Abstract
Background and Purpose:
Stroke induces the expression of several long noncoding RNAs (lncRNAs) in the brain. However, their functional significance in post-stroke outcome is poorly understood. We recently observed that a brain-specific lncRNA called Fos downstream transcript (FosDT) is induced rapidly in the rodent brain following focal ischemia. Using FosDT knockout rats, we presently evaluated the role of FosDT in post-stroke brain damage.
Methods:
FosDT knockout rats were generated using CRISPR-Cas9 genome editing on a Sprague-Dawley background. Male and female FosDT−/− and FosDT+/+ cohorts were subjected to transient middle cerebral artery occlusion. Post-ischemic sensorimotor deficits were evaluated between days 1 and 7, and lesion volume on day 7 of reperfusion. The developmental expression profile of FosDT was determined with real-time PCR and mechanistic implications of FosDT in the ischemic brain were conducted with RNA-seq analysis and immunostaining of pathological markers.
Results:
FosDT expression is developmentally regulated, with the adult cerebral cortex showing significantly higher FosDT expression than neonates. FosDT−/− rats did not show any anomalies in growth and development, fertility, brain cytoarchitecture and cerebral vasculature. However, when subjected to transient focal ischemia, FosDT−/− rats of both sexes showed enhanced sensorimotor recovery and reduced brain damage. RNA-seq analysis showed that improved post-stroke functional outcome in FosDT−/− rats is partially associated with curtailed induction of inflammatory genes, reduced apoptosis, mitochondrial dysfunction, and oxidative stress.
Conclusions:
Our study shows that FosDT is developmentally dispensable, mechanistically important, and a functionally promising target to reduce ischemic brain damage and facilitate neurologic recovery.
Keywords: Ischemic stroke, lncRNA, Development, Motor function, Lesion volume
INTRODUCTION
Stroke alters the profiles of various classes of noncoding RNAs (ncRNAs), including microRNAs (miRNAs), long ncRNAs (lncRNAs), circular RNAs and transcribed ultraconserved regions.1–5 The lncRNAs (200 nt to >100 kb) are the largest class of ncRNAs involved in transcriptional and translational regulation in a developmental-stage and a disease-specific manner.6–8 Many lncRNAs are organ- and cell-type specific. It was reported that <10% of the lncRNAs in humans ubiquitously express in different cell types, while ~30% are seen in only one cell type.9 Furthermore, ~40% of the lncRNAs are expressed specifically in the brain.6 Particularly, lncRNAs transcribed within the vicinity of protein-coding genes are preferentially expressed in the brain and share overlapping transcription factor binding sites with protein-coding genes.2,10 We previously showed that induction of a highly conserved brain-enriched lncRNA called Fos downstream transcript (FosDT, MRAK159688) promotes ischemic brain damage by interacting with RE1-silencing transcription factor (REST)-associated chromatin-modifying proteins.2,11 FosDT gene is cogenic to the Fos gene, which is a marker of cellular stress.12 FosDT and Fos genes are located within a gene desert of ~240,000 nt on chromosome 6 in rats (chromosome 14 in humans).11 We currently evaluated the functional significance of FosDT in brain development and post-ischemic outcome by using FosDT−/− rats developed by CRISPR-Cas9 genome editing.
We also evaluated the reproducibility of our data by analyzing the role of FosDT in ischemic brain damage by another lab. We further conducted RNA-seq analysis of FosDT−/− and FosDT+/+ rats following transient focal ischemia to understand the mechanistic implications of FosDT in the ischemic brain.
MATERIALS AND METHODS
The data supporting results are available within the article and its online supplementary files. Experimental protocols using animals were approved by the University of Wisconsin Research Animal Resources and Care Committee and Center for Laboratory Animal Medicine and Care at the University of Texas Health Science Center at Houston in accordance with the Institutional Animal Care and Use Committee (IACUC) guidelines. Animals were cared in accordance with the Guide for the Care and Use of Laboratory Animals [U.S. Department of Health and Human Services publication no. 86–23 (revised)]. Experiments were conducted in compliance with the Animal Research: Reporting of In Vivo Experiments (ARRIVE) guidelines.13 Animals were randomly assigned to study groups and outcome parameters were evaluated blindly.
FosDT knockout rats
FosDT−/− rats were generated using CRISPR-cas9 genome editing on the SD background by deleting 539 nt that do not overlap with the Fos gene. The target sequence having no perfect matches elsewhere in the genome within the PAM-proximal 12 bp seed region and >3 mismatches in the distal 8 bp region on each flanking end was selected. A single-stranded DNA donor (ssODN) was designed to integrate 75 bp of homology across both sides of the region targeted for excision. Two purified guide RNAs (50 ng/μl), a ssODN (50 ng/μl) and a Cas9 protein (40 ng/μl) were microinjected into the pronucleus of fertilized one-cell SD rat embryos and surgically implanted into the oviducts of 2 pseudo-pregnant SD females. Offspring were genotyped and further bred to obtain homozygous FosDT knockouts. Tail DNA was genotyped using the primer set (5′-to-3′) ACTCCAGTCCTCACCTCTTC (sense) and AATACCCTGAACTAGCGTTTC (antisense).
