Abstract
Fibroblasts are the most common cell type of connective tissues. In the CNS, fibroblast-like cells are mainly located in the meninges and perivascular Virchow-Robin space. The origins of these fibroblast-like cells and their functions in both CNS development and pathological conditions remain largely unknown. In this review, we first introduce the anatomical location and molecular markers of CNS fibroblast-like cells. Next, the functions of fibroblast-like cells in CNS development and neurological disorders, including stroke, CNS traumatic injuries, and other neurological diseases, are discussed. Third, current challenges and future directions in the field are summarized. We hope to provide a synthetic review that stimulates future research on CNS fibroblast-like cells.
Keywords: Meningeal fibroblasts, perivascular fibroblasts, stroke, neurological disroders
Introduction
Fibroblasts are mesenchymal cells that exist in the interstitial space of all organs.1 They have flattened nuclei and thin cytoplasm with tenuous processes. As the most common cell type of connective tissues, fibroblasts play important roles in structure support, injury repair, and fibrosis.2, 3 Our knowledge on fibroblast functions mainly comes from skin fibroblasts. Recent studies demonstrate that fibroblast-like cells also exist in the meninges and perivascular Virchow-Robin space, and exert important functions both during central nervous system (CNS) development and in pathological conditions.4, 5 There are, however, controversial findings on the source and function of fibroblast-like cells after CNS injury. For example, it is unclear whether meningeal or perivascular fibroblast-like cells accumulate at injury site after CNS injury. In addition, it is in debate whether fibroblast-like cells play a neuroprotective and detrimental role after CNS injury. In this review, we first introduce the anatomical location and molecular markers of CNS fibroblast-like cells. Next, the functions of fibroblast-like cells in CNS development and neurological disorders, including stroke, CNS traumatic injuries, and other neurological diseases, are discussed. Third, current challenges and future directions in the field are summarized. We hope to provide a synthetic review that stimulates future research on CNS fibroblast-like cells.
Location of CNS fibroblast-like cells
In the CNS, fibroblast-like cells are found in the meninges and perivascular Virchow-Robin space in both mice and humans.5–7 Meninges, which cover the brain and spinal cord, are an important connective tissue derived from the neural crest and mesoderm. Neural crest-derived cells form meninges of the forebrain, while mesoderm-derived cells form meninges of the midbrain and hindbrain. Meninges differentiate into dura (outmost layer), arachnoid, and pia (innermost layer) starting at ~E13 in mouse.8, 9 Dura is a collagenous membrane containing loosely packed dural fibroblast-like cells, arachnoid is a translucent sheet containing closely packed arachnoid fibroblast-like cells joined by tight junctions and desmosomes, and pia consists of a thin layer of pial fibroblast-like cells mainly joined by gap junctions10–12 (Figure 1). Between arachnoid and pia is the cerebrospinal fluid-containing subarachnoid space (SAS).8, 13 Trabeculae, collagen fiber cores coated with meningothelial cells (fibroblast-like cells), divide the SAS into compartments10–12 (Figure 1). The SAS also contains blood vessels, which are covered by meningothelial cells.10, 12 Together with penetrating blood vessels, pia also runs into brain parenchyma. Therefore, fibroblast-like cells are also found in perivascular Virchow-Robin space, sandwiched between vascular smooth muscle cells and astrocytes endfeet5, 6, 10, 14–16 (Figure 1). These perivascular fibroblasts are exclusively found in large blood vessels, including arteries, arterioles, veins and venules. The various subtypes of fibroblast-like cells reflect a complex system, highlighting important and diverse functions of these cells in the CNS.
Figure 1.
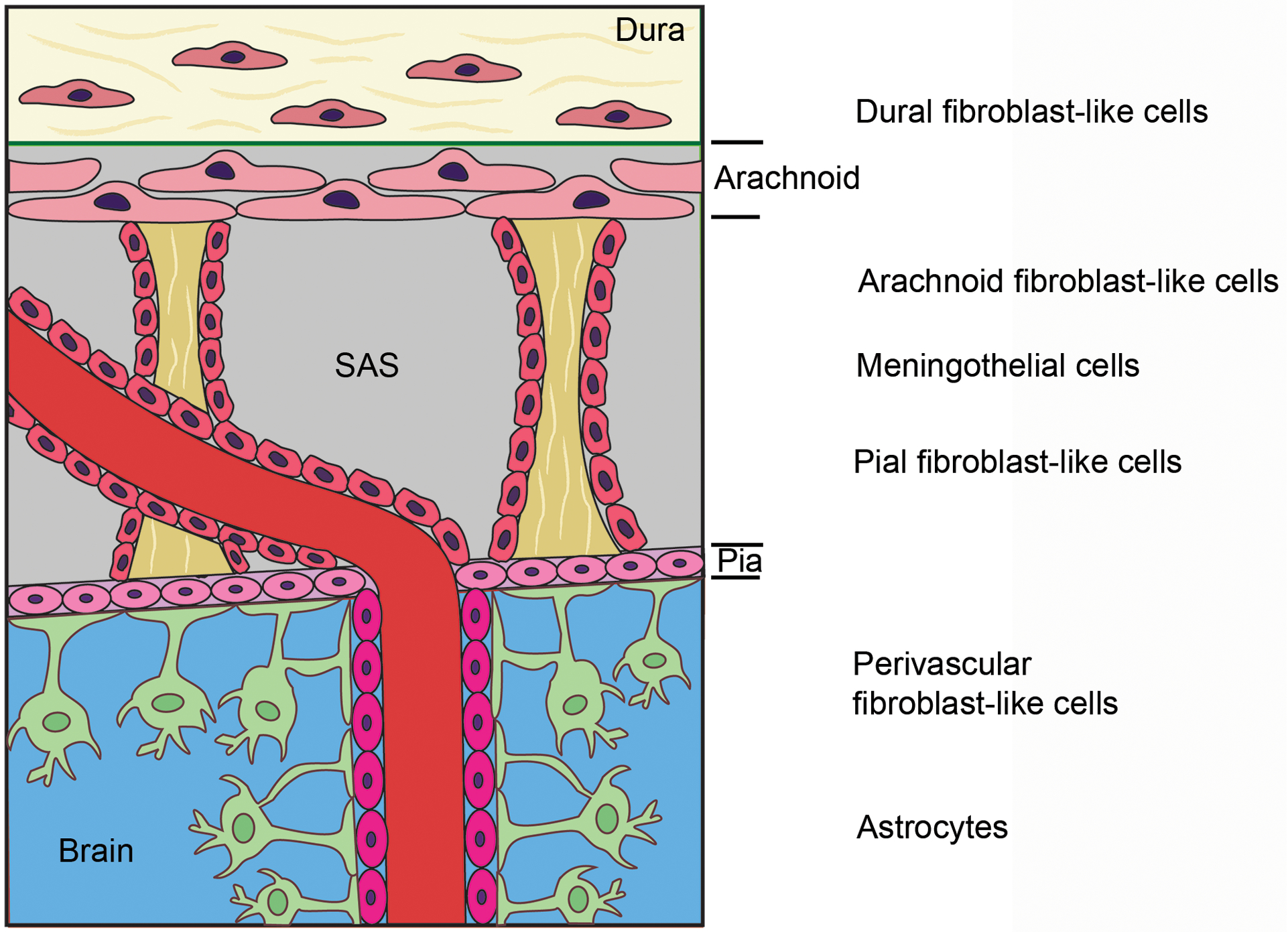
Diagram illustration of meningeal and perivascular fibroblast-like cells. SAS, subarachnoid space.
