Abstract
Objective:
Investigate relationship between management of patent ductus arteriosus (PDA) and acute kidney injury (AKI) in very low birthweight neonates.
Study Design:
Retrospective cohort study of neonates, <1500g, admitted to 24 NICUs, 1/1/14 – 3/31/14. AKI diagnosed using the neonatal modified KDIGO definition; diagnosis and treatment of PDA extracted from the medical record. Demographics, clinical characteristics, and AKI stage compared using chi-square and analysis of variance. A general estimating equation logistic regression used to estimate adjusted odds ratios.
Results:
Of 526 neonates with sufficient data to diagnose AKI, 157 (30%) had PDA (61 conservative management, 62 pharmacologic treatment only, 34 surgical ligation). In analyses adjusted for sex, birthweight, gestational age, caffeine, nephrotoxin exposure, vasopressor and mechanical ventilation use, with conservative management as reference, there were no differences among treatment cohorts in the odds of AKI.
Conclusion:
The underlying physiology of PDA, not management strategy, may determine the likelihood of AKI in neonates <1500 grams.
Acute kidney injury (AKI) occurs commonly in critically ill neonatal populations and is associated with adverse outcomes (1–2). Very low birthweight infants (VLBW) are particularly prone to AKI with an incidence of 20 – 30% (1–4). Several factors contribute to the vulnerability of VLBW neonates to AKI, including factors intrinsic to the kidney (incomplete development/low nephron numbers at birth), unique neonatal physiology (immature skin resulting in excessive fluid losses, changes in circulation during transition, nutritional needs), nephrotoxic medicine exposures, and critical illness (necrotizing enterocolitis, sepsis). The unique physiology of the ductus arteriosus (DA) and management strategies employed in neonates with clinically significant patent ductus arteriosus (PDA) may also impact neonatal kidney function.
The PDA represents a challenge to clinicians as both the natural physiology (physiologic shunting of blood flow decreasing kidney perfusion) and/or active treatment (using nephrotoxic medication or surgical treatment) have the potential to result in AKI. In term and late preterm infants, the DA constricts shortly after birth resulting in closure of the DA within minutes to a few days in most healthy neonates. Infants born preterm, including VLBW neonates, commonly have delayed PDA closure, which may result in pulmonary over-circulation and decreased blood flow to the gastrointestinal tract and kidneys (5, 6). This hypoperfusion has the potential to increase the risk of AKI. Previous studies have suggested an increased risk of AKI among infants with hemodynamically significant PDA (5).
In neonates with a clinically significant PDA, a variety of management strategies have evolved [expectant/conservative management, cyclooxygenase inhibitors (indomethacin or ibuprofen), surgical ligation]. Each of these options has the potential for negatively impacting the neonatal kidney. While indomethacin and ibuprofen are known nephrotoxic drugs, prolonged hypoperfusion may also compromise kidney function and cause injury. Published comparisons of treatment strategy provide conflicting results. Aygun et al. (7) reported that AKI was highest in neonates with surgical closure of their PDA, with or without ibuprofen. Majed et al. (8) found an increased risk of AKI in neonates with a PDA, and nonsteroidal antinflamatory drug treatment was associated with an increased incidence of stage 1 AKI but a significant reduction in stages 2 and 3 AKI. Hammerman et al. (9) reported no difference in creatinine levels comparing ductal closure with ibuprofen vs. indomethacin.
This secondary analysis of data collected for the AWAKEN study aimed to evaluate the association of management strategy for PDA with AKI in a subset of AWAKEN subjects, those infants <1500 grams. We hypothesized that PDA management strategy would be associated with the incidence of AKI.
METHODS
This is a secondary analysis of the retrospective, international cohort study, “AWAKEN”. The protocol and methodology of the AWAKEN study have been published (10). Briefly, the records of all neonates admitted to level 2-4 neonatal intensive care units (NICUs) at 24 participating Neonatal Kidney Collaborative centers over a three-month period (1/1/14 – 3/31/14) were reviewed. To be eligible for inclusion, the neonate had to have received at least 48 hours of IV hydration, been admitted to the NICU within the first 14 days after birth and survived at least 48 hours after admission, not have congenital heart disease requiring surgery before day 7, and not have severe congenital kidney anomalies. This secondary analysis included only neonates who were < 1500 grams at birth. Each center involved in the study received approval from their Institutional Review Board or Human Research Ethics Committee.
Data Collection
Data collection for AWAKEN included baseline demographics; daily data collection for the first week (physiologic parameters, laboratory results, and specified drug exposures); weekly snapshots until discharge, transfer, 120 days of age or death; all clinically obtained creatinine determinations; and discharge disposition and diagnoses. Diagnoses were those recorded by the local care team, extracted by AWAKEN study personnel, and entered on clinical report forms for inclusion in the database. All data were those that were collected according to local standards of care; laboratory values, including creatinine, were based on institution-specific assays and reporting conventions.
Acute kidney injury (AKI)
Acute kidney injury was ascertained using the modified neonatal KDIGO definition (11). Based on these criteria, stage 1 AKI was defined as a serum creatinine rise ≥ 0.3 mg/dL or 50% increase from a previous creatinine level up to <2-fold increase. The urinary output threshold was set at 1 mL/kg/h or less averaged over 24 hours and normalized for the weight on the day urine output was measured. Stage 2 AKI was diagnosed if the serum creatinine increased by 2 times up to <3 times the previous value and/or 24 hour urine output > 0.3 ml/kg/h to ≤0.5 ml/kg/h; stage 3 AKI was defined as a serum creatinine increase by ≥ 3 times the previous level, serum creatinine ≥ 2.5 mg/dL, receipt of dialysis or daily urine output ≤ 0.3 mL/kg/h (2).
Patent Ductus Arteriosus (PDA)
Presence or absence of confirmed PDA and its management (conservative, pharmacologic, or ligation with or without pharmacologic therapy) were extracted from the electronic medical record as documented by the clinical care team and entered in the AWAKEN Discharge Form. Criteria for diagnosis were not specified; measurements obtained from echocardiograms, if performed, were not extracted from the medical record.
Subjects were classified into four groups (no PDA, conservative management, medical treatment and surgical treatment). We assumed “no PDA” if it was not listed as a discharge diagnosis. PDA without either pharmacologic or surgical treatments was “PDA – conservative treatment”, a neonate was categorized as “PDA – medical treatment” if given indomethacin or ibuprofen, and “PDA – surgical treatment” included neonates with and without pharmacologic treatment prior to ligation.
Statistical analysis
Demographics, clinical characteristics, creatinine levels, and AKI stage were compared among PDA management groups using a chi-square and analysis of variance for categorical and continuous variables, respectively. A general estimating equation logistic regression—to account for clustering by study site—was used to estimate odds ratios (ORs) and associated 95% confidence intervals (CIs) for the association between PDA management and AKI. Potential covariates were selected based on having a significant p-value in bivariate analyses. We then utilized a backwards selection process in order to select the most parsimonious model. The final, most parsimonious models were adjusted for size for gestational age and vasopressor use. Additionally, in a post hoc analysis due to differences in the distribution of gestational age and birthweight by whether vasopressors were administered, the adjusted models further included an interaction between PDA and vasopressor administration and associations reported by vasopressor administration. For all analyses, an alpha of 5% was used, p-values were two-sided, and SAS v9.4 was used to conduct the analyses.
RESULTS
Demographics
Of the 2162 infants enrolled in AWAKEN, 526 infants < 1500 grams birth weight were included in this study. Of the 157 neonates diagnosed with PDA, 61 (39%) had a PDA but were managed without medications or surgery, 62 (39%) were treated pharmacologically, and 34 (22%) were treated with surgical ligation with or without prior pharmacologic treatment (Figure 1). The demographics and clinical characteristics of this cohort are presented in Table 1. A total of 157 (29.8%) neonates in this study were diagnosed with PDA by their care teams. There were significant differences between neonates without a diagnosis of PDA and those with a PDA. Most notably, infants with a PDA were of younger gestational age, of lower birthweight, and were more likely to be exposed to nephrotoxic drugs and mechanical ventilation. Among the VLBW neonates with a PDA, those who were conservatively managed were of more advanced gestational age at birth, of higher birthweight, less likely to require invasive ventilation on day 7 and had a shorter length of stay compared with neonates who received active interventions. Those requiring surgery were of lower gestational age, of lower birthweight, more likely to be exposed to vancomycin and vasopressors, and had a longer length of stay (Table 1). Of the neonates managed surgically, 24 had prior indomethacin treatment. There were significant differences in birthweight and gestational age among the cohorts as well as exposure to vancomycin and vasopressors. Neonates with either medical or surgical treatment of PDA were more likely to require mechanical ventilation on day 7 (p<0.0001) and had longer lengths of stay (p<0.0001). Of note, mortality was not different by PDA group (p=0.14).
Figure 1:
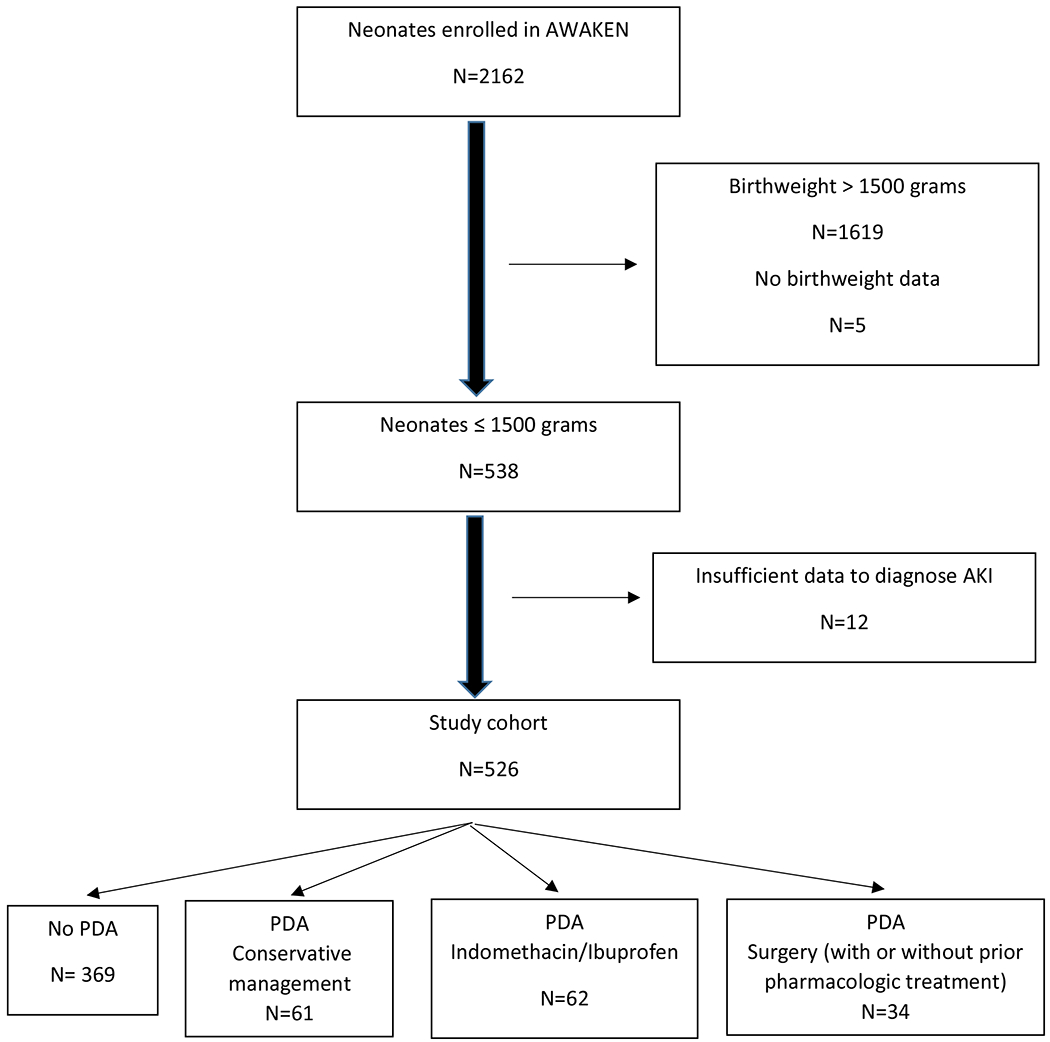
Study population CONSORT diagram
Table 1:
Comparison of demographics, clinical characteristics by PDA diagnosis and management strategy
| PDA Management Strategy |
p-value* | ||||
|---|---|---|---|---|---|
| No PDA (n=369) |
Conservative management (n=61) |
Indomethacin/Ibuprofen (n=62) |
Surgery (with or without pharmacologic treatment) (n=34) |
||
| DEMOGRAPHICS | |||||
| Birthweight (g), mean ± SD | 1094±276 | 1007±306 | 907±243 | 729±187 | <0.0001 |
| GA (wk), mean ± SD | 29.1±3.1 | 27.3±3.0 | 26.4±2.2 | 24.8±1.2 | <0.0001 |
| Male (n, %) | 179 (48.5) | 37 (60.7) | 31 (50.0) | 21 (61.8) | 0.18 |
| CLINICAL | |||||
| SGA status (n, %) | 127 (34.4) | 12 (19.7) | 8 (12.9) | 4 (11.8) | 0.0001 |
| Caffeine (n, %) | 271 (73.4) | 54 (88.5) | 62 (100.0) | 32 (94.1) | <0.0001 |
| Aminoglycosides (n, %) | 285 (77.2) | 55 (90.2) | 61 (98.4) | 34 (100) | <0.0001 |
| Vancomycin (n, %) | 84 (22.8) | 18 (29.5) | 23 (37.1) | 24 (70.6) | <0.0001 |
| Vasopressors (n, %) | 57 (15.5) | 14 (23.0) | 18 (29.0) | 25 (73.5) | <0.0001 |
| Invasive Vent d7 (n, %) | 206 (55.8) | 45 (73.8) | 58 (93.6) | 33 (97.1) | <0.0001 |
| Length of stay (d), mean ± SD | 55±42.3 | 65±38.8 | 89.4±35.5 | 116.6±48.9 | <0.0001 |
| Mortality (n, %) | 32 (8.7) | 7 (11.5) | 1 (1.6) | 3 (8.8) | 0.14 |
GA, gestational age; SGA, small for gestational age; PDA, patent ductus arteriosus
Estimated from chi-square and analysis of variance for categorical and continuous variables, respectively
Creatinine Concentrations/Acute Kidney Injury
Neonates with a PDA had a higher maximum creatinine concentration than those without (p=0.0001). (Table 2) Compared to those without a PDA, neonates with a PDA were more likely to have AKI (OR 3.74, 95% CI 2.17-6.44, p< 0.0001.
Table 2:
Comparison of maximum serum creatinine values and acute kidney injury status as a function of patent ductus arteriosus management strategy
| PDA Management Strategy |
p-value* | ||||
|---|---|---|---|---|---|
| No PDA (N=369) |
Conservative management (n=61) |
Pharmacologic management (n=62) |
Surgery (with or without pharmacologic treatment) (n=34) |
||
| Maximum Creatinine (mg/dL), mean ± SD | 1 (0.6) | 1.1 (0.4) | 1 (0.3) | 1.4 (1.1) | <0.0001 |
| AKI Status | |||||
| No AKI (n, %) | 279 (75.6) | 26 (42.6) | 32 (51.6) | 13 (38.2) | <0.0001 |
| AKI (all) (n, %) | 90 (24.4) | 35 (57.4) | 30 (48.4) | 21 (61.8) | |
| Stage (among AKIs) | |||||
| Stage 1 (n, %) | 49 (54.4) | 18 (51.4) | 11 (36.7) | 7 (33.3) | 0.11 |
| Stage 2 (n, %) | 18 (20.0) | 12 (34.3) | 13 (43.3) | 7 (33.3) | |
| Stage 3 (n, %) | 23 (25.6) | 5 (14.3) | 6 (20.0) | 7 (33.3) | |
PDA patent ductus arteriosus; AKI acute kidney injury
Estimated from an analysis of variance for maximum creatinine and a chi-square test for AKI status
Examining associations between PDA management and AKI and using conservative PDA management as the referent group, there was no statistically significant difference in the crude or adjusted ORs for AKI among the three management cohorts (Table 3). Among those without administration of a vasopressor, neonates with PDA who underwent surgical ligation were 71% less likely to have AKI (aOR 0.29, 95% CI 0.09-0.95) while those with administration of vasopressors and had their PDA managed by indomethacin or ibuprofen were 80% less likely to have AKI (OR 0.20, 95% CI 0.04-0.97). That said, there was a large overlap of the associations between vasopressor groups, and there were no statistically significant differences in the associations by vasopressor administration (p=0.2847).
Table 3.
Crude and adjusted odds ratios and associated 95% confidence intervals for the association between AKI and patent ductus arteriosus management strategy*
| Overall |
Without pressors |
With pressors |
||||
|---|---|---|---|---|---|---|
| cOR (95% CI) | aOR† (95% CI) | cOR (95% CI) | aOR† (95% CI) | cOR (95% CI) | aOR† (95% CI) | |
| No PDA | 0.23 (0.12-0.43) | 0.24 (0.13-0.44) | 0.25 (0.13-0.47) | 0.30 (0.16-0.56) | 0.14 (0.03-0.68) | 0.20 (0.04-1.08) |
| PDA – no treatment | Reference | Reference | Reference | Reference | Reference | Reference |
| PDA – medical treatment | 0.61 (0.29-1.29) | 0.51 (0.24-1.09) | 0.77 (0.34-1.75) | 0.50 (0.17-1.45) | 0.17 (0.04-0.75) | 0.20 (0.04-0.97) |
| PDA – surgical treatment | 1.15 (0.49-2.67) | 0.53 (0.22-1.28) | 0.68 (0.21-2.13) | 0.29 (0.09-0.95) | 0.30 (0.05-1.80) | 0.26 (0.04-1.89) |
cOR crude odds ratio; aOR adjusted odds ratio; CI confidence interval; AKI acute kidney injury; PDA patent ductus arteriosus
Estimated from general estimating equation logistic regression to adjust for clustering by study site; analyses were adjusted for sex, birthweight, gestational age, caffeine, nephrotoxin exposure, vasopressor use, and mechanical ventilation use
Adjusted for size for an interaction of birthweight and gestational age and (for the overall model only) vasopressor use
DISCUSSION
The current study provides an analysis of a large multicenter cohort of VLBW with PDA evaluating the association between treatment strategy and AKI. We have confirmed, using a database that includes over 500 VLBW neonates, that PDA is associated with an increased odds of AKI. However, we found no differences in AKI incidence among medical, surgical, or conservative management strategies for PDA in the AWAKEN VLBW cohort.
PDA among preterm infants has been associated with poorer systemic perfusion predisposing these neonates to AKI (12,13). To date, reports on the association of PDA with AKI have yielded conflicting results, particularly when it comes to the impact of treatment (3,5,8,). Non-steroidal anti-inflammatory drugs, including indomethacin and ibuprofen, commonly utilized to treat PDA, affect kidney arterial perfusion (14, 15, 16, 17). Investigators have reported an association between these drugs and biomarkers of kidney injury, including urinary podocyte numbers (18) and albuminuria (19). An association between non-steroidal anti-inflammatory drugs and AKI is less clear, with several recent studies showing inconsistent results (3, 7, 8). Our data failed to show an association between nonsteroidal anti-inflammatory drug administration for PDA and increased incidence of AKI compared with the presence of PDA alone. We postulate that, although the medications used to promote ductal closure may have an adverse impact on kidney perfusion and kidney function, the PDA itself may be the major factor associated with the development of AKI.
There are certain inherent limitations related to the retrospective nature of this current study. The presence or absence of PDA was derived from medical documentation and lacked details, such as the echocardiographic results. Therefore, the diagnosis of PDA entered on the discharge form may have included either a small PDA or hemodynamically significant PDA. Current practice varies among providers and institutions regarding treatment of hemodynamically significant PDA. We speculate that the neonates who were treated for PDA were more likely to have hemodynamically significant lesions. If that was the case, use of nephrotoxic medications did not exacerbate kidney injury, but may have ameliorated it.
Since we do not know whether PDA was diagnosed or treated before or after AKI was diagnosed and there are other factors that can affect the incidence and severity of AKI, the association between hemodynamically significant PDA and occurrence of AKI may be less than suggested by our analyses. The presence and absence of AKI in association with PDA can only be determined after the diagnosis of PDA is made. These limitations can be resolved with a prospective study. Prospective, randomized trials of PDA management are needed to provide unbiased estimates of the benefits of available therapies. These trials would benefit from the incorporation of near infrared spectroscopy (NIRS), use of biomarkers, and radiological studies (13,14). The addition of NIRS may provide information on the impact of both the PDA and NSAIDs on kidney blood flow.
The single-center nature of previous reports makes it difficult to generalize the results. Although our data have the advantage of being drawn from a wide variety of international centers, the data remain observational; further, due to the small sample size of PDAs managed pharmacologically and surgically, conclusions about causative associations between PDA, PDA management, and AKI should be tempered. Furthermore, because the sample size is small, the associations are imprecise and it is possible that the true association is actually null. Despite our data suggesting that use of non-steroidal anti-inflammatory drugs to manage PDA may not be deleterious, it would be premature to base clinical care on the observational findings of this and other studies.
Given the recent understanding in animal and epidemiology studies of the role of acute kidney injury on the development and progression of chronic kidney disease (CKD), reduction in AKI incidence in the NICU could have an important impact on reduction of CKD in former premature infants. Thus it is imperative that prospective studies be done to clarify the associations identified in this manuscript between clinically significant PDA, its management, and acute kidney injury in at-risk neonates.
Acknowledgements:
The authors thank all the members of the Neonatal Kidney Collaborative who participated in the AWAKEN study:
University of Alabama, Birmingham: David Askenazi, MD; N. Ambalavanan, MD; Russell Griffin, PhD; Cincinnati Children’s Hospital: Stuart Goldstein, MD; Amy Nathan, MD; James Greenberg, MD; Canberra Hospital: Alison Kent, MD (currently at the University of Rochester); Jeffrey Fletcher, MD; Farah Sethna, MD; Children’s Hospital of Colorado: Danielle Soranno, MD; Jason Gien, MD; Katja Gist, MD; Children’s Hospital at Montefiore/Albert Einstein: Mamta Fuloria, MD; Kim Reidy, MD; Frederick Kastel, MD; Natalie Uy, MD; Children’s National Medical Center: Mary Revenis, MD; Sofia Perrazo, MD; Shantanu Rastogi, MD; Golisano Children’s Hospital University of Rochester: George Schwartz, MD; Carl T. D’Angio, MD; Ronnie Guillet, MD, PhD; Erin Rademacher, MD; Ahmed El Samra, MD (currently Union Hospital, Terre Haute); Ayesa Mian, MD; Maimonides Medical Center: Juan Kupferman, MD; Alok Bhutada, MD; McGill University: Michael Zappitelli, MD; Pia Wintermark, MD; Medanta, Medicity The Cradle: Sanjay Wazir, MD; Sidharth Sethi, MD; Sandeep Dubey, MD; Metrohealth Medical Center: Maroun Mhanna, MD; Deepak Kumar, MD; Rupesh Raina, MD; Nationwide Children’s Hospital: Susan Ingraham, MD; Arwa Nada, MD; Elizabeth Bonachea, MD; Stonybrook University: Richard Fine, MD; Robert Woroniecki, MD; Shanthy Sridhar, MD; Texas Children’s Hospital: Ayse Ariken, MD; Christopher Rhee, MD; Tufts Medical Center: Lawrence Milner, MD; Alexandra Smith, MD; Julie Nicoletta, MD; University of British Columbia: Cherry Mammen, MD; Avash Jeet Singh, MD; Anne Synnes, MD; University of Iowa: Jennifer Jetton, MD; Tarah Colaizy, MD; Jonathan Klein, MD; Patrick Brophy (currently University of Rochester); University of Kentucky: Aftab Chishti, MD; Mina Hanna, MD; University of Miami: Carolyn Abitbol, MD; Marissa Defreitas, MD; Shahnaz Duara, MD; Salih Yasin, MD; University of Michigan: David Selewski, MD (currently Medical University of South Carolina); Subrata Sarker, MD; University of New Mexico: Craig Wong, MD; A. Staples, MD; Robin Ohls, MD; Catherine Joseph, MD (currently Texas Children’s Hospital); Tara Dupont, MD (currently University of Utah); University of Virginia: Jennifer Charlton, MD; Jonathan Swanson, MD; Matthew Harer (currently University of Wisconsin); Patricio Ray, MD; University of Washington: Sangeeta Hingorani, MD; Christine Hu, MD; Sandra Juul, MD
Footnotes
Financial Disclosure Statement:
All authors declare no real or perceived conflicts of interest that could affect the study design, collection, analysis and interpretation of data, writing of the report, or the decision to submit for publication.
REFERENCES
- 1.Elmas AT, Tabel Y, Ozdemir R. Risk factors and mortality rate in premature babies with acute kidney injury. J Clin Lab Anal 2018; 32 (7):e22441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jetton JG, Guillet R, Askenazi DJ, Dill L, Jacobs J, Kent AL, et al. Assessment of Worldwide Acute Kidney Injury Epidemiology in Neonates: Design of a retrospective cohort study. Front Pediatr 2016; 4:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Srinivasan N, Schwartz A, John E, Price R, Amin S. Acute kidney injury impairs postnatal renal adaptation and increases morbidity and mortality in very low-birth-weight infants. Am J Perinatol. 2018; 35(1):39–47. [DOI] [PubMed] [Google Scholar]
- 4.Weintraub AS, Connors J, Carey A, Blanco V, Green RS. The spectrum of onset of acute kidney injury in preterm infants less than 30 weeks gestation. J Perinatol 2016; 36:47–480. [DOI] [PubMed] [Google Scholar]
