Abstract
Objectives:
To understand the epidemiology of acute hematogenous osteomyelitis and septic arthritis, including clinical and demographic features, microbiology, treatment approaches, treatment-associated complications, and outcomes.
Study Design:
Retrospective cohort study of 453 children with AHO and/or SA from 2009 – 2015.
Results:
Among the 453 patients, 218 (48%) had AHO, 132 (29%) had SA, and 103 (23%) had concurrent AHO/SA. Treatment failure/recurrent infection occurred in 41 (9%). Patients with concurrent AHO/SA had longer hospital stays, longer duration of antibiotic therapy, and were more likely to have prolonged bacteremia and require intensive care. Staphylococcus aureus was identified in 228 (51%) of patients, of which 114 (50%) were methicillin-resistant S. aureus (MRSA). Compared with SA, AHO and concurrent AHO/SA were associated with higher odds of treatment failure (OR 8.19, 95% CI 2.02, 33.21; p=.003; and OR 14.43, 95% CI 3.39, 61.37; p<.001, respectively). Need for more than one surgical procedure was also associated with higher odds of treatment failure (OR 2.98, 95% CI 1.18, 7.52; P = .021). Early change to oral antibiotic therapy was not associated with treatment failure (OR 0.64, 95% CI 0.24, 1.74; p=.386). Most (73%) medically-attended treatment complications occurred while on parenteral therapy.
Conclusions:
Musculoskeletal infections are challenging pediatric infections. S. aureus remains the most common pathogen, with MRSA accounting for 25% of all cases. Concurrent AHO/SA is associated with more severe disease and worse outcomes. Fewer treatment-related complications occurred while on oral therapy. Early transition to oral therapy was not associated with treatment failure.
Pediatric musculoskeletal infections), including acute hematogenous osteomyelitis and septic arthritis, are common invasive infections, occurring in up to 80 per 100,000 children in the US [1]. These infections require prolonged antibiotics and are often complicated by bacteremia, septic thrombophlebitis, and myositis [2]. Despite the frequency and severity of pediatric MSKI, there is a paucity of contemporary US data regarding clinical characteristics, management strategies, and outcomes.
Though Staphylococcus aureus has remained the most common causative pathogen of MSKI for decades [3], recent data suggest decreasing rates of once ubiquitous community-associated methicillin-resistant S. aureus (CA-MRSA), and resistance to other commonly used antimicrobials, such as clindamycin, has increased [4–6]. The addition of pneumococcal conjugate (PCV7 and PCV13) and Haemophilus influenzae type B (Hib) vaccines to the childhood immunization schedule also resulted in substantial declines in invasive disease due to these pathogens [7–11]. Epidemiologic data for other important pathogens in MSKI, including Streptococcus pyogenes and Kingella kingae, are limited [1, 12].
Changing epidemiologic trends, along with limited evidence for the optimal therapeutic approaches have led to considerable variability in the management of MSKI in children [13]. Several studies suggest that early transition to oral antibiotics may not be associated with increased risk of treatment failure [14–18] and obviates the risk of frequently observed catheter-related complications [14, 19]. A contemporary evaluation of pediatric MSKI in the US is needed.
We sought to define and compare the epidemiology of AHO and SA at two large US children’s hospitals, focusing on clinical and demographic features, microbiology, treatment, medically-attended complications, and outcomes.
Methods
We performed a retrospective review of children with MSKI who were treated at Egleston Hospital at Children’s Healthcare of Atlanta (Georgia) and the Monroe Carell Jr. Children’s Hospital at Vanderbilt University Medical Center (Nashville, Tennessee). This study was approved by each site’s Institutional Review Board. Patients were identified through a query of ICD-9 codes (Table I; available at www.jpeds.com) used for the diagnosis of AHO and SA, and the diagnosis was confirmed by chart review. Inclusion criteria were age 6 months – 18 years (inclusive) and a discharge diagnosis of AHO or SA between 1 January 2009 – 30 September 2015. Exclusion criteria were evidence of subacute or chronic osteomyelitis (symptoms >14 days), history of deep penetrating trauma in or adjacent to the site, presence of a foreign body or hardware in or adjacent to the site, infection that occurred within 12 months of a surgical procedure involving the affected bone or joint, osteomyelitis of skull or jaw, and osteomyelitis of the clavicle (unless a pathogen associated with AHO was recovered as these sites are commonly implicated in chronic recurrent multifocal osteomyelitis). Patients with a primary bone abnormality syndrome (such as osteogenesis imperfecta) and those with compromised immune systems (primary diagnosis or secondary to systemic immunomodulators) were excluded; however, children with sickle cell anemia-associated functional asplenia and selective IgA deficiency were included.
Table 1.
ICD-9 codes to identify AHO and septic arthritis
| ICD-9 Code | Diagnosis |
|---|---|
| Osteomyelitis codes | |
| 730 | Acute osteomyelitis |
| 730.0 | Acute osteomyelitis |
| 730.2 | Unspecified osteomyelitis |
| 730.8 | Other infections involving the bone in diseases classified elsewhere |
| 730.9 | Unspecified infection of bone |
| Septic arthritis codes | |
| 711.0 | Pyogenic arthritis |
| 711.4 | Arthropathy associated with other bacterial diseases |
| 711.8 | Arthropathy associated with infection and other parasitic disease |
| 711.9 | Unspecified infective arthritis |
| 713.7 | Other general diseases with articular involvement |
| 713.8 | Arthropathy associated with other conditions classified elsewhere |
Data abstraction and definitions
Data were abstracted through review of the electronic medical record by trained study personnel and recorded in a standardized electronic case report form into a central database (Research Electronic Data Capture, REDCap). Final review and adjudication of discrepancies was performed by a pediatric infectious disease physician at each site.
Subjective data were recorded directly from clinical documentation during electronic medical record review. If no information was provided, the data were considered missing and the denominator was noted.
Definitions were specified a priori. Concurrent AHO/SA was defined as disease of both the bone and contiguous joint identified by examination, imaging and/or surgical procedure and documented in the chart; disseminated infection was defined as presence of visceral abscess, pulmonary nodules, endocarditis, and/or thrombophlebitis; and persistent bacteremia was defined as a positive blood culture >72 hours after initiation of antibiotics. Delayed surgical procedure was defined as the first surgical procedure occurring >3 days after admission. Adherence to a prescribed antibiotic course was determined by the clinician’s observation as documented in the chart. Treatment failure was defined as documentation of the following events: worsening AHO or SA while on antibiotic therapy prompting an antibiotic change (not including change to narrow antibiotic activity, adjustment for patient convenience, or changes related to toxicity or reaction); new onset swelling, erythema, or pain in the original site of AHO/SA within 1 month of completing the initial antibiotic course that required an additional course of antimicrobials; or diagnosis of chronic osteomyelitis (identified by notation in the medical record of chronic osteomyelitis/infection or radiographic evidence of chronic infection, such as a sequestrum or Brodie’s abscess) in the same extremity after completion of initial antibiotic course.
