Abstract
Successful allogeneic transplantation has been made possible by suppressing activation of the adaptive immune system. Current immunosuppressive therapy prevents rejection by targeting T and B cells. Despite this effective treatment, it is the innate immune system, which includes dendritic cells, monocytes, NK cells, that is responsible for the initiation of the adaptive immune response. Recent work has described that the innate immune system is capable of recognizing allogeneic nonself and some of the mechanisms of innate allorecognition have been uncovered. Better understanding of the role of the innate immune system in initiation and maintenance of the allo-immune response has potential to lead to better treatment strategies for transplant patients, prolonging allograft survival. Here, we review advances in our understanding of innate allorecognition in transplantation.
Keywords: Allorecognition, Innate Immunity, Transplantation, Monocyte, Dendritic Cell, Monocyte Memory
Introduction
The alloimmune response is a main hurdle towards reaching rejection free survival of transplanted organs. Allorecognition, the discrimination between self and non-self by the immune system (1), is required prior to initiating an allogeneic response. In transplantation, nonself major histocompatibility complex (MHC) molecules and minor histocompatibility antigens are recognized by T cells (2, 3). Microbial non-self is recognized by innate cells through Pattern Recognition Receptors (PRR), causing activation and maturation of antigen presenting cells (such as monocytes, dendritic cells, and macrophages) which leads to subsequent T cell activation (4, 5). These pathways have also been studied in transplantation (6-11). Toll Like Receptors (TLR) have been shown to be required for minor mismatch rejection and their absence diminishes major mismatch rejection (6). However, major mismatch rejection still occurs despite lack of TLR signaling (12, 13). Likewise, disruption of common downstream microbial sensing pathways did not alleviate rejection (7-9). An alternate explanation was provided by the danger hypothesis which explains innate immune activation by release of Danger Associated Molecular Patterns (DAMP) from cells during and soon after the transplant procedure. However, it does not explain why, long after danger and injury of grafts have subsided, rejection responses can still be initiated (10, 11). Likewise, T-cell deficient recipients of healed allografts reconstituted with T cells initiated rapid rejection despite the absence of inflammation and danger. The shortcomings of these studies have encouraged work to address if innate immune cells can distinguish between self and allogeneic non-self. Here, we will provide an overview of recent work on innate allorecognition in transplantation.
Innate Allorecognition in Transplantation
The ability to distinguish self from non self is evolutionarily conserved and is not restricted to vertebrates. Invertebrate organisms such as corals, sponges, sea mats and squirts that lack an adaptive immune system can still, upon contact, identify and reject genetically different organisms belonging to the same species (14). The genetic basis of these allorecognition systems has been identified in two of those marine organisms (15, 16). However, the concept of self – non-self recognition by innate immune cells was introduced three decades ago by Charles Janeway (4). Pathogens express conserved Pathogen Associated Molecular Patterns (PAMP), which are recognized by Pattern Recognition Receptors (PRR), such as the Toll-like receptors (TLR), expressed by antigen presenting cells (APC) (17-19). This step provides an activation signal that matures the APC which can in turn activate the adaptive immune system by presenting antigen to T cells. Although it was clear that transplantation of allogeneic non-self activates the innate immune system, it was not known what the specific signals are that do so. This has inspired testing whether similar innate pathways impact the allo-immune response and whether their disruption can lead to better graft survival.
Early work on allorecognition studied whether PRR can contribute to the innate alloimmune response. Disruption of TLR signaling, using targeted deletion of the downstream TLR signal adaptor protein, MyD88, prevents minor antigen-mismatched (HY-mismatched) skin allograft rejection (6). Impaired DC maturation in draining lymph nodes was the cause of lack of rejection in this model, suggesting that TLRs are involved in DC activation after an allogeneic transplant. Follow up studies demonstrated that TLR signaling did not prevent MHC or multiple minor antigen-mismatched allograft rejection (7). Other studies in models of islet, skin, or heart grafts that are fully allogeneic showed acute rejection by recipients in the absence of TLR signaling (12, 13).