Cerebral vasculature and histological evaluation
FosDT+/+ and FosDT−/− cohorts were observed for any signs of disability at regular intervals and euthanized at 3 months of age (300±20 g; n=3/group) by transcardiac paraformaldehyde perfusion. Brain, lung, heart, liver, kidney, spleen and muscle were embedded in paraffin, sectioned (10 μm) and stained with hematoxylin and eosin (H&E), luxol fast blue and silver stain. Sections were analyzed for histological changes by a trained pathologist.3 To study the cerebral vasculature, cohorts of FosDT−/− and FosDT+/+ rats (3 months; 300±20 g; n=3/group) were transcardially perfused, injected with 25% India ink in PBS containing 6% gelatin, post-fixed and cerebral vasculature was observed.14
Focal ischemia
Rats were subjected to 90 min of transient middle cerebral artery occlusion (MCAO) using a silicone-coated nylon monofilament (4-0 Doccol, USA) under isoflurane anesthesia as described earlier.3,11,15 Rats were euthanized at various reperfusion time points between 12h and 7 days as needed. Sham-operated rats underwent the same surgical procedure, except for MCAO. Physiological parameters (pH, PaO2 and PaCO2,) were monitored, and rectal temperature was maintained at 37.0 ± 0.5°C during surgery. The impact of the estrous cycle on functional outcomes in females was minimized by randomly assigning to groups. Rats that showed no signs of neurological deficits during the acute phase after MCAO and or a hemorrhage after euthanasia were excluded.
RNA-seq and real-time PCR
Total RNA was extracted using the mirVana™ miRNA Isolation Kit (ThermoFisher Scientific, USA). Following ribosomal RNA depletion, samples were fragmented, DNA library was prepared and sequenced on Illumina’s NovaSeq 6000 system. The differentially expressed mRNAs were selected with log2 (fold change) ≥1 or log2 (fold change) ≤-1, and with p<0.05. FosDT and Fos expression was estimated with real-time PCR with SYBR Green and TaqMan methods. Relative gene expression was normalized to 18s rRNA by the comparative Ct method (2−ΔΔCt). See Supplemental Material for additional details
Sensorimotor function testing
Sensory and motor functions were evaluated on days 1, 3, 5, and 7 of reperfusion by rotarod test (cylinder rotating at 8 rpm for 4 min), beam walk test (foot faults while crossing a tapered 120 cm long beam) and adhesive sticker removal test as described earlier.3,11,15 Animals were pretrained in these tasks for 3 days. The spontaneous locomotor activity was evaluated by an open field test.16 Rats were prescreened for baseline spontaneous motor activity and post-stroke motor behavior on days 3 and 7 of reperfusion. Each rat was placed in a separate arena (16”×16”), locomotion was tracked for 20 min and automatically analyzed by video tracking and analysis software (Noldus Information Technology Inc., USA).
Lesion volume analysis
Coronal brain sections (40 μm) from each rat were stained with cresyl violet and scanned with NIH ImageJ software. Ischemic lesion volume was calculated by numeric integration of data from 6 serial coronal sections with respect to sectional interval and corrected for edema and differential shrinkage.3,11,15,17
Immunostaining
Brain sections from FosDT−/− and FosDT+/+ rats subjected to transient MCAO and 1 day of reperfusion were stained with antibodies against cleaved caspase-3, pDrp-1, 3-NT and NeuN followed by suitable secondary antibodies and homologous areas were used to analyze immunostaining as described earlier.3 See Supplemental Material for details.
RESULTS
FosDT is highly localized in the brain and regulated developmentally
In postnatal day 7 (P7) rats, FosDT expression was observed in all brain regions tested (cerebral cortex, striatum, hippocampus, cerebellum and thalamus) with the highest level in the striatum and the lowest level in the cerebral cortex (Fig. 1A). In the adult rats, the cerebral cortex and cerebellum showed a significant increase in FosDT levels over P7 by ~20 fold and ~8 fold, respectively (Fig. 1B and C). Fos expression mirrored FosDT expression in all brain areas in P7 and adult rats (Fig. 1A, B and C). Although the intra-relative abundance of FosDT and Fos were higher in muscle, spleen and lung in comparison to the liver, only the heart showed a significant age-dependent increase in FosDT and Fos abundance (Supplemental Fig. I).
Fig. 1: Developmental expression pattern of FosDT and Fos in the rat brain.