To investigate differences during the study period, we analyzed the cohort over two time periods: January 2009 – June 2012 and July 2012 – September 2015, as they had a comparable number of patients identified, 229 vs. 224 respectively.
Both institutions follow Infectious Diseases Society of America outpatient parenteral antimicrobial therapy guidelines for laboratory monitoring while receiving intravenous antibiotics [20].
Statistical Analyses
Descriptive statistics were used to describe the epidemiology of AHO and SA. The Fisher exact and Wilcoxon rank-sum tests were used to compare categorical and continuous variables, respectively. For pairwise comparisons between the three infection types (AHO, SA, AHO/SA) or between causative organisms, a Bonferroni correction was applied to penalize for multiple comparisons.
Multiple logistic regression was used to evaluate the independent association of duration of parenteral therapy with treatment failure. Model covariates included patient age, sex, race, ethnicity, clinic site, clinical (parenteral antibiotic duration, surgical procedure, myositis, multifocal infection, infection type) and microbiological variables (positive pathogen culture, bacteremia) significantly associated at the 0.05 level in bivariate analyses with either short course parenteral (≤7 days) or treatment failure. Odds ratios for clinically relevant variables were reported in addition to Wald 95% confidence intervals (CI) and p values.
Results
Patient Demographics and Characteristics
Patients, 268 from Vanderbilt and 185 from Emory, met criteria for inclusion. Demographic and clinical data are shown in Table 2. The majority of patients were non-Hispanic white (55%), male (63%), and had no pre-existing medical conditions (71%). The median age was 6.0 years (IQR 2.2 – 10.5). Children with SA were younger (2.9 years; IQR 1.3–7.7) than children with AHO (8.1 years; IQR 3.3–11.2, p≤.001) (Table 3). AHO was the most common infection type (48%), followed by SA (29%), and concurrent AHO/SA (23%). The pelvis and lower extremities were the most commonly affected sites in children with AHO (92%) with the femur most commonly affected (Table 2). Similarly, the lower extremities and sacroiliac joints were the most commonly affected sites in children with SA (89%) with the knee being the most commonly affected joint. Half of all patients had MSKI-associated myositis, most commonly in children with concurrent AHO/SA (64%). Disseminated infection was present in 7%, most commonly in children with concurrent AHO/SA, occurring in 15% of those children.
Table 2.
Population Characteristics
| Characteristic | ||
|---|---|---|
| Na | ||
| Age at admission (years, median [IQR]) | 453 | 6.00 (2.20, 10.50) |
| Female, No. (%) | 453 | 168 (37) |
| Race/Ethnicity, No. (%) | 392 | |
| Non-Hispanic White | 215 (55) | |
| Non-Hispanic Black | 127 (32) | |
| Hispanic | 26 (7) | |
| Other | 24 (6) | |
| Pre-existing medical conditionb, No. (%) | 437 | |
| None | 311 (71) | |
| Respiratory | 32 (7) | |
| Neurologic | 17 (4) | |
| Blood | 11 (3) | |
| Ortho | 12 (3) | |
| GI | 7 (2) | |
| Prematurity | 10 (2) | |
| Kidney | 3 (1) | |
| Cardiac | 5 (1) | |
| Liver | 1 (0) | |
| Other | 76 (17) | |
| Infection type and locationc, No. (%) | ||
| Acute Hematogenous Osteomyelitis | 218 | |
| Femur | 53 (24) | |
| Fibula | 16 (7) | |
| Foot | 39 (18) | |
| Hand | 9 (4) | |
| Humerus | 9 (4) | |
| Pelvis | 39 (18) | |
| Radius | 1 (<1) | |
| Tibia | 43 (20) | |
| Ulna | 3 (1) | |
| Vertebral | 8 (4) | |
| Otherd | 6 (3) | |
| Septic Arthritis | 132 | |
| Ankle | 11 (8) | |
| Elbow | 9 (7) | |
| Foot | 4 (3) | |
| Hand | 2 (2) | |
| Hip | 51 (39) | |
| Knee | 54 (41) | |
| Sacro-iliac | 1 (1) | |
| Shoulder | 3 (2) | |
| Wrist | 3 (2) | |
| Pubic symphysis | 1 (1) | |
| Concurrent AHO/SAe | 103 | |
| Lower Extremity | 88 (85) | |
| Upper Extremity | 20 (19) | |
| Associated Findings, No. (%) | 453 | |
| Myositis/pyomyositis | 229 (51) | |
| Septic Arthritis | 132 | 41 (31) |
| Acute Hematogenous Osteomyelitis | 218 | 122 (56) |
| Concurrent AHO/SA | 103 | 66 (64) |
| Disseminated infectionf | 30 (7) | |
| Septic Arthritis | 132 | 3 (2) |
| Acute Hematogenous Osteomyelitis | 218 | 12 (6) |
| Concurrent AHO/SA | 103 | 15 (15) |
| Fasciitis | 22 (5) | |
| Septic Arthritis | 132 | 3 (2) |
| Acute Hematogenous Osteomyelitis | 218 | 11 (5) |
| Concurrent AHO/SA | 103 | 8 (8) |
Some subjects had missing data
Subjects may have multiple pre-existing conditions
Subjects may be infected at multiple sites
Other AHO sites- rib, clavicle, scapula, sternum
Five subjects with both upper and lower extremity infections
Defined as visceral abscess, pulmonary nodules, or endocarditis)
Table 3.
