Abstract
Background:
In contrast to pulmonary vascular resistance (PVR), PVR index (PVRI) accounts for variations in body habitus. We tested the association of PVRI compared to PVR with clinical outcomes in lean and obese (BMI ≥30 kg/m2) patients with pulmonary arterial hypertension (PAH).
Methods:
This retrospective study included adult patients with PAH who underwent right heart catheterization at Cleveland Clinic between February 1992 and November 2019.
Results:
We included 644 patients (mean age, 53 ± 16 years, and 74 % females). PAH was idiopathic or heritable in 44% of patients. Cardiac output increased (p <0.0001), while PVR decreased (p <0.0001) with increasing body weight. Both PVR and PVRI were associated with markers of disease severity, with more pronounced association for PVRI. Both PVR and PVRI were risk factors for first PAH hospitalization, mortality and mortality or lung transplant in the whole cohort and the group of patients with BMI < 30 kg/m2. However, PVRI (HR (95% CI): 1.06 (1.02 -1.11)), but not PVR (HR (95% CI): 1.03 (0.99-1.07)), was a risk factor for first PAH hospitalization in obese patients. In the obese group, neither PVR nor PVRI were risk factors for mortality.
Conclusions:
PVRI appears to have a stronger association than PVR with disease severity markers in PAH; however, both PVR and PVRI were similarly associated with hospitalizations and survival in the overall cohort. We found no strong evidence to recommend a change from PVR to PVRI in the definition of PAH.
Keywords: Pulmonary arterial hypertension, Pulmonary vascular resistance, Pulmonary vascular resistance index, Outcome, Mortality
Introduction:
Pulmonary arterial hypertension (PAH) is a type of pulmonary hypertension (PH) characterized by progressive narrowing of the small pulmonary arteries, that if left untreated, can lead to right heart failure and death (1). The diagnosis of PAH requires a distinct pre-capillary hemodynamic profile, characterized by a mean pulmonary artery pressure (mPAP) > 20 mmHg, pulmonary artery wedge pressure (PAWP) ≤ 15 mmHg and pulmonary vascular resistance (PVR) ≥ 3 Wood units (WU) (2).
In addition to being crucial for the diagnosis of PAH (2), PVR carries important prognostic implications as it has been associated with survival in some studies (3, 4). In the calculation of PVR, cardiac output (CO) is used in the denominator (PVR= (mPAP – PAWP) / CO); nevertheless, CO does not account for the body habitus of the patient and might not be a reliable indicator of cardiac performance. In fact, cardiac index (CI) is routinely used in clinical practice since it provides information on how the heart functions relative to the body size and not in isolation. Therefore, pulmonary vascular resistance index (PVRI= (mPAP – PAWP) / CI) might be a better hemodynamic parameter in predicting outcomes in patients with PAH. Despite these potential advantages, PVRI has not been routinely adopted in the adult population; and pulmonary hypertension (PH) guidelines and proceedings continue to use PVR (2).
There is no consensus on whether the use of indexed values of PVR by body surface area (BSA) is more appropriate, particularly as PVR and PVRI have not been directly compared. The use of PVRI may be particularly important in patients with obesity (BMI ≥ 30 kg/m2), a condition that continues to increase worldwide, with an age-adjusted prevalence among US adults of 42.4% (5). Obese individuals have higher CO, associated with bigger stroke volumes, given the larger BSA (6, 7), a condition that magnifies the differences between PVR and PVRI determinations; and therefore results in relatively lower values of PVR in comparison with PVRI. This gap may have a direct impact on determining the prognosis in patients with PAH and may explain why PVR failed to predict outcomes in some studies (8-11). Pulmonary vascular resistance index could be a better predictor of the severity of PAH, particularly in obese individuals (12, 13).
There is a paucity of data on the use of PVRI in adult patients with PAH. Benza et al. identified a baseline PVRI cut-off value of 30 WU.m2 as predictor of three-year survival in patients with PAH (12). However, no prior study, to our knowledge, compared the performance of PVR and PVRI in assessing disease severity and predicting survival in a large cohort of PAH patients, when stratified by the presence of obesity. We hypothesize that PVRI is a stronger risk factor than PVR for adverse clinical outcomes, especially in obese individuals with PAH.
Methods:
a). Study subjects:
This retrospective study was approved by the Cleveland Clinic institutional review board (study number 19-1602). Written informed consent was waived given the retrospective study design. Patients with PAH (PH group 1)(14) were identified from the Cleveland Clinic PH Registry. All patients had pre-capillary PH and two PH experts agreed on the PH etiology based on the proceedings of the 5th World Symposium in PH (15). We included unique PAH patients who had the initial right heart catheterization (RHC) at Cleveland Clinic between February 1992 and November 2019.
b). Hemodynamic determinations:
We recorded right atrial (RA) pressure, mPAP and PAWP in the supine position with the pressure transducer located at the mid-thoracic line (4th intercostal space). Pulmonary artery pressures were recorded at end expiration using waveform tracings and calibers. Based on current recommendations (16), we favored the use of thermodilution method (when available) to estimate CO. In 18% of the patients, the CO used in the PVR calculation was obtained by indirect Fick methodology using Dehmer formula (17) because thermodilution CO measurements were not available. We calculated CI (CO / BSA by Du Bois et al. formulae (18)), PVR and PVRI. We also recorded mixed venous oxygen saturation (SvO2).
c). Other measurements:
At the time of the initial RHC we collected data on demographics, New York Heart Association (NYHA) functional class. We recorded the distance walked during the six-minute walk test (6MWD), diffusion lung capacity for carbon monoxide (DLCO) and several echocardiographic variables including right atrial area, presence and severity of right ventricular dysfunction and presence of pericardial effusion (19). Right ventricular function was determined subjectively by visual inspection and objectively by several echocardiographic determinations including tricuspid annular plane systolic excursion (19). We documented the level of N-terminal pro-B type natriuretic peptide (NT-proBNP) and when not available, we used brain natriuretic peptide (BNP). In order to use NT-proBNP and BNP determinations simultaneously, we categorized their plasma concentrations into risk groups (low, intermediate and high) based on the cut-offs proposed by the ESC/ERS PH guidelines (16).