Recent work has focused on the possibility that innate receptor systems, aside from those recognizing microbial non-self, can sense allogeneic non-self and activate APC. The first evidence that allogeneic non-self can be sensed comes from comparing the response of the innate immune system to syngeneic and allogeneic cells in RAG−/− mice that lack the two main lymphoid cell types, T and B cells . In these recipients, allogeneic splenocytes, but not syngeneic ones, can elicit a delayed-type hypersensitivity-like response (20). A set of adoptive transfer and depletion experiments established that monocytes mediate this response. A later, independent study demonstrated that macrophages in alloimmunized hosts were capable of allorecognition, gaining the ability to destroy allogeneic cells with the help of CD4+ T cells in a CD40 dependent manner (21). This allocytotoxic response could be elicited via administration of an agonistic anti-CD40 antibody at the time of alloimmunization in the absence of lymphocytes. Further support for innate allorecognition by monocytes comes from studies in RAG−/−γc−/− mice, which lack T, B, NK and innate lymphoid cells, using kidney, heart and bone marrow plug transplantation models. It was reported that the innate response to allogeneic grafts was different from syngeneic grafts (22, 23). Host-derived, mature MHC-IIhiCD80hi, IL-12+ monocyte-derived DCs (mo-DCs) persistently infiltrated allografts, which was detectable for several weeks after transplantation. Syngeneic grafts only transiently harbored a smaller numbers of less mature mo-DCs that failed to produce IL-12 and did not induce a Th1 (IFNγ+) T cell response. Similar observations were made in wild type (WT) recipients transplanted with allogeneic and syngeneic grafts (22). This alloresponse required a non-MHC mismatch and was not dependent on MHC disparities. Similar observations were made by Chow et al, using a model of allogeneic cell transferred to Rag−/−γc−/− recipients (23). These studies therefore demonstrated that monocytes and macrophages have the ability to sense allogeneic non-self and become activated to mediate allocytotoxicity and differentiate into mature DC that subsequently activate adaptive immune responses.
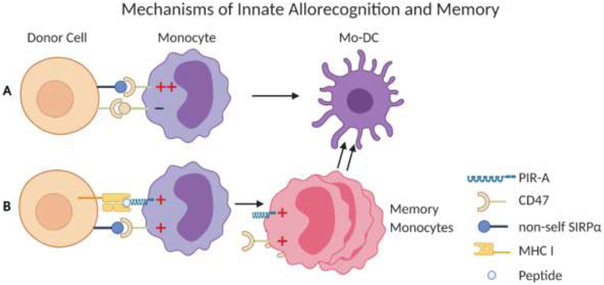
The recognition of non-MHC allodeterminants was further investigated using a genetic mapping study based on differences in the monocyte response elicited by donors from NOD and NOR grafts. These mouse strains share the majority of the genome, but differ in about 12 percent. While NOD donors evoke a strong monocyte response in the recipient, NOR mice do not. These studies identified Signal Regulatory Protein Alpha (SIRPα) as a donor allodeterminant. SIRPα is a polymorphic protein of the immunoglobulin super family (IgSF) that is expressed in myeloid and parenchymal cells and binds to CD47, which is non-polymorphic and expressed on all cells (24). Under steady state conditions, SIRPα mediates an inhibitory signal and CD47 an activating signal in monocytes preventing their activation. Variation in donor SIRPα can modulate binding affinity to CD47 resulting in monocyte activation (25). For example, the SIRPα variant from NOD mice elicits a stronger CD47 activating signal (25-27). The proposed model of allorecognition is that allografts expressing non-self SIRPα are activating the recipient monocytes by inducing more robust CD47 signals (25, 28). In contrast, syngeneic grafts were both donors and recipients express self-SIRPα keep the balance of CD47 and SIRPα signaling undisturbed and monocytes are not activated (Figure 1).
Figure 1: Mechanisms of innate allorecognition and memory.
This model illustrates the role of SIRPα - CD47 (A) and MHC-I - PIR-A (B) pathways in generating mature dendritic cells and monocyte memory. (A) The balance of activating and inhibitory signals mediated by CD47 and SIRPa on recipient monocytes is skewed towards activation if allogeneic SIRPa is recognized resulting in monocyte maturation to dendritic cells (Mo-DC). (B) Memory monocytes are formed if both PIR-A and CD47 pathways on a subset of monocytes are activated. This leads to clonal expansion and formation of Mo-DC.