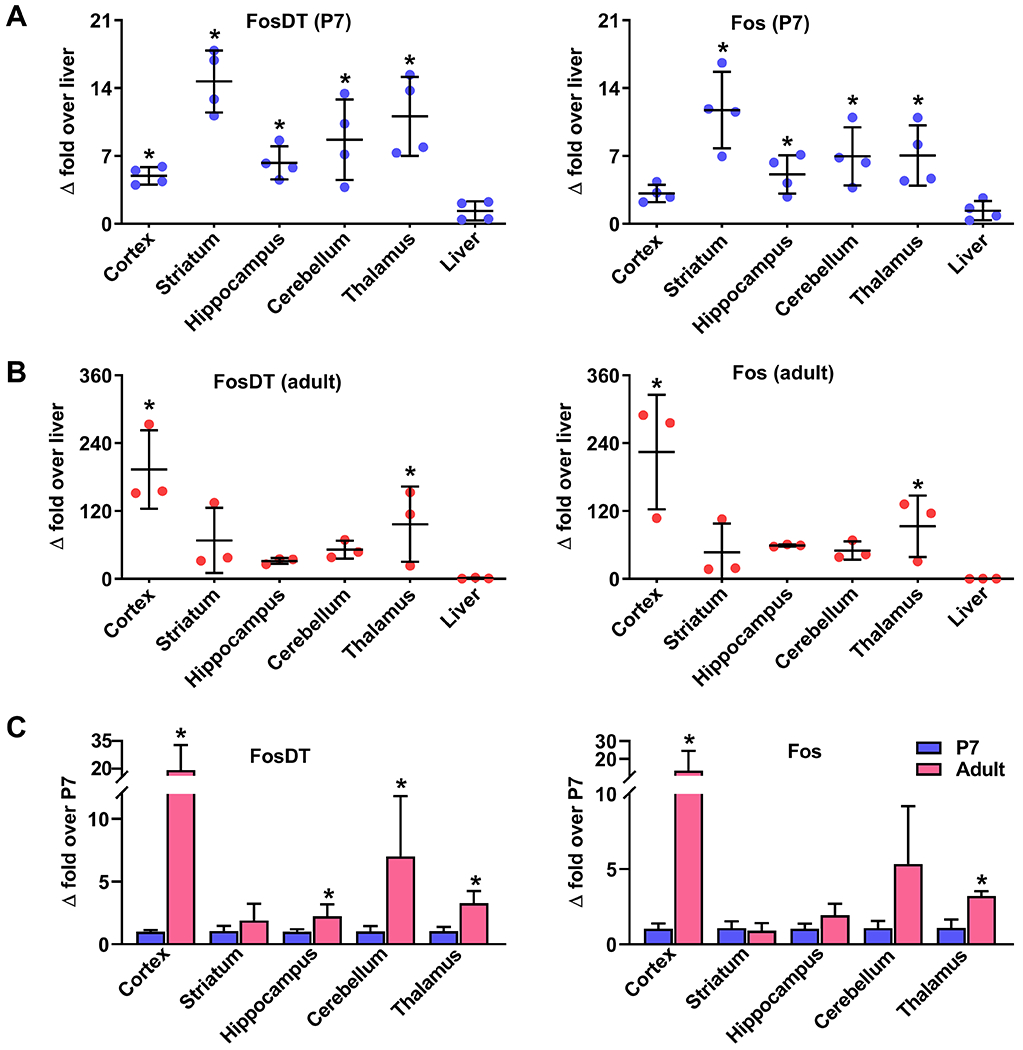
At postnatal day 7 (P7), all brain regions (cerebral cortex, striatum, hippocampus, cerebellum, and thalamus) showed significant FosDT expression (A). At the adult stage, the cerebral cortex and thalamus showed a significant increase in the FosDT expression compared to P7 (B). Fos expression mirrored FosDT expression at both P7 and adult stages (A and B). As the liver showed the lowest expression of FosDT and Fos, we presented the values as a fold-over liver. FosDT expression is significantly higher in the adult cerebral cortex compared to P7 (C). Fos expression mirrored the developmental pattern seen for FosDT in the brain (A, B and C). Values are mean ± SD (n=3-4/group). *p<0.05 compared to liver or P7 by Mann-Whitney U test. P7, postnatal day 7.
FosDT deletion had no effect on development, cytoarchitecture and cerebral vasculature
Tail DNA genotyping showed a 821 nt band for FosDT+/+ and a 282 nt band for FosDT−/− cohorts confirming the knockout (Fig. 2A2). PCR analysis showed a 142 nt band of FosDT RNA in the brains of the FosDT+/+ cohort, which was absent in the FosDT−/− cohort (Fig. 2A3). However, both the cohorts showed similar expression of Fos mRNA (Fig. 2A3). FosDT+/+ and FosDT−/− rats showed no visible phenotypic defects at the P2 or adult (2 months) stage (Fig. 2B). The growth pattern and fertility of FosDT−/− rats are similar to FosDT+/+ rats (Fig. 2B). Adult FosDT−/− rats showed no neuronal degeneration in the grey matter (in the cerebral cortex and hippocampus) (Fig. 2C) or white matter (corpus callosum), compared with the FosDT+/+ rats (Supplemental Fig. IIA and Supplemental Fig. IIB). No noticeable difference was observed between FosDT+/+ and FosDT−/− rats in major cerebral blood vessel structures, including MCA, anterior cerebral artery (ACA) and posterior cerebral artery (PCA) (Fig. 2D). None of the peripheral organs evaluated (lung, liver, heart, spleen, muscle and kidney) showed any cytoarchitectural differences between the adult FosDT+/+ and FosDT−/− rats (Supplemental Fig. IIC). When subjected to transient MCAO, the FosDT+/+ cohort showed significant induction of both FosDT and Fos expression in the cerebral cortex (Supplemental Fig. III). In contrast, FosDT−/− cohort subjected to transient MCAO showed no induction of FosDT expression and a significantly curtailed induction of Fos (~60% lower) in the cerebral cortex compared to FosDT+/+ (Supplemental Fig. III).
Fig. 2: FosDT deletion had no effect on development, brain cytoarchitecture or vasculature.