Statistical Analysis:
Patients’ data were summarized as means and standard deviations for continuous variables, and as counts and percentages for categorical variables. Continuous data were compared with Student’s t test. Linear regression analysis was used to address the relationship between CO, PVR, and body weight or BSA and assess the association between PVR, PVRI, and markers of PAH disease severity. For linear regression analysis, we provided the coefficient estimates, standard errors and p-values. Cox proportional-hazard regression was performed for time-to-event outcomes. The proportional hazards assumption was examined using the Schoenfeld residuals test. In order to compare the association of PVRI and PVR with clinical outcomes, we built 2 regression models that included the same predefined covariates (age, sex, race, PAH etiology, and being on PAH specific therapy). Model discrimination was examined using Harrell's C-indices. Spline models (B-spline with 3 knots) were created to provide a detailed description of the non-linear relationship between PVRI or PVR and HR for mortality and mortality or lung transplant. Subgroup analyses were conducted to evaluate the impact of body weight on PVR and PVRI in association with clinical outcomes in lean and obese patients. We also tested the interaction between weight as a continuous variable and PVR or PVRI in association with clinical outcomes All models were adjusted for predefined covariates including age, sex, race, PAH etiology, and being on PAH specific therapy. In addition, we created models that also included variables known to predict outcomes in PAH (20). Hazard ratio (HR) plots with 95% confidence interval were generated using a reference point (HR=1) of 3 WU for PVR and 4 WU.m2 for PVRI. When specified; we used the fully conditional specification (FCS) method for multiple imputation for missing data (100 times). All analyses were performed using SAS 9.4 software (SAS Institute, Cary, NC) and R software. The level of statistical significance was set at p < 0.05 (two-tailed).
Results:
a). Patient Characteristics:
We included a total of 644 patients with a mean ± SD age of 53 ± 16 years, of whom 476 (74 %) were females. The etiology of PAH was idiopathic or heritable in 286 (44%), connective tissue disease associated in 185 (29%), congenital heart disease associated in 81 (13%) and due to other etiologies (drug and toxin induced, associated with portal hypertension and HIV infection) in 92 (14%) patients. Weight distribution of our cohort (BMI 29 ± 7.7 kg/m2) followed that of the US population (5). Patients’ characteristics are shown in Table 1. At time of first RHC done at our center, the diagnosis of PAH was new (incident cases) in 568 (88%) patients and already established (prevalent cases) in 76 (12%) patients. The mean PVR and PVRI were 10 ± 6 WU and 18.6 ± 10.6 WU.m2, respectively.
Table 1:
Baseline patient characteristics.
| Variable | N* | Mean ± SD, n (%) |
|---|---|---|
| Age (years) | 644 | 53 ± 16 |
| Female sex | 644 | 476 (74) |
| Race | 644 | |
| Caucasian | 537 (83) | |
| Black | 82 (13) | |
| Other | 25 (4) | |
| BMI (kg/m2) | 644 | 29 ± 7.7 |
| BSA (m2) | 644 | 1.88 ± 0.36 |
| Etiology of PAH | 644 | |
| Idiopathic or heritable | 286 (44) | |
| CTD associated | 185 (29) | |
| CHD associated | 81 (13) | |
| Other | 92 (14) | |
| Comorbidities | 644 | |
| Hypertension | 294 (45.7) | |
| Diabetes mellitus | 105 (16.3) | |
| Coronary artery disease | 80 (12.4) | |
| Obstructive sleep apnea | 112 (17.4) | |
| Hypothyroidism | 109 (16.9) | |
| PAH specific therapy | 644 | |
| No | 568 (88) | |
| Yes ¶ | 76 (12) | |
| NYHA functional class | 462 | |
| I | 12 (2.6) | |
| II | 137 (29.7) | |
| III | 239 (51.7) | |
| IV | 74 (16) | |
| 6MWD (m) | 534 | 312 ± 117 |
| BNP/NT-pro BNP risk category (16) | 493 | |
| Low | 123 (25) | |
| Intermediate | 182 (37) | |
| High | 188 (38) | |
| DLCO (% predicted) | 507 | 42 ± 36 |
| Echocardiogram | ||
| RV dysfunction | 522 | |
| Normal | 121 (23) | |
| Mild dysfunction | 90 (17) | |
| Moderate dysfunction | 95 (18) | |
| Severe dysfunction | 216 (41) | |
| Pericardial effusion | 606 | |
| Yes | 468 (77) | |
| No | 138 (23) | |
| RHC | ||
| RA (mmHg) | 622 | 10 ± 6 |
| mPAP (mmHg) | 644 | 49 ± 13 |
| PAWP (mmHg)† | 621 | 10 ± 3 |
| Thermodilution CO (L/min/m) | 526 | 4.7 ± 1.8 |
| Thermodilution CI (L/min/m2) | 526 | 2.5 ± 0.85 |
| PVR (WU) | 644 | 10 ± 6 |
| PVRI (WU.m2) | 644 | 18.6 ± 10.6 |
| SvO2 (%) | 505 | 64 ± 9.5 |
Definition of Abbreviations: BMI: body mass index, BNP: brain natriuretic peptide, CHD: congenital heart disease, CI: cardiac index, CTD: connective tissue disease, DLCO: diffusion lung capacity for carbon monoxide, mPAP: mean pulmonary artery pressure, NT-pro BNP: N-terminal pro B-type natriuretic peptide, NYHA: New York Heart Association, PAH: pulmonary arterial hypertension, PAWP: pulmonary artery wedge pressure, PVR: pulmonary vascular resistance, PVRI: pulmonary vascular resistance index, RA: right atrial, RHC: right heart catheterization, RV: right ventricle, SvO2: mixed venous oxygen saturation, 6MWD: distance walked during six-minute walk test.
