To the Editor
The increasing prevalence of Methicillin-resistant Staphylococcus aureus over the last two decades has led to increased vancomycin use and vancomycin-associated infusion reactions. Immediate IgE-independent hypersensitivity reactions (HSRs) to vancomycin, known as “Red Man Syndrome,” are commonly seen in clinical practice and, in the majority of cases, avoided by slowing the infusion and/or premedication with antihistamines [1]. Conversely, IgE-dependent HSRs to vancomycin are rare, only described in case reports, and vancomycin-specific IgE measurement has not been reported in the literature [2, 3]. In some cases, the two types of immediate HSRs are indistinguishable, and patients are assumed to have an IgE-mediated HSR resulting in vancomycin avoidance even when needed [4].
In clinical practice, the evaluation of immediate HSRs to vancomycin is challenging as skin testing is not validated and the positive- and negative-predictive values of the results are not known [2]. Published approaches are based on single-subject case reports where the methodologies significantly differed [1, 5, 6]. Moreover, the recommendations for nonirritating intradermal skin test concentrations for immediate vancomycin HSRs are based on studies using sterile water (SW) as the vancomycin diluent following the manufacturer’s recommendations [1, 3, 7]. However, a major limitation of these recommendations is underlying irritative potential of intradermal testing with SW. In the absence of reliable in vitro tests for drug allergy testing, diagnosis commonly relies on skin testing; however, guidelines for optimal drug diluents for skin testing are not stated by the Joint Task Force [8]. It is unknown whether the use of alternative diluents, such as human serum albumin-based sterile saline (HSS), might influence the irritant potential and solubility of irritative drugs like vancomycin. Therefore, the purpose of this study was to evaluate the effect of diluents on vancomycin skin test responses in vancomycin-naïve subjects.
Vancomycin-naïve subjects (n = 11) were enrolled between July 2019 and February 2020 (Table E1). Our objective was to establish a range for non-irritating doses for intradermal vancomycin skin testing without having a potential confounder of pre-existing antibodies. Subjects were excluded <18 years old, had a previous exposure to vancomycin, or had taken antihistamines within seven days prior to skin testing. The study was approved by the Johns Hopkins Medicine Institutional Review Board and all study subjects provided written informed consent.
Vancomycin Hydrochloride for Injection, USP in 500-mg vials (Alvogen, Inc.) was reconstituted in SW, normal saline (NS), lactated ringer’s solution (RL), or HSS, and serially diluted to yield a range of concentrations of 1 to 3000 μg/mL. The pH of each solution was measured using a glass-bulb probe digital pH meter. Test solutions (0.02 to 0.04 ml) were injected intradermally in duplicates with a 27-gauge needle to produce a bleb measuring 2 to 4 mm in diameter on the volar aspect of the forearm. Histamine, 0.1 μg/mL, was used as the positive control, and the respective diluent served as the negative control. The longest wheal diameter at 20 min assessed by visual measurements and short-wave infrared camera. Local symptoms produced at different concentrations were recorded. To avoid suggestion bias and because irritation and pruritis are subjective complaints, these were only documented when volunteered by the subject.
The statistical analyses were conducted using GraphPad Prism 8.0 (GraphPad Software, Inc., CA, US). The dose response curve (DRC) for vancomycin skin responses were calculated and the EC50 for the different test solutions were compared using one-way analysis of variance (ANOVA) with post-hoc Tukey honest significant difference (HSD) test. A two-sided p-value of <0.05 was considered statistically significant.
Based on the prior methods currently referenced in the literature for nonirritating intradermal skin test concentrations for immediate vancomycin HSRs, we skin tested intradermally three vancomycin-naïve subjects to vancomycin diluted in SW at 1 to 1000 μg/mL [1, 7]. All subjects had positive skin responses and a wheal diameter size plateau at 25 μg/mL. All subjects reported immediate sensations of pain to each concentration including the SW negative control. This outcome confirmed the irritative characteristics of SW for intradermal skin testing and suggested that other diluents might mitigate this problem. Three diluents were tested: two with no intrinsic pH buffering capacity (NS, HSS) and one with pH buffering capacity (LR) to counter the acidity of vancomycin hydrochloride. Vancomycin diluted in SW and NS lowered the pH more than in HSS and LR (Figure E1). The inclusion of lactate buffered the acidity of vancomycin even at the highest concentration tested; therefore, LR was expected to generate the least irritation. This expectation was not met and both LR and NS caused pain in the majority of subjects (Table 1). The best-tolerated diluent was HSS: this diluent elicited no complaints of pain but did produce a sensation of pruritus at the highest concentrations reported by all subjects (n = 11).
Table I.
Vancomycin intradermal skin responses in vancomycin- naïve subjects tested with vancomycin diluted in LR, NS, and HSS.
| Test Solution (μg/mL) | Wheal diameter, mm Mean (SD) | Wheal diameter ≥ 3 mm Total n (%) | Pain/Irritation Total n (%) | Pruritus§ Total n (%) |
|---|---|---|---|---|
| Vancomycin in LR (n = 6) | ||||
| 10 | 0 | 0 | 0 | |
| 30 | 3.2 (3.5) | 3 (50) | 0 | |
| 100 | 7.4 (1.3) | 6 (100) | 2 (33) | |
| 300 | 9.5 (1.0) | 5 (83) | ||
| 1000 | 9.3 (1.0) | |||
| 3000 | 9.6 (0.7) | |||
| Vancomycin in NS (n = 4) | ||||
| 10 | 0 | 0 | 0 | |
| 30 | 4.6 (3.2) | 3 (75) | 0 | |
| 100 | 6.5 (0.9) | 4 (100) | 1 (25) | |
| 300 | 8.1 (1.7) | 3 (75) | ||
| 1000 | 8.5 (1.5) | |||
| 3000 | 10.4 (2.9) | |||
| Vancomycin in HSS (n = 11) | ||||
| 10 | 0 | 0 | 0 | |
| 30 | 0 | 0 | 0 | |
| 100 | 1.4 (3.1) | 2 (22) | 0 | |
| 300 | 4.1 (3.7) | 7 (77) | 1 (11) | |
| 1000 | 9.3 (2.0) | 11 (100) | 11 (100) | |
| 3000 | 11.3 (2.9) | |||
LR, Lactated ringer’s solution; NS, Normal saline; HSS, Human serum albumin in sterile saline in LR, and NS.
Pruritus was the only symptom reported by the subjects tested to vancomycin diluted in HSS.
The mean EC50 for LR, NS, and HSS were 50 μg/mL (95% CI 38-65), 60 μg/mL (95% CI 39-95), and 370 μg/mL (95% CI 298–464) respectively (Figure 1A). Wheal diameter performed by the short-wave infrared camera DRC generated similar EC50 results. LogEC50 of vancomycin diluted in LR, NS, and HSS were compared by one-way ANOVA [F(2, 18) 39, p<0.001). Tukey HSD exhibited a significant difference in the logEC50 for vancomycin diluted in HSS vs. LR (p<0.001) and NS (p<0.001) but not for vancomycin diluted in LR vs. NS (p=0.98). There were only two subjects who had a positive response to vancomycin in HSS at 100 μg/mL (Figure E2). Figure 1B shows the differences in EC50 among the 11 tested subjects. These dose relationships generally achieved a similar plateau for all subjects at their mean highest tested concentration that averaged at 76 ± 16% of the positive control (Figure E2).
Figure 1:
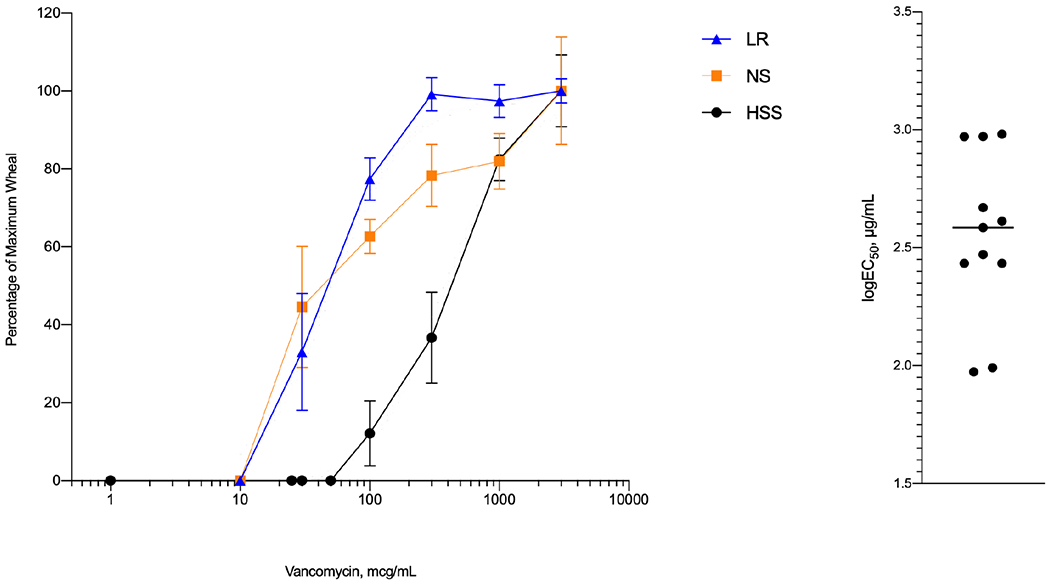
Vancomycin intradermal skin test responses. Panel A: Non-paired comparisons of the dose-response curve skin responses for vancomycin resuspended in LR (black), NS (orange), and HSS (blue). LR, Lactated ringer’s solution; NS, Normal saline; HSS, Human serum albumin based sterile saline. Values are given as mean ± SEM. Panel B: Individual (n = 11) logEC50 values for vancomycin diluted in HSS at concentrations of 1 to 3000 μg/mL.
Information for non-irritative doses and optimal diluent for skin tests with non-beta-lactam antibiotics including vancomycin is sparse despite their high irritant potential [1, 7, 9]. In contrast, data support the use of buffers with stabilizers (HSS) for aeroallergens to avoid unexplained variability [10]. In preliminary testing it became apparent that SW potentiates vancomycin’s irritant effects and leads to immediate pain and scant biological variability [1, 3, 7]. Nonetheless, the current nonirritating intradermal skin test concentrations for immediate vancomycin HSRs are based on vancomycin diluted in SW [3, 9, 11].
Therefore, we suspected that the diluent might be sub-optimal for testing the mast cell’s biological response in skin and found that vancomycin diluted in HSS produces skin responses at concentrations consistent with a mast cell activation response. Notably, these findings were consistent with reports of vancomycin-induced mast cell responses in vitro (i.e., showing a plateau with isolated mast cells at concentrations ≥ 500 μg/mL but no responses ≤100 μg/mL) [12]. The skin test response follows this pattern and elicits a sensation of pruritus instead of irritation, another suggestive characteristic for a mast cell response. While LR and NS generated DRCs (Figure 1) that were graded like HSS, although at lower concentrations, the sensation of irritation was reported by the majority of subjects. The pH of the different test solutions appeared to be unrelated to irritation.
An important feature of the skin test response when using HSS may be the spread in the EC50 among the subjects, which varied approximately 30-fold. Future studies will determine if this heterogeneity reflects a highly variable immediate IgE-independent HSR to vancomycin. Another interesting feature of the DRCs of vancomycin diluted in HSS was that the plateau skin response was lesser than the histamine positive control. This is unlike the IgE-mediated skin test response for aeroallergens that often exceeds the histamine control and may reflect the mechanism of the stimulus, possibly a limitation of the mast cell response through the Mas-Related G Protein-Coupled Receptor-X2 (MRGPRX2) [13]. IgE-independent activation of humanized mast cells by vancomycin was recently described via the MRGPRX2 receptor; however future human studies are needed to further evaluate this hypothesis [12]. A study comparing patients with immediate IgE-independent HSR to vancomycin with appropriate controls will generate a better understanding of the mechanism and clinical application.
Our work has some limitations that need consideration. Our findings were based on a small sample size; nevertheless, except for a couple of unexplained and interesting outliers, the observed results, with vancomycin diluted in HSS, showed a reasonable degree of variability. We studied only vancomycin-naïve subjects as our objective was to establish a range for non-irritating doses without having a potential confounder of pre-existing antibodies. Nevertheless, the DRCs for patients with a history of vancomycin immediate IgE-independent HSR may differ yet testing this possibility in future studies is the reason for first establishing a biologically relevant skin test procedure.
This re-defined DRC for vancomycin skin responses is compatible with a biological mast cell response and could lead to an understanding of immediate IgE-independent HSR and distinguish its risk from IgE-mediated HSRs. Thus, clinicians should consider variability in skin test responses based on the diluent and consider diluting vancomycin in HSS when testing for immediate vancomycin hypersensitivity reactions.
Supplementary Material
Acknowledgments
Supported by: S.A.A. receives support from the National Institutes of Health (NIH)-National Institute of General Medical Sciences (grant no. T32GM066691). E.O. receives career development support from the NIH-National Institute of Allergy and Infectious Diseases (NIAID, grant no. K23AI139394). O.T. receives support from the NIH-National Institute of Diabetes and Digestive and Kidney (grant no. 5F30DK120160).
Abbreviations:
- ANOVA
Analysis of variance
- CI
confidence interval
- DRC
Dose response curve
- EC50
Half maximal effective concentration
- HSD
Honest significant difference
- HSR
Hypersensitivity reactions
- HSS
Human serum albumin-based sterile saline
- Ig
Immunoglobulin
- LR
Lactated ringer’s solution
- MRGPRX2
Mas-Related G-protein-coupled Receptor X2
- NS
Normal saline
- SW
Sterile water
Footnotes
Conflict of Interest statement: The authors declare that they have no relevant conflicts of interest.
Ethical approval: The study was approved by the Johns Hopkins Medicine Institutional Review Board (IRB00153292).
Disclosures: None
REFERENCES
- 1.Polk RE, Israel D, Wang J, Venitz J, Miller J, Stotka J. Vancomycin skin tests and prediction of “red man syndrome” in healthy volunteers. Antimicrob Agents Chemother. 1993;37(10):2139–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Minhas JS, Wickner PG, Long AA, Banerji A, Blumenthal KG. Immune-mediated reactions to vancomycin: A systematic case review and analysis. Ann Allergy Asthma Immunol. 2016;116(6):544–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Broyles AD, Banerji A, Barmettler S, Biggs CM, Blumenthal K, Brennan PJ, et al. Practical Guidance for the Evaluation and Management of Drug Hypersensitivity: Specific Drugs. The Journal of Allergy and Clinical Immunology: In Practice. 2020;8(9):S16–S116. [DOI] [PubMed] [Google Scholar]
- 4.McAuley MA. Allergic reaction or adverse drug effect: correctly classifying vancomycin-induced hypersensitivity reactions. J Emerg Nurs. 2012;38(1):60–2. [DOI] [PubMed] [Google Scholar]
- 5.Anne S, Middleton E Jr., Reisman RE. Vancomycin anaphylaxis and successful desensitization. Ann Allergy. 1994;73(5):402–4. [PubMed] [Google Scholar]
- 6.Otani IM, Kuhlen JL Jr., Blumenthal KG, Guyer A, Banerji A. A role for vancomycin epicutaneous skin testing in the evaluation of perioperative anaphylaxis. J Allergy Clin Immunol Pract. 2015;3(6):984–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Empedrad R, Darter AL, Earl HS, Gruchalla RS. Nonirritating intradermal skin test concentrations for commonly prescribed antibiotics. J Allergy Clin Immun. 2003;112(3):629. [DOI] [PubMed] [Google Scholar]
- 8.Joint Task Force on Practice P, American Academy of Allergy A, Immunology, American College of Allergy A, Immunology, Joint Council of Allergy A, et al. Drug allergy: an updated practice parameter. Ann Allergy Asthma Immunol. 2010;105(4):259–73. [DOI] [PubMed] [Google Scholar]
- 9.Broz P, Harr T, Hecking C, Grize L, Scherer K, Jaeger KA, et al. Nonirritant intradermal skin test concentrations of ciprofloxacin, clarithromycin, and rifampicin. Allergy. 2012;67(5):647–52. [DOI] [PubMed] [Google Scholar]
- 10.Norman PS, Marsh DG. Human serum albumin and Tween 80 as stabilizers of allergen solutions. J Allergy Clin Immunol. 1978;62(5):314–9. [DOI] [PubMed] [Google Scholar]
- 11.Empedrad R, Darter AL, Earl HS, Gruchalla RS. Nonirritating intradermal skin test concentrations for commonly prescribed antibiotics. J Allergy Clin Immunol. 2003;112(3):629–30. [DOI] [PubMed] [Google Scholar]
- 12.Navines-Ferrer A, Serrano-Candelas E, Lafuente A, Munoz-Cano R, Martin M, Gastaminza G. MRGPRX2-mediated mast cell response to drugs used in perioperative procedures and anaesthesia. Sci Rep. 2018;8(1):11628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Malling HJ. Diagnosis and immunotherapy of mould allergy. II. Reproducibility and relationship between skin sensitivity estimated by end-point titration and histamine equivalent reaction using skin prick test and intradermal test. Allergy. 1985;40(5):354–62. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


