68 |
−0.09 0.16 |
−0.07 0.30 |
−0.03 0.68 |
−0.08 0.25 |
0.02 0.74 |
−0.06 0.37 |
| CRP |
r p |
0.19 0.004 |
0.06 0.38 |
−0.02 0.83 |
−0.08 0.25 |
0.05 0.48 |
0.39 <.0001 |
−0.02 0.80 |
0.20 0.002 |
−0.05 0.44 |
Shown are Pearson’s correlation coefficients and p-values and includes patients from placebo and statin group combined (N=220–226).
Discussion
In this large, multicenter, placebo-controlled trial in moderate-to severe COPD patients at risk for exacerbations of COPD but without cardiovascular disease, diabetes mellitus or an indication for a statin, we found that simvastatin (40 mg daily) administered for 12 months did not decrease circulating TF-PCA, but was associated with a small increase in TF-PCA levels (Figure 1). In addition, we observed no decrease in plasma fibrinogen, FVIIIC, FVIIa, and markers of thrombin generation with the use of statins as reported by others (4, 20, 22, 23). There was a decrease at 12 months in plasma FVIIC in the statin group (Figure 1), which has been observed in some but not other studies (4). FVII binds to lipid membranes and the decrease may reflect the lower lipid levels in the statin group. We observed a relationship between FVIIC with total cholesterol and LDL levels. Moreover, the decrease in plasma FVII may reflect increased binding to membrane-bound TF and removal from plasma secondary to the increase in TF-PCA. We have observed a decline in plasma FVIIC associated with an increase in TF-PCA in healthy human volunteers during hyperglycemic clamps (33). Plasma TFPI decreased in the statin group (Figure 1), as also observed in previous studies (4). TFPI in plasma circulates bound to lipids (34); the decline likely reflects the lipid lowering effect of statin. In the present study, basal TFPI levels correlated with total, LDL and HDL cholesterol levels.
Our results differ from reports that statins decrease expression of TF and blood coagulation biomarkers. Most studies were small in size and more importantly, focused on patients with hypercholesterolemia and/or patients with or at high risk for cardiovascular disease (4, 20, 22). Hypercholesterolemia is associated with upregulated monocytes (a source of TF) and blood coagulation mechanisms (4, 9, 16). Thus, an unrecognized lipid lowering statin effect may be a confounding factor in studies where a statin was administered to patients with hyperlipidemia or diabetes mellitus. In STATCOPE, patients with cardiovascular disease, diabetes mellitus and those with an indication statin therapy were excluded (32) and this enabled us to define the effects of simvastatin unencumbered by the effects of increased lipids and glucose, or manifest atherothrombotic disease, all of which are associated with elevation in TF and coagulation biomarkers (4, 5, 8, 9, 12). The strengths of this study include the relatively large sample size, the prospective design and the presence of a placebo group.
Previous studies have suggested that statins have anti-inflammatory effects (3, 4). In our study, TF-PCA levels correlated with plasma fibrinogen, FVIIIC, and CRP (Tables 2 and 3), three recognized acute phase reactants indicating that TF-PCA also functions as an acute phase reactant. We found no decrease in the statin group in fibrinogen, FVIII, TF-PCA or CRP, particularly relevant in COPD because it is an intense pro-inflammatory state. Moreover, plasma fibrinogen and FVIIIC were higher in those with COPD exacerbations at 1 year compared to those without (Figure 2), indicating ongoing inflammation. Interestingly, this was observed only in the statin group and the reasons are unclear. In our moderate to severe COPD patients, simvastatin did not decrease CRP levels (current study) or the rate of acute exacerbations (32). In the JUPITER trial of apparently healthy persons without hyperlipidemia but with elevated high-sensitivity CRP levels, rosuvastatin significantly reduced CRP levels and the incidence of major cardiovascular events (35). The lack of effect in our study may be related to the ongoing, greater inflammatory state in COPD patients compared to the healthy persons in the JUPITER trial, in addition to the difference in the statin used.