While the total number of patients in our cohort was 644, some patient had missing variables. N represent the number of patients with available data regarding each variable.
In subjects with missing PAWP, left ventricular diastolic pressure was used for calculation of PVR.
Of the 76 patients treated, 54, 16 and 6 received 1, 2 and 3 PAH-specific therapies, respectively (calcium channel blocker in 6, phosphodiestearase-5 inhibitors in 24, soluble guanylate cyclase stimulator in 4, endothelin receptor blocker in 30, and prostacyclin analogues in 40 patients).
b). Impact of body weight and BSA on CO and PVR:
A total of 239 (37%) patients were obese. When comparing hemodynamic determinations between obese and lean PAH patients we noted that mPAP (51 ± 12 mmHg vs 48 ± 14 mmHg (p=0.002)), PAWP (11 ± 3.0 mmHg vs 9 ± 3 mmHg (p<0.001)), CO (5.1 ± 2.0 L/min vs 4.5 ± 1.6 L/min (p<0.001)), and stroke volume (62 ± 24 vs 57 ± 23 mL (p=0.007)) were higher in obese individuals.
The results of univariate linear regression analysis showed that CO increased (t value = 9.8, p <0.0001), while PVR decreased (t value = −5, p <0.0001) with increasing body weight. Similarly, CO increased (t value = 10.6, p <0.0001), while PVR decreased (t value = −6.1, p <0.0001) with increasing BSA. These results remained statistically significant after adjusting for prespecified covariates. As expected, there was no significant association between PVRI and body weight (t value = 1.09, p = 0.27) or BSA (t value = 0.17, p = 0.86), or between CI and body weight (t value = 1.51, p = 0.13) or BSA (t value = 1.64, p = 0.1).
c). Association between PVR, PVRI and markers of disease severity:
When adjusted for prespecified covariates, both PVR and PVRI were significantly associated with markers of disease severity (Table 2). Overall, significant but similar estimates were observed for PVR and PVRI and BNP/NT-proBNP risk category, 6MWD, pericardial effusion, RV dysfunction, mean RA pressure and SvO2. PVRI showed a higher estimate for differentiating NYHA functional class 3 or 4 from 1. PVR but not PVRI was significantly associated with DLCO (% predicted).
Table 2:
Association between PVR, PVRI and markers of disease severity in linear regression analysis adjusted for age, sex, race, PAH etiology and being on PAH specific therapy
| Disease severity marker | PVR (WU) | PVRI (WU.m2) | |||||
|---|---|---|---|---|---|---|---|
| Estimate | Standard Error |
P value | Estimate | Standard Error |
P value | ||
| NYHA class | 2 vs 1 | 0.89 | 1.64 | 0.58 | 1.79 | 1.42 | 0.21 |
| 3 vs 1 | 2.49 | 1.62 | 0.12 | 3.44 | 1.41 | 0.015 | |
| 4 vs 1 | 3.6 | 1.72 | 0.04 | 4.3 | 1.47 | 0.004 | |
| BNP/NT-proBNP risk category * | Intermediate vs low | 2.20 | 0.6 | 0.0002 | 1.99 | 0.53 | 0.0002 |
| High vs low | 4.75 | 0.61 | <0.0001 | 4.32 | 0.52 | <0.0001 | |
| 6MWD (m) | −0.006 | 0.002 | 0.01 | −0.006 | 0.002 | 0.004 | |
| DLCO (% predicted) | −0.02 | 0.007 | 0.035 | −0.006 | 0.006 | 0.33 | |
| Pericardial effusion | Yes vs no | 1.1 | 0.55 | 0.047 | 1.26 | 0.48 | 0.008 |
| RV dysfunction | Yes vs no | 3.41 | 0.53 | <0.0001 | 4.16 | 0.44 | <0.0001 |
| Severity of RV dysfunction | Mild vs none | 1.02 | 0.87 | 0.24 | 1.52 | 0.72 | 0.036 |
| Moderate vs none | 3.31 | 0.8 | <0.0001 | 3.89 | 0.67 | <0.0001 | |
| Severe vs none | 4.2 | 0.66 | <0.0001 | 4.93 | 0.56 | <0.0001 | |
| RA area (cm2) | 0.083 | 0.035 | 0.019 | 0.094 | 0.03 | 0.002 | |
| RA mean pressure (mmHg) | 0.25 | 0.04 | <0.0001 | 0.27 | 0.03 | <0.0001 | |
| SvO2 (%) | −0.28 | 0.02 | <0.0001 | −0.24 | 0.02 | <0.0001 | |
Definition of Abbreviations: BNP: brain natriuretic peptide, DLCO: diffusion lung capacity for carbon monoxide, NT-pro BNP: N-terminal pro B-type natriuretic peptide, NYHA: New York Heart Association, PVR: pulmonary vascular resistance, PVRI: pulmonary vascular resistance index, RA: right atrial, RV: right ventricle, SvO2: mixed venous oxygen saturation, 6MWD: distance walked during six-minute walk test.