An additional driver of innate allorecognition necessary for the formation of memory monocytes has recently been described. Immunological memory – the rapid and more protective immune response against previously experienced antigen – is a main driver of transplant rejection (29-31). Initially thought to be a property of the adaptive immune system, it has recently been demonstrated that innate cells, specifically NK cells, macrophages (32-38) and DCs (39) can acquire memory-like mechanisms and protective functions. Ly49D+ NK cells expand and can mount a memory response in an H-2Dd specific manner (37). Our group recently established that, independently of lymphoid cells, monocytes and macrophages mount a donor-specific anamnestic memory response to allogeneic donor cells upon secondary exposure (40). The response was heightened and specific to donor, but not third party, cells, displaying features of memory similar to what is observed in T and B cells (29-31). This specific response differed from previously described concept of “trained” immunity, where an encounter with one antigen does lead to a heightened response to other antigens (41-45). The memory response was maintained for 4 to 7 weeks after immunization, making the response long-lived compared to the average monocyte life span of 3 days (46, 47). Further experiments showed that memory specificity was determined by recognition of MHC-I by paired immunoglobulin-like receptors (PIR). PIR, like SIRPa, belong to the IgSF and are orthologs of human leukocyte immunoglobulin-like receptors (LILRs). PIR are expressed on monocytes and macrophages (48), and are comprised of six linked stimulatory PIR-A and one inhibitory PIR-B (49-51). Individual PIR-A differentially bind to distinct MHC-I molecules (52). Manipulating PIR-A through knock out or treatment with blocking agents prevented monocytes and macrophages from mounting a memory response (40). Single-cell RNA-seq and MHC class I tetramer binding experiments demonstrated that PIR are differentially expressed on monocytes and that Ly-6Chi monocytes that express certain PIR-A clonally expand after allogeneic antigen exposure supporting a mechanism of memory similar to that seen in NK cells (37, 40). The SIRPα-CD47 allorecognition pathway is not only important in initial innate allorecognition (25), but also necessary during priming in order to elicit a memory response (40).
Role of innate allorecognition in transplantation
Early studies suggested that the existence of innate allorecognition is required for T cell mediated rejection. OT-II CD4+ T cells specific to Ovalbumin (OVA) were adoptively transferred to RAG−/− hosts transplanted with either B6.OVA or F1.OVA (B6 x Balb/c) heart allografts. Only allogeneic but not syngeneic OVA expressing grafts were rejected (22). Further investigation demonstrated that only allogeneic grafts induced maturation of mono-DCs and proliferation and IFNg production by OT-II T cells. Short-term depletion of mono-DCs abolished acute rejection in wild type mice whereas depletion of neutrophils had no effect on rejection (22). This work emphasizes the importance of innate allorecognition as a prerequisite for T cell activation and rejection. A second set of studies demonstrated that innate allorecognition not only initiates, but also locally sustains, T cell mediated rejection (53).
Recipient-derived DCs rapidly infiltrate allogeneic transplants, replace donor-derived DC, and maintain contact with antigen specific effector T cells. The stable T cell - DC interactions are associated with increased local T cell proliferation and activation. The local role of DCs in allorecognition is evident by the lack of rejection after depleting DCs in splenectomized LTBR−/− mice (which lack secondary lymphoid organs) that were adoptively transferred with effector T cells. In wild type recipients, depletion of DCs 5 days after receiving a heart allograft significantly delayed rejection (>30 days) (53). Finally, innate allorecognition also contributes to chronic rejection. Interfering with innate allorecognition by using recipients that lack CD47 or PIR-A resulted in reduced features of chronic rejection in a model of mouse renal allograft transplantation. In heart transplants, blocking the PIR-A pathway in recipients simultaneously treated with low-dose co-stimulation blockade (CTL4-Ig), led to long-term allograft survival with minimal pathology whereas recipients treated only with CTLA4-Ig rejected their allografts. However, absence of CD47 or PIR-A had no or only modest effects on acute rejection. Absence of inhibitory PIR-B on the other hand accelerated rejection. The major effect of the SIRPα-CD47 and MHC-I-PIR-A pathways, therefore, seem to be on chronic allograft rejection.
Implications for clinical transplantation
Although no clinical-translational studies have investigated the role of innate allorecognition, there is some data demonstrating that similar pathways exist in humans, suggesting that these pathways may have a similar role in clinical transplantation. The SIRPa – CD47 pathway is very similar in humans and has been mostly investigated in the context of tumors (54). Just like in mice, human SIRPa is polymorphic, while CD47 is not. Preliminary data confirmed that the amino-terminal ligand binding domain of human SIRPα is highly polymorphic (55). Studies investigating the role of donor SIRPa polymorphism in transplantation are underway. In xenotransplantation models, combined bone marrow and solid organ transplantation of human CD47 expressing tissues such as lungs or skin prolongs the solid organ’s survival (56, 57). While this is utilizing the same pathway, it is not interfering with innate allorecognition but utilizing CD47 expression to protect from phagocytosis.