Clinical Characteristics and Outcomes
| Characteristica | All | Septic Arthritis | Osteomyelitis | Both | ||||
|---|---|---|---|---|---|---|---|---|
| Clinical Presentation | N | N | N | N | ||||
| Age at admission (years) | 453 | 6.00 (2.20, 10.50) | 132 | 2.90 (1.30, 7.70) | 218 | 8.05 (3.30, 11.20)° | 103 | 8.00 (2.90, 11.50)° |
| Symptoms prior to presentation, days | 452 | 4.0 (2.0, 7.0) | 132 | 3.0 (2.0, 5.0) | 217 | 5.0 (3.0, 7.0) | 103 | 4.0 (2.0, 7.0) |
| Fever prior to presentation, days | 346 | 3.0 (1.0, 5.0) | 91 | 2.0 (1.0, 4.0) | 174 | 3.0 (2.0, 5.0) | 81 | 3.0 (2.0, 5.0) |
| Antibiotic pretreatment, No. (%) | 453 | 133 (29%) | 132 | 24 (18%) | 218 | 71 (33%) | 103 | 38 (37%) |
| Antibiotic pretreatment, days | 123 | 3.0 (1.0, 5.0) | 22 | 2.5 (1.0, 5.0) | 67 | 3.0 (1.0, 6.0) | 34 | 3.0 (1.0, 5.0) |
| Fever at admission, No. (%) | 453 | 112 (25%) | 132 | 23 (17%) | 218 | 46 (21%) | 103 | 43 (42%)°* |
| Symptoms at presentation, No. (%) | ||||||||
| Erythema | 438 | 146 (33%) | 129 | 31 (24%) | 210 | 73 (35%) | 99 | 42 (42%) |
| Swelling | 438 | 248 (57%) | 127 | 68 (54%) | 212 | 114 (54%) | 99 | 66 (67%) |
| Tenderness | 451 | 361 (80%) | 132 | 97 (73%) | 217 | 176 (81%) | 102 | 88 (86%) |
| Functional status at presentation, No. (%) | ||||||||
| Full range of motion | 451 | 143 (32%) | 132 | 30 (23%) | 217 | 91 (42%) | 102 | 22 (22%) |
| Normal ambulation | 377 | 12 (3%) | 112 | 2 (2%) | 181 | 7 (4%) | 84 | 3 (4%) |
| Limp | 377 | 221 (59%) | 112 | 71 (63%) | 181 | 106 (59%) | 84 | 44 (52%) |
| Not ambulatingb | 377 | 144 (38%) | 112 | 39 (35%) | 181 | 68 (38%) | 84 | 37 (44%) |
| Ambulatory with assistancec | 333 | 71 (21%) | 104 | 12 (12%) | 157 | 40 (25%) | 72 | 19 (26%) |
| Characteristics During Hospitalization | ||||||||
| Hospitalization duration, days | 453 | 5.0 (4.0, 8.0) | 132 | 4.0 (3.0, 6.0) | 218 | 5.0 (4.0, 7.0)° | 103 | 8.0 (5.0, 14.0)°* |
| ICUd required, No. (%) | 453 | 48 (11%) | 132 | 4 (3%) | 218 | 18 (8%) | 103 | 26 (25%)°* |
| Mechanical ventilation required | 450 | 16 (4%) | 132 | 3 (2%) | 215 | 6 (3%) | 103 | 7 (7%) |
| Surgery performed, No. (%) | 453 | 345 (76%) | 132 | 126 (95%) | 218 | 129 (59%)° | 103 | 90 (87%)* |
| Laboratory Characteristics | ||||||||
| CRPe at admission (mg/L) | 449 | 71.0 (29.0, 152.0) | 130 | 51.5 (18.0, 88.0) | 217 | 65.0 (23.0, 148.0)° | 102 | 137.5 (74.0, 235.0)°* |
| Peak CRP (mg/L) | 450 | 92.0 (42.0, 184.0) | 130 | 66.5 (33.0, 117.0) | 217 | 85.0 (36.0, 172.0) | 103 | 180.0 (92.0, 270.0)°* |
| WBCf at admission (K/mm3) | 444 | 12.0 (9.0, 15.0) | 130 | 13.0 (10.0, 15.0) | 214 | 11.0 (8.0, 14.0) | 100 | 12.5 (9.5, 16.0) |
| Peak WBC count (K/mm3) | 444 | 13.0 (10.0, 16.0) | 130 | 13.0 (11.0, 16.0) | 214 | 12.0 (9.0, 16.0) | 100 | 14.0 (11.0, 21.0) |
| ESRg at admission (mm/hr) | 412 | 47.0 (27.0, 67.0) | 124 | 44.0 (24.5, 64.5) | 195 | 42.0 (25.0, 66.0) | 93 | 52.0 (33.0, 78.0) |
| Peak ESR (mm/hr) | 408 | 59.0 (36.0, 80.0) | 122 | 53.5 (33.0, 71.0) | 196 | 52.0 (32.0, 79.0) | 90 | 70.0 (53.0, 96.0) |
| Antibiotic Therapy, No. (%) | ||||||||
| Initial parenteral antibiotics | 452 | 445 (98%) | 132 | 130 (98%) | 217 | 214 (99%) | 103 | 101 (98%) |
| Oral therapy at discharge | 451 | 304 (67%) | 131 | 94 (72%) | 217 | 149 (69%) | 103 | 61 (59%) |
| Parenteral therapy exclusively | 444 | 37 (8%) | 130 | 15 (12%) | 213 | 14 (7%) | 101 | 8 (8%) |
| PICC line placed | 450 | 184 (41%) | 130 | 37 (28%) | 218 | 81 (37%) | 102 | 66 (65%) |
| Total duration of antibiotic treatment (days) | 445 | 34 (28, 46) | 131 | 25 (21,31) | 213 | 37 (30, 48) | 101 | 43 (33, 57) |
| Outcomes, No. (%) | ||||||||
| Function at end of therapy | ||||||||
| Cast | 362 | 4 (1%) | 109 | 0 (0%) | 175 | 2 (1 %) | 78 | 2 (3%) |
| Full range of motion | 359 | 314 (87%) | 108 | 103 (95%) | 172 | 160 (93%) | 79 | 51 (65%) |
| Pain at end of therapy | ||||||||
| None | 361 | 286 (79%) | 109 | 93 (85%) | 174 | 140 (80%) | 78 | 53 (68%) |
| Mild | 361 | 66 (18%) | 109 | 15 (14%) | 174 | 30 (17%) | 78 | 21 (27%) |
| Moderate | 361 | 9 (2%) | 109 | 1 (1%) | 174 | 4 (2%) | 78 | 4 (5%) |
| Pathologic fracture | 442 | 12 (3%) | 130 | 0 (0%) | 212 | 4 (2%) | 100 | 8 (8%) |
| Treatment failure/Recurrent infection | 444 | 41 (9%) | 130 | 4 (3%) | 212 | 17 (8%) | 102 | 20 (20%) |
| Nonadherence with outpatient antibioticsh | 378 | 31 (8%) | 113 | 7 (6%) | 183 | 15 (8%) | 82 | 9 (11 %) |
Data are presented as median (interquartile range) unless otherwise stated
Ambulation status only collected on patients with lower extremity infection
Among subjects not ambulating normally
Intensive care unit
C-reactive protein
White blood cell count
Erythrocyte sedimentation rate
Suspected or confirmed by documentation in medical record
Tests for significance among all three groups were conducted for a priori selected categorical or continuous variables using Fisher’s exact test or Wilcoxon rank sum test, respectively. If the group differences were significant at p<0.05, pairwise comparisons were computed and Bonferroni-adjusted. Significant pairwise differences between groups are marked based on the comparator
= vs. SA
= vs. AHO
Characteristics During Hospitalization
The duration of hospitalization was similar between children with SA and AHO, and children with concurrent AHO/SA had a significantly longer hospitalization (AHO/SA median 8.0 days [IQR 5.0–14.0] versus SA 4.0 days [IQR 3.0–6.0], p<.001; AHO 5.0 days [IQR 4.0–7.0], p<.001) (Table 3). Eleven percent of patients required care in the intensive care unit (ICU), and 4% required mechanical ventilation. A higher proportion of children with concurrent AHO/SA required ICU care (25%) compared with children with AHO (8%, p<.001) or SA (3%, p<.001). Surgery was performed in 76% of patients, and a higher proportion of children with SA underwent at least one surgical procedure compared with AHO (95% vs 59%, p<.001).