NT-proBNP and BNP plasma concentrations were categorized into risk groups (low, intermediate and high) based on the cut-offs proposed by the ESC/ERS PH guidelines (16).
d). Association of PVR and PVRI with clinical outcomes:
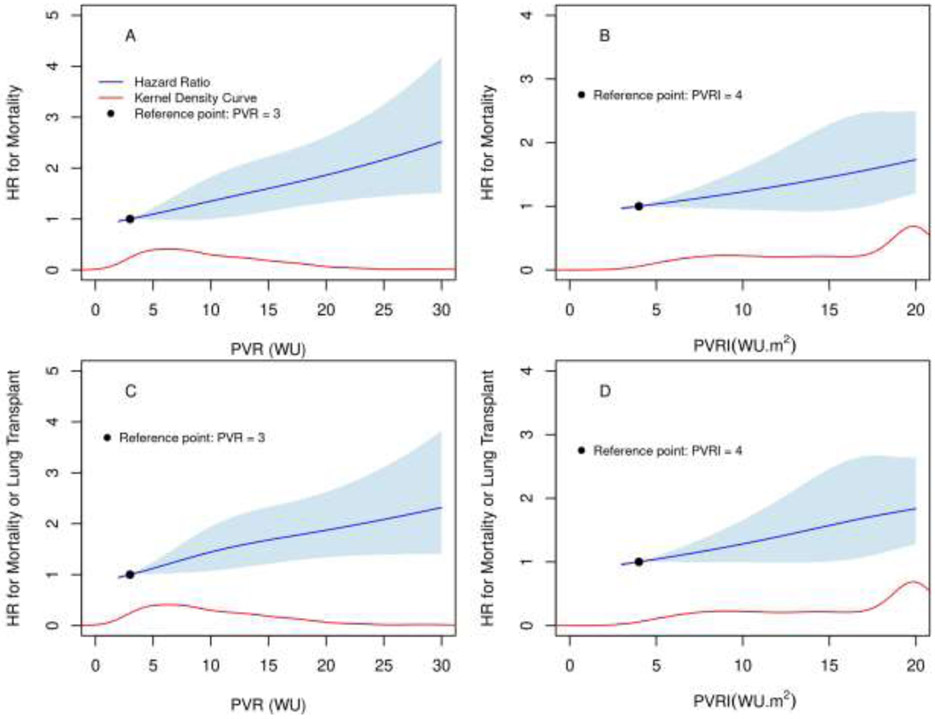
During a median (IQR) follow-up of 50.5 (20 - 103) months, 370 (57.5%) patients died and 31 (5%) received a lung transplant. Of the 510 patients in whom we had information regarding hospitalizations, 266 (52%) were hospitalized at least once for PAH. In time-to-event analyses, adjusted for prespecified covariates, PVR was a risk factor for first PAH hospitalization (HR (95% CI): 1.03 (1.-1.05), p= 0.02), mortality (HR (95% CI): 1.04 (1.02-1.06), p= 0.0002) (figure 1, panel A) and mortality or lung transplantation (HR (95% CI): 1.03 (1.02 −1.05), p= 0.0002) (figure 1, panel C). Using the same analysis, PVRI was also a risk factor for first PAH hospitalization (HR (95% CI): 1.05 (1.02-1.08), p= 0.0004), mortality (HR (95% CI): 1.04 (1.01-1.06), p= 0.0017) (figure 1, panel B) and mortality or lung transplantation (HR (95% CI): 1.04 (1.02 −1.06), p= 0.0006) (figure 1, panel D).
Figure 1: Risk of death or death or lung transplantation based on PVR and PVRI.
Figures show the adjusted hazard ratio (HR) for mortality and mortality or lung transplant (y-axis) against PVR (Panel A and Panel C) and PVRI (Panel B and Panel D) in the x-axis. The blue line represents the HR and the blue shade represents the 95% CI. The red lines represent the Kernel density. For the PVRI figures, the upper value was truncated at 20 WU.m2. Panel A: HR (95% CI): 1.04 (1.02-1.06), p= 0.0002. Panel B: HR (95% CI): 1.04 (1.01-1.06), p= 0.0017. Panel C: HR (95% CI): 1.03(1.02 -1.05), p= 0.0002. Panel D: HR (95% CI): 1.04 (1.02 -1.06), p= 0.0006.
We also adjusted the time to event analysis to include variables known to predict outcomes in PAH(20) including NYHA functional class, 6MWD, NT-pro BNP, RA pressure, and SvO2 (21). We noted that both PVR and PVRI lost their significance in association with outcomes in a similar degree; without any clinically important difference between these indices (Table 4). We also performed the same analyses with imputations for missing variables and observed similar results overall, including the Harrell’s C-indices. The association between PVRI and time to first hospitalization became significant with imputation, but with minimal changes in HR or Harrel’s C-index (with imputation: HR: 1.04(1.01-1.08), p=0.02 and Harrell’s C-index of 0.64; without imputation: 1.04(0.99-1.09), p=0.07, Harrell’s C-index of 0.67).
Table 4:
Time to event analysis including variables known to predict outcomes in PAH*
| Outcome | N | PVR | PVRI | ||||
|---|---|---|---|---|---|---|---|
| HR (95% CI) |
P value | Harrell’s C-index |
HR (95% CI) |
P value | Harrell’s C-index |
||
| Time to first hospitalization | 260 | 0.99(0.96-1.03) | 0.77 | 0.66 | 1.04(0.99-1.09) | 0.07 | 0.67 |
| Time to lung transplant | 284 | 0.92(0.78-1.09) | 0.33 | 0.88 | 0.99(0.82-1.21) | 0.93 | 0.88 |
| Time to death | 285 | 1.0(0.97-1.05) | 0.76 | 0.77 | 1.0(0.96-1.04) | 0.98 | 0.77 |
| Time to lung transplant or death | 285 | 1.0(0.97-1.04) | 0.95 | 0.76 | 1.0(0.96-1.04) | 0.95 | 0.76 |
Definition of Abbreviations: BMI: body mass index, CI: confidence interval, HR: hazard ratio, PVR: pulmonary vascular resistance, PVRI: pulmonary vascular resistance index.