The homologs of PIR in humans are LILR (leukocyte immunoglobulin like receptors) (58). Human LILRs are comprised of a set of 6 stimulatory, ITAM-motif containing, PIR-A and five inhibitory, ITIM-motif containing, PIR-B expressed on a wide spectrum of immune cells including T, B, NK, DC and myeloid subsets. Both LILR-As and LILR-Bs bind a diverse range of MHC-I molecules (55, 59). Interest in LILR primarily stems from their expression on MDSC in patients with cancer. So far, in transplantation, increased frequencies of circulating MDSC have been detected in kidney recipients (60). In vitro studies suggest that they have an immunosuppressive role. In intestinal transplant recipients, MDSC were also detected in allograft intestinal mucosa (61). If MDSC formation is allo-specific or a non-specific phenomenon remains to be investigated. Both, the SIRPa – CD47 and LILR – MHCI pathways will need to be investigated in transplantation and could be potential targets to improve transplant outcomes.
Concluding Remarks
Recent work has demonstrated that monocytes and macrophages directly recognize allogeneic non-self. This innate allorecognition is independent of lymphoid cells, induces the maturation of APCs which initiate and sustain the adaptive alloimmune response. It also generates a protective innate response by enabling macrophages to selectively recognize and eliminate allogeneic non-self targets. So far, two mechanisms of innate allorecognition have been identified: Recognition of polymorphic donor SIRPα by CD47 and binding of allogeneic MHC-I by PIR-A. Manipulating these pathways alleviated chronic rejection in mouse models. Future studies need to establish whether these pathways are clinically relevant.
Acknowledgements
This work was supported by NIH grant AI145881 (MHO) and Stuart K. Patrick Grant for Transplant Innovation (KIA). The authors have nothing to disclose. Figures were created using Biorender.com.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Klein J, in Biology of the Mouse Histocompatibility-2 Complex. (Springer-Verlag, New York, Heidelberg, Berlin, 1975), pp. 521–536. [Google Scholar]
- 2.Felix NJ et al. , Alloreactive T cells respond specifically to multiple distinct peptide-MHC complexes. Nat Immunol 8, 388–397 (2007). [DOI] [PubMed] [Google Scholar]
- 3.Sykes M, Wood K, Sachs DH, in Fundamental Immunology, Paul WE, Ed. (Woleters Kluwer/Lippincott Williams & Wilkins, Philadelphia, 2008), chap. 44, pp. 1426–1488. [Google Scholar]
- 4.Janeway CA Jr., Approaching the asymptote? Evolution and revolution in immunology. Cold Spring Harb Symp Quant Biol 54 Pt 1, 1–13 (1989). [DOI] [PubMed] [Google Scholar]
- 5.Iwasaki A, Medzhitov R, Regulation of adaptive immunity by the innate immune system. Science 327, 291–295 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goldstein DR, Tesar BM, Akira S, Lakkis FG, Critical role of the Toll-like receptor signal adaptor protein MyD88 in acute allograft rejection. J Clin Invest 111, 1571–1578 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McKay D, Shigeoka A, Rubinstein M, Surh C, Sprent J, Simultaneous deletion of MyD88 and Trif delays major histocompatibility and minor antigen mismatch allograft rejection. Eur. J. Immunol 36, 1994–2002 (2006). [DOI] [PubMed] [Google Scholar]
- 8.Oberbarnscheidt MH et al. , Type I interferons are not critical for skin allograft rejection or the generation of donor-specific CD8+ memory T cells. Am J Transplant 10, 162–167 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li H et al. , Graft-versus-host disease is independent of innate signaling pathways triggered by pathogens in host hematopoietic cells. J Immunol 186, 230–241 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matzinger P, Tolerance, danger, and the extended family. Annu. Rev. Immunol 12, 991–1045 (1994). [DOI] [PubMed] [Google Scholar]
- 11.Gallucci S, Lolkema M, Matzinger P, Natural adjuvants: endogenous activators of dendritic cells. Nat. Med 5, 1249–1255 (1999). [DOI] [PubMed] [Google Scholar]
- 12.Tesar BM, Zhang J, Li Q, Goldstein DR, TH1 immune responses to fully MHC mismatched allografts are diminished in the absence of MyD88, a toll-like receptor signal adaptor protein. Am J Transplant 4, 1429–1439 (2004). [DOI] [PubMed] [Google Scholar]
- 13.Hutton MJ et al. , Islet allograft rejection is independent of toll-like receptor signaling in mice. Transplantation 88, 1075–1080 (2009). [DOI] [PubMed] [Google Scholar]