- 5.Velazquez DM, Reidy KJ, Sharma M, Kim M, Vega M, Havranek T. The effect of hemodynamically significant patent ductus arteriosus on acute kidney injury and systemic hypertension in extremely low gestational age newborns. J Matern Fetal Neonatal Med. 2019; 32(19):3209–3214. [DOI] [PubMed] [Google Scholar]
- 6.Bomelburg T, Jorch G. Abnormal blood flow patterns in renal arteries of small preterm infants with patent ductus arteriosus detected by Doppler ultrasonography Eur J Pediatr. 1989;148(7):660–4. [DOI] [PubMed] [Google Scholar]
- 7.Aygün A, Poryo M, Wagenpfeil G, Wissing A, Ebrahimi-Fakhari D, Zemlin M, et al. Birth weight, Apgar scores and gentamicin were associated with acute kidney injuries in VLBW neonates requiring treatment for patent ductus arteriosus. Acta Paediatr. 2019;108(4):645–653. [DOI] [PubMed] [Google Scholar]
- 8.Majed B, Bateman DA, Uy N, Lin F. Patent ductus arteriosus is associated with acute kidney injury in the preterm infant. Pediatr Nephrol 2019;34(6):1129–1139. [DOI] [PubMed] [Google Scholar]
- 9.Hammerman C, Shchors I, Jacobson S, Schimmel MS, Bromiker R, Kaplan M, et al. Ibuprofen versus continuous indomethacin in premature neonates with patent ductus arteriosus: is the difference in the mode of administration? Pediatr Res. 2008; 64(3):291–7. [DOI] [PubMed] [Google Scholar]
- 10.Jetton JG, Boohaker LJ, Sethi SK, Wazir S, Rohatgi S, Soranno DE, et al. Incidence and outcomes of neonatal acute kidney injury (AWAKEN): a multicenter, multinational, observational cohort study. Lancet Child Adolesc Health 2017; 1:184–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Selewski DT, Charlton JR, Jetton JG, Guillet R, Mhanna MJ, Askenazi DJ, et al. Neonatal acute kidney injury. Pediatrics 2015; 136 (2), e463–e473. [DOI] [PubMed] [Google Scholar]
- 12.Radicioni M, Bini V, Campus GM, Camerini PG. Terlipressin-induced modifications of Doppler ultrasound signals of systemic arteries in preterm infants with vasoactive-resistant patent ductus arteriosus: A pilot study. J Clin Ultrasound. 2018; 46(3):202–208. [DOI] [PubMed] [Google Scholar]
- 13.Bonsante F, Ramful D, Binquet C, Samperiz S, Daniel S, Gouyon JB, et al. Low Renal Oxygen Saturation at Near-Infrared Spectroscopy on the First Day of Life Is Associated with Developing Acute Kidney Injury in Very Preterm Infants. Neonatology. 2019; 115(3):198–204. [DOI] [PubMed] [Google Scholar]
- 14.Guignard JP. The adverse renal effects of prostaglandin-synthesis inhibitors in the newborn rabbit. Semin Perinatol. 2002; 26(6):398–405. [DOI] [PubMed] [Google Scholar]
- 15.Van-Bel F, Van-Zoeren D, Schipper J, Guit GL, Baan J. Effect of indomethacin on superior mesenteric artery blood flow velocity in preterm infants. J Pediatr 1990; 116: 965–970. [DOI] [PubMed] [Google Scholar]
- 16.Van-Bel F, Guit GL, Schipper J, van-de-Bor M, Baan J. Indomethacin-induced changes in renal blood flow velocity waveform in premature infants investigated with color Doppler imaging. J Pediatr 1991; 118: 621–626 [DOI] [PubMed] [Google Scholar]
- 17.Bensman A Non-steroidal anti-inflammatory drugs (NSAIDs) systemic use: The risk of renal failure. Front. Pediatr 2020; 7:517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kent AL, Brown L, Broom M, Broomfield A, Dahlstrom JE. Increased urinary podocytes following indomethacin suggests drug-induced glomerular injury. Pediatr Nephrol. 2012; 27(7):1111–7. [DOI] [PubMed] [Google Scholar]
- 19.Sellmer A, Bech BH, Bjerre JV, Schmidt MR, Hjortdal VE, Esberg G, et al. Urinary neutrophil gelatinase-associated lipocalin in the evaluation of patent ductus arteriosus and AKI in very preterm neonates: a cohort study. BMC Pediatr. 2017;17(1):7. [DOI] [PMC free article] [PubMed] [Google Scholar]