In addition to age, sex, race, PAH etiology, and being on PAH specific therapy, we added NYHA functional class, 6MWD, NT-pro BNP, right atrial pressure, and mixed venous oxygen saturation (20) to the time to event analysis.
e). Subgroup Analysis:
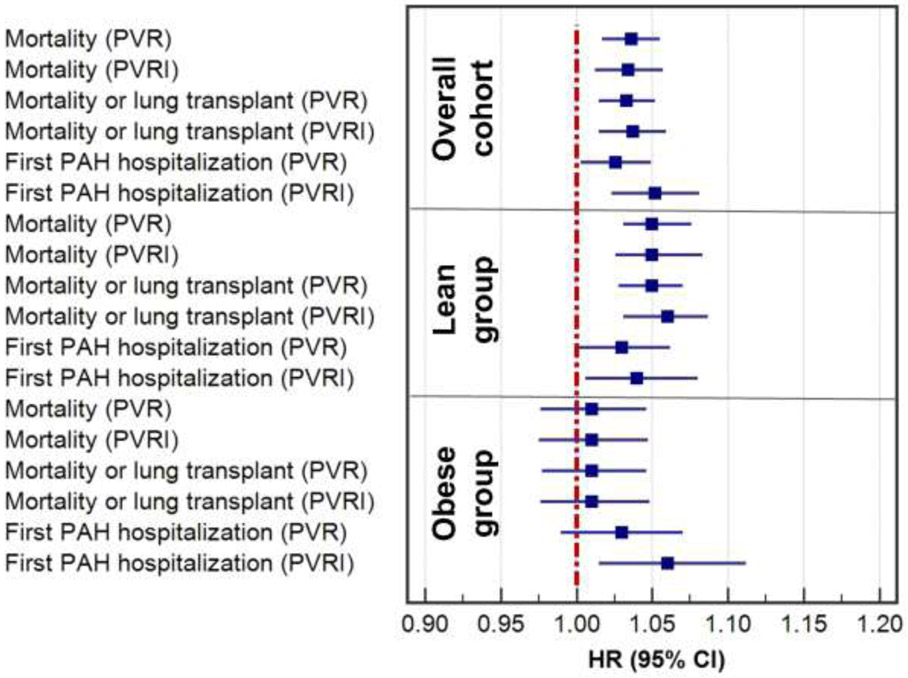
To assess the impact of body weight on the association of PVR and PVRI with clinical outcomes, we divided our cohort into lean (BMI < 30 kg/m2) and obese (BMI ≥ 30 kg/m2) group. Mean ± SD (range) BSA was 1.75 ± 0.2 (1.2-2.40) m2 and 2.07 ± 0.26 (1.44-3.06) m2 in the group of patients with BMI <30 kg/m2 and ≥ 30 kg/m2, respectively. In obese patients, PVRI (HR (95% CI): 1.06 (1.02 −1.11), p=0.001) but not PVR (HR (95% CI): 1.03 (0.99-1.07), p= 0.15) was a risk factor for first PAH hospitalization, after adjusting for prespecified covariates (figure 2). In the obese group, neither PVR nor PVRI were risk factors for mortality or lung transplant (Table 3). Meanwhile, in lean patients, both PVR (HR: 1.03 (1-1.06), p= 0.034) and PVRI (HR: 1.04 (1-1.08), p= 0.02) were risk factors for first PAH hospitalization. In addition, both PVR (HR: 1.05 (1.03-1.07), p< 0.0001) and PVRI (HR: 1.06(1.03-1.09), p< 0.0001) were risk factors for death or lung transplantation (figure 2). Harrell's C-indices showed that PVRI were similar to PVR in strength of association with these time-to-event outcomes, in the entire cohort and in the groups of lean or obese patients (Table 3).
Figure 2:
Forest plots of hazard ratios (HR) for first PAH hospitalization, mortality and mortality or lung transplant based on PVR and PVRI in the whole cohort, obese (BMI ≥ 30 kg/m2) and lean patients BMI (< 30 kg/m2)
Table 3:
Time to event analysis adjusted for age, sex, race, PAH etiology, and being on PAH specific therapy in the subgroups of patients with BME < 30 and ≥ 30 kg/m2
| Outcome | N | PVR | PVRI | ||||
|---|---|---|---|---|---|---|---|
| HR (95% CI) |
P value | Harrell’s C-index |
HR (95% CI) |
P value | Harrell’s C-index |
||
| BMI < 30 kg/m2 | |||||||
| Time to first hospitalization | 307 | 1.03(1-1.06) | 0.03 | 0.62 | 1.04(1.01-1.08) | 0.02 | 0.62 |
| Time to lung transplant | 382 | 0.97(0.91-1.05) | 0.46 | 0.83 | 0.97(0.91-1.09) | 0.94 | 0.82 |
| Time to death | 383 | 1.05(1.03-1.08) | <0.0001 | 0.71 | 1.05(1.03-1.08) | <0.0001 | 0.7 |
| Time to lung transplant or death | 383 | 1.05(1.03-1.07) | <0.0001 | 0.69 | 1.06(1.03-1.09) | <0.0001 | 0.69 |
| BMI ≥ 30 kg/m2 | |||||||
| Time to first hospitalization | 181 | 1.03(0.99-1.07) | 0.15 | 0.6 | 1.06(1.02-1.11) | 0.001 | 0.62 |
| Time to lung transplant | 230 | 1.07(0.94-1.21) | 0.31 | 0.79 | 1.06(0.89-1.26) | 0.54 | 0.78 |
| Time to death | 230 | 1.01(0.98-1.05) | 0.55 | 0.66 | 1.01(0.98-1.05) | 0.58 | 0.66 |
| Time to lung transplant or death | 230 | 1.01(0.98-1.05) | 0.54 | 0.66 | 1.01(0.98-1.05) | 0.54 | 0.66 |
Definition of Abbreviations: BMI: body mass index, CI: confidence interval, HR: hazard ratio, PVR: pulmonary vascular resistance, PVRI: pulmonary vascular resistance index.