- 14.Rosengarten RD, Nicotra ML, Model systems of invertebrate allorecognition. Curr Biol 21, R82–92 (2011). [DOI] [PubMed] [Google Scholar]
- 15.Nicotra ML et al. , A hypervariable invertebrate allodeterminant. Curr Biol 19, 583–589 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosa SF et al. , Hydractinia allodeterminant alr1 resides in an immunoglobulin superfamily-like gene complex. Curr Biol 20, 1122–1127 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Broz P, Monack DM, Newly described pattern recognition receptors team up against intracellular pathogens. Nat Rev Immunol 13, 551–565 (2013). [DOI] [PubMed] [Google Scholar]
- 18.Medzhitov R, PrestonHurlburt P, Janeway CA, A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature 388, 394–397 (1997). [DOI] [PubMed] [Google Scholar]
- 19.Poltorak A et al. , Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science 282, 2085–2088 (1998). [DOI] [PubMed] [Google Scholar]
- 20.Zecher D, van Rooijen N, Rothstein D, Shlomchik W, Lakkis F, An innate response to allogeneic nonself mediated by monocytes. J Immunol 183, 7810–7816 (2009). [DOI] [PubMed] [Google Scholar]
- 21.Liu W, Xiao X, Demirci G, Madsen J, Li XC, Innate NK cells and macrophages recognize and reject allogeneic nonself in vivo via different mechanisms. J Immunol 188, 2703–2711 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oberbarnscheidt MH et al. , Non-self recognition by monocytes initiates allograft rejection. J Clin Invest 124, 3579–3589 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chow KV et al. , Innate Allorecognition Results in Rapid Accumulation of Monocyte-Derived Dendritic Cells. J Immunol 197, 2000–2008 (2016). [DOI] [PubMed] [Google Scholar]
- 24.Barclay AN, Van den Berg TK, The interaction between signal regulatory protein alpha (SIRPalpha) and CD47: structure, function, and therapeutic target. Annu. Rev. Immunol 32, 25–50 (2014). [DOI] [PubMed] [Google Scholar]
- 25.Dai H et al. , Donor SIRPa polymorphism modulates the innate immune response to allogeneic grafts. Science Immunology 2, eaam6202 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yamauchi T et al. , Polymorphic Sirpa is the genetic determinant for NOD-based mouse lines to achieve efficient human cell engraftment. Blood 121, 1316–1325 (2013). [DOI] [PubMed] [Google Scholar]
- 27.Wong AS et al. , Polymorphism in the innate immune receptor SIRPalpha controls CD47 binding and autoimmunity in the nonobese diabetic mouse. J. Immunol 193, 4833–4844 (2014). [DOI] [PubMed] [Google Scholar]
- 28.Menon MC, Heeger PS, Donor SIRP-alpha polymorphisms: widening the innate-to-adaptive continuum in allograft rejection. Kidney Int. 92, 1305–1308 (2017). [DOI] [PubMed] [Google Scholar]
- 29.Espinosa JR, Samy KP, Kirk AD, Memory T cells in organ transplantation: progress and challenges. Nature reviews. Nephrology 12, 339–347 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ford ML, Larsen CP, Overcoming the memory barrier in tolerance induction: molecular mimicry and functional heterogeneity among pathogen-specific T-cell populations. Curr Opin Organ Transplant 15, 405–410 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Macedo C et al. , Contribution of naïve and memory T-cell populations to the human alloimmune response. Am J Transplant 9, 2057–2066 (2009). [DOI] [PubMed] [Google Scholar]
- 32.O'Leary JG, Goodarzi M, Drayton DL, von Andrian UH, T cell- and B cell-independent adaptive immunity mediated by natural killer cells. Nat Immunol 7, 507–516 (2006). [DOI] [PubMed] [Google Scholar]
- 33.Paust S et al. , Critical role for the chemokine receptor CXCR6 in NK cell-mediated antigen-specific memory of haptens and viruses. Nat Immunol 11, 1127–1135 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sun JC, Beilke JN, Lanier LL, Adaptive immune features of natural killer cells. Nature 457, 557–561 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cooper MA et al. , Cytokine-induced memory-like natural killer cells. Proc Natl Acad Sci USA 106, 1915–1919 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Quintin J et al. , Candida albicans infection affords protection against reinfection via functional reprogramming of monocytes. Cell Host Microbe 12, 223–232 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nabekura T, Lanier LL, Antigen-specific expansion and differentiation of natural killer cells by alloantigen stimulation. J Exp Med 211, 2455–2465 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weavers H, Evans IR, Martin P, Wood W, Corpse Engulfment Generates a Molecular Memory that Primes the Macrophage Inflammatory Response. Cell 165, 1658–1671 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hole CR et al. , Induction of memory-like dendritic cell responses in vivo. Nature communications 10, 2955 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dai H et al. , PIRs mediate innate myeloid cell memory to nonself MHC molecules. Science 368, 1122–1127 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Netea MG, Quintin J, van der Meer JW, Trained immunity: a memory for innate host defense. Cell host & microbe 9, 355–361 (2011). [DOI] [PubMed] [Google Scholar]