FosDT−/− rats were generated using CRISPR-Cas9 genome editing (A1). Scissors represent Cas9 cutting sites. The transcription start site for FosDT is located within the Fos 3’ untranslated region (3’UTR) (A1). A portion of FosDT that does not overlap with Fos was selected for precise excision. Cas9 target sites at the regions flanking the excision region were selected (A1). Tail DNA genotyping showed 821 bp and 282 bp PCR products in FosDT+/+ and FosDT−/− rats, respectively (A2). FosDT deletion abolished FosDT without affecting Fos (A3). FosDT+/+ and FosDT−/− rats showed similar phenotype and growth up to 1 year (B). H&E stained brain sections showed no structural and cellular differences between adult male FosDT−/− and FosDT+/+ cohorts in the cerebral cortex and hippocampal CA1, CA2 CA3 and DG layers (C). Cerebral vasculature demarcated by India ink shows no observable differences between FosDT−/− and FosDT+/+ rats in the major arterial organization, including MCA, ACA and PCA (D). n=3/group. Scale bar=50 μm. P2, postnatal day 2; MCA, anterior cerebral artery; ACA, anterior cerebral artery and PCA, posterior cerebral artery.
FosDT deletion ameliorated post-stroke functional outcome in both sexes
Following transient MCAO, post-stroke motor function recovery was significantly faster and higher in adult male and female FosDT−/− rats compared with the sex-matched FosDT+/+ rats assessed by rotarod test, beam walk test and adhesive removal test between reperfusion days 1 and 7 (Fig. 3A and B). Post-ischemic lesion volume was also significantly smaller at 7 days of reperfusion in both male and female FosDT−/− rats compared with the sex-matched FosDT+/+ rats (by ~42%; p<0.05; n=8 to 10/group) (Fig 3C and D).
Fig. 3: FosDT deletion is neuroprotective in both sexes.

Rotarod test (upper), beam walk test (middle), and adhesive removal test (lower panel) show better motor function recovery between days 1 and 7 following transient MCAO in the male (A) and female (B) FosDT−/− rats compared to FosDT+/+ rats. FosDT−/− rats showed smaller infarcts following transient MCAO in both males and females compared to sex-matched FosDT+/+ rats (C and D). Cresyl violet-stained sections are from representative FosDT−/− and FosDT+/+ rats subjected to transient MCAO and 7 days of reperfusion (C). Lesion volume was computed from the numerical integration of the infarct area and distance between coronal sections (D). Values are mean±SEM (A and B) and mean±SD (D) of n=8-10/group. *p<0.05 compared to respective reperfusion time point by repeated-measures ANOVA followed by Bonferroni′s multiple comparisons test (multiple groups) or by Mann-Whitney U test (two groups).
Neuroprotection in FosDT knockouts is reproducible
We validated the improved functional recovery and smaller infarcts in FosDT−/− rats independently in a second laboratory at the University of Texas-Houston. All experiments were performed blinded to genotype. Focal ischemia led to a similar magnitude of weight loss between FosDT−/− and FosDT+/+ rats at 3 days of reperfusion in both sexes (Fig. 4A1 and B1). However, at 7 days of reperfusion, FosDT−/− rats of both sexes showed significantly better recovery of body weight compared to FosDT+/+ rats (Fig. 4A1 and B1). In the FosDT−/− rats of both sexes, somatosensory dysfunction evaluated by the adhesive removal test was significantly lower (Fig. 4A2 and B2) and the spontaneous locomotor activity assessed by the open field test was significantly higher (Fig. 4A3 and B3) compared to the FosDT+/+ rats at days 3 and 7 of reperfusion following transient MCAO. Both male and female FosDT−/− rats showed significantly smaller infarcts at 7 days of reperfusion compared to FosDT+/+ rat (by ~28%; p<0.05; n=6 to 8/group) (Fig. 4C and D).
Fig. 4: Post-stroke neuroprotection in FosDT−/− rats was independently validated by a second lab.

Male and female adult FosDT−/− rats showed better post-stroke weight gain on day 7 (A1 and B1), improved motor function recovery assessed with adhesive removal test (latency) (A2 and B2) and open field test (total distance) (A3 and B3) on day 3 and day 7 of reperfusion following transient MCAO compared to FosDT+/+ rats. FosDT−/− rats of both sexes also showed decreased lesion volume compared to FosDT+/+ rats estimated at day 7 of reperfusion following transient MCAO (C and D). Cresyl violet-stained sections are from representative rats of each group. Values are mean±SD (A1, B1, C and D) and mean±SEM (A2, B2, A3 and B3) of n=6–8/group. *p<0.05 compared to respective reperfusion time point by repeated-measures ANOVA followed by Bonferroni's test (multiple groups) or by Mann-Whitney U test (two groups).