We tested the impact of obesity on time to event analyses with and without the incorporation of PVR and PVRI. We noted that BMI as a continuous variable had a direct association with time to first hospitalization (HR:1.02, 95% CI: 1.01-1.04, p=0.004), but no significant association with time to death (HR: 1.00, 95% CI: 0.99-1.01, p=0.91), time to lung transplant (HR: 0.98, 95% CI: 0.93-1.03, p=0.44), or time to lung transplant or death (HR: 1.00, 95% CI: 0.98-1.01, p=0.67). Similar relationships were observed when using BMI as a dichotomous variable (<30 or ≥ 30 kg/m2). When we added BMI either as a continuous variable or dichotomous variable, we did not observe a significant impact in our PVR and PVRI time to event analyses.
We also tested the interaction between weight as continuous variable with PVR/PVRI and its association with clinical outcomes, while adjusting for age, sex, race, PAH etiology and being on PAH specific therapy. We noted no interaction between weight and PVR or PVRI with time to death, time to lung transplant or time to lung transplant or death. However, there was a significant interaction between weight and PVR or PVRI in regards to time to first hospitalization (weight and PVR: p=0.009; weight and PVRI: p=0.016), suggesting that the impact of PVR and PVRI on hospitalizations is more pronounced in patients with higher weight.
Discussion
In a large cohort of patients with PAH, we noted that CO was directly related, while PVR was inversely associated with body weight. Both PVR and PVRI were associated with markers of disease severity in PAH, but in general, some associations were stronger for PVRI than PVR. Both PVR and PVRI were risk factors for first PAH hospitalization and death or death or lung transplant in the whole cohort and the group of patients with BMI < 30 kg/m2, without any apparent superiority between the two measurements. However, PVRI, but not PVR, was a risk factor for first PAH hospitalization in obese subjects.
The inverse correlation between PVR and body weight is explained by the higher CO in obese individuals given the increase in body mass (6, 22, 23). Cardiac output is the denominator in the PVR equation (24); therefore, a higher CO in obese individuals relative to the transpulmonary gradient (TPG: mPAP-PAWP) leads to an inverse relationship between PVR and weight. A large study by Frank et al. showed that adult obese patients who underwent RHC, had higher mPAP, PAWP and TPG than individual with normal weight; however, the TPG was only 1 to 2 mmHg higher, depending on the severity of obesity (25). Furthermore, the potential increase in intrathoracic pressure related to obesity, should affect in the same proportion both components of the TPG, hence not affecting its absolute value (26).
An approach to adjust for the hemodynamic changes seen in obesity is to index PVR to BSA, by using CI instead of CO in the PVR calculation. In fact, the clinical importance and application of PVRI are demonstrated in American Heart Association and American Thoracic Society guidelines for the treatment of pediatric pulmonary hypertension (27) and the recent updates on diagnosis and management of pediatric pulmonary hypertension (28), where the use PVRI is recommended for making the diagnosis of PH in children, who certainly have a wide variation in BSA. Given the obesity epidemic, adult patients also have a wide variation in BSA. There are also significant variations in BSA depending on the sex (with average values of 2.1 m2 in adult males and 1.83 m2 in adult females) or presence of comorbidities. For instance, in a patient with a TPG of 20 mmHg and a CO of 7 L/min, the PVR is 2.6 WU. However, the PVRI can vary from 3.1 to 8.1 WU.m2 if we use a BSA of 1.2 or 3.1 m2 (which is the range of BSA in our cohort), a PVRI variation that can be as high as 2.6 times. A relatively higher PVRI in relation to PVR may increase the recognition of precapillary PH at the cost of including more patients with postcapillary PH; however, our study was not designed to test this hypothesis.
Studies showed mixed results regarding the prognostic value of PVR and PVRI in PAH. In the REVEAL study (3), PVRI was associated with 1-year survival only in univariate analysis, while a PVR > 32 WU was associated with survival in the final multivariate model (29). The REVEAL 2.0 incorporated PVR < 5 WU as a predictor of lower 12-month mortality (subtracting one point from the final score); however, no points are added for higher PVR values (30). Benza et al. showed that a PVRI > 30 WU.m2 was associated with reduced 3-year survival in a cohort of patients with PAH treated with subcutaneous treprostinil (12). Conversely, other studies showed that PVR and PVRI were not predictors of survival in PAH (8-11). In our study, we demonstrated that both PVR and PVRI were risk factors for death, death or lung transplant as well as first PAH hospitalization, both in the entire cohort and the group of patients with BMI < 30 kg/m2. While we hypothesized that PVRI would be a stronger risk factor than PVR for adverse clinical outcomes particularly in obese patients, neither PVRI nor PVR, were risk factors for mortality, mortality or lung transplantation in obese subjects. PVRI but not PVR, was a risk factor for first PAH hospitalization in obese individuals. Possible explanations are the several inherent assumptions in calculating PVR (31), the overall limited ability of PVR and PVRI in predicting outcomes as shown by other studies, particularly when incorporated in multivariable models (8-11), the limited correction in PVR when indexing by BSA (the average BSA was 1.75 ± 0.20 m2 in lean compared to 2.07 ± 0.26 m2 in obese patients) and the reduced sample size when performing subgroup analyses. As expected, when we added other variables known to affect outcomes in PAH (20) to our time to event analyses, both PVR and PVRI were not significantly associated with outcomes; however, this reinforced the main finding of our study, that PVRI does not provide a major advantage over PVR.