Markers of inflammation (C-reactive protein [CRP], erythrocyte sedimentation rate [ESR] and white blood cell [WBC] count) were elevated. Children with concurrent AHO/SA had significantly higher CRP at admission (137.5 mg/L; IQR 74.0–235.0) than those with AHO (65.0 mg/L; IQR 23.0–148.0, p<.001) or SA (51.5 mg/L; IQR 18.0–88.0, p<.001). Peak CRP was higher in those with concurrent AHO/SA (180.0 mg/L; IQR 92.0–270.0) than in those with AHO (85.0 mg/L; IQR 36.0–172.0, p=.007) and SA (66.5 mg/L; IQR 33.0–117.0, p<.001).
Nearly all (98%) initial antibiotic therapy was administered intravenously. Clindamycin was the most common initial and final antibiotic used (Table 4; available at www.jpeds.com). When comparing trends over the study period (Jan 2009 – June 2012 vs. July 2012 – September 2015), empiric vancomycin use decreased from 54% to 35% (p<.001) and use of a PICC decreased from 56% to 26% (p<.001). The majority of patients (67%) were transitioned to oral antibiotics at discharge, a trend that increased over the study period from 58% to 77% (p<.001). Overall, 8% of patients received exclusively parenteral therapy for the duration of treatment. The median duration of total antibiotic therapy for all patients was 34.0 days (IQR 28.0, 46.0); for patients with SA 25.0 days (IQR 21.0–31.0), AHO 37.0 days (IQR 30.0–48.0) and AHO/SA 43.0 days (IQR 33.0–57.0).
Table 4.
Antibiotic use by Infection Type
| Characteristic | All (N=453) | Septic Arthritis (N=132) | Osteomyelitis (N=217) | Both (N=104) | ||||
|---|---|---|---|---|---|---|---|---|
| Initial antibiotics | ||||||||
| Vancomycin | 452 | 203 (45%) | 132 | 54 (41%) | 216 | 81 (38%) | 104 | 68 (65%) |
| Clindamycin | 452 | 321 (71%) | 132 | 91 (69%) | 216 | 164 (76%) | 104 | 66 (63%) |
| 1st generation cephalosporin | 452 | 14 (3%) | 132 | 5 (4%) | 216 | 7 (3%) | 104 | 2 (2%) |
| 2+ generation cephalosporin | 452 | 184 (41%) | 132 | 85 (64%) | 216 | 62 (29%) | 104 | 37 (36%) |
| Anti-staphylococcal penicillin | 452 | 11 (2%) | 132 | 1 (1%) | 216 | 5 (2%) | 104 | 5 (5%) |
| Other penicillin | 452 | 9 (2%) | 132 | 2 (2%) | 216 | 3 (1%) | 104 | 4 (4%) |
| Fluroquinolone | 452 | 5 (1%) | 132 | 0 (0%) | 216 | 5 (2%) | 104 | 0 (0%) |
| Linezolid | 452 | 1 (0%) | 132 | 0 (0%) | 216 | 1 (0%) | 104 | 0 (0%) |
| Other | 452 | 29 (6%) | 132 | 2 (2%) | 216 | 11 (5%) | 104 | 16 (15%) |
| Final antibiotics | ||||||||
| Vancomycin | 452 | 32 (7%) | 131 | 3 (2%) | 217 | 22 (10%) | 104 | 7 (7%) |
| Clindamycin | 452 | 242 (54%) | 131 | 78 (60%) | 217 | 110 (51%) | 104 | 54 (52%) |
| 1st generation cephalosporin | 452 | 97 (21%) | 131 | 23 (18%) | 217 | 49 (23%) | 104 | 25 (24%) |
| 2+ generation cephalosporin | 452 | 82 (18%) | 131 | 48 (37%) | 217 | 27 (12%) | 104 | 7 (7%) |
| Anti-staphylococcal penicillin | 452 | 30 (7%) | 131 | 5 (4%) | 217 | 17 (8%) | 104 | 8 (8%) |
| Other penicillin | 452 | 29 (6%) | 131 | 13 (10%) | 217 | 9 (4%) | 104 | 7 (7%) |
| Fluroquinolone | 452 | 20 (4%) | 131 | 6 (5%) | 217 | 12 (6%) | 104 | 2 (2%) |
| Linezolid | 452 | 15 (3%) | 131 | 2 (2%) | 217 | 9 (4%) | 104 | 4 (4%) |
| Other | 452 | 11 (2%) | 131 | 5 (4%) | 217 | 4 (2%) | 104 | 2 (2%) |
Microbiology
A pathogen was identified by sterile site sampling (e.g., blood or surgical site culture or polymerase-chain reaction [PCR]) in 62% of all patients (Table 5). A pathogen was identified more commonly in children with concurrent AHO/SA (81%) compared with AHO (68%, p=.050) or SA (38%, p<.001). Of patients with a pathogen identified (n=277), blood culture alone identified the pathogen in 22%, surgical site sampling in 27%, and blood culture plus surgical site sampling in 50%. Overall, 29% of patients received antibiotic pretreatment prior to admission. Of those, a pathogen was identified in 64%, similar to those that did not receive antibiotic pre-treatment (61%, p=.593).
Table 5.
Microbiologic Characteristics
| Characteristic | All | Septic Arthritis | Osteomyelitis | Both | ||||
|---|---|---|---|---|---|---|---|---|
| N | Median (IQR) | N | Median (IQR) | N | Median (IQR) | N | Median (IQR) | |
| Duration of bacteremia (days) | 171 | 2.0 (1.0, 4.0) | 16 | 1.5 (1.0, 2.0) | 92 | 2.0 (1.0, 4.0) | 63 | 3.0 (2.0, 4.0) |
| Bacteremia | 419 | 171 (41%) | 118 | 16 (14%) | 203 | 92 (45%) | 98 | 63 (64%) |
| Persistent bacteremia (>72 h) | 419 | 53 (13%) | 118 | 3 (3%) | 203 | 26 (13%) | 98 | 24 (24%) |
| Organism identified by blood or infection site culturea | 448 | 277 (62%) | 132 | 50 (38%) | 213 | 144 (68%) | 103 | 83 (81%) |
| None | 171 (38%) | 82 (62%) | 69 (32%) | 20 (19%) | ||||
| S. aureus | 228 (51%) | 25 (19%) | 127 (60%) | 76 (74%) | ||||
| S. pyogenes | 21 (5%) | 9 (7%) | 7 (3%) | 5 (5%) | ||||
| K. kingae | 5 (1%) | 3 (2%) | 2 (1%) | 0 (0%) | ||||
| S. pneumoniae | 6 (1%) | 4 (3%) | 2 (1%) | 0 (0%) | ||||
| H. influenzae | 2 (0%) | 2 (2%) | 0 (0%) | 0 (0%) | ||||
| Salmonella spp. | 4 (1%) | 0 (0%) | 3 (1%) | 1 (1%) | ||||
| Other organismb | 14 (3%) | 8 (6%) | 4 (2%) | 2 (2%) | ||||
| S. aureus antibiotic resistance | 228 | 25 | 127 | 76 | ||||
| Methicillin | 114 (50%) | 10 (40%) | 61 (48%) | 43 (57%) | ||||
| Clindamycin | 19 (8%) | 3 (12%) | 11 (9%) | 5 (7%) | ||||
| TMP/SMX | 3 (1%) | 1 (4%) | 1 (1%) | 1 (1%) | ||||
| Erythromycin | 126 (55%) | 11 (44%) | 70 (55%) | 45 (59%) | ||||
| MRSA resistance | 114 | 10 | 61 | 43 | ||||
| Clindamycin | 8 (7%) | 2 (20%) | 4 (7%) | 2 (5%) | ||||
| TMP/SMX | 1 (1%) | 0 (0%) | 0 (0%) | 1 (2%) | ||||
| Doxycycline | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | ||||
| Erythromycin | 90 (79%) | 9 (90%) | 49 (80%) | 32 (74%) | ||||
| MSSA resistance | 114 | 15 | 66 | 33 | ||||
| Clindamycin | 11 (10%) | 1 (7%) | 7 (11%) | 3 (9%) | ||||
| TMP/SMX | 2 (2%) | 1 (7%) | 1 (2%) | 0 (0%) | ||||
| Erythromycin | 36 (32%) | 2 (13%) | 21 (32%) | 13 (39%) | ||||
Three subjects had polymicrobial infections
Other organisms include coagulase negative Staphylococcus, Group C Streptococcus, Group G Streptococcus, viridans group Streptococcus, Leclercia adecarboxylata, Enterobacter asbunae, Enterobacter cloacae, Pseudomonas fluorescens, Bacillus spp.