Based on the anatomical distribution of fibroblast-like cells, it is speculated that they regulate brain influx/efflux function via the glymphatic system, a specialized fluid-transporting system that functions predominantly during sleep.17 Cerebrospinal fluid from the SAS flows into brain parenchyma via the perivascular Virchow-Robin space, where it crosses the glia limitans in an aquaporin-4-dependent manner.18–21 In the interstitium, cerebrospinal fluid mixes with interstitial fluid and leaves the brain via perivenous space and along cranial and spinal nerves.18–21 Although how exactly fibroblast-like cells affect the glymphatic system remains unknown, there are a few interesting hypotheses. For example, fibroblast-like cells may modulate glymphatic function via regulating aquaporin-4 expression on astrocytic endfeet. Alternatively, fibroblast-like cells may directly affect fluid-transporting conduits---the basement membrane by up-/down-regulating numerous extracellular matrix (ECM) proteins. Additionally, fibroblast-like cells may regulate the glymphatic system via acting on mural cells and/or astrocytes. These hypotheses should be tested in future research.
Markers of CNS fibroblast-like cells
Molecular characterization of fibroblasts is challenging. Although several markers, including retinaldehyde dehydrogenase type 2 (Raldh2),22, 23 fibroblast-specific protein 1 (FSP1),24, 25 vimentin,23, 26 platelet-derived growth factor receptor-alpha (PDGFRα),5, 15, 27 platelet-derived growth factor receptor-beta (PDGFRβ),28 ER-TR7,6, 29–31 fibronectin,23, 32, 33 and collagen type I (Col1α1),5, 14, 22 have been used to identify fibroblasts, these markers have several major limitations. First, none of these markers are fibroblast-specific. For example, vimentin is also expressed in arachnoid/meningothelial cells, astrocytes and endothelial cells, in addition to fibroblasts.23, 26 PDGFRα labels both fibroblasts and oligodendrocyte progenitor cells (OPCs) in the CNS.5, 15, 27 PDGFRβ marks fibroblasts and mural cells.28, 34 Although FSP1 labels fibroblasts in physiological conditions, its expression is induced in hematopoietic cells, endothelial cells, and vascular smooth muscle cells after injury.24, 25 Second, many fibroblast markers are ECM proteins, whose expression is substantially upregulated after injury. Therefore, these markers do not always reflect fibroblast changes in pathological conditions. For instance, increased fibronectin expression does not necessarily mean more fibroblasts. Third, there are no universal or subtype/organ-specific fibroblast markers. All currently used fibroblast markers label mixed populations and their specificity for different subtypes of fibroblasts remains largely unknown. Col1α1 is a well-characterized fibroblast marker in the brain.5, 14, 22 However, it cannot distinguish dural/arachnoid/pial fibroblasts or discern meningeal fibroblasts from perivascular fibroblasts.5 In addition, ER-TR7 mainly labels meningeal fibroblasts, pericytes, and reticular fibers.6, 29–31 The advantages and disadvantages of commonly used markers for fibroblast-like cells are summarized in Table 1.
Table 1.
Comparison of commonly used markers for fibroblast-like cells
| Fibroblast markers | Expression in other cell types | ECM protein | Comments | References |
|---|---|---|---|---|
| Raldh2 | Oligodendrocytes, Astrocytes | No | Expressed in both meningeal and perivascular fibroblasts, but not specific for fibroblasts in the CNS. Upregulated after CNS injury. | 22, 23 |
| FSP1 | Hematopoietic cells, Endothelial cells, Vascular smooth muscle cells | No | Expressed in both meningeal and perivascular fibroblasts, but not specific for fibroblasts in the CNS. Expressed outside the CNS. | 24, 25 |
| Fibronectin | Macrophages, Astrocytes | Yes | Expressed in both meningeal and perivascular fibroblasts, but not specific for fibroblasts in the CNS. Upregulated after CNS injury. Expressed in other cells after injury. | 23, 32, 33 |
| Vimentin | Arachnoid/meningothelial cells, Astrocytes, Pericyte, Endothelial cells | No | Expressed in both meningeal and perivascular fibroblasts, but not specific for fibroblasts in the CNS. Expressed outside the CNS. | 23, 26 |
| PDGFRα | Oligodendrocyte Precursor Cells | No | Expressed in both meningeal and perivascular fibroblasts, but not specific for fibroblasts in the CNS. Expressed outside the CNS. | 5, 15, 27 |
| ER-TR7 | Pericytes, Reticular fibers |
Yes | Predominantly expressed in meningeal fibroblasts and pericytes in the CNS. Expressed outside the CNS. | 6, 29–31 |
| Col1α1 | Podocytes, Pericytes | Yes | Specifically expressed in meningeal and perivascular fibroblasts in the CNS. Upregulated after CNS injury. Expressed in pericyte and podocytes outside the CNS. | 5, 14, 22 |
| PDGFRβ | Mural cells, Some neurons | No | Expressed in both meningeal and perivascular fibroblasts, but not specific for fibroblasts in the CNS. Expressed outside the CNS. | 28, 34 |
Together, these findings support the use of both anatomical location and multiple markers to identify fibroblasts. Future research should focus on identifying pan- and subtype/organ-specific fibroblast markers. These markers will open doors for new research and substantially move the field forward.
Fibroblast-like cells in CNS development
Expression studies suggest that meningeal fibroblast-like cells play important roles during brain development. Single-cell RNA sequencing analysis revealed distinct gene expression profiles of mouse fibroblasts in the pia, arachnoid, and dura at E14.5, suggesting different functions of these cells.16 Immunohistochemistry validated that arachnoid and pial markers are conserved between mouse and human fetal meninges.16 Meningeal fibroblast-like cells, in particular pial fibroblasts, are the main source of pial ECM proteins, including collagens and laminins, which serve as an attachment point for radial glial cells and physical barrier for migrating neurons.16, 35–37 Pial fibroblasts are also a major source of CXCL12, which regulates neuronal migration and positioning during development. In addition, fibroblast-like cells in the pia and arachnoid show enriched expression of retinoic pathway genes, indicating that these cells may contribute to the production of retinoic acid (RA), which induces the differentiation of neural progenitors into neurons in the cortex.16, 38
Loss-of-function studies reveal an indispensable role of meningeal fibroblast-like cells in CNS development. Mice with hypomorphic mutations in Foxc1, a transcription factor mainly expressed in meningeal cells, show meningeal defects, cerebral basement membrane breakdown, cortical dysplasia, and neuronal over-migration during development.35, 39 Similarly, Foxc1-deficient mice exhibit reduced meningeal fibroblast-like cells and RA production during development, resulting in decreased neurons and severe CNS defects.16, 40 These Foxc1 knockout mice also display cerebrovascular growth defect and cerebral hemorrhages due to diminished meningeal RA and abnormal neocortical vasculature growth.41 Consistent with these findings, ablation of Raldh2, which mediates RA synthesis, leads to abnormal neurogenesis and cerebrovascular development in mice.42 In addition, mice lacking PDGF-C, a major ligand for PDGFRα, show abnormal meninges, neuronal over-migration in the cortex, and cerebral bleeding,15 possibly due to reduced PDGFRα+ meningeal fibroblast-like cells and decreased ECM proteins. Like Foxc1 mutant mice, humans with Foxc1 mutations develop similar developmental defects in the CNS.16, 43, 44 Together, these results highlight critical roles of meningeal fibroblast-like cells in brain development and meninges formation.