Our study results are aligned with those of Weatherald et al., who reported that when compared to total pulmonary resistant (TPR: mPAP/CO), the use of values indexed by BSA did not improve the diagnostic accuracy to define exercise PH in obese subjects with mPAP ≤ 20 mmHg who underwent exercise hemodynamic testing. Importantly, TPR indexed by BSA had a higher sensitivity at the expense of considerably lower specificity (32).
Our study has several limitations that include the single-center setting, retrospective design, and the inclusion of patients with PVR ≥ 3 WU, a traditional cut-off for PAH. It remains unclear whether using a lower PVR cut off value could have altered the association of PVR and PVRI with clinical outcomes. Our study was not designed to answer this question. In addition, we could not test the impact of different classes of obesity on the prognostic value of PVR and PVRI given the relatively small sample size for each subgroup. Nevertheless, this is the first study to compare the association of PVRI to that of PVR with strong clinical outcomes in PAH, with particular focus on studying the effect of body weight on the performance of these hemodynamic indexes. We showed that CO increases, while PVR decreases with increasing body weight. Clinicians managing patients with PAH should be aware that the higher CO measurements in obese patients could lead to a lower PVR relative to PVRI. PVRI appeared to perform better than PVR in the association with some markers of disease severity in the overall cohort and time to first PAH hospitalization in obese patients, however, no significant differences were noted in association with mortality or time to first PAH hospitalization in the overall cohort. Although we hypothesized that PVRI would be a stronger risk factor than PVR for adverse clinical outcomes especially in obese individuals, our findings showed insufficient clinical significance, to recommend a change from PVR to PVRI in the definition of PAH. Further studies are needed to determine whether standardizing PVR for BSA further improves the discrimination between pre and postcapillary PH and whether the performance of PVR and PVRI in predicting clinical outcomes is different in patients with PVR < 3 WU.
Conclusions:
PVR is inversely associated with body weight and therefore may be relatively lower than PVRI in obese individuals. PVRI appears to have a stronger association with surrogate markers of disease severity in PAH; however, both PVR and PVRI were similarly associated with PAH hospitalizations and overall survival. In obese individuals, PVRI but not PVR, was a risk factor for first hospitalization, although both PVR and PVRI were not associated with survival. We found no strong evidence to recommend a change from PVR to PVRI in the definition of PAH.
Acknowledgments:
Funding sources: A.R.T is supported by NIH grant # R01HL130307.
Footnotes
Conflict of interest statements:
Ghaleb Khirfan MD: The author has no significant conflicts of interest with any companies or organization whose products or services may be discussed in this article.
Manshi Li, MS: The author has no significant conflicts of interest with any companies or organization whose products or services may be discussed in this article.
Xiaofeng Wang, PHD: The author has no significant conflicts of interest with any companies or organization whose products or services may be discussed in this article.
Raed A. Dweik MD: The author has no significant conflicts of interest with any companies or organization whose products or services may be discussed in this article.
Gustavo A. Heresi MD, MS: Gustavo A. Heresi received personal fees for being a member in Bayer Healthcare – Advisory Board and Speaking.
Adriano R. Tonelli MD: The author has no significant conflicts of interest with any companies or organization whose products or services may be discussed in this article.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Ghaleb Khirfan, Department of Pulmonary and Critical Care Medicine. Respiratory Institute, Cleveland Clinic, Cleveland, OH, USA..
Manshi Li, Department of Quantitative Health Sciences, Cleveland Clinic, Cleveland, OH, USA..
Xiaofeng Wang, Department of Quantitative Health Sciences, Cleveland Clinic, Cleveland, OH, USA..
Raed A. Dweik, Department of Pulmonary, Allergy and Critical Care Medicine. Respiratory Institute, Cleveland Clinic, Cleveland, OH, USA..
Gustavo A. Heresi, Department of Pulmonary and Critical Care Medicine. Respiratory Institute, Cleveland Clinic, Cleveland, OH, USA..
Adriano R. Tonelli, Department of Pulmonary and Critical Care Medicine. Respiratory Institute, Cleveland Clinic, Cleveland, OH, USA..