Bacteremia was identified in 41% of patients that had at least one blood culture (n=419). Bacteremia was most common among patients with concurrent AHO/SA (64%). The median duration of bacteremia was 2.0 days (IQR 1.0 – 4.0). Persistent bacteremia of at least three days was observed in 13%, most commonly in patients with concurrent AHO/SA (24%).
S. aureus was the most commonly identified organism, isolated in 51% of all patients, a proportion that remained similar over time (55% vs. 47%, p=.130). S. aureus accounted for 81% of all identified pathogens. S. aureus was recovered more frequently in concurrent SA+AHO (74%) than either SA (19%) or AHO (60%) alone (p<.001). Of those with S. aureus, 54% were identified by both blood and surgical site cultures, 23% only from surgical specimens, and 23% by blood culture alone. S. aureus was the causative pathogen in the majority of patients with bacteremia (91%). Methicillin resistance was observed in 50% of all S. aureus isolates and remained similar over time (52% vs. 48%, p=.595), across infection types (p=.278), and within treatment centers (47% vs. 51% [p=0.714] and 58% vs. 44% [p=0.181]). Clindamycin resistance was observed in 8% of all S. aureus isolates and was stable over time (8% vs. 9%, p>.999). Trimethoprim-sulfamethoxazole (TMP-SMX) resistance was observed in 1% of S. aureus isolates.
S. pyogenes, S. pneumoniae, and K. kingae accounted for 5%, 1%, and 1% of infections, respectively. Demographics, and clinical characteristics of patients with S. pyogenes compared with S. aureus are shown in Table 6 (available at www.jpeds.com). For non-S. aureus pathogens, 45% were identified only in surgical site specimens, 35% in both blood and surgical site cultures, and 18% only by blood culture.
Table 6:
Staphylococcus aureus (SA) vs Streptococcus pyogenes (GAS)
| Characteristic | SA (N=228) | GAS(N=21) | ||
|---|---|---|---|---|
| N | Median (IQR) | N | Median (IQR) | |
| Age at admission (yrs) | 228 | 8.80 (3.60, 11.60) | 21 | 6.10 (2.90, 7.80) |
| Female, n (%) | 228 | 76 (33%) | 21 | 10 (48%) |
| Duration of hospitalization (days) | 228 | 7.00 (5.00, 10.50) | 21 | 4.00 (3.00, 8.00) |
| Total (IV + PO) duration of antibiotic treatment (days) | 227 | 42.00 (31.00, 52.00) | 21 | 36.00 (29.00, 47.00) |
| Peak CRP (mg/L) during hospitalization | 228 | 158.00 (76.00, 245.00) | 21 | 129.00 (62.00, 184.00) |
| Peak WBC count (*103) during hospitalization | 222 | 13.00 (10.00, 17.00) | 21 | 14.00 (12.00, 22.00) |
| Peak ESR (mm/hr) during hospitalization | 202 | 65.50 (38.00, 88.00) | 19 | 57.00 (44.00, 80.00) |
| Infection Type, n (%) | ||||
| Both | 228 | 76 (33%) | 21 | 5 (24%) |
| Osteomyelitis | 228 | 127 (56%) | 21 | 7 (33%) |
| Septic Arthritis | 228 | 25 (11%) | 21 | 9 (43%) |
| Bacteremia, n (%) | 214 | 156 (73%) | 18 | 7 (39%) |
| Persistent bacteremia (>72 h), n (%) | 214 | 52 (24%) | 18 | 0 (0%) |
| Surgery performed, n (%) | 228 | 187 (82%) | 21 | 18 (86%) |
| ICU required, n (%) | 228 | 41 (18%) | 21 | 4 (19%) |
| PO therapy at discharge, n (%) | 228 | 135 (59%) | 21 | 15 (71%) |
| Function at end of therapy, n (%) | ||||
| Cast | 185 | 4 (2%) | 19 | 0 (0%) |
| Full ROM | 185 | 151 (82%) | 18 | 16 (89%) |
| Pain at end of therapy, n (%) | ||||
| None | 185 | 135 (73%) | 19 | 17 (89%) |
| Mild | 185 | 43 (23%) | 19 | 2 (11%) |
| Moderate | 185 | 7 (4%) | 19 | 0 (0%) |
| Pathologic fracture, n (%) | 225 | 12 (5%) | 21 | 0 (0%) |
| Concern for recurrence of infection/treatment failure, n (%) | 225 | 26 (12%) | 21 | 1 (5%) |
Outcomes
The majority of children (87%) had full range of motion at the end of therapy, though this differed by causative organism: 94% of children without S. aureus infection had full range of motion at the end of therapy, compared with 87% with MSSA and 76% with MRSA (p<.001 for MRSA vs no S. aureus). Nearly all (98%) had mild or no pain at the end of therapy. The overall rate of treatment failure or recurrent infection was 9%, and there was no difference in the frequency over time (7% vs. 11%, p=.140). Of those with treatment failure, 39% required a surgical procedure after their original hospital admission.