Unlike meningeal fibroblast-like cells, the function of perivascular fibroblast-like cells in brain development remains unknown. This is an important area that needs future research.
Fibroblast-like cells in neurological disorders
After CNS injury, fibroblast-like cells actively proliferate, migrate, and secrete excessive ECM proteins (e.g. collagen and fibronectin) and bioactive molecules (e.g. axon growth inhibitors), contributing to the formation of fibrotic scar. These changes are predominantly induced by transforming growth factor-β1 (TGF-β1) signaling, which promotes fibroblast activation and ECM protein expression.32, 45 Fibroblast-like cells and fibrotic scar exert both detrimental and neuroprotective roles depending on the timing after CNS injury. The process of CNS injury repair can be broadly categorized into three stages: inflammation, new tissue formation, and remodeling.3, 46 It has been shown that fibrotic scar generally inhibits neuronal regeneration in the remodeling stage due to the physical barrier and axon growth inhibitors in the scar.4, 47, 48 At the new tissue formation stage, however, fibrotic scar plays a beneficial role due to its effect on junctional integrity.46 Here, we discuss the functional significance of fibroblast-like cells in various neurological disorders, including stroke, CNS traumatic injuries, and others. The origins and functions of fibroblast-like cells in stroke and CNS traumatic injuries are summarized in Figure 2.
Figure 2.
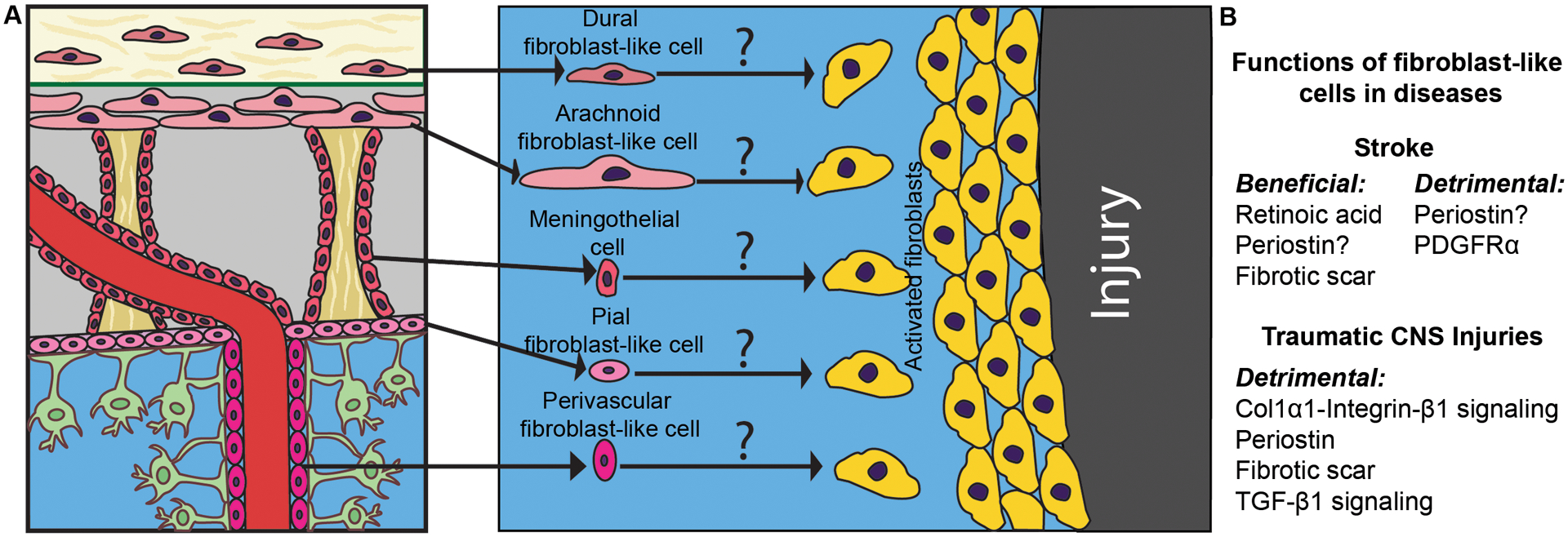
Origins and functions of fibroblast-like cells in stroke and CNS traumatic injuries. A, Schematic illustration of possible origins of fibroblast-like cells in CNS injury. Question marks indicate the exact origin of activated fibroblast-like cells remains unknown. B, The functions and potential mechanisms of fibroblast-like cells in stroke and CNS traumatic injuries.
Stroke
There is evidence suggesting that fibroblast-like cells migrate to injury site after stroke. For example, PDGFRβ+ and CD105+ stromal cells derived from large vessels but not capillaries proliferate, migrate to injury site, deposit ECM proteins, and contribute to scar formation after ischemic stroke in both mice and humans.49 Since these cells do not express pericyte markers (NG2 and CD13), but show increased fibronectin expression,49 it is believed that these PDGFRβ+ and CD105+ cells are fibroblast-like cells. Using Col1α1-GFP transgenic mice, it has been shown that Col1α1+ cells, which express multiple fibroblast markers and reside in the meninges and along large blood vessels in the brain, accumulate in stroke lesion.22
Most studies support a neuroprotective role of fibroblast-like cells in stroke. First, Col1α1+ fibroblasts act as an important source of RA, which induces neural progenitor differentiation and promotes recovery in rodents.22, 50 Next, nestin+ cells with features of fibroblasts are found in ischemic core at day 14 but not day 3 after stroke in rats,51 suggesting that fibroblast-like cells may contribute to stroke recovery. Additionally, at the chronic stage in a mouse ischemic stroke model, Col1α1+ and fibronectin+ fibroblast-like cells in the peri-infarct regions strongly express periostin,52, 53 which induces proliferation/differentiation of neural stem cells and improves functional recovery in neonatal hypoxic-ischemic rats.54 Furthermore, mice lacking AKAP12, which is heavily expressed in meninges and fibrotic scar, exhibit impaired fibrotic scar structure, increased leakage from lesions, extended inflammation, and aggravated tissue damage in the photothrombotic stroke model.46
It should be noted, however, that there is also evidence suggesting that fibroblast-like cells play a detrimental role in stroke. Unlike in ischemic stroke, neutralization of periostin attenuates early brain injury, whereas administration of recombinant periostin exacerbates early brain injury after subarachnoid hemorrhage in mice,55 In addition, activation of PDGFRα, which is expressed in fibroblast-like cells and OPCs, increases cerebrovascular permeability in both ischemic and hemorrhagic stroke in mice.56, 57 The relative contributions of fibroblast- and OPC-mediated PDGFRα activation in vascular damage after stroke, however, need future research.
These results suggest that fibroblast-like cells can be either beneficial or deleterious in stroke. One possible explanation for this disparity is that different subpopulations of fibroblast-like cells, including dural, arachnoid, pial, perivascular fibroblasts and meningothelial cells, may have distinct biological properties and functions in stroke. If this is true, the overall functions of fibroblast-like cells may depend on the location of injury. Specifically, if the injury predominantly occurs in deep brain regions without affecting meninges, the outcome is mainly determined by perivascular fibroblast-like cells. On the contrary, if the lesion occurs in superficial brain regions, where meninges are injured, both meningeal and perivascular fibroblast-like cells contribute to the outcome. It should be noted that we cannot exclude the possibility that fibroblast-like cells from one location may have both neuroprotective and detrimental functions. In this case, the overall outcome largely depends on the specific microenvironment rather than the location of injury. In addition, injury models and disease stages may also affect the functions of fibroblast-like cells. Future studies should elucidate the functions of different fibroblast populations in stroke and other neurological disorders.
A key question in the field is which subtypes of fibroblast-like cells participate in stroke pathogenesis. Controversial findings exist probably due to the lack of molecular markers to distinguish these cells. On one hand, there is evidence supporting that meningeal fibroblast-like cells contribute to stroke repair. Fibronectin+ and ET-TR7+ mouse meningeal cells have epithelial properties under normal condition.58 After photothrombotic stroke, however, these cells show epithelial–mesenchymal transition and migrate into the lesion site,58 indicating a pro-recovery role of meningeal fibroblast-like cells in stroke. However, it remains unclear whether these fibronectin+/ET-TR7+ meningeal cells are originated from dural, arachnoid, pial fibroblasts or meningothelial cells.
On the other hand, perivascular fibroblast-like cells have also been shown to regulate stroke pathogenesis. A time-course study reports that PDGFRβ is expressed only in blood vessel walls at day 3 after ischemic stroke and it co-localizes with both pericyte marker Desmin and fibroblast marker Col1α1 in mouse brains.34 At day 7 after injury, however, PDGFRβ is detected in both blood vessel walls and nonvessel-associated fibroblast-like cells.34 Although PDGFRβ co-localizes with both Desmin and Col1α1 in blood vessel walls, it only merges with Col1α1 in nonvessel-associated fibroblast-like cells.34 These findings suggest that PDGFRβ+Col1α1+ fibroblasts in ischemic brains may come from blood vessel walls. The accumulation of PDGFRβ+Col1α1+ fibroblast-like cells in ischemic brain may be caused by proliferation of perivascular fibroblast-like cells and/or differentiation of pericytes. Although the underlying mechanisms remain largely unknown, there is evidence suggesting that PDGFRβ signaling may be involved. First, PDGFRβ+/− mice exhibit enlarged infarct volume and decreased expression of fibroblast markers (fibronectin and Col1α1) after ischemic stroke.34 Next, PDGFR (PDGFRα and PDGFRβ) signaling regulates pericyte proliferation and their differentiation into myofibroblasts in two mouse models of kidney fibrosis.59 It is difficult to distinguish fibroblast-like cells and pericytes, especially after brain injury, due to the large number of molecular markers they share. Future research should focus on identifying markers that are able to differentiate these cells and addressing their functional significance in stroke pathogenesis.
CNS traumatic injuries
Fibroblast-like cells are identified at injury core in rodents after spinal cord injury (SCI) by electron microscopy.60, 61 Subsequent studies show that invading fibroblast-like cells express CTGF, a profibrotic and angiogenic protein, in rats after SCI.62 It has also been reported that fibroblast-like cells substantially upregulate fibronectin63 and Col1α164 expression after SCI in rodents, suggesting that these cells contribute to fibrotic scar formation after SCI. In addition, fibroblast-like cells also contribute to glial scar formation after SCI. It has been shown that meningeal fibroblast-like cells enhance astrocyte reactivity and glial scar marker expression in an astrocyte-fibroblast co-culture system.65 In a rat SCI model, ER-TR7+ and PDGFRβ+ meningeal fibroblast-like cells upregulate the expression of Col1α1, which induces glial scar formation via integrin-β1 receptor on astrocytes.66 Like in SCI, fibroblast-like cells are also involved in the pathogenesis of traumatic brain injury (TBI). It has been shown that fibronectin+ fibroblast-like cells migrate to injury site after TBI and substantially upregulate TGF-β receptor, whose activation promotes fibroblast proliferation, ECM production, and fibrotic scar formation.32 This result indicates that fibroblast-like cells are a major target of TGF-β after TBI. In addition, ER-TR7+ fibroblast-like cells synthesize chondroitin sulfate proteoglycans, which strongly inhibit axon growth and regeneration, at the injury site after TBI in mice.67
Functional studies support a detrimental role of fibroblast-like cells in SCI. First, inhibiting Col1α1-integrin-β1 signaling promotes functional recovery after SCI in mice.68 Next, mice deficient in periostin, an ECM protein mainly expressed by scar-forming cells, display diminished PDGFRβ+ cell proliferation, reduced collagen expression, decreased fibrotic scar formation, and better functional recovery after SCI.69 Consistent with this finding, administration of periostin neutralizing antibody leads to similar changes in mice after SCI.69 Deferoxamine treatment, which inhibits the expression of ECM proteins and axon growth inhibitory molecules, reduces fibrotic scar formation and promotes axon regeneration in rats after SCI.70 Suppression of fibrous scarring promotes neuronal and axonal regeneration, and induces functional recovery in a rat model of SCI.71 These results suggest that fibroblast-derived ECM proteins may be targeted in SCI therapies. In addition, knockdown of MiR-21–5p, which mediates TGF-β1-induced fibrosis-related gene expression, improves motor functional recovery in a mouse model of SCI.45 Echoed with this report, microtubule stabilizing drugs, such as Epothilone B and Taxol, inhibit fibrotic scar formation and promote axon regeneration in rodent models of SCI by reducing TGF-β signaling.72, 73 These findings indicate that blocking TGF-β1 signaling may have a therapeutic effect in SCI. Furthermore, depletion of hematogenous macrophages results in decreased fibroblast-like cell number and increased axonal growth in a mouse model of SCI,74 suggesting important roles of hematogenous macrophages in fibroblast-like cell recruitment and injury repair in SCI. Similarly, a detrimental role of fibroblast-like cell-derived ECM proteins is observed in TBI. For instance, suppressing fibrotic scar formation by inhibiting collagen type IV triple-helix formation promotes axonal regeneration without disturbing the healing process in TBI.75 Additionally, local administration of chondroitinase-ABC, which degrades chondroitin sulfate proteoglycans, has been shown to significantly promote functional recovery in rats after SCI.76 These findings suggest that inhibiting ECM deposition may be a reliable strategy to promote axonal regeneration after TBI.
Whether fibroblast-like cells at lesion sites are from meninges or blood vessels has been controversial and seems to depend on the types of injury. Using Col1α1-GFP transgenic mice, it has been demonstrated that Col1α1+ fibroblast-like cells predominantly come from blood vessels in the contusion model, in which the dura of meninges is intact.14 In contrast, there is also evidence suggesting that fibroblast-like cells migrate into injury sites from adjacent meninges in a rat spinal cord contusion model.77 This discrepancy may be explained by different fibroblast markers used in these studies. In the penetrating SCI model that breaks the meninges, on the other hand, fibroblast-like cells seem to come from meninges rather than blood vessels.14 It is thus important to perform SCI research using both contusion and penetrating models. It is speculated that fibroblast-like cells predominantly come from meninges in TBI, which shares similar pathology as penetrating SCI injury. Future research should elucidate the origins of fibroblast-like cells in CNS traumatic injuries.
Other neurological disorders
Multiple sclerosis is an autoimmune disease. It has been reported that both PDGFRα+PDGFRβ+ meningeal fibroblast-like cells and PDGFRα−PDGFRβ+ pericytes increase and form a network in mouse meninges in the experimental autoimmune encephalitis (EAE) model of multiple sclerosis.78 Upon inflammatory stimuli, such as TH17 cells and IL-17/IL-22, these cells secrete ECM proteins and cytokines, leading to enhanced immune response and demyelination.78 These findings suggest that meningeal fibroblast-like cells may play a detrimental role in EAE. Using Col1α1-GFP transgenic mice, it has been shown that perivascular fibroblast-like cells in the spinal cord become activated and infiltrate into neural tissue in EAE.79 Interestingly, this change is associated with animal behavioral deficits, demyelination, myeloid cell accumulation, and ECM deposition,79 indicating a detrimental role of perivascular fibroblast-like cells in EAE. In addition, fibroblast-conditioned media and fibroblast-derived ECM have been found to inhibit OPC differentiation in vitro.79 Given that abnormal ECM deposition is observed in multiple sclerosis in both mice80 and humans,81, 82 it is believed that fibroblast-like cells affect multiple sclerosis pathology and outcomes at least partially via their ECM proteins. This hypothesis and the underlying molecular mechanisms, however, need further investigation.
Fibroblast-like cells also contribute to scar formation in 3-nitropropionic acid-induced Huntington’s disease model. It has been shown that collagen-producing PDGFRβ+ fibroblast-like cells increase and extend into extravascular space in the lesion core over time in 3-nitropropionic acid-treated rats.83 Ultrastructural analysis demonstrates that these PDGFRβ+ fibroblast-like cells have close interaction with macrophages,83 suggesting a possible role of macrophages in fibroblast migration and fibrotic scar formation. Biochemical analysis shows that these PDGFRβ+ fibroblast-like cells are induced to express nestin and vimentin in the lesion core,83 highlighting important roles of nestin and vimentin in the structural dynamics of fibroblast-like cells. The functional significance of fibroblast-like cells in Huntington’s disease and other neurodegenerative disorders, however, remains unknown and needs future investigation.
Conclusions
Fibroblast-like cells are stromal cells with high plasticity. With the advance of imaging and next generation sequencing techniques, CNS fibroblast-like cells and their subpopulations have been identified. Several challenges, however, prevent the study of these cells. First, there are no pan- or subtype-specific fibroblast markers available currently, which makes isolation of pure fibroblast populations and investigation of their functions impossible. Second, fibroblast-like cells are highly plastic. They are able to differentiate and/or de-differentiate into other cell types after CNS injury, which makes it difficult to define and characterize their functions. Third, there are no genetic tools that allow for loss-of-function studies. The development of such tools (e.g. mice lacking a subpopulation of fibroblasts) relies on subpopulation-specific markers. Fourth, the origins of fibroblast-like cells in CNS injuries are largely unknown. It remains unknown if fibroblast-like cells at lesion sites are originated from dural, arachnoid, pial, perivascular fibroblasts and/or meningothelial cells (Figure 2). It seems the location and nature of injury may affect the types of fibroblast-like cells accumulated at lesion sites. Fifth, the functions of fibroblast-like cells in development and pathological conditions are largely unknown. Current knowledge on the functions of fibroblast-like cells mainly comes from association studies, in vitro experiments, and/or non-CNS fibroblasts. It remains unclear if different subtypes of fibroblast-like cells have distinct functions, and if one subtype of fibroblast-like cells can exert both beneficial and deleterious effects in CNS injuries. Fifth, the molecular mechanisms responsible for the migration and functions of different subtypes of fibroblast-like cells in physiological and pathological conditions are unclear. Answering these important questions will address key challenges and substantially move the field forward.
Acknowledgments
LX searched the literature and drafted the article. YY gave suggestions and edited the article. Both authors read and approved the final article.
Sources of Funding
This work was supported by NIH grants (R01HL146574 and R21AG064422) to YY and American Heart Association Predoctoral Fellowship (20PRE35210605) to LX.
Non-standard Abbreviations and Acronyms:
- CNS
Central nervous system
- Col1α1
Collagen type I
- ECM
Extracellular matrix
- FSP1
Fibroblast specific protein 1
- OPCs
Oligodendrocyte progenitor cells
- PDGFRα
Platelet-derived growth factor receptor-alpha
- PDGFRβ
Platelet-derived growth factor receptor-beta
- Raldh2
Retinaldehyde dehydrogenase type 2
- RA
Retinoic acid
- SCI
Spinal cord injury
- SAS
Subarachnoid space
- TGF-β1
Transforming growth factor-β1
- TBI
Traumatic brain injury
Footnotes
Disclosures
None.
References
- 1.Di Carlo SE, Peduto L. The perivascular origin of pathological fibroblasts. The Journal of clinical investigation. 2018;128:54–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thulabandu V, Chen D, Atit RP. Dermal fibroblast in cutaneous development and healing. Wiley interdisciplinary reviews. Developmental biology. 2018;7:e307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gurtner GC, Werner S, Barrandon Y, Longaker MT. Wound repair and regeneration. Nature. 2008;453:314–321 [DOI] [PubMed] [Google Scholar]
- 4.Fernandez-Klett F, Priller J. The fibrotic scar in neurological disorders. Brain pathology (Zurich, Switzerland). 2014;24:404–413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vanlandewijck M, He L, Mae MA, Andrae J, Ando K, Del Gaudio F, Nahar K, Lehouvier T, Lavina B, Gouveia L, et al. A molecular atlas of cell types and zonation in the brain vasculature. Nature. 2018;554:475–480 [DOI] [PubMed] [Google Scholar]
- 6.Hannocks MJ, Pizzo ME, Huppert J, Deshpande T, Abbott NJ, Thorne RG, Sorokin L. Molecular characterization of perivascular drainage pathways in the murine brain. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2018;38:669–686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dragunow M Meningeal and choroid plexus cells--novel drug targets for cns disorders. Brain research. 2013;1501:32–55 [DOI] [PubMed] [Google Scholar]
- 8.Dasgupta K, Jeong J. Developmental biology of the meninges. Genesis (New York, N.Y. : 2000). 2019;57:e23288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jiang X, Iseki S, Maxson RE, Sucov HM, Morriss-Kay GM. Tissue origins and interactions in the mammalian skull vault. Developmental biology. 2002;241:106–116 [DOI] [PubMed] [Google Scholar]
- 10.Mastorakos P, McGavern D. The anatomy and immunology of vasculature in the central nervous system. Sci Immunol. 2019;4:eaav0492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alcolado R, Weller RO, Parrish EP, Garrod D. The cranial arachnoid and pia mater in man: Anatomical and ultrastructural observations. Neuropathology and applied neurobiology. 1988;14:1–17 [DOI] [PubMed] [Google Scholar]
- 12.Weller RO, Sharp MM, Christodoulides M, Carare RO, Mollgard K. The meninges as barriers and facilitators for the movement of fluid, cells and pathogens related to the rodent and human cns. Acta neuropathologica. 2018;135:363–385 [DOI] [PubMed] [Google Scholar]
- 13.Patel N, Kirmi O. Anatomy and imaging of the normal meninges. Seminars in ultrasound, CT, and MR. 2009;30:559–564 [DOI] [PubMed] [Google Scholar]
- 14.Soderblom C, Luo X, Blumenthal E, Bray E, Lyapichev K, Ramos J, Krishnan V, Lai-Hsu C, Park KK, Tsoulfas P, et al. Perivascular fibroblasts form the fibrotic scar after contusive spinal cord injury. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33:13882–13887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Andrae J, Gouveia L, Gallini R, He L, Fredriksson L, Nilsson I, Jahansson BR, Eriksson U, Betsholtz C. A role for pdgf-c/pdgfralpha signaling in the formation of the meningeal basement membranes surrounding the cerebral cortex. Biology open. 2016;5:461–474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DeSisto J, O’Rourke R, Jones HE, Pawlikowski B, Malek AD, Bonney S, Guimiot F, Jones KL, Siegenthaler JA. Single-cell transcriptomic analyses of the developing meninges reveal meningeal fibroblast diversity and function. Developmental cell. 2020;54:43–59 e44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xie L, Kang H, Xu Q, Chen MJ, Liao Y, Thiyagarajan M, O’Donnell J, Christensen DJ, Nicholson C, Iliff JJ, et al. Sleep drives metabolite clearance from the adult brain. Science. 2013;342:373–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iliff JJ, Wang M, Liao Y, Plogg BA, Peng W, Gundersen GA, Benveniste H, Vates GE, Deane R, Goldman SA, et al. A paravascular pathway facilitates csf flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid beta. Sci Transl Med. 2012;4:147ra111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rasmussen MK, Mestre H, Nedergaard M. The glymphatic pathway in neurological disorders. The Lancet. Neurology. 2018;17:1016–1024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mestre H, Mori Y, Nedergaard M. The brain’s glymphatic system: Current controversies. Trends Neurosci. 2020;43:458–466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mestre H, Hablitz LM, Xavier AL, Feng W, Zou W, Pu T, Monai H, Murlidharan G, Castellanos Rivera RM, Simon MJ, et al. Aquaporin-4-dependent glymphatic solute transport in the rodent brain. eLife. 2018;7:e40070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kelly KK, MacPherson AM, Grewal H, Strnad F, Jones JW, Yu J, Pierzchalsko K, Kane MA, Herson PS, Siegenthaler JA. Col1a1+ perivascular cells in the brain are a source of retinoic acid following stroke. BMC neuroscience. 2016;17:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Niclou SP, Franssen EH, Ehlert EM, Taniguchi M, Verhaagen J. Meningeal cell-derived semaphorin 3a inhibits neurite outgrowth. Molecular and cellular neurosciences. 2003;24:902–912 [DOI] [PubMed] [Google Scholar]
- 24.Strutz F, Okada H, Lo CW, Danoff T, Carone RL, Tomaszewski JE, Neilson EG. Identification and characterization of a fibroblast marker: Fsp1. The Journal of cell biology. 1995;130:393–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kong P, Christia P, Saxena A, Su Y, Frangogiannis NG. Lack of specificity of fibroblast-specific protein 1 in cardiac remodeling and fibrosis. American journal of physiology. Heart and circulatory physiology. 2013;305:H1363–1372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schnitzer J, Franke WW, Schachner M. Immunocytochemical demonstration of vimentin in astrocytes and ependymal cells of developing and adult mouse nervous system. The Journal of cell biology. 1981;90:435–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dang TC, Ishii Y, Nguyen V, Yamamoto S, Hamashima T, Okuno N, Nguyen QL, Sang Y, Ohkawa N, Saitoh Y, et al. Powerful homeostatic control of oligodendroglial lineage by pdgfralpha in adult brain. Cell reports. 2019;27:1073–1089.e1075 [DOI] [PubMed] [Google Scholar]
- 28.Armulik A, Genove G, Betsholtz C. Pericytes: Developmental, physiological, and pathological perspectives, problems, and promises. Developmental cell. 2011;21:193–215 [DOI] [PubMed] [Google Scholar]
- 29.Van Vliet E, Melis M, Foidart JM, Van Ewijk W. Reticular fibroblasts in peripheral lymphoid organs identified by a monoclonal antibody. The journal of histochemistry and cytochemistry : official journal of the Histochemistry Society. 1986;34:883–890 [DOI] [PubMed] [Google Scholar]
- 30.Cupovic J, Onder L, Gil-Cruz C, Weiler E, Caviezel-Firner S, Perez-Shibayama C, Rulicke T, Bechmann I, Ludewig B. Central nervous system stromal cells control local cd8(+) t cell responses during virus-induced neuroinflammation. Immunity. 2016;44:622–633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang Y, Zhang P, Xiong Y, Li X, Qi Y, Hu H. Ectopia of meningeal fibroblasts and reactive gliosis in the cerebral cortex of the mouse model of muscle-eye-brain disease. The Journal of comparative neurology. 2007;505:459–477 [DOI] [PubMed] [Google Scholar]
- 32.Komuta Y, Teng X, Yanagisawa H, Sango K, Kawamura K, Kawano H. Expression of transforming growth factor-beta receptors in meningeal fibroblasts of the injured mouse brain. Cellular and molecular neurobiology. 2010;30:101–111 [DOI] [PubMed] [Google Scholar]
- 33.Shearer MC, Niclou SP, Brown D, Asher RA, Holtmaat AJ, Levine JM, Verhaagen J, Fawcett JW. The astrocyte/meningeal cell interface is a barrier to neurite outgrowth which can be overcome by manipulation of inhibitory molecules or axonal signalling pathways. Molecular and cellular neurosciences. 2003;24:913–925 [DOI] [PubMed] [Google Scholar]
- 34.Makihara N, Arimura K, Ago T, Tachibana M, Nishimura A, Nakamura K, Matsuo R, Wakisaka Y, Kuroda J, Sugimori H, et al. Involvement of platelet-derived growth factor receptor beta in fibrosis through extracellular matrix protein production after ischemic stroke. Experimental neurology. 2015;264:127–134 [DOI] [PubMed] [Google Scholar]
- 35.Hecht JH, Siegenthaler JA, Patterson KP, Pleasure SJ. Primary cellular meningeal defects cause neocortical dysplasia and dyslamination. Annals of neurology. 2010;68:454–464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Halfter W, Dong S, Yip YP, Willem M, Mayer U. A critical function of the pial basement membrane in cortical histogenesis. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2002;22:6029–6040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hartmann D, Sievers J, Pehlemann FW, Berry M. Destruction of meningeal cells over the medial cerebral hemisphere of newborn hamsters prevents the formation of the infrapyramidal blade of the dentate gyrus. The Journal of comparative neurology. 1992;320:33–61 [DOI] [PubMed] [Google Scholar]
- 38.Siegenthaler JA, Pleasure SJ. We have got you ‘covered’: How the meninges control brain development. Current opinion in genetics & development. 2011;21:249–255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zarbalis K, Siegenthaler JA, Choe Y, May SR, Peterson AS, Pleasure SJ. Cortical dysplasia and skull defects in mice with a foxc1 allele reveal the role of meningeal differentiation in regulating cortical development. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:14002–14007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Siegenthaler JA, Ashique AM, Zarbalis K, Patterson KP, Hecht JH, Kane MA, Folias AE, Choe Y, May SR, Kume T, et al. Retinoic acid from the meninges regulates cortical neuron generation. Cell. 2009;139:597–609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mishra S, Choe Y, Pleasure SJ, Siegenthaler JA. Cerebrovascular defects in foxc1 mutants correlate with aberrant wnt and vegf-a pathways downstream of retinoic acid from the meninges. Developmental biology. 2016;420:148–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Haushalter C, Schuhbaur B, Dolle P, Rhinn M. Meningeal retinoic acid contributes to neocortical lamination and radial migration during mouse brain development. Biology open. 2017;6:148–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Aldinger KA, Lehmann OJ, Hudgins L, Chizhikov VV, Bassuk AG, Ades LC, Krantz ID, Dobyns WB, Millen KJ. Foxc1 is required for normal cerebellar development and is a major contributor to chromosome 6p25.3 dandy-walker malformation. Nature genetics. 2009;41:1037–1042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Haldipur P, Dang D, Aldinger KA, Janson OK, Guimiot F, Adle-Biasette H, Dobyns WB, Siebert JR, Russo R, Millen KJ. Phenotypic outcomes in mouse and human foxc1 dependent dandy-walker cerebellar malformation suggest shared mechanisms. eLife. 2017;6:e20898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang W, Liu R, Su Y, Li H, Xie W, Ning B. Microrna-21–5p mediates tgf-beta-regulated fibrogenic activation of spinal fibroblasts and the formation of fibrotic scars after spinal cord injury. International journal of biological sciences. 2018;14:178–188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cha JH, Wee HJ, Seo JH, Ahn BJ, Park JH, Yang JM, Lee SW, Kim EH, Lee OH, Heo JH, et al. Akap12 mediates barrier functions of fibrotic scars during cns repair. PloS one. 2014;9:e94695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dias DO, Goritz C. Fibrotic scarring following lesions to the central nervous system. Matrix biology : journal of the International Society for Matrix Biology. 2018;68–69:561–570 [DOI] [PubMed] [Google Scholar]
- 48.Kawano H, Kimura-Kuroda J, Komuta Y, Yoshioka N, Li HP, Kawamura K, Li Y, Raisman G. Role of the lesion scar in the response to damage and repair of the central nervous system. Cell and tissue research. 2012;349:169–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fernandez-Klett F, Potas JR, Hilpert D, Blazej K, Radke J, Huck J, Engel O, Stenzel W, Genove G, Priller J. Early loss of pericytes and perivascular stromal cell-induced scar formation after stroke. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2013;33:428–439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shearer KD, Fragoso YD, Clagett-Dame M, McCaffery PJ. Astrocytes as a regulated source of retinoic acid for the brain. Glia. 2012;60:1964–1976 [DOI] [PubMed] [Google Scholar]
- 51.Shin YJ, Kim HL, Park JM, Cho JM, Kim SY, Lee MY. Characterization of nestin expression and vessel association in the ischemic core following focal cerebral ischemia in rats. Cell and tissue research. 2013;351:383–395 [DOI] [PubMed] [Google Scholar]
- 52.Shimamura M, Taniyama Y, Nakagami H, Katsuragi N, Wakayama K, Koriyama H, Kurinami H, Tenma A, Tomioka H, Morishita R. Long-term expression of periostin during the chronic stage of ischemic stroke in mice. Hypertens Res. 2014;37:494–499 [DOI] [PubMed] [Google Scholar]
- 53.Shimamura M, Taniyama Y, Katsuragi N, Koibuchi N, Kyutoku M, Sato N, Allahtavakoli M, Wakayama K, Nakagami H, Morishita R. Role of central nervous system periostin in cerebral ischemia. Stroke. 2012;43:1108–1114 [DOI] [PubMed] [Google Scholar]
- 54.Ma SM, Chen LX, Lin YF, Yan H, Lv JW, Xiong M, Li J, Cheng GQ, Yang Y, Qiu ZL, et al. Periostin promotes neural stem cell proliferation and differentiation following hypoxic-ischemic injury. PLoS One. 2015;10:e0123585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu L, Kawakita F, Fujimoto M, Nakano F, Imanaka-Yoshida K, Yoshida T, Suzuki H. Role of periostin in early brain injury after subarachnoid hemorrhage in mice. Stroke. 2017;48:1108–1111 [DOI] [PubMed] [Google Scholar]
- 56.Su EJ, Fredriksson L, Geyer M, Folestad E, Cale J, Andrae J, Gao Y, Pietras K, Mann K, Yepes M, et al. Activation of pdgf-cc by tissue plasminogen activator impairs blood-brain barrier integrity during ischemic stroke. Nature medicine. 2008;14:731–737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ma Q, Huang B, Khatibi N, Rolland W 2nd, Suzuki H, Zhang JH, Tang J. Pdgfr-alpha inhibition preserves blood-brain barrier after intracerebral hemorrhage. Annals of neurology. 2011;70:920–931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cha JH, Wee HJ, Seo JH, Ahn BJ, Park JH, Yang JM, Lee SW, Lee OH, Lee HJ, Gelman IH, et al. Prompt meningeal reconstruction mediated by oxygen-sensitive akap12 scaffolding protein after central nervous system injury. Nature communications. 2014;5:4952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen YT, Chang FC, Wu CF, Chou YH, Hsu HL, Chiang WC, Shen J, Chen YM, Wu KD, Tsai TJ, et al. Platelet-derived growth factor receptor signaling activates pericyte-myofibroblast transition in obstructive and post-ischemic kidney fibrosis. Kidney international. 2011;80:1170–1181 [DOI] [PubMed] [Google Scholar]
- 60.Zhang SX, Geddes JW, Owens JL, Holmberg EG. X-irradiation reduces lesion scarring at the contusion site of adult rat spinal cord. Histology and histopathology. 2005;20:519–530 [DOI] [PubMed] [Google Scholar]
- 61.Kostyk SK, Popovich PG, Stokes BT, Wei P, Jakeman LB. Robust axonal growth and a blunted macrophage response are associated with impaired functional recovery after spinal cord injury in the mrl/mpj mouse. Neuroscience. 2008;156:498–514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Conrad S, Schluesener HJ, Adibzahdeh M, Schwab JM. Spinal cord injury induction of lesional expression of profibrotic and angiogenic connective tissue growth factor confined to reactive astrocytes, invading fibroblasts and endothelial cells. Journal of neurosurgery. Spine. 2005;2:319–326 [DOI] [PubMed] [Google Scholar]
- 63.Zhu Y, Soderblom C, Trojanowsky M, Lee DH, Lee JK. Fibronectin matrix assembly after spinal cord injury. Journal of neurotrauma. 2015;32:1158–1167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Okada M, Miyamoto O, Shibuya S, Zhang X, Yamamoto T, Itano T. Expression and role of type i collagen in a rat spinal cord contusion injury model. Neuroscience research. 2007;58:371–377 [DOI] [PubMed] [Google Scholar]
- 65.Wanner IB, Deik A, Torres M, Rosendahl A, Neary JT, Lemmon VP, Bixby JL. A new in vitro model of the glial scar inhibits axon growth. Glia. 2008;56:1691–1709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bundesen LQ, Scheel TA, Bregman BS, Kromer LF. Ephrin-b2 and ephb2 regulation of astrocyte-meningeal fibroblast interactions in response to spinal cord lesions in adult rats. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2003;23:7789–7800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yi JH, Katagiri Y, Susarla B, Figge D, Symes AJ, Geller HM. Alterations in sulfated chondroitin glycosaminoglycans following controlled cortical impact injury in mice. The Journal of comparative neurology. 2012;520:3295–3313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hara M, Kobayakawa K, Ohkawa Y, Kumamaru H, Yokota K, Saito T, Kijima K, Yoshizaki S, Harimaya K, Nakashima Y, et al. Interaction of reactive astrocytes with type i collagen induces astrocytic scar formation through the integrin-n-cadherin pathway after spinal cord injury. Nature medicine. 2017;23:818–828 [DOI] [PubMed] [Google Scholar]
- 69.Yokota K, Kobayakawa K, Saito T, Hara M, Kijima K, Ohkawa Y, Harada A, Okazaki K, Ishihara K, Yoshida S, et al. Periostin promotes scar formation through the interaction between pericytes and infiltrating monocytes/macrophages after spinal cord injury. The American journal of pathology. 2017;187:639–653 [DOI] [PubMed] [Google Scholar]
- 70.Vogelaar CF, Konig B, Krafft S, Estrada V, Brazda N, Ziegler B, Faissner A, Muller HW. Pharmacological suppression of cns scarring by deferoxamine reduces lesion volume and increases regeneration in an in vitro model for astroglial-fibrotic scarring and in rat spinal cord injury in vivo. PloS one. 2015;10:e0134371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Klapka N, Hermanns S, Straten G, Masanneck C, Duis S, Hamers FP, Muller D, Zuschratter W, Muller HW. Suppression of fibrous scarring in spinal cord injury of rat promotes long-distance regeneration of corticospinal tract axons, rescue of primary motoneurons in somatosensory cortex and significant functional recovery. The European journal of neuroscience. 2005;22:3047–3058 [DOI] [PubMed] [Google Scholar]
- 72.Ruschel J, Hellal F, Flynn KC, Dupraz S, Elliott DA, Tedeschi A, Bates M, Sliwinski C, Brook G, Dobrindt K, et al. Axonal regeneration. Systemic administration of epothilone b promotes axon regeneration after spinal cord injury. Science (New York, N.Y.). 2015;348:347–352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hellal F, Hurtado A, Ruschel J, Flynn KC, Laskowski CJ, Umlauf M, Kapitein LC, Strikis D, Lemmon V, Bixby J, et al. Microtubule stabilization reduces scarring and causes axon regeneration after spinal cord injury. Science (New York, N.Y.). 2011;331:928–931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhu Y, Soderblom C, Krishnan V, Ashbaugh J, Bethea JR, Lee JK. Hematogenous macrophage depletion reduces the fibrotic scar and increases axonal growth after spinal cord injury. Neurobiology of disease. 2015;74:114–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yoshioka N, Hisanaga S, Kawano H. Suppression of fibrotic scar formation promotes axonal regeneration without disturbing blood-brain barrier repair and withdrawal of leukocytes after traumatic brain injury. The Journal of comparative neurology. 2010;518:3867–3881 [DOI] [PubMed] [Google Scholar]
- 76.Bradbury EJ, Moon LD, Popat RJ, King VR, Bennett GS, Patel PN, Fawcett JW, McMahon SB. Chondroitinase abc promotes functional recovery after spinal cord injury. Nature. 2002;416:636–640 [DOI] [PubMed] [Google Scholar]
- 77.Mey J, D JM, Brook G, Liu RH, Zhang YP, Koopmans G, McCaffery P. Retinoic acid synthesis by a population of ng2-positive cells in the injured spinal cord. The European journal of neuroscience. 2005;21:1555–1568 [DOI] [PubMed] [Google Scholar]
- 78.Pikor NB, Astarita JL, Summers-Deluca L, Galicia G, Qu J, Ward LA, Armstrong S, Dominguez CX, Malhotra D, Heiden B, et al. Integration of th17- and lymphotoxin-derived signals initiates meningeal-resident stromal cell remodeling to propagate neuroinflammation. Immunity. 2015;43:1160–1173 [DOI] [PubMed] [Google Scholar]
- 79.Yahn SL, Li J, Goo I, Gao H, Brambilla R, Lee JK. Fibrotic scar after experimental autoimmune encephalomyelitis inhibits oligodendrocyte differentiation. Neurobiology of disease. 2020;134:104674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Haist V, Ulrich R, Kalkuhl A, Deschl U, Baumgartner W. Distinct spatio-temporal extracellular matrix accumulation within demyelinated spinal cord lesions in theiler’s murine encephalomyelitis. Brain Pathol. 2012;22:188–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mohan H, Krumbholz M, Sharma R, Eisele S, Junker A, Sixt M, Newcombe J, Wekerle H, Hohlfeld R, Lassmann H, et al. Extracellular matrix in multiple sclerosis lesions: Fibrillar collagens, biglycan and decorin are upregulated and associated with infiltrating immune cells. Brain pathology (Zurich, Switzerland: ). 2010;20:966–975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.van Horssen J, Bo L, Dijkstra CD, de Vries HE. Extensive extracellular matrix depositions in active multiple sclerosis lesions. Neurobiology of disease. 2006;24:484–491 [DOI] [PubMed] [Google Scholar]
- 83.Riew TR, Choi JH, Kim HL, Jin X, Lee MY. Pdgfr-beta-positive perivascular adventitial cells expressing nestin contribute to fibrotic scar formation in the striatum of 3-np intoxicated rats. Frontiers in molecular neuroscience. 2018;11:402. [DOI] [PMC free article] [PubMed] [Google Scholar]