References:
- 1.Tonelli AR, Arelli V, Minai OA, et al. : Causes and circumstances of death in pulmonary arterial hypertension. American journal of respiratory and critical care medicine 2013;188:365–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Simonneau G, Montani D: Haemodynamic definitions and updated clinical classification of pulmonary hypertension. The European respiratory journal 2019;53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benza RL, Miller DP, Gomberg-Maitland M, et al. : Predicting survival in pulmonary arterial hypertension: insights from the Registry to Evaluate Early and Long-Term Pulmonary Arterial Hypertension Disease Management (REVEAL). Circulation 2010;122:164–72. [DOI] [PubMed] [Google Scholar]
- 4.Maron BA, Brittan EL, Hess E, et al. : Pulmonary vascular resistance and clinical outcomes in patients with pulmonary hypertension: a retrospective cohort study. The Lancet Respiratory medicine 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hales CM, Carroll MD, Fryar CD, Ogden CL: Prevalence of Obesity and Severe Obesity Among Adults: United States, 2017-2018. NCHS data brief 2020:1–8. [PubMed] [Google Scholar]
- 6.Collis T, Devereux RB, Roman MJ, et al. : Relations of stroke volume and cardiac output to body composition: the strong heart study. Circulation 2001;103:820–5. [DOI] [PubMed] [Google Scholar]
- 7.de Divitiis O, Fazio S, Petitto M, Maddalena G, Contaldo F, Mancini M: Obesity and cardiac function. Circulation 1981;64:477–82. [DOI] [PubMed] [Google Scholar]
- 8.D'Alonzo GE, Barst RJ, Ayres SM, et al. : Survival in patients with primary pulmonary hypertension. Results from a national prospective registry. Annals of internal medicine 1991;115:343–9. [DOI] [PubMed] [Google Scholar]
- 9.Humbert M, Sitbon O, Chaouat A, et al. : Survival in patients with idiopathic, familial, and anorexigen-associated pulmonary arterial hypertension in the modern management era. Circulation 2010;122:156–63. [DOI] [PubMed] [Google Scholar]
- 10.McLaughlin VV, Shillington A, Rich S: Survival in primary pulmonary hypertension: the impact of epoprostenol therapy. Circulation 2002;106:1477–82. [DOI] [PubMed] [Google Scholar]
- 11.Weatherald J, Boucly A, Chemla D, et al. : Prognostic Value of Follow-Up Hemodynamic Variables After Initial Management in Pulmonary Arterial Hypertension. Circulation 2018;137:693–704. [DOI] [PubMed] [Google Scholar]
- 12.Benza RL, Gomberg-Maitland M, Naeije R, Arneson CP, Lang IM: Prognostic factors associated with increased survival in patients with pulmonary arterial hypertension treated with subcutaneous treprostinil in randomized, placebo-controlled trials. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation 2011;30:982–9. [DOI] [PubMed] [Google Scholar]
- 13.Kwan WC, Shavelle DM, Laughrun DR: Pulmonary vascular resistance index: Getting the units right and why it matters. Clinical cardiology 2019;42:334–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Simonneau G, Montani D: Haemodynamic definitions and updated clinical classification of pulmonary hypertension. 2019;53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Simonneau G, Gatzoulis MA, Adatia I, et al. : Updated clinical classification of pulmonary hypertension. Journal of the American College of Cardiology 2013;62:D34–41. [DOI] [PubMed] [Google Scholar]
- 16.Galie N, Humbert M, Vachiery JL, et al. : 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). European heart journal 2016;37:67–119. [DOI] [PubMed] [Google Scholar]
- 17.Dehmer GJ, Firth BG, Hillis LD: Oxygen consumption in adult patients during cardiac catheterization. Clinical cardiology 1982;5:436–40. [DOI] [PubMed] [Google Scholar]
- 18.Du Bois D, Du Bois EF: A formula to estimate the approximate surface area if height and weight be known. 1916. Nutrition 1989;5:303–11; discussion 12-3. [PubMed] [Google Scholar]
- 19.Ahmed M, Dweik RA, Tonelli AR: What is the best approach to a high systolic pulmonary artery pressure on echocardiography? Cleveland Clinic journal of medicine 2016;83:256–60. [DOI] [PubMed] [Google Scholar]
- 20.Boucly A, Weatherald J: Risk assessment, prognosis and guideline implementation in pulmonary arterial hypertension. The European respiratory journal 2017;50. [DOI] [PubMed] [Google Scholar]
- 21.Khirfan G, Almoushref A, Naal T, et al. : Mixed Venous Oxygen Saturation Is a Better Prognosticator Than Cardiac Index in Pulmonary Arterial Hypertension. Chest 2020;158:2546–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alpert MA, Omran J, Bostick BP: Effects of Obesity on Cardiovascular Hemodynamics, Cardiac Morphology, and Ventricular Function. Current obesity reports 2016;5:424–34. [DOI] [PubMed] [Google Scholar]
- 23.Poirier P, Giles TD, Bray GA, et al. : Obesity and cardiovascular disease: pathophysiology, evaluation, and effect of weight loss: an update of the 1997 American Heart Association Scientific Statement on Obesity and Heart Disease from the Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Circulation 2006;113:898–918. [DOI] [PubMed] [Google Scholar]
- 24.Chemla D, Lau EM, Papelier Y, Attal P, Hervé P: Pulmonary vascular resistance and compliance relationship in pulmonary hypertension. The European respiratory journal 2015;46:1178–89. [DOI] [PubMed] [Google Scholar]
- 25.Frank RC, Min J, Abdelghany M, et al. : Obesity Is Associated With Pulmonary Hypertension and Modifies Outcomes. Journal of the American Heart Association 2020;9:e014195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jawad A, Tonelli AR: Impact of Intrathoracic Pressure in the Assessment of Pulmonary Hypertension in Overweight Patients. 2017;14:1861–3. [DOI] [PubMed] [Google Scholar]
- 27.Abman SH, Hansmann G, Archer SL, et al. : Pediatric Pulmonary Hypertension: Guidelines From the American Heart Association and American Thoracic Society. Circulation 2015;132:2037–99. [DOI] [PubMed] [Google Scholar]
- 28.Rosenzweig EB, Abman SH, Adatia I, et al. : Paediatric pulmonary arterial hypertension: updates on definition, classification, diagnostics and management. The European respiratory journal 2019;53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Benza RL, Gomberg-Maitland M, Miller DP, et al. : The REVEAL Registry risk score calculator in patients newly diagnosed with pulmonary arterial hypertension. Chest 2012;141:354–62. [DOI] [PubMed] [Google Scholar]
- 30.Benza RL, Gomberg-Maitland M, Elliott CG, et al. : Predicting Survival in Patients With Pulmonary Arterial Hypertension: The REVEAL Risk Score Calculator 2.0 and Comparison With ESC/ERS-Based Risk Assessment Strategies. Chest 2019;156:323–37. [DOI] [PubMed] [Google Scholar]
- 31.Suresh K, Shimoda LA: Lung Circulation. Comprehensive Physiology 2016;6:897–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weatherald J, Boucly A, Lau E, et al. : Are indexed values better for defining exercise pulmonary hypertension? The European respiratory journal 2017;50. [DOI] [PubMed] [Google Scholar]