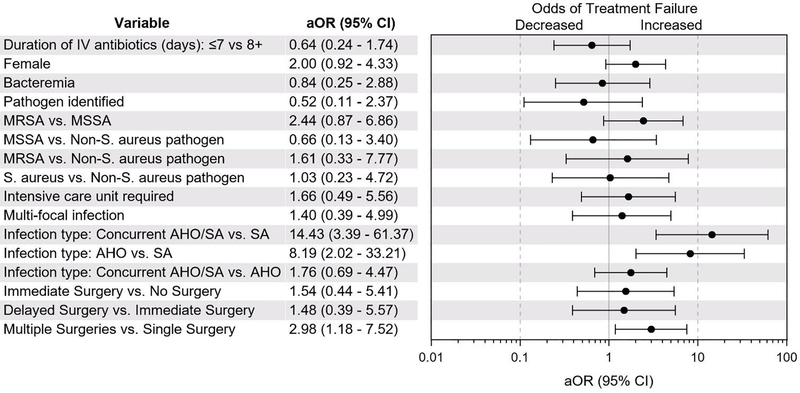
Compared with SA, AHO (OR 8.19, 95% CI 2.02, 33.21 p=.003) and concurrent AHO/SA (OR 14.43, 95% CI 3.39, 61.37; p<.001) were associated with higher odds of treatment failure (Figure). Additionally, requiring >1 surgical procedure was associated with higher odds of treatment failure (OR 2.98, 95% CI 1.18, 7.52; p=.02), Hospital location was also associated with higher odds of treatment failure (OR 2.84, 95% CI 1.00, 8.00; p= .049). In contrast, short course parenteral antibiotic therapy (≤7 days), organism, including MRSA, and delayed surgical procedure were not associated with treatment failure.
Figure.
Odds of treatment failure by clinical characteristic. Infection type (concurrent AHO/SA vs. SA and AHO vs. SA) and need for multiple surgical procedures was associated with treatment failure. Duration of parenteral antibiotics, causative organism and delayed surgical procedure were not associated with treatment failure.
Non-adherence with outpatient antibiotic therapy was suspected in 8% of patients. Of these, 43% had recurrent infection or treatment failure, compared with 8% in those with no suspicion of non-adherence (p<.001).
Overall, 103 medically-attended events associated with antibiotic complications occurred in 84 (19%) patients (Table 7; available at www.jpeds.com). Seventy-four percent of the complications occurred while on parenteral therapy, with PICC-associated complications being most common. Gastrointestinal intolerance was the most common complication while on oral therapy.
Table 7.
Treatment-associated complications at follow up
| Number of complications | |||
|---|---|---|---|
| Complication Typea | Total | IV | PO |
| Any medically attended events due to complications | 103 | 76 | 27 |
| Medication intolerance | 58 | 31 | 27 |
| Hypersensitivity reaction/rash | 22 | 13 | 9 |
| GI intolerance/vomiting/diarrhea | 16 | 6 | 10 |
| Drug fever | 8 | 8 | 0 |
| Hematologic abnormality | 6 | 2 | 4 |
| Kidney injury | 4 | 4 | 0 |
| Transaminase elevation | 2 | 2 | 0 |
| C. diff colitis | 1 | 0 | 1 |
| Anaphylaxis | 1 | 1 | 0 |
| Other | 6 | 2 | 4 |
| PICC/catheter problem | 46 | 46 | |
| Catheter malfunction | 16 | ||
| Catheter pulled out | 11 | ||
| Line-associated infection | 7 | ||
| Thrombus | 5 | ||
| Catheter leak | 3 | ||
| Dermatitis | 3 | ||
| Catheter fracture | 2 | ||
| Other | 2 | ||
Some patients had multiple complications during a medically attended event
Discussion
In this contemporary epidemiologic study of children with MSKI, we found that empiric vancomycin use and PICC placement decreased over time, and oral antimicrobial therapy at discharge increased over time; importantly, these trends were not associated with increased odds of treatment failure. S. aureus remains the most commonly isolated organism, with MRSA causing half of these infections. Our data suggest that children with concurrent AHO/SA had more severe disease than those with either AHO or SA alone.
For decades, the clinical features and epidemiology of MSKI in children changed very little [21]. S. aureus has long been reported as the most common cause of MSKI in children. With the emergence of CA-MRSA in the early 2000s, however, these characteristics, as well as management approaches and outcomes, substantially changed [22]. Recent studies of S. aureus isolates recovered from children (primarily skin and soft tissue infections) suggest a decreasing proportion of infections due to MRSA, with increasing numbers of MSSA and resistance to clindamycin [4, 23, 24]. In our study, we found that the frequency of MRSA and clindamycin resistance was stable over these 7 years. Our study provides updated epidemiologic and clinical care trends at two large US children’s hospitals in the SE US, which is essential to optimize management decisions and resource utilization. In addition, these findings underscore the importance of understanding local epidemiology and consulting antibiograms to inform empiric antibiotic selection.
Additionally, literature regarding the severity of MRSA compared with MSSA infections is conflicting [25]. Although several studies have found infection due to MRSA to be associated with higher inflammatory markers, complications, and longer durations of hospitalization in children with MSKI [26–29], other studies have shown no differences [25, 30]. We found infections due to MRSA were not associated with higher odds of treatment failure than MSSA infection, when controlling for other variables. Numerous variables affect the severity of MSKI. Further investigation into the mechanisms, including host and pathogen factors, is needed.
The frequency of confirmed non-S. aureus infection in our study was low. Despite high rates of K. kingae infections in children <5 years of age reported in prior studies [27, 28], K. kingae accounted for only 1% of infections in our cohort. Notably, patients with SA, in which K.kingae is an important pathogen, were less likely to have an identified pathogen. This finding may be attributed to low rates of molecular testing at both study sites during this time period. Previous studies have shown significantly higher yield of K. kingae detection with the use of PCR-based techniques [27, 28], and this may represent a portion of the 38% of cases in this series in which no pathogen was identified. In the post-PCV13 and Hib vaccine era, rates of invasive infections caused by S. pneumoniae and H. influenzae remain low. Interestingly, we found that surgical site sampling was required for pathogen identification in 27% of all patients, increasing to 45% for non-staphylococcal infections. This observation highlights the importance of surgical sampling for diagnostic purposes, as pathogen identification has significant treatment and antimicrobial stewardship implications. Of note, antibiotic pretreatment was not associated with reduced rates of pathogen identification, consistent with prior studies [31].
Our study supports a growing body of literature suggesting that early transition to oral antibiotics is not associated with adverse outcomes in children with MSKI [14–18]. In 2009, Zaoutis et al published a multicenter, retrospective study of children with culture-positive osteomyelitis in the US and found that early transition to oral therapy was not associated with an increased risk of treatment failure [15]. Keren et al published a study finding that the rates of treatment failure were the same in the oral therapy (5.0%) and parenteral therapy (6.0%) groups [14]. In our study, the frequency of treatment failure was 9%. In multivariable regression analysis, early transition to oral antibiotics was not associated with increased risk of treatment failure. Additionally, consistent with prior studies [14, 32], we found most antibiotic-associated complications occur while on parenteral therapy, with PICC complications being most common. The proportion of treatment failure in patients with documented suspicion for medication nonadherence was 43%, a finding that may be useful for counseling families about the importance of adherence with oral therapy. Overall, our study confirms that early transition to oral antibiotic therapy for children with MSKI is safe and effective with proper adherence, avoiding the potential complications of parenteral therapy.
When investigating other factors associated with severe infection and treatment failure, we found infection type to be a significant factor. Concurrent AHO/SA was associated with increased markers of inflammation, bacteremia, prolonged bacteremia, duration of hospitalization, need for ICU-level care, and a 14-fold increased risk in treatment failure compared with SA alone. These findings are similar to recent reports of patients with concurrent AHO/SA [2, 33, 34]. Alhinai et al described factors that predicted an acute complicated course of osteomyelitis, which included associated suppurative arthritis [33]. Carillo et al found that a CRP of ≥100mg/L along with fever that persisted >2 days after starting treatment and bacteremia correlated with an increased likelihood for concurrent AHO/SA [2]. Branson et al found patients with concurrent infection had longer duration of symptoms on presentation, had S. aureus or MRSA as a causative pathogen, had positive blood cultures, a longer duration of fever after admission, higher peak temperature at the time of presentation, higher peak and admission inflammatory markers and positive synovial fluid Gram stain and culture [34]. Further studies on this subset of patients are needed to validate these findings and improve outcomes in these children.
Hospital site was also associated with increased odds of treatment failure in this study though rates of MRSA, antibiotic selection, hospitalization duration was similar between sites. Variables associated with increased severity of disease such as concurrent AHO/SA and need for ICU level care occurred less in the center with higher treatment failures. Thus, reasons for site specific differences are unclear. Although we controlled for a variety of covariates, it is possible that unmeasured patient- or hospital-level confounding factors underly this finding.
We recognize several limitations of our study. First, as a retrospective review, all data, including clinical symptoms, are reliant upon provider documentation. Additionally, by using ICD-9 codes to identify those with SA and AHO, it is possible that some patients with AHO or SA were missed due to incorrect coding. Also, as examination, imaging, and surgical procedure were used to identify patients with concurrent AHO/SA, patients that did not have obvious signs of concomitant infection on examination, or those that did not have imaging or surgery could have been misclassified. Medically-attended events that occurred outside of the study centers were not captured which could underestimate rates of treatment failure and complications. However, for these two comprehensive pediatric healthcare centers with few patients lost to follow up, this limitation is likely minimal. Relying on provider documentation to assess medication nonadherence has the potential to introduce bias as providers are more likely to document nonadherence in patients who fail therapy. Finally, both centers serve metropolitan areas in the southeastern US, and generalizability to other centers and geographic regions may be limited.
In summary, we found that S. aureus remains the major cause of MSKI. Rates of MRSA remained stable over time. Additionally, we found the frequency of non-S. aureus pathogens was low, but recovery of these pathogens often necessitates surgical source sampling. We also found that early transition to oral antibiotics was not associated with treatment failure, and carries less risk of treatment complications compared with outpatient parenteral antimicrobial therapy. Finally, we found that concurrent AHO/SA and need for >1 surgery were associated with a high-risk of treatment failure. These findings are consistent with data in smaller studies [5, 6, 33]. This study is unique in that the large sample size across multiple institutions allows for more generalizability.
Acknowledgements:
We thank the Emory and Vanderbilt VTEU administrative, clinical, regulatory, and finance cores for their support, including Nadine Rouphael, Dean Kleinhenz, Hannah Huston, Michele Paine McCullough, Amy Muchinsky, Kathy Stephens, Kate Sokolow, Shanda Phillips, and Kelsey Phipps (no financial conflicts or industry relations exist among the acknowledgees). We thank Children’s Healthcare of Atlanta and the Monroe Carell Jr. Children’s Hospital at Vanderbilt for their support.
Supported by awards from the Division of Microbiology and Infectious Diseases, National Institute of Allergy and Infectious Diseases at the National Institutes of Health to the Emory Vaccine and Treatment Evaluation Unit (HHSN272201300018I), Vanderbilt University Medical Center (HHSN272201300023I), and Emmes (HHSN272201500002C). REDCap database support for Emory University was provided through UL1 TR000424, and for Vanderbilt University Medical Center through UL1 TR000445 from NCATS/NIH.
Footnotes
The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Arnold JC, Bradley JS. Osteoarticular Infections in Children. Infect Dis Clin North Am. 2015; 29:557–574. [DOI] [PubMed] [Google Scholar]
- 2.Carrillo-Marquez MA, Hulten KG, Hammerman W, Mason EO, Kaplan SL. USA300 is the predominant genotype causing Staphylococcus aureus septic arthritis in children. Pediatr Infect Dis J. 2009; 28:1076–1080. [DOI] [PubMed] [Google Scholar]
- 3.Harik NS, Smeltzer MS. Management of acute hematogenous osteomyelitis in children. Expert Rev Anti Infect Ther. 2010; 8:175–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sutter DE, Milburn E, Chukwuma U, Dzialowy N, Maranich AM, Hospenthal DR. Changing susceptibility of Staphylococcus aureus in a US pediatric population. Pediatrics. 2016; 137. [DOI] [PubMed] [Google Scholar]
- 5.Hulten KG, Mason EO, Linda BL, Forbes AR, Revell PA, Kaplan SL. Analysis of Invasive Community-Acquired Methicillin-Susceptible Staphylococcus aureus Infections During a Period of Declining Community Acquired Methicillin-Resistant Staphylococcus aureus Infections at a Large Children’s Hospital. Pediatr Infect Dis J, 2018; 37: 235–241. [DOI] [PubMed] [Google Scholar]
- 6.Weiss L, Lansell A, Figueroa J, Suchdev PS, Kirpalani A. Declining Prevalence of Methicillin-Resistant Staphylococcus aureus Septic Arthritis and Osteomyelitis in Children: Implications for Treatment. Antibiotics. 2020; 9:101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Whitney CG, Pilishvili T, Farley MM, Schaffner W, Craig A, LYnfield R, et al. Effectiveness of seven-valent pneumococcal conjugate vaccine against invasive pneumococcal disease: a matched case-control study. Lancet. 2006; 368:1495–1502. [DOI] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention. Prevention of pneumococcal disease among infants and children --- use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine: recommendations of the advisory committee on immunization practices (ACIP). MMWR. 2010;59(RR11):1–8. [PubMed] [Google Scholar]
- 9.Moore MR, Link-Gelles R, Schaffner W, Lynfield R, Lexau C, Bennett N, et al. Effect of use of 13-valent pneumococcal conjugate vaccine in children on invasive pneumococcal disease in children and adults in the USA: analysis of multisite, population-based surveillance. Lancet Infect Dis. 2015; 15:301–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Olarte L, Romero J, Barson W, Bradley J, Lin PL, Givner L, et al. Osteoarticular infections caused by Streptococcus pneumoniae in children in the post-pneumococcal conjugate vaccine era. Pediatr Infect Dis J. 2017; 36:1201–1204. [DOI] [PubMed] [Google Scholar]
- 11.Wood JB, Thomsen IP, Creech CB, Newland JG. Best practices for treatment of invasive methicillin-susceptible Staphylococcus aureus infections: the case for oxacillin. J Pediatric Infect Dis Soc. 2016; 5:480–482. [DOI] [PubMed] [Google Scholar]
- 12.Gutierrez K. Bone and joint infections in children. Pediatr Clin N Am. 2005; 52:779–794. [DOI] [PubMed] [Google Scholar]
- 13.Wood JB, Fricker GP, Beekmann SE, Polgreen P, Buddy Creech C. Practice patterns of providers for the management of Staphylococcus aureus bacteremia in children: results of an emerging infections network survey. J Pediatric Infect Dis Soc. 2018; 7:e152–e155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Keren R, Shah SS, Srivastava R, Rangel S, Bendel-Stenzel M, Harik N, et al. Comparative effectiveness of intravenous vs oral antibiotics for postdischarge treatment of acute osteomyelitis in children. JAMA Pediatr. 2015; 169:120–128. [DOI] [PubMed] [Google Scholar]
- 15.Zaoutis T, Localio AR, Leckerman K, Saddlemire S, Bertoch D, Keren R. Prolonged intravenous therapy versus early transition to oral antimicrobial therapy for acute osteomyelitis in children. Pediatrics. 2009; 123:636–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Paakkonen M, Kallio PE, Kallio MJ, Peltola H. Does bacteremia associated with bone and joint infections necessitate prolonged parenteral antimicrobial therapy? Journal of the Pediatric Infectious Diseases Society. 2015; 4:174–177. [DOI] [PubMed] [Google Scholar]
- 17.Peltola H, Paakkonen M, Kallio P, Kallio MJT, Grp O-SS. Prospective, randomized trial of 10 days versus 30 days of antimicrobial treatment, including a short-term course of parenteral therapy, for childhood septic arthritis. Clinical Infectious Diseases. 2009; 48:1201–1210. [DOI] [PubMed] [Google Scholar]
- 18.Peltola H, Paakkonen M, Kallio P, Kallio MJT, Arthritis O-S. Short-versus long-term antimicrobial treatment for acute hematogenous osteomyelitis of childhood prospective, randomized trial on 131 culture-positive cases. Pediatric Infectious Disease Journal. 2010; 29:1123–1128. [DOI] [PubMed] [Google Scholar]
- 19.Ruebner R, Keren R, Coffin S, Chu J, Horn D, Zaoutis TE. Complications of central venous catheters used for the treatment of acute hematogenous osteomyelitis. Pediatrics. 2006; 117:1210–1215. [DOI] [PubMed] [Google Scholar]
- 20.Tice AD, Rehm SJ, Daloviso JR, Bradley JS, Martinelli LP, Graham DR, et al. , Practice guidelines for outpatient parenteral antimicrobial therapy. IDSA guidelines. Clin Infect Dis, 2004; 38:1651–72. [DOI] [PubMed] [Google Scholar]
- 21.Moumile K, Merckx J, Glorion C, Pouliquen JC, Berche P, Ferroni A. Bacterial aetiology of acute osteoarticular infections in children. Acta Paediatr. 2005; 94:419–422. [DOI] [PubMed] [Google Scholar]
- 22.Arnold SR, Elias D, Buckingham SC, Thomas ED, Novias E, Arkader A, et al. Changing patterns of acute hematogenous osteomyelitis and septic arthritis: emergence of community-associated methicillin-resistant Staphylococcus aureus. J Pediatr Orthop. 2006; 26:703–708. [DOI] [PubMed] [Google Scholar]
- 23.Vicetti Miguel CP, Mejias A, Leber A, Sanchez PJ. A decade of antimicrobial resistance in Staphylococcus aureus: A single center experience. PLoS One. 2019; 14:e0212029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Spaulding AB, Thurm C, Courter JD, Banerjee R, Gerber JS, Newland JG, et al. Epidemiology of Staphylococcus aureus infections in patients admitted to freestanding pediatric hospitals, 2009–2016. Infect Control Hosp Epidemiol. 2018; 39:1487–1490. [DOI] [PubMed] [Google Scholar]
- 25.An TJ, Benvenuti MA, Mignemi ME, Martus J, Wood J, Thomsen I et al. Similar clinical severity and outcomes for methicillin-resistant and methicillin-susceptible Staphylococcus aureus pediatric musculoskeletal infections. Open Forum Infect Dis. 2017; 4:ofx013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Davis WT, Gilbert SR. Comparison of methicillin-resistant versus susceptible Staphylococcus aureus pediatric osteomyelitis. J Pediatr Orthop. 2018; 38:e285–e291. [DOI] [PubMed] [Google Scholar]
- 27.Martinez-Aguilar G, Avalos-Mishaan A, Hulten K, Hammerman W, Mason EO Jr., Kaplan SL. Community-acquired, methicillin-resistant and methicillin-susceptible Staphylococcus aureus musculoskeletal infections in children. Pediatr Infect Dis J. 2004; 23:701–706. [DOI] [PubMed] [Google Scholar]
- 28.Hawkshead JJ 3rd, Patel NB, Steele RW, Heinrich SD. Comparative severity of pediatric osteomyelitis attributable to methicillin-resistant versus methicillin-sensitive Staphylococcus aureus. J Pediatr Orthop. 2009; 29:85–90. [DOI] [PubMed] [Google Scholar]
- 29.Gafur OA, Copley LA, Hollmig ST, Browne RH, Thornton LA, Crawford SE. The impact of the current epidemiology of pediatric musculoskeletal infection on evaluation and treatment guidelines. J Pediatr Orthop. 2008; 28:777–785. [DOI] [PubMed] [Google Scholar]
- 30.Saavedra-Lozano J, Falup-Pecurariu O, Faust SN, Girschick H, Hartwig N, Kaplan S, et al. Bone and Joint Infections. Pediatr Infect Dis J. 2017; 36:788–799.v [DOI] [PubMed] [Google Scholar]
- 31.Benvenuti MA, An TJ, Mignemi ME, Martus JE, Thomsen IP, Schoenecker JG. Effects of Antibiotic Timing on Culture Results and Clinical Outcomes in Pediatric Musculoskeletal Infection. J Pediatr Orthop. 2019; 39:158–162. [DOI] [PubMed] [Google Scholar]
- 32.Barrier A, Williams DJ, Connelly M, Creech CB. Frequency of peripherally inserted central catheter complications in children. Pediatr Infect Dis J. 2012; 31:519–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alhinai Z, Elahi M, Park S, Foo B, Lee B, Chapin K. Prediction of Adverse Outcomes in Pediatric Acute Hematogenous Osteomyelitis. Clin Infect Dis. 2020; 71: e454–e464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Branson J, Vallejo J, Flores A, Hulten K, Mason EO, Kaplan S, et al. The Contemporary Microbiology and Rates of Concomitant Osteomyelitis in Acute Septic Arthritis. Pediatr Infect Dis J. 2017; 36: 267–273. [DOI] [PubMed] [Google Scholar]