FosDT deletion altered post-ischemic cerebral transcriptome
RNA-seq analysis showed that 288 protein-coding transcripts were differentially expressed in the cerebral cortex of the adult FosDT−/− rats compared with the FosDT+/+ rats (122 upregulated >3-fold and 166 downregulated <0.3 fold; n=4/group) (Fig. 5A, B and C; Supplemental Fig. IV and Table I). RNA expression profiles were evaluated subacutely at 12h of reperfusion following transient MCAO when post-stroke transcription changes are high. The peri-infarct cortex of adult male FosDT+/+ rats at 12h of reperfusion following transient MCAO showed altered expression of 810 transcripts (526 upregulated >3-fold and 284 downregulated <0.3 fold) compared to sham control (n=4/group) (Fig. 5A, B and C; Supplemental Fig. IV and Table II). Whereas, in the FosDT−/− rats subjected to 12h reperfusion following transient MCAO, of the 526 transcripts induced in the FosDT+/+ cohort, 294 showed either curtailed induction or no induction and one showed downregulation (Fig. 5A, B and C; Supplemental Fig. IV and Table II). Furthermore, of the 284 transcripts downregulated in the post-ischemic FosDT+/+ cohort, 9 showed upregulation, 36 downregulation and 239 showed reduced or no down-regulation in the FosDT−/− cohort (Fig. 5A, B and C; Supplemental Fig. IV and Table II). In addition, FosDT−/− rats showed altered expression of 409 transcripts (235 upregulated >3-fold and 174 downregulated <0.3 fold; n=4/group) that were not altered in FosDT+/+ rats after transient MCAO and 12h reperfusion (Fig. 5A, B and C; Supplemental Fig. IV and Table III). FosDT−/− rats subjected to sham surgery or transient MCAO showed switch-on and/or switch-off of specific genes. Figure 5 shows 15 up-(Fig. 5D1 and D2 upper panel) and 15 down-regulated (Fig. 5D1 and D2 lower panel) unique transcripts in sham control (Fig. 5D1) and transient MCAO (Fig. 5D2) groups of FosDT−/− compared to respective FosDT+/+ rats.
Fig. 5: FosDT knockdown altered the cerebral RNA expression profiles.

Total RNA isolated from peri-infarct cortical tissue of adult male FosDT−/− and FosDT+/+ rats subjected to 90 min of transient MCAO and 12h of reperfusion or equivalent area in sham groups was analyzed by RNA-seq. When the 4 groups were compared in different permutations, FosDT+/+ (MCAO vs sham) group showed highest numbers (810) of differentially expressed transcripts (DETs) followed by FosDT−/− (MCAO vs sham, 686), FosDT−/− vs FosDT+/+ (MCAO, 312) and FosDT−/− and FosDT+/+ (Sham, 288) (A). Venn diagrams show the distribution of common and independent DETs among the 4 groups (B). Volcano plots show the up- (red dots) and downregulated (blue dots) DETs between different comparisons (C). The plots are represented as log2 fold change against -log10 of the p-value (n=4/group). FosDT−/− sham showed induction or suppression of several transcripts compared with FosDT+/+ sham (D1). FosDT−/− MCAO also showed induction or suppression of many transcripts compared with FosDT+/+ MCAO (D2) (n=4/group).
Gene Set Enrichment Analysis (GSEA) using the set of transcripts that were differentially expressed after stroke showed that inflammation mediated by cytokines and interleukins is the major downregulated category, and metabolism, transport, neuronal system, post-translational protein modification and adaptive immune system were the major upregulated categories in the FosDT−/− compared with the FosDT+/+ rats following focal ischemia (Fig. 6A). GO analysis indicated that the neutrophil chemotaxis, inflammatory response, cytokine production, and extracellular exosome functions were significantly changed in FosDT−/− rats compared to FosDT+/+ rats following focal ischemia (Fig. 6B). KEGG pathway analysis also showed that inflammation and metabolism are the significant functions altered due to FosDT deletion following focal ischemia (Fig. 6B).
Fig. 6: FosDT deletion regulated post-stroke pathological changes.

Gene Set Enrichment Analysis (GSEA) of DETs between FosDT−/− MCAO vs. FosDT+/+ MCAO groups showed various up- and downregulated pathways (A). Gene ontology (GO) analysis and KEGG pathways analysis also showed the response of various pathways, including inflammation in FosDT−/− rats compared to FosDT+/+ rats after focal ischemia (B). Immunostaining decreased apoptosis (cleaved casp-3), mitochondrial dysfunction (pDrp1), and oxidative stress (3-NT) in the NeuN+ cells (neuronal) in the cortical peri-infarct region of FosDT−/− and FosDT+/+ rats at 24h of reperfusion after 60 min of transient MCAO (n=3/group) (C). Blue is DAPI for nuclei. PTM, post-translation modification; Cleaved casp-3, Cleaved caspase-3; pDrp1, phosphorylated dynamin-related protein-1 and 3-NT, 3-nitrotyrosine. Scale bar=50 μm.
FosDT deletion curtailed post-ischemic pathological changes.
Following transient MCAO and 1 day of reperfusion, FosDT−/− rats showed curtailed immunostaining of cleaved caspase-3 (apoptosis), phosphorylated dynamin-related protein-1 (pDrp1; mitochondrial dysfunction) and 3-nitrotyrosine (3-NT; oxidative stress) compared with the FosDT+/+ rats (Fig. 6C).
DISCUSSION
Focal ischemia is known to extensively alter the cerebral lncRNAome.2,18 However, the functional significance of lncRNAs in promoting ischemic brain damage is yet to be evaluated in detail. We previously showed that lncRNA FosDT is significantly induced in the post-ischemic brain and its knockdown is neuroprotective.11 FosDT is primarily a brain-specific lncRNA. In adult rats, except the heart, other peripheral organs (spleen, liver, kidney, muscle and lung) showed low FosDT expression. Although FosDT is expressed in all areas of the brain analyzed (cerebral cortex, striatum, hippocampus, cerebellum and thalamus), the cerebral cortex showed >20 times more expression than other brain regions of adult rats. Furthermore, FosDT−/− rats showed normal growth and no observable phenotypic changes or any discernible differences in the cytoarchitecture of the brain or peripheral organs compared with the FosDT+/+ rats. The FosDT−/− rats also showed no changes in the cerebral vasculature. It is intriguing that a highly expressed lncRNA had no noticeable function in the adult body. However, FosDT function might be masked by compensatory mechanisms during normal physiologic states but activated when neurons are stressed. In support of this notion, FosDT−/− rats showed a higher level of recovery in motor function and acquired smaller infarcts than FosDT+/+ rats when subjected to focal ischemia. Although this effect was observed in both sexes, there were overall smaller infarcts in the females, which is consistent with the literature and may explain (ceiling effects) why females had no significant differences between FosDT+/+ and FosDT−/− at day 5 or 7 of reperfusion in the rotarod test.19 As data reproducibility is of paramount importance, we confirmed our results independently in a second laboratory at the University of Texas. They also observed that FosDT−/− rats subjected to transient focal ischemia show smaller infarcts and better neurological functional outcome in both sexes.
To understand the molecular mechanisms that might be responsible for the post-stroke neuroprotection observed in the FosDT−/− rats, RNA-seq analysis was conducted using the ipsilateral cortical tissue from adult rats subjected to transient MCAO and 12h of reperfusion. As both sexes showed improved post-stroke functional outcome and recovery, we used only males for this. Many studies showed that the dominant neurotoxic mechanisms, including inflammation and apoptosis, are activated within 24h of transient MCAO.20 Inflammatory genes constitute a significant category of genes induced during the acute phase following focal ischemia.21–23 Uncontrolled inflammation is also a known precipitator of secondary brain damage after stroke.23 Interestingly, FosDT−/− rats showed curtailed post-ischemic induction of many inflammatory genes compared to FosDT+/+ rats. Specifically, genes that regulate neutrophil chemotaxis, inflammatory response, cytokine production, and exosome function were significantly down-regulated in the FosDT−/− rats compared to FosDT+/+ rats following focal ischemia. In addition, following transient MCAO, FosDT−/− rats showed significant induction of several genes that modulate metabolism, transport, post-translational protein modifications and adaptive immune response compared to FosDT+/+ rats. Thus, FosDT significantly influences a set of genes that might be responsible for the pathological events that lead to secondary brain damage after stroke.
In mammals, lncRNAs are the largest class of noncoding RNAs discovered so far.24 Importantly, many lncRNAs are brain-specific.6,25 They are functionally diverse and control cell fate during neural differentiation and synaptogenesis, neurite outgrowth and synapse maturation by mechanisms including scaffolding of epigenetic machinery, recruiting translational repression machinery, and controlling transcription factors and splicing regulators.25,26 Post-stroke outcome in rodents was shown to be altered by modulating several lncRNA, including C2dat1, Malat1, Maclpil, MEG3, and Nespas.27–31 FosDT gene is located in a gene desert of nearly a quarter-million nucleotides with only the Fos gene in its upstream proximity.11 We observed that the expression pattern of FosDT mirrors that of the Fos in various organs of both P7 and adult rats. Fos, which is a marker of cellular stress and is induced rapidly after brain injury, forms a complex with Jun/AP1 transcription factor and regulates cell development by modulating signal transduction, cell proliferation and differentiation.11,12,32,33 We previously showed that both FosDT and Fos are induced after focal ischemia in the rat brain.11 Both FosDT−/− and FosDT+/+ rats showed induction of Fos expression following focal ischemia, but the fold induction was much lower in FosDT−/− than FosDT+/+ rats indicating that FosDT controls Fos expression. Many genes observed to be dysregulated in lncRNA Malat1 knockout mice are its neighboring genes and hence Malat1 is thought to play a cis-regulatory role.34 Similarly, FosDT might control post-ischemic induction of Fos, indicating that intragenic lncRNAs such as FosDT can modulate the expression of their host genes.11 Similar to FosDT, Malat1 is also developmentally and functionally dispensable in the normal brain.34 However, some lncRNA knockouts (Gomafu, Fendrr, Peril and Mdgt) are developmentally normal but behaviorally impaired and show postnatal lethality.35,36
FosDT interacts with transcription factor REST-associated chromatin-modifying proteins to modulate the transcription of downstream genes, suggesting that FosDT acts as a scaffold and guides protein complexes to target genomic loci.11,37 Our previous studies showed that knockdown of FosDT allows the expression of the NFkB2 gene.11 NFkB2 (p100/p52) protein is known to prevent the nuclear translocation of NFkB RelA/p65 owing to its IkB property and thereby minimizes inflammation and cell death after ischemia.38,39 FosDT shares the characteristic of controlling genes by collaborating with transcription factors with other lncRNAs. For example, lncRNA HOTAIR regulates chromatin dynamics and silencing of epigenetic target genes/loci by recruiting polycomb repressive complex 2 and the LSD1/coREST/REST complex.40,41 This implies that lncRNAs like FosDT and HOTAIR coordinate multilevel gene regulation in a cell.
Overall, we show that the lncRNA FosDT is not essential for the development of the brain and peripheral organs, but its induction after stroke promotes ischemic brain damage. This is confirmed by the observation that FosDT−/− rats show reduced brain damage and better recovery of motor function following focal ischemia. We further showed that FosDT promotes ischemic brain damage by inducing the expression of many inflammatory genes. Future studies will establish FosDT as a novel therapeutic target for stroke.
Supplementary Material
Sources of Funding:
Supported in part by the NIH RO1 NS099531 and by the Department of Neurological Surgery, University of Wisconsin-Madison.
Non-standard Abbreviations and Acronyms:
- 3-NT
3-nitrotyrosine
- ACA
anterior cerebral artery
- FosDT
Fos downstream transcript
- GO
Gene ontology
- GSEA
Gene Set Enrichment Analysis
- H&E
hematoxylin and eosin
- lncRNAs
long noncoding RNAs
- MCA
middle cerebral artery
- MCAO
middle cerebral artery occlusion
- miRNAs
microRNAs
- P7
postnatal day 7
- PCA
posterior cerebral artery
- pDrp1
phosphorylated dynamin-related protein-1
- REST
RE1-silencing transcription factor
- ssODN
single-stranded DNA donor
Footnotes
Conflict(s)-of-Interest/Disclosure(s). None.
Supplemental Materials: Figures I –V and Tables I-III
Please see https://www.ahajournals.org/journal/str.
REFERENCES
- 1.Dharap A, Bowen K, Place R, Li LC, Vemuganti R. Transient focal ischemia induces extensive temporal changes in rat cerebral micrornaome. J Cereb Blood Flow Metab. 2009;29:675–687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dharap A, Nakka VP, Vemuganti R. Effect of focal ischemia on long noncoding rnas. Stroke. 2012;43:2800–2802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim T, Mehta SL, Morris-Blanco KC, Chokkalla AK, Chelluboina B, Lopez M, et al. The microrna mir-7a-5p ameliorates ischemic brain damage by repressing alpha-synuclein. Sci Signal. 2018;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mehta SL, Pandi G, Vemuganti R. Circular rna expression profiles alter significantly in mouse brain after transient focal ischemia. Stroke. 2017;48:2541–2548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mehta SL, Vemuganti R. Ischemic stroke alters the expression of the transcribed ultraconserved regions of the genome in rat brain. Stroke. 2018;49:1024–1028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Derrien T, Johnson R, Bussotti G, Tanzer A, Djebali S, Tilgner H, et al. The gencode v7 catalog of human long noncoding rnas: Analysis of their gene structure, evolution, and expression. Genome Res. 2012;22:1775–1789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sarropoulos I, Marin R, Cardoso-Moreira M, Kaessmann H. Developmental dynamics of lncrnas across mammalian organs and species. Nature. 2019;571:510–514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Adams BD, Parsons C, Walker L, Zhang WC, Slack FJ. Targeting noncoding rnas in disease. J Clin Invest. 2017;127:761–771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Djebali S, Davis CA, Merkel A, Dobin A, Lassmann T, Mortazavi A, et al. Landscape of transcription in human cells. Nature. 2012;489:101–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ponjavic J, Oliver PL, Lunter G, Ponting CP. Genomic and transcriptional co-localization of protein-coding and long non-coding rna pairs in the developing brain. PLoS Genet. 2009;5:e1000617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mehta SL, Kim T, Vemuganti R. Long noncoding rna fosdt promotes ischemic brain injury by interacting with rest-associated chromatin-modifying proteins. J Neurosci. 2015;35:16443–16449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shimazu M, Mizushima H, Sasaki K, Arai Y, Matsumoto K, Shioda S, et al. Expression of c-fos in the rat cerebral cortex after focal ischemia and reperfusion. Brain Res Bull. 1994;33:689–697 [DOI] [PubMed] [Google Scholar]
- 13.Percie du Sert N, Hurst V, Ahluwalia A, Alam S, Avey MT, Baker M, et al. The arrive guidelines 2.0: Updated guidelines for reporting animal research. PLoS Biol. 2020;18:e3000410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ma Y, Mehta SL, Lu B, Li PA. Deficiency in the inner mitochondrial membrane peptidase 2-like (immp21) gene increases ischemic brain damage and impairs mitochondrial function. Neurobiol Dis. 2011;44:270–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim T, Mehta SL, Kaimal B, Lyons K, Dempsey RJ, Vemuganti R. Poststroke induction of alpha-synuclein mediates ischemic brain damage. J Neurosci. 2016;36:7055–7065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee J, d′Aigle J, Atadja L, Quaicoe V, Honarpisheh P, Ganesh BP, et al. Gut microbiota-derived short-chain fatty acids promote post-stroke recovery in aged mice. Circ Res. 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Swanson RA, Morton MT, Tsao-Wu G, Savalos RA, Davidson C, Sharp FR. A semiautomated method for measuring brain infarct volume. J Cereb Blood Flow Metab. 1990;10:290–293 [DOI] [PubMed] [Google Scholar]
- 18.Bhattarai S, Pontarelli F, Prendergast E, Dharap A. Discovery of novel stroke-responsive lncrnas in the mouse cortex using genome-wide rna-seq. Neurobiol Dis. 2017;108:204–212 [DOI] [PubMed] [Google Scholar]
- 19.Alkayed NJ, Harukuni I, Kimes AS, London ED, Traystman RJ, Hurn PD. Gender-linked brain injury in experimental stroke. Stroke. 1998;29:159–165; discussion 166 [DOI] [PubMed] [Google Scholar]
- 20.Lopez MS, Dempsey RJ, Vemuganti R. Resveratrol neuroprotection in stroke and traumatic cns injury. Neurochem Int. 2015;89:75–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu Q, Johnson EM, Lam RK, Wang Q, Bo Ye H, Wilson EN, et al. Peripheral trem1 responses to brain and intestinal immunogens amplify stroke severity. Nat Immunol. 2019;20:1023–1034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yamaguchi A, Jitsuishi T, Hozumi T, Iwanami J, Kitajo K, Yamaguchi H, et al. Temporal expression profiling of damps-related genes revealed the biphasic post-ischemic inflammation in the experimental stroke model. Mol Brain. 2020;13:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Flemming A Calming inflammation to prevent stroke damage. Nat Rev Immunol. 2019;19:473. [DOI] [PubMed] [Google Scholar]
- 24.Iyer MK, Niknafs YS, Malik R, Singhal U, Sahu A, Hosono Y, et al. The landscape of long noncoding rnas in the human transcriptome. Nat Genet. 2015;47:199–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ramos AD, Andersen RE, Liu SJ, Nowakowski TJ, Hong SJ, Gertz C, et al. The long noncoding rna pnky regulates neuronal differentiation of embryonic and postnatal neural stem cells. Cell Stem Cell. 2015;16:439–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Briggs JA, Wolvetang EJ, Mattick JS, Rinn JL, Barry G. Mechanisms of long non-coding rnas in mammalian nervous system development, plasticity, disease, and evolution. Neuron. 2015;88:861–877 [DOI] [PubMed] [Google Scholar]
- 27.Deng Y, Chen D, Wang L, Gao F, Jin B, Lv H, et al. Silencing of long noncoding rna nespas aggravates microglial cell death and neuroinflammation in ischemic stroke. Stroke. 2019;50:1850–1858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang Y, Luo Y, Yao Y, Ji Y, Feng L, Du F, et al. Silencing the lncrna maclpil in pro-inflammatory macrophages attenuates acute experimental ischemic stroke via lcp1 in mice. J Cereb Blood Flow Metab. 2020;40:747–759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu Q, Deng F, Xing Z, Wu Z, Cen B, Xu S, et al. Long non-coding rna c2dat1 regulates camkiiδ expression to promote neuronal survival through the nf-κb signaling pathway following cerebral ischemia. Cell Death Dis. 2016;7:e2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yan H, Rao J, Yuan J, Gao L, Huang W, Zhao L, et al. Long non-coding rna meg3 functions as a competing endogenous rna to regulate ischemic neuronal death by targeting mir-21/pdcd4 signaling pathway. Cell Death Dis. 2017;8:3211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang X, Tang X, Liu K, Hamblin MH, Yin KJ. Long noncoding rna malat1 regulates cerebrovascular pathologies in ischemic stroke. J Neurosci. 2017;37:1797–1806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chiu R, Boyle WJ, Meek J, Smeal T, Hunter T, Karin M. The c-fos protein interacts with c-jun/ap-1 to stimulate transcription of ap-1 responsive genes. Cell. 1988;54:541–552 [DOI] [PubMed] [Google Scholar]
- 33.Shaulian E, Karin M. Ap-1 as a regulator of cell life and death. Nat Cell Biol. 2002;4:E131–136 [DOI] [PubMed] [Google Scholar]
- 34.Zhang B, Arun G, Mao YS, Lazar Z, Hung G, Bhattacharjee G, et al. The lncrna malat1 is dispensable for mouse development but its transcription plays a cis-regulatory role in the adult. Cell Rep. 2012;2:111–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ip JY, Sone M, Nashiki C, Pan Q, Kitaichi K, Yanaka K, et al. Gomafu lncrna knockout mice exhibit mild hyperactivity with enhanced responsiveness to the psychostimulant methamphetamine. Sci Rep. 2016;6:27204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sauvageau M, Goff LA, Lodato S, Bonev B, Groff AF, Gerhardinger C, et al. Multiple knockout mouse models reveal lincrnas are required for life and brain development. Elife. 2013;2:e01749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dharap A, Pokrzywa C, Vemuganti R. Increased binding of stroke-induced long non-coding rnas to the transcriptional corepressors sin3a and corest. ASN Neuro. 2013;5:283–289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schneider A, Martin-Villalba A, Weih F, Vogel J, Wirth T, Schwaninger M. Nf-kappab is activated and promotes cell death in focal cerebral ischemia. Nat Med. 1999;5:554–559 [DOI] [PubMed] [Google Scholar]
- 39.Zhang W, Potrovita I, Tarabin V, Herrmann O, Beer V, Weih F, et al. Neuronal activation of nf-kappab contributes to cell death in cerebral ischemia. J Cereb Blood Flow Metab. 2005;25:30–40 [DOI] [PubMed] [Google Scholar]
- 40.Khalil AM, Guttman M, Huarte M, Garber M, Raj A, Rivea Morales D, et al. Many human large intergenic noncoding rnas associate with chromatin-modifying complexes and affect gene expression. Proc Natl Acad Sci U S A. 2009;106:11667–11672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tsai MC, Manor O, Wan Y, Mosammaparast N, Wang JK, Lan F, et al. Long noncoding rna as modular scaffold of histone modification complexes. Science. 2010;329:689–693 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