In the statin group there was a small increase in circulating TF-PCA associated with a decrease in its primary inhibitor TFPI. Elevated levels of circulating TF-PCA are thrombogenic (5) and reported in patients with diseases associated with increased predisposition to thrombosis (8–11, 33). The STATCOPE trial included patients with moderate-to severe COPD at increased risk for exacerbations based on their prior exacerbation history and/or the need for use of home oxygen therapy, but without known cardiovascular disease or an indication for a statin. TF is both prothrombotic and pro-inflammatory (5). COPD patients have an increased predisposition to cardiovascular events (25, 36). In the STATCOPE trial, there were no differences in the fatal or nonfatal cardiovascular events between the placebo and statin groups (32). Further studies are needed to clarify the impact of the statin-induced changes in TF and coagulation mechanisms on clinical thrombotic events in patients with moderate to severe COPD who are prone to increased exacerbation risk.
Our study has some limitations. The participants had moderate-to-severe airway disease and it is unclear whether the observation that simvastatin did not decrease TF-PCA levels would apply to patients with less pulmonary impairment or those treated with a higher simvastatin dose. Moreover, the findings may not be applicable to a broader population with hyperlipidemias and other comorbidities.
In conclusion, in contrast to previous studies on statins, in COPD patients without diabetes, cardiovascular disease or requiring a statin treatment, simvastatin (40 mg per day) did not decrease TF or factors VIIa and VIII, fibrinogen, TAT or D-dimer. The decreases observed in TFPI and factor VII reflect the decrease in serum lipids.
Supplementary Material
Essentials.
Studies in vitro and in patients with hyperlipidemias show statins inhibit tissue factor and coagulation.
We studied effects of simvastatin (40 mg daily, 12 months) vs placebo on TF and coagulation in 227 COPD patients.
Simvastatin did not decrease membrane-bound TF, or plasma factor VIIa, factor VIII, fibrinogen, TAT or D-dimer.
Simvastatin decreased plasma TFPI and factor VII, which correlated with decreases in lipids.
Acknowledgments
Supported by grants from the National Heart, Lung, and Blood Institute of the National Institutes of Health (U10 HL074407 U10 HL074408, U10HL074409, U10 HL074416, U10 HL074418, U10 HL074422, U10 HL074424, U10 HL074428, U10 HL074431, U10 HL074439, and U10 HL074441) and the Canadian Institutes of Health Research (115074); STATCOPE ClinicalTrials.gov number, NCT01061671
Footnotes
Conflict of Interest Statement
None of the authors have any conflicts of interest to declare with respect to this manuscript.
References
- 1.Brugts JJ, Yetgin T, Hoeks SE, Gotto AM, Shepherd J, Westendorp RG, et al. The benefits of statins in people without established cardiovascular disease but with cardiovascular risk factors: meta-analysis of randomised controlled trials. BMJ. 2009;338:b2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ross SD, Allen IE, Connelly JE, Korenblat BM, Smith ME, Bishop D, et al. Clinical outcomes in statin treatment trials: a meta-analysis. Arch Intern Med. 1999;159:1793–802. [DOI] [PubMed] [Google Scholar]
- 3.Oesterle A, Laufs U, Liao JK. Pleiotropic Effects of Statins on the Cardiovascular System. Circ Res. 2017;120:229–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Undas A, Brummel-Ziedins KE, Mann KG. Statins and blood coagulation. Arterioscler Thromb Vasc Biol. 2005;25:287–94. [DOI] [PubMed] [Google Scholar]
- 5.Grover SP, Mackman N. Tissue Factor: An Essential Mediator of Hemostasis and Trigger of Thrombosis. Arterioscler Thromb Vasc Biol. 2018;38:709–25. [DOI] [PubMed] [Google Scholar]
- 6.Giesen PL, Rauch U, Bohrmann B, Kling D, Roque M, Fallon JT, et al. Blood-borne tissue factor: another view of thrombosis. Proc Natl Acad Sci U S A. 1999;96:2311–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Singh A, Boden G, Homko C, Gunawardana J, Rao AK. Whole-blood tissue factor procoagulant activity is elevated in type 1 diabetes: effects of hyperglycemia and hyperinsulinemia. Diabetes Care. 2012;35(6):1322–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boden G, Vaidyula VR, Homko C, Cheung P, Rao AK. Circulating tissue factor procoagulant activity and thrombin generation in patients with type 2 diabetes: effects of insulin and glucose. J Clin Endocrinol Metab. 2007;92:4352–8. [DOI] [PubMed] [Google Scholar]
- 9.Sambola A, Osende J, Hathcock J, Degen M, Nemerson Y, Fuster V, et al. Role of risk factors in the modulation of tissue factor activity and blood thrombogenicity. Circulation. 2003;107:973–7. [DOI] [PubMed] [Google Scholar]
- 10.Gentile NT, Vaidyula VR, Kanamalla U, DeAngelis M, Gaughan J, Rao AK. Factor VIIa and tissue factor procoagulant activity in diabetes mellitus after acute ischemic stroke: impact of hyperglycemia. Thromb Haemost. 2007;98:1007–13. [DOI] [PubMed] [Google Scholar]
- 11.Key NS, Slungaard A, Dandelet L, Nelson SC, Moertel C, Styles LA, et al. Whole blood tissue factor procoagulant activity is elevated in patients with sickle cell disease. Blood. 1998;91:4216–23. [PubMed] [Google Scholar]
- 12.Vaidyula VR, Criner GJ, Grabianowski C, Rao AK. Circulating tissue factor procoagulant activity is elevated in stable moderate to severe chronic obstructive pulmonary disease. Thromb Res. 2009;124:259–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bruni F, Puccetti L, Pasqui AL, Pastorelli M, Bova G, Cercignani M, et al. Different effect induced by treatment with several statins on monocyte tissue factor expression in hypercholesterolemic subjects. Clin Exp Med. 2003;3:45–53. [DOI] [PubMed] [Google Scholar]
- 14.Ferro D, Basili S, Alessandri C, Cara D, Violi F. Inhibition of tissue-factor-mediated thrombin generation by simvastatin. Atherosclerosis. 2000;149:111–6. [DOI] [PubMed] [Google Scholar]
- 15.Aikawa M, Rabkin E, Sugiyama S, Voglic SJ, Fukumoto Y, Furukawa Y, et al. An HMG-CoA reductase inhibitor, cerivastatin, suppresses growth of macrophages expressing matrix metalloproteinases and tissue factor in vivo and in vitro. Circulation. 2001;103:276–83. [DOI] [PubMed] [Google Scholar]
- 16.Owens AP 3rd, Passam FH, Antoniak S, Marshall SM, McDaniel AL, Rudel L, et al. Monocyte tissue factor-dependent activation of coagulation in hypercholesterolemic mice and monkeys is inhibited by simvastatin. J Clin Invest. 2012;122:558–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Steiner S, Speidl WS, Pleiner J, Seidinger D, Zorn G, Kaun C, et al. Simvastatin blunts endotoxin-induced tissue factor in vivo. Circulation. 2005;111:1841–6. [DOI] [PubMed] [Google Scholar]
- 18.Undas A, Brummel-Ziedins KE, Potaczek DP, Stobierska-Dzierzek B, Bryniarski L, Szczeklik A, et al. Atorvastatin and quinapril inhibit blood coagulation in patients with coronary artery disease following 28 days of therapy. J Thromb Haemost. 2006;4:2397–404. [DOI] [PubMed] [Google Scholar]
- 19.Undas A, Celinska-Lowenhoff M, Brummel-Ziedins KE, Brozek J, Szczeklik A, Mann KG. Simvastatin given for 3 days can inhibit thrombin generation and activation of factor V and enhance factor Va inactivation in hypercholesterolemic patients. Arterioscler Thromb Vasc Biol. 2005;25:1524–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Undas A, Celinska-Lowenhoff M, Domagala TB, Iwaniec T, Dropinski J, Lowenhoff T, et al. Early antithrombotic and anti-inflammatory effects of simvastatin versus fenofibrate in patients with hypercholesterolemia. Thromb Haemost. 2005;94:193–9. [DOI] [PubMed] [Google Scholar]
- 21.Undas A, Brummel KE, Musial J, Mann KG, Szczeklik A. Simvastatin depresses blood clotting by inhibiting activation of prothrombin, factor V, and factor XIII and by enhancing factor Va inactivation. Circulation. 2001;103:2248–53. [DOI] [PubMed] [Google Scholar]
- 22.Sanguigni V, Pignatelli P, Lenti L, Ferro D, Bellia A, Carnevale R, et al. Short-term treatment with atorvastatin reduces platelet CD40 ligand and thrombin generation in hypercholesterolemic patients. Circulation. 2005;111:412–9. [DOI] [PubMed] [Google Scholar]
- 23.Adams NB, Lutsey PL, Folsom AR, Herrington DH, Sibley CT, Zakai NA, et al. Statin therapy and levels of hemostatic factors in a healthy population: the Multi-Ethnic Study of Atherosclerosis. J Thromb Haemost. 2013;11:1078–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Undas A, Kaczmarek P, Sladek K, Stepien E, Skucha W, Rzeszutko M, et al. Fibrin clot properties are altered in patients with chronic obstructive pulmonary disease. Beneficial effects of simvastatin treatment. Thromb Haemost. 2009;102:1176–82. [DOI] [PubMed] [Google Scholar]
- 25.Andre S, Conde B, Fragoso E, Boleo-Tome JP, Areias V, Cardoso J, et al. COPD and Cardiovascular Disease. Pulmonology. 2019;25:168–76. [DOI] [PubMed] [Google Scholar]
- 26.Barba R, Zapatero A, Marco J, Losa JE, Plaza S, Casas JM, et al. Venous thromboembolism in COPD hospitalized patients. J Thromb Thrombolysis. 2012;33:82–7. [DOI] [PubMed] [Google Scholar]
- 27.Moua T, Wood K. COPD and PE: a clinical dilemma. Int J Chron Obstruct Pulmon Dis. 2008;3(2):277–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Borvik T, Braekkan SK, Enga K, Schirmer H, Brodin EE, Melbye H, et al. COPD and risk of venous thromboembolism and mortality in a general population. Eur Respir J. 2016;47:473–81. [DOI] [PubMed] [Google Scholar]
- 29.Akpinar EE, Hosgun D, Akpinar S, Atac GK, Doganay B, Gulhan M. Incidence of pulmonary embolism during COPD exacerbation. J Bras Pneumol. 2014;40:38–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Beghe B, Verduri A, Roca M, Fabbri LM. Exacerbation of respiratory symptoms in COPD patients may not be exacerbations of COPD. Eur Respir J. 2013;41(:993–5. [DOI] [PubMed] [Google Scholar]
- 31.He Y, Li X, Gasevic D, Brunt E, McLachlan F, Millenson M, et al. Statins and Multiple Noncardiovascular Outcomes: Umbrella Review of Meta-analyses of Observational Studies and Randomized Controlled Trials. Ann Intern Med. 2018;169:543–53. [DOI] [PubMed] [Google Scholar]
- 32.Criner GJ, Connett JE, Aaron SD, Albert RK, Bailey WC, Casaburi R, et al. Simvastatin for the prevention of exacerbations in moderate-to-severe COPD. N Engl J Med. 2014;370:2201–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vaidyula VR, Rao AK, Mozzoli M, Homko C, Cheung P, Boden G. Effects of hyperglycemia and hyperinsulinemia on circulating tissue factor procoagulant activity and platelet CD40 ligand. Diabetes. 2006;55:202–8. [PubMed] [Google Scholar]
- 34.Mast AE. Tissue Factor Pathway Inhibitor: Multiple Anticoagulant Activities for a Single Protein. Arterioscler Thromb Vasc Biol. 2016;36:9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM Jr., Kastelein JJ, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359:2195–207. [DOI] [PubMed] [Google Scholar]
- 36.Curkendall SM, DeLuise C, Jones JK, Lanes S, Stang MR, Goehring E Jr., et al. Cardiovascular disease in patients with chronic obstructive pulmonary disease, Saskatchewan Canada cardiovascular disease in COPD patients. Ann Epidemiol. 2006;16:63–70. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