- 42.Netea MG, Training innate immunity: the changing concept of immunological memory in innate host defence. Eur. J. Clin. Invest 43, 881–884 (2013). [DOI] [PubMed] [Google Scholar]
- 43.Netea MG et al. , Trained immunity: A program of innate immune memory in health and disease. Science 352, aaf1098 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gardiner CM, Mills KH, The cells that mediate innate immune memory and their functional significance in inflammatory and infectious diseases. Semin. Immunol 28, 343–350 (2016). [DOI] [PubMed] [Google Scholar]
- 45.Pradeu T, Du Pasquier L, Immunological memory: What's in a name? Immunol. Rev 283, 7–20 (2018). [DOI] [PubMed] [Google Scholar]
- 46.Patel AA et al. , The fate and lifespan of human monocyte subsets in steady state and systemic inflammation. J. Exp. Med 214, 1913–1923 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yona S et al. , Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity 38, 79–91 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kubagawa H et al. , Biochemical nature and cellular distribution of the paired immunoglobulin-like receptors, PIR-A and PIR-B. J Exp Med 189, 309–318 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kubagawa H, Burrows PD, Cooper MD, A novel pair of immunoglobulin-like receptors expressed by B cells and myeloid cells. Proc. Natl. Acad. Sci. U. S. A 94, 5261–5266 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Takai T, Paired immunoglobulin-like receptors and their MHC class I recognition. Immunology 115, 433–440 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tun T et al. , Genomic structure of mouse PIR-A6, an activating member of the paired immunoglobulin-like receptor gene family. Tissue Antigens 61, 220–230 (2003). [DOI] [PubMed] [Google Scholar]
- 52.Nakamura A, Kobayashi E, Takai T, Exacerbated graft-versus-host disease in Pirb−/− mice. Nat Immunol 5, 623–629 (2004). [DOI] [PubMed] [Google Scholar]
- 53.Zhuang Q et al. , Graft-infiltrating host dendritic cells play a key role in organ transplant rejection. Nat Commun 7, 12623 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Murata Y, Kotani T, Ohnishi H, Matozaki T, The CD47-SIRPalpha signalling system: its physiological roles and therapeutic application. J Biochem 155, 335–344 (2014). [DOI] [PubMed] [Google Scholar]
- 55.Friday A et al. , Identification of human SIRPa diversity that could regulate innate allorecognition [abstract]. Am J Transplant 17, (2017). [Google Scholar]
- 56.Watanabe H et al. , Intra-bone bone marrow transplantation from hCD47 transgenic pigs to baboons prolongs chimerism to >60 days and promotes increased porcine lung transplant survival. Xenotransplantation 27, e12552 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tena AA et al. , Prolonged Survival of Pig Skin on Baboons After Administration of Pig Cells Expressing Human CD47. Transplantation 101, 316–321 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hudson LE, Allen RL, Leukocyte Ig-Like Receptors - A Model for MHC Class I Disease Associations. Front Immunol 7, 281 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.van der Touw W, Chen HM, Pan PY, Chen SH, LILRB receptor-mediated regulation of myeloid cell maturation and function. Cancer Immunol. Immunother 66, 1079–1087 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Luan Y et al. , Monocytic myeloid-derived suppressor cells accumulate in renal transplant patients and mediate CD4(+) Foxp3(+) Treg expansion. Am J Transplant 13, 3123–3131 (2013). [DOI] [PubMed] [Google Scholar]
- 61.Okano S et al. , Myeloid-derived suppressor cells increase and inhibit donor-reactive T cell responses to graft intestinal epithelium in intestinal transplant patients. Am J Transplant 18, 2544–2558 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]