Abstract
Implantable biomaterials are essential surgical devices, extending and improving the quality of life of millions of people globally. Advances in materials science, manufacturing, and in our understanding of the biological response to medical device implantation over several decades have resulted in improved safety and functionality of biomaterials. However, post-operative infection and immune responses remain significant challenges that interfere with biomaterial functionality and host healing processes. The objective of this review is to provide an overview of the biology of post-operative infection and the physiological response to implanted biomaterials, and to discuss emerging strategies utilizing local drug delivery and surface modification to improve the long-term safety and efficacy of biomaterials.
Keywords: Biomaterials, bacterial adhesion, surface modification, sustained release, inflammation, infection, fibrosis
Graphical Abstract
1. Introduction
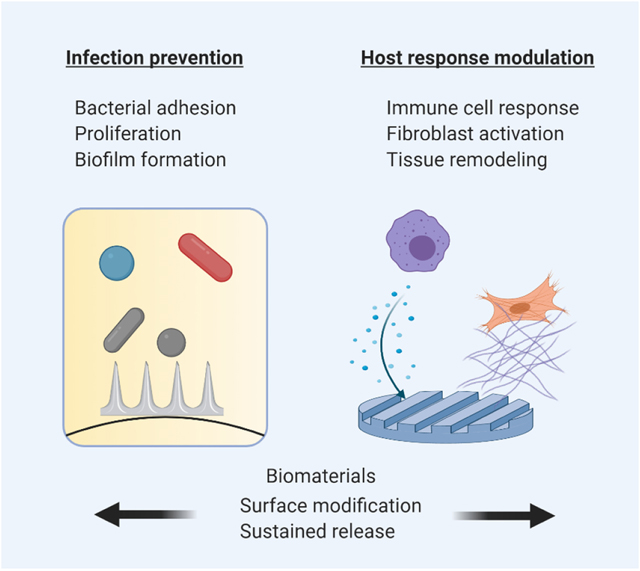
Biomaterials are implanted in tens of millions of patients globally, with more than 13 million procedures occurring annually in the U.S. alone (1). Biomedical implants have been employed in almost every domain of surgery, ranging from microscale implants in microvascular and ophthalmic surgery to macroscopic devices in orthopedic and general surgery. Interaction between the host environment and implanted biomaterials begins immediately upon introduction, after which biomaterials may become coated with serum proteins, aqueous humor, mucosal secretions, microbiota, and/or extracellular fluids depending on their location in the body (2). This makes the biomaterial surface highly susceptible to microbial as well as host cellular adhesion. In 1987, orthopedic surgeon, Dr. Anthony Gristina postulated that “a race to the surface” would result in: (1) bacterial adhesion to the surface of the material and proliferation into a biofilm leading to infection, or (2) host cell encapsulation of the biomaterial with extracellular matrix to isolate it from host tissues (3). Accordingly, infection and inflammation are the two primary causes of medical device complications and failure, both of which are mediated by cellular interaction with the biomaterial. Thus, it is desirable to engineer biomaterials that are both resistant to contamination/biofouling and can successfully integrate with host tissue. Biomaterial associated infections lead to an increased annual economic burden of more than $11 billion (4, 5). The first half of this review focuses on mechanisms involved in post-operative bacterial infection of long-term implants which are highly susceptible to bacterial adhesion and biofilm formation such as orthopedic prostheses, ureteral stents and sutures. We discuss advances in biomaterial design that may help prevent bacterial adhesion and proliferation. The latter half of this review discusses host responses to implanted biomaterials, particularly the role of innate immune cells and stromal cells in implant fibrosis. Recent approaches for modulating cellular responses to implanted biomaterials are also examined. Collectively, medical implants that are highly biocompatible and impervious to infection will significantly improve clinical outcomes and reduce healthcare costs.
2. Implants are susceptible to infection
Nearly half of all healthcare-associated infections in the U.S. occur with implanted biomaterials (6). Implant-associated infections increase duration of hospitalization, as well as rates of re-hospitalization and re-intervention (7–9). Microbiota from the patient, the operating room, and the surface of the implant itself have all been implicated in post-operative infections (10, 11). Bacterial adhesins interact with host proteins that coat the implant’s surface, aiding in bacterial adhesion to the surface of the device. Left unchecked, certain species of bacteria (predominantly commensal bacteria like Staphylococcus aureus and Staphylococcus epidermidis) can secrete signaling molecules to communicate and alter their metabolic states to form a biofilm (12–14). A biofilm is a matrix composed of proteins, DNA, and membrane structures from dead cells that protects the bacteria within the biofilm, including limiting penetration of small molecule drugs (15, 16). Systemic administration of antibiotics is often the first-line mode of prophylaxis and treatment of infections, although poor bioavailability and systemic toxicity may limit efficacy (17–19). Systemic antibiotic therapy has been associated with risk of allergic reactions, nephritis and enteric dysbiosis (20). Further, the lack of patient adherence to prescribed dosing regimen further limits both the safety and efficacy of antibiotics (21, 22). Subtherapeutic drug exposure contributes to the number of bacterial species that have become resistant to widely used antibiotics such as gentamicin and methicillin (23–25), as well as antibacterial agents such as triclosan (26). Thus, rather than relying on adjunct antibiotic administration for preventing post-operative infections, engineering functionality into the implant itself may lead to improved surgical outcomes. Figure 1 provides an overview of biomaterial-associated infection and strategies reviewed herein. In this section, we describe mechanisms of bacterial adhesion and biofilm formation, followed by approaches for engineering implantable biomaterials that provide local, sustained antibiotic release and/or prevent bacterial adhesion.
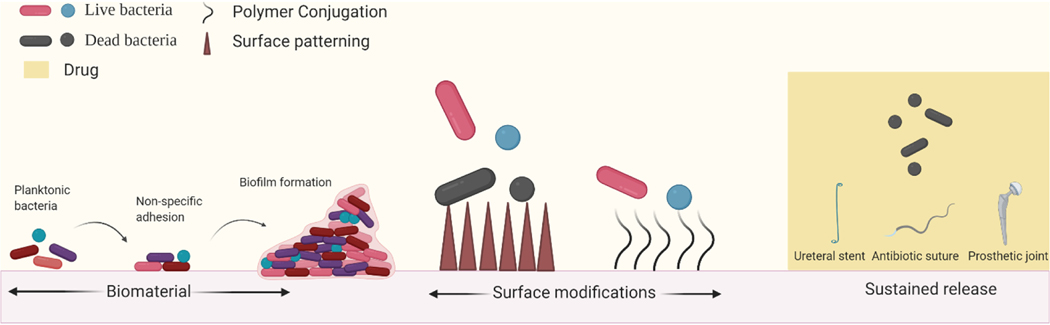
Figure 1.
Stages of biofilm formation and strategies to prevent infection. Bacteria adhere to biomaterial surfaces via non-specific adhesion and proliferate to form biofilms. Modifying surface properties of the biomaterial such as biocidal patterning and polymer conjugation have been shown to be effective in preventing biomaterial associated infections in animal models. Biomaterials can also act as drug depots which can release bactericidal small molecules locally circumventing systemic delivery barriers.
2.1. Mechanisms of infection
Bacteria colonize a diverse range of surfaces, including host tissues and abiotic biomaterials (27). Bacteria may adhere to biomaterials via non-specific interactions, such as electrostatic interactions with the negatively charged bacterial cell wall, or by hydrophobic interactions. Alternatively, bacteria may interact with host tissue in a ligand specific manner (28, 29). Most bacteria have evolved to survive in a community adherent to solid surfaces where nutrient availability tends to be highest, and there is also positive selection for bacteria that form biofilms as a protection (30, 31). Biofilm formation on implants presents a challenge for effective treatment both because of poor penetration of antibiotics and metabolic heterogeneity within the biofilm which can allow some cells to survive high doses of antibiotics (32). Such resistant bacterial cells can proliferate once they sense a decline in the bacterial population, causing a recurrent infection (33). Below, we discuss strategies to prevent infections by eliminating early planktonic bacteria or by preventing adhesion.
2.2. Sustained release technologies for prevention of infection
Numerous anatomical barriers and clearance mechanisms significantly reduce drug concentrations at intended locations (34, 35). Local delivery of drugs overcomes these challenges and is a promising alternative to systemic therapies. There is increasing use of polymers for manufacturing medical implants due to the tailorable textural, mechanical, and physical properties (36). Moreover, a wide range of natural and synthetic polymers have demonstrated high strength, flexibility, and durability together with a favorable safety profile and long-term biocompatibility (37–40). More recently, the potential for loading polymeric implants with therapeutics for sustained, local release to prevent infection, as well as surface patterning to prevent bacterial adhesion, have been explored. Specific clinical applications for modulating implantable biomaterials properties to prevent post-surgical infection are described below (Table 1).
Table 1.
Summary of manufacturing methods, materials and active ingredients used in implants described in section 2.2.1–2.2.3.
| Device | Implant material | Processing method | Active ingredient | Clinical application | Reference |
|---|---|---|---|---|---|
| Prosthetic joint | LDPE | Compression molding | Rifampin, Vancomycin | Recurrent PJI prevention | [41] |
| Kirschner Wire | PLGA, PCL | Electrospinning | Vancomycin, Daptomycin, Linezolid, Rifampin | Preventing infection after bone fractures | [44] |
| Ureteral stent | Biosoft® polymer blend | - | Tachyplesin III | Preventing ureteral stent infection | [51] |
| Device coating | Poly(HPMA) | Surface-initiated atom transfer radical polymerization | None | Urinary catheter coating to prevent biofilm formation | [52] |
| Device coating | Biosoft® polymer blend | Dip coating | Vancomycin, BMAP-28 | Ureteral stent coating to prevent bacterial adhesion | [53] |
| Antibacterial suture | Nylon, Polyglactin | High temperature extrusion | Triclosan | Preventing post-operative infection-general surgery | [57] |
| Antibacterial suture | PCL,PEG | Electrospinning | Levofloxacin | Preventing post-operative infection-ophthalmic surgery | [59] |
| Antibacterial suture | PCL | Electrospinning | Levofloxacin | Preventing post-operative infection-ophthalmic surgery | [65] |
2.2.1. Orthopedic implants
Joint replacement surgery is performed more than a million times annually in the U.S. (41). Prosthetic joint infection is a major cause of revision surgery with a recurrence rate of 16% (42). The current standard of care to treat prosthetic joint infections involves explanting the prosthesis and using bone cement spacers loaded with antibiotics as filler materials for several weeks before implanting a new prosthesis, a timeframe in which mobility is limited (43). To obviate the need for intermediate bone cement spacers, Suhardi, et al. manufactured prosthetic joint implants containing eccentric drug clusters, which resemble highly elongated ellipsoids (41). They demonstrated that the in vitro release kinetics of the drug was dependent on the eccentricity of the drug clusters. Polymer processing methods that result in spherical drug clusters, such as solvent casting, can entrap drug for longer durations resulting in slow release kinetics. The authors demonstrate that compression molding results in eccentric drug clusters in the polymer matrix, which had faster elution rates. Implants loaded with a combination of vancomycin and rifampin released drug in a sustained manner for up to 6 months in vitro. In a rabbit joint S. aureus biofilm infection model, 100% of the animals that received drug loaded prosthetic joints remained uninfected at day 21, while only 20% of animals receiving vancomycin-loaded bone cement spacers remained uninfected (41). Another strategy that has been used successfully in smaller load bearing implants is the use of drug-loaded polymeric coatings to provide local delivery of antibiotics. Ashbaugh, et al. coated Kirschner-wire implants with poly(lactic-co-glycolic acid) (PLGA) and poly(ε-caprolactone) (PCL) nanofibers separately loaded with combinations of rifampin, vancomycin, linezolid or daptomycin. Subsequently, they selectively melted the PCL nanofibers to create a diffusion matrix composed of PLGA fibers surrounded by PCL (44). This created a structure where PLGA nanofibers were embedded within bulk PCL. Using this strategy, drug release was tuned by selectively loading antibiotic combinations in either PCL, PLGA nanofibers or both. Release kinetics of vancomycin, daptomycin, linezolid in combination with rifampicin were studied in vitro. In a prosthetic joint S. aureus infection model in mice, they observed that the antibiotic-releasing coatings successfully reduced the bacterial load in the K-wire implants as compared to no drug treatment. Further they showed that the systemic antibiotic exposure was modest, underscoring a safety benefit of locally delivered therapeutics (44). These results show that the use of novel manufacturing and coating methods and design of biomaterials which enable local and sustained delivery of drugs may provide protection against bacterial infections.
2.2.2. Ureteral stents
Ureteral stents are commonly used devices to maintain urological patency. The constitutive materials in early iterations of ureteral stents were synthetic polymers such as poly(ethylene) and silicones. Later versions of the stents were made from metals and metal-alloys such as titanium and nitinol. The most pressing issue concerning both metallic and polymer ureteral stents is the formation of crystalline and bacterial biofilms. Ureteral stents often have a limited indwelling time before removal due to the development of symptoms such as local pain, fever and frequent urination resulting from infection and biofilm formation. Nearly 90% of removed indwelling stents test positive for cultures of common ureteral pathogens such as Pseudomonas aeruginosa, S. aureus, Escherichia coli and Proteus mirabilis (45).
Protein deposits from urine are thought to enable bacterial adhesion via interaction with bacterial adhesins (46). Boston Scientific developed a triclosan-coated ureteral stent in 2006 to prevent bacterial adhesion. Triclosan is a broad spectrum antibacterial agent that does not interfere in the normal wound healing response. In rabbits, triclosan-coated stents prevented a P. mirabilis infection in over 50% of animals within a week of implantation (47). However, common pathogens including E. coli, P. aeruginosa, and S. aureus are growing increasingly resistant to triclosan due to widespread use as an antiseptic over the past several decades (48–50), limiting the potential clinical use of triclosan-coated stents. More recently, coating ureteral stents with anti-bacterial peptides has been explored. Tachyplesin III conjugated to biomaterial surfaces has been shown to sterically inhibit bacterial adhesion and subsequent colonization (51). Tachyplesin III peptide coating on ureteral stents, when used in combination with piperacillin-tazobactam (TZP) has been shown to reduce the in vitro TZP minimum inhibitory concentration (MIC) against P. aeruginosa 8-fold. In a rat subcutaneous infection model, the combination of intraperitoneally delivered TZP and a Tachyplesin III peptide-coated stent reduced stent surface colonization by 400-fold. The steric hindrance/antifouling effect has also been shown for synthetic polymers such as poly[N-(2-hydroxypropyl) methacrylamide] (poly(HPMA)). In a study conducted by Gomes, et al. (52), poly(HPMA) was conjugated to a glass surface to form brush-like structures to characterize the attachment and biofilm forming capacity of E. coli on bare glass, poly(dimethylsiloxane) (PDMS), and brush-like poly(HPMA) surfaces. They observed 80% reductions in both total biofilm forming cells and viable cells on the poly(HPMA) coatings compared to bare glass surfaces in-vitro (52). Furthermore, BMAP-28, a cathelicidin peptide with intrinsic antimicrobial activity, was tested by Orlando, et al as a coating alone and in combination with antibiotics (53). When stents coated with either vancomycin or BMAP-28 were challenged with S. aureus, the bacterial burden was significantly reduced compared to uncoated stents. However, near complete elimination of infection was achieved when the BMAP-28-coated stents were used in conjunction with intraperitoneally administered vancomycin (53). Another study investigated the synergistic effects of combining antibiotics (54). They showed in vitro that there was an eight-fold reduction in the minimum bactericidal concentration of amikacin when used in combination with clarithromycin against adherent P. aeruginosa. In a rat model of P. aeruginosa bladder infection, they found that either systemically administered amikacin with an uncoated stent or clarithromycin-coated stents provided a reduction in bacterial colonization compared to an uncoated stent alone. However, near elimination of infection was achieved in rats that received systemic amikacin in combination with clarithromycin-coated ureteral stents (54). These results demonstrate that sensitization of bacteria to antibiotics by deterring attachment and the use of combinatorial drugs are significantly advantageous in preventing ureteral infections of biomaterials.
2.2.3. Antibacterial sutures
Surgical site infections (SSIs) are one of the most common post-operative complications, and have an incidence of 5.6 per 100 surgeries in developing countries and 2.6 per 100 surgeries in the U.S. (55). Additionally, in procedures like colorectal surgeries, incidence of post-operative infection can be as high as 17.5%, even in developed countries (56). While sutures are routinely used for wound closure in a wide variety of surgical procedures, the presence of sutures at a wound site has been associated with infection. This has led to the investigation, development, and approval of surgical sutures with antibacterial properties. In 2002, Ethicon received regulatory clearance for coated antibacterial sutures containing triclosan in general surgery (57). However, as mentioned previously, there is growing concern over bacterial resistance to triclosan (26, 48). One alternative strategy that has gained attention is employing sustained-release technologies to deliver antibiotics locally at the surgical site. Chen et al. (58) developed PCL-based levofloxacin hydrochloride coatings for silk sutures (sizes 2–0 and 0–0). The PCL/levofloxacin coatings demonstrated sustained release for up to 5 days in vitro, leading to measurable zones of inhibition against S. aureus and E. coli in agar diffusion assays for 7 days (58). However, suture coatings may require multifilament thread and increased suture diameter to achieve sufficient drug release, which limits use in surgeries requiring thin sutures (e.g., microsurgery). For example, Ethicon’s antibacterial sutures are only available in diameters suitable for general surgery (70–339 μm) One potential solution is to form a suture entirely out of polymer with drug embedded, which is achievable via electrospinning. In one study, Kashiwabuchi et al. manufactured poly(L-lactide) (PLA) nanofiber sutures (size 8–0) loaded with the antibiotic levofloxacin, using a wet electrospinning method. By incorporating poly(ethylene glycol) within the PLA nanofibers, they achieved sustained release kinetics of levofloxacin for over a period of 70 days in vitro. Further, agar diffusion tests showed that the drug released from the electrospun sutures successfully inhibited S. epidermidis for a period of 7 days (59).
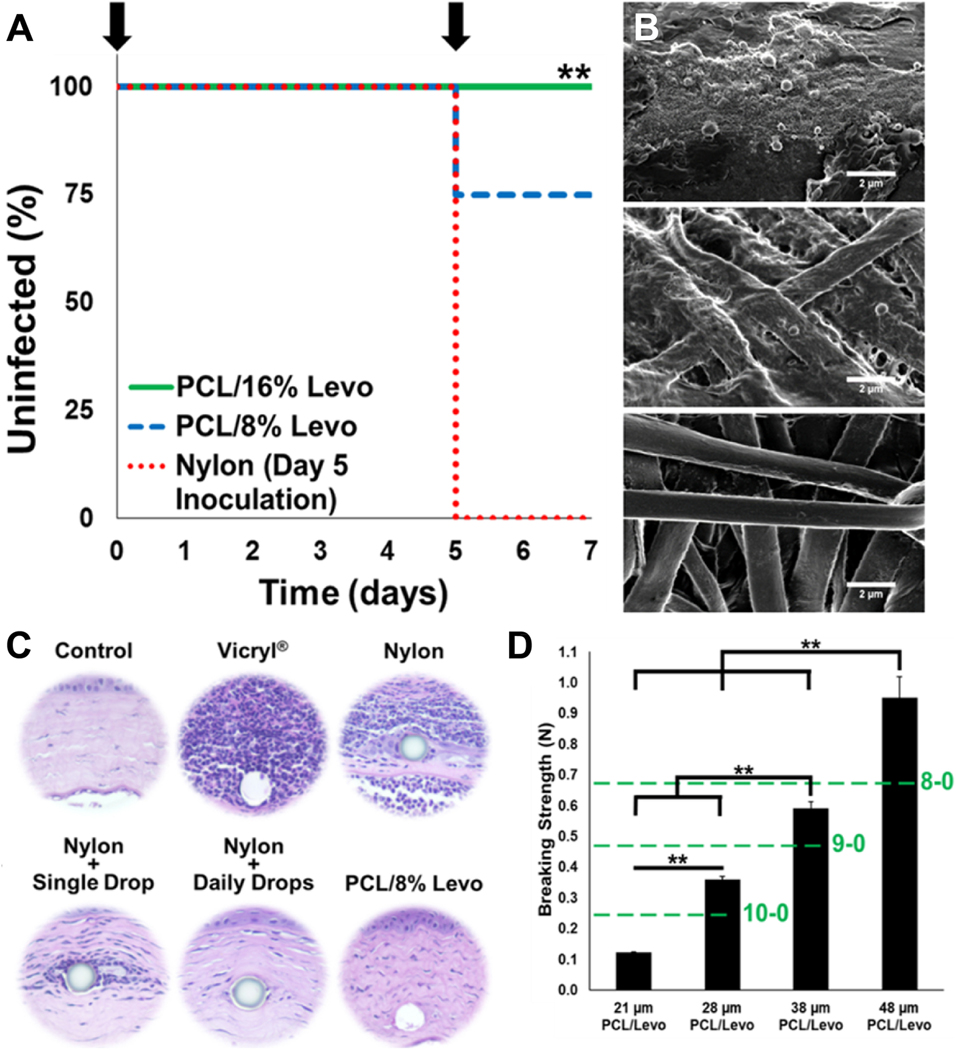
Historically, drug-loaded electrospun suture materials have failed to demonstrate sufficient mechanical strength as stipulated by the United States Pharmacopeia guidelines for clinical use (60–64). Parikh et al.(65) designed a novel electrospinning apparatus that was capable of manufacturing highly twisted nanofibers loaded with levofloxacin. Increasing the number of twists greatly improved tensile properties of the multifilament sutures. Sutures that were highly twisted (~1575 twists per 10 centimeters) surpassed United States Pharmacopeia breaking strength requirements (0.24 N) for a 28 μm absorbable suture with a maximum tensile strength of 0.35 N. Further, the sutures exhibited a direct dependency on the number of twists imparted to the nanofibers for breaking strength. This is the first report of electrospun suture materials demonstrating sufficiently high breaking strength required for use in clinic. In a rat model of corneal keratitis, the levofloxacin-loaded sutures successfully prevented multiple consecutive inoculations of S. Aureus over one week (see Figure 2), and detectable levels of levofloxacin in rat eyes for a month (65). Overall, the general approach of loading small molecules into polymeric suture materials to enable local and sustained delivery of antibiotics by means of direct loading of drug in the suture or via surface coatings have shown great promise in abrogating bacterial adhesion and proliferation.
Figure 2.
(A) Antibiotic-eluting ophthalmic sutures prevent S. aureus infection in a rat model of corneal keratitis (** indicates p<0.01). (B) Scanning electron micrographs (scalebars represent 2 μm) of commercial nylon sutures (top), 8% (middle) and 16% Levofloxacin loaded nanofiber sutures (bottom) explanted from the corneal stroma after bacterial challenge with S. aureus. (C) H&E stained corneal tissue showing reduced inflammation in eyes which received drug eluting suture 48 h after bacterial inoculation. (D) Breaking strength of drug loaded sutures scaled with suture diameter. Dashed green lines indicate minimum breaking strength requirement for absorbable sutures of 10–0, 9–0 and, 8–0 diameters. Modified with permission from [68].
2.3. Modulating material surface properties to prevent bacterial adhesion
Bacterial adhesion to materials is highly dependent on surface topography, chemistry, and hydrophobicity (66–68). Surface roughness has been shown to affect bacterial adhesion in dental implants (69), leading to many attempts to modify surface roughness to reduce the risk of infection (70–72). The rationale is that bacteria have evolved to detect patterns that are dimensionally similar to their own size (~1 μm), and thus, creating smoother surfaces (surface roughness <1 nm) reduces adhesion (73). For example, one study showed that titanium surfaces blasted with micronized aluminum oxide particles to produce a nanoscale surface roughness (>100 nm) had increased adhesion of bacteria derived from whole saliva. In contrast, naturally occurring nanopatterns, such as the self-cleaning hydrophobic surfaces on lotus leaves, and dragonfly and cicada wing patterns are highly resistant to bacterial adhesion and biofouling (74). Bhadra et al. (75) used hydrothermal etching to nanopattern titanium surfaces, and evaluated the attachment of S. aureus and P. aeruginosa compared to unmodified titanium surfaces. They noted a significant reduction in the total number of adherent bacteria and specifically live bacteria on the nanopatterned surfaces, indicating that certain nanopatterned surfaces have an inherent bactericidal nature (75).
Linklater, et al. (76) compared the bactericidal nature of dragonfly wings to a synthetic nanomaterial developed for use in photovoltaic applications and biosensors, termed black silicon (77–79). Black silicon was manufactured using a reactive ion-etching process to achieve nanoscale protrusions on the surface of silicon wafers. The authors then studied the adhesion and bactericidal properties of these surfaces using S. aureus, P. aeruginosa, and S. subtilis. Nanotopographical features on black silicon resemble sharp protrusions and are capable of causing indentations and mechanical stress to the bacterial cell wall. The authors postulate that an imbalance between the hydrostatic pressure caused by the cytoplasm on the bacterial cell wall and the indentation stress caused by the nanopatterned substrate caused cell wall rupture and death of the bacteria. Further, certain bacteria such as S. subtilis are spore forming, and this property protects the bacteria from environmental stress and enhances their rigidity. Nanopatterned black silicon surfaces not only disrupted spores of S. subtilis, indicating an anti-adhesive nature, but also required a significantly higher number of bacterial cells of all three tested model organisms in order to achieve colonization of the material suggesting a bactericidal nature (76). Further research and validation is required in order to translate these technologies for clinical use.
3. Implants are susceptible to immunologic reactions
Implanted biomaterials generally illicit a chronic inflammatory response that eventually results in fibrosis, isolating the material from the rest of the body. This fate is common to materials made from natural and synthetic sources, and the speed and extent of the fibrotic response determines the functional lifespan of the implant. Surgical procedures and biomaterial implantation cause injury to the surrounding tissue, facilitating interactions with host proteins and cells. Non-specific protein adsorption to the material surface forms a “provisional matrix” which makes the microenvironment surrounding the biomaterial conducive to cellular adhesion (80). A complex set of infiltration, proliferation, fusion and degranulation events associated with various types of innate immune cells and stromal cells, such as smooth muscle cells and fibroblasts, culminates in fibrotic encapsulation (Figure 3). This physiological response to the implant is interlinked with bacterial colonization, as misguided host defenses can create a niche for bacterial adhesion and proliferation (10, 81). In particular, neutrophils and macrophages form part of an acute response to infection as well as abiotic implants and thus, dysregulation of their activity can result in infection, inflammation, and fibrosis (82, 83). In this section we provide an overview of recent advances in controlling the biological response to biomaterials, including providing local drug release, cell capture, and modifying surface topography. Lastly, we outline this evolution of concepts in the design and implementation of endovascular devices with improved surgical outcomes.
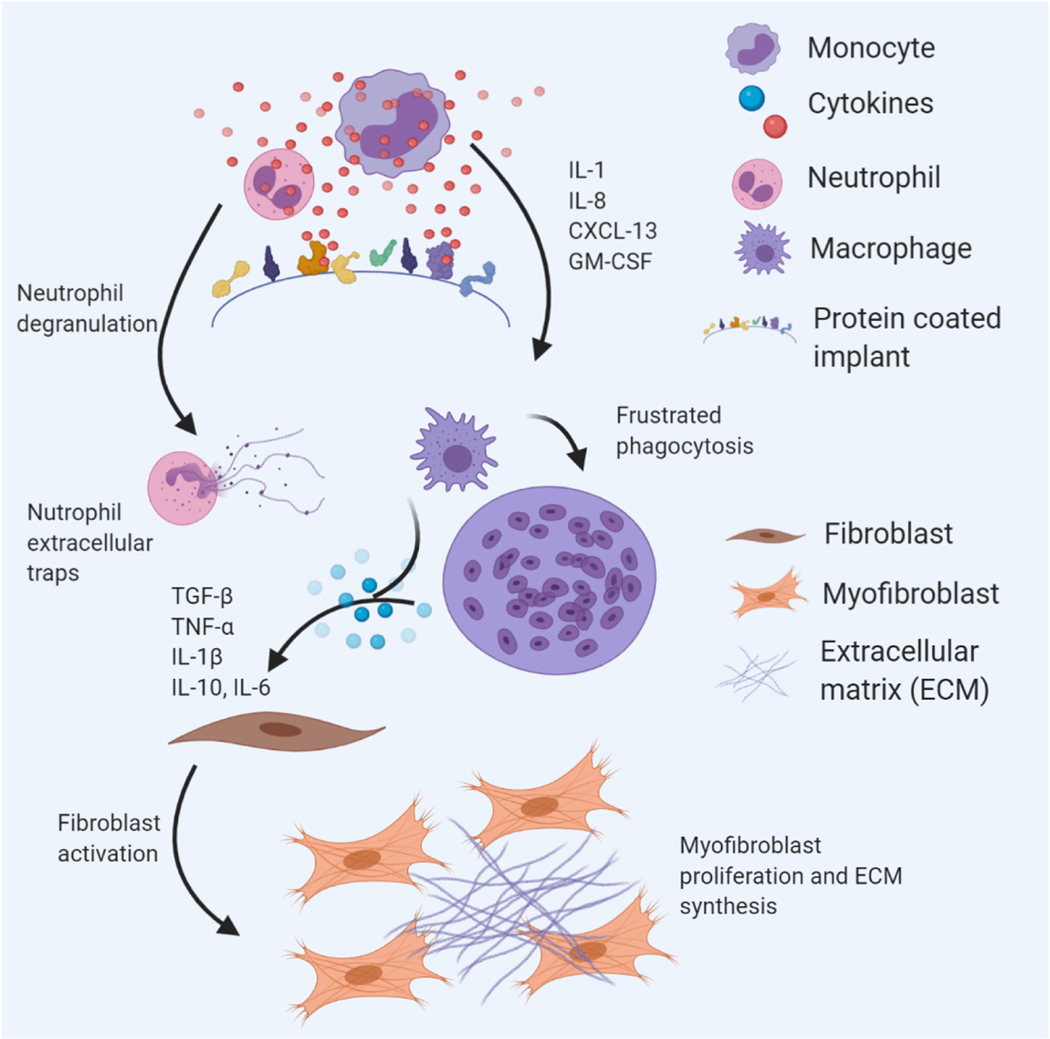
Figure 3.
Local cellular response to implanted biomaterials. Proteins from blood or extracellular fluids adsorb to biomaterial surfaces upon implantation. Innate immune cells like neutrophils and monocytes adhere to protein coated surfaces through non-specific binding interactions. Neutrophils undergo degranulation and deposit neutrophil extracellular traps. Monocytes differentiate into macrophages which can fuse to form giant cells in an attempt to phagocytose biomaterials. Crosstalk between immune cells and stromal cells is an important step in wound healing and is a promising target for engineering biomaterials that can modulate host response.
3.1. Immune cell responses to biomaterials
Immune cells are first responders that influence the nature of the microenvironment surrounding the biomaterial. Neutrophils are the predominant immune cell type at the site of implantation in the first hours (84). The role of neutrophils in generating reactive oxygen species (ROS) and vasodilation has been well studied (85, 86). While the primary function of neutrophils is to phagocytose foreign material and release inflammatory cytokines to recruit monocytes and macrophages, most implants are too large to be phagocytosed. In this case, neutrophils undergo degranulation and deposit neutrophil extracellular traps (NETs), or networks made from DNA, histones, and neutrophil elastase (84, 87, 88). NETs function to trap pathogens like bacteria and viruses and drive the sterile inflammatory response to implant materials. Jhunjhunwala, et al. (89) used a mouse model of peritoneal fibrosis to examine the role of NETs in the fibrotic response to foreign materials. They implanted microcapsules made from alginate, glass, poly(lactic-co-glycolic)acid (PLGA) and poly(methyl methacrylate) (PMMA) in the peritoneal cavity of mice and observed an increase in histone H1 and neutrophil elastases on the surfaces of glass, PMMA and alginate microcapsules. Interestingly, PLGA microcapsules that degrade in 2 weeks showed lower levels of neutrophil recruitment as compared to PLGA microcapsules that take longer to degrade (89). Further, Cohen et al. (90) showed that cell adhesion has a deterministic role in neutrophil fate. The authors showed that neutrophil adhesion to polyethylene glycol (PEG) scaffolds resulted in increased secretion of ROS, matrix metalloprotease-9 (MMP-9), and myeloperoxidase molecules in comparison to polydimethylsiloxane PDMS scaffolds (90). Further studies are required to elucidate the mechanisms underlying neutrophil-biomaterial interactions and device centric approaches to modulate neutrophil response to biomaterials.
Macrophage plasticity and diversity in phenotypes are essential to tissue restoration, repair and remodeling (91, 92). Macrophages begin to accumulate on biomaterial surfaces a few hours after implantation, and can remain there for several months (93). Macrophage recruitment is thought to be mediated by monocytes to enable further recruitment of immune cells (94–96). Monocytes can also differentiate into macrophages of many different phenotypes based on physical and chemical microenvironmental cues, typically classified as M1 or M2. M1 macrophages are involved in secretion of proinflammatory cytokines and are associated with host defense (97), while subtypes of M2 macrophages are generally associated with constructive tissue remodeling (98, 99). Therefore, it is generally desirable to skew macrophages surrounding biomedical implants to an M2 phenotype. Multiple studies have successfully demonstrated macrophage polarization to M1/M2 phenotypes by using immunomodulatory small molecule therapies (100–102). Recent data in RAG-2/γ KO mice showed that macrophages are central to host fibrotic response and that macrophage depletion leads to the near absence of the foreign body reactions surrounding intraperitoneal implants in rodents and non-human primates (103). Further, inhibiting colony stimulating factor-1 (CSF-1) receptors on macrophages can lead to an equivalent effect in suppressing foreign body reaction to multiple classes of materials, including polymers, hydrogels, and glass microcapsules (Figure 4). Recent work has also implicated IL-17, a pro-inflammatory cytokine associated with Th-17 helper T cells and macrophage activation, in biomaterial mediated fibrosis (104–107). The absence of IL-17 signaling in mice was shown to mitigate fibrotic response to intraperitoneally implanted PCL (108).
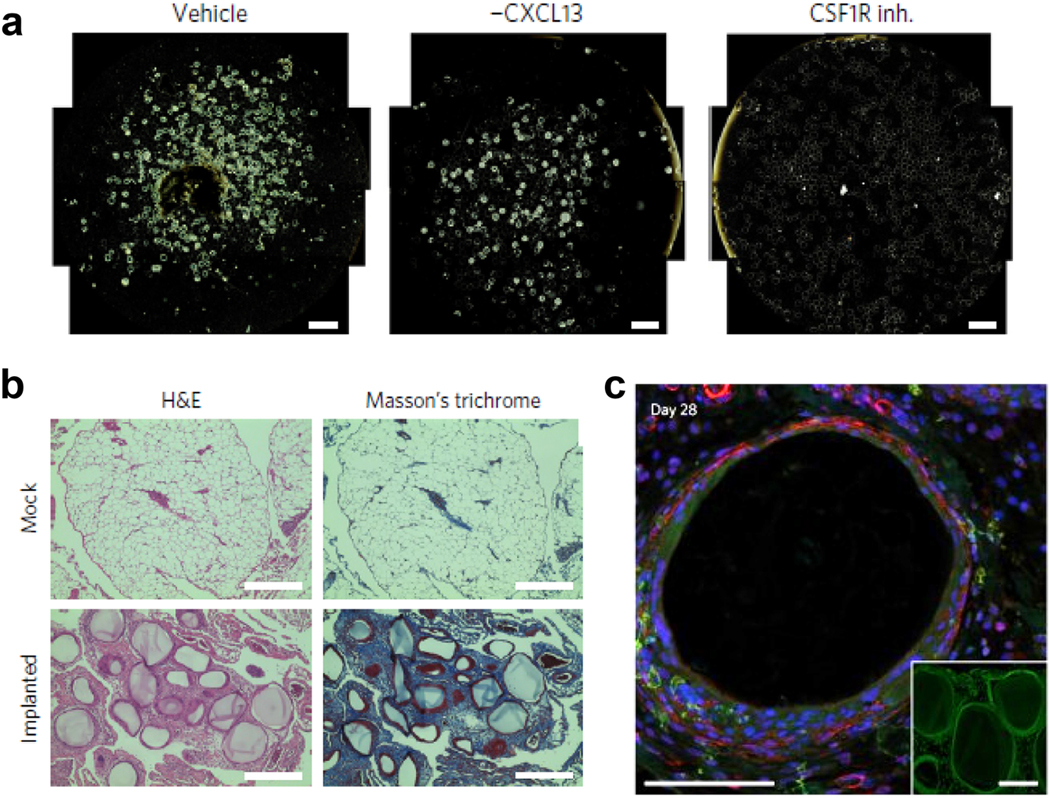
Figure 4.
(A) Colony stimulating factor-1 receptor inhibition (CSF1R inh.) mitigated fibrotic overgrowth on intraperitoneally injected alginate spheres in mice as compared to vehicle only and CXCL13 neutralized wild type mice. (B) Histological examination of intraperitoneal alginate spheres in non-human primates. (C) Macrophage populations (green) and fibrosis associated α-smooth muscle actin (red) around explanted alginate spheres from non-human primates as visualized by confocal imaging. Modified with permission from [113].
Fibroblasts are the majority cell type in the healing stage of tissue restoration, and play a key role in inflammation, angiogenesis, and fibrosis. Fibroblasts are thought to be present in the neo-tissue for years, reprogramming their phenotype to become quiescent once tissue restoration is complete (109–111). Upon injury, a deluge of pro-inflammatory molecules activate fibroblasts present in connective tissue (112). Fibroblasts then differentiate into either fibrocytes, myofibroblasts or other phenotypes based on microenvironmental and chemical cues. Myofibroblasts are associated with highly dense ECM deposition in implant associated fibrosis pathologies (113, 114). Many pharmacological and materials-centric strategies to restrict fibroblast proliferation and activation have been successfully employed in preclinical models. Pitha et al. (115) detailed the use of Rho-kinase (ROCK) inhibitors (Y27632, fasudil, H1152) in suppressing the myofibroblast phenotype in scleral fibroblasts. All three inhibitors reduced TGF-β-induced α-SMA expression and associated collagen contraction in primary scleral fibroblast cells in vitro. Further, in mouse models of bead-induced glaucoma, they showed that scleral fibroblast proliferation was restricted in mice treated with Y27632 and fasudil by sub-conjunctival injection (115). Biomaterial properties including implant size, surface topography, and porosity also influence fibroblast behavior. Veiseh, et al. (116) injected crosslinked alginate and glass spheres of 8 different diameters (0.3–1.9 mm) intraperitoneally in C57BL/6 mice to study the effect of size on fibrotic response. Interestingly, increasing sphere diameter inversely correlated with immune cell recruitment and cellular overgrowth on explanted spheres after 14 days in vivo as measured by flow cytometry and phase contrast imaging, respectively. Furthermore, expression of fibrotic markers such as α-SMA, collagen 1 (COL1A1) and collagen 2 (COL1A2) genes in cellular deposits on the sphere surfaces was significantly downregulated for 1.8 mm spheres as compared to 0.3 mm spheres. Additionally, when alginate spheres either 0.5 or 1.55 mm diameter were implanted subcutaneously in non-human primates, the 1.55 mm spheres were devoid of cellular growth, whereas the 0.5 mm spheres were encapsulated in fibrotic capsules up to 100 μm thick (116). Taken together, these studies suggest that modulating fibroblast behavior either by material design or by using small molecules may deliver outcomes desirable for non-fibrogenic tissue remodeling.
3.2. Engineering biomaterials to modulate immune response
Restoration of function to injured tissues is crucial to the survival of organisms. The role of innate and adaptive immune responses to implanted materials, particularly shaping tissue architecture surrounding biomaterials in becoming increasingly clear. Early responders such as neutrophils have a very transient presence and therefore it is difficult to understand and modulate their behavior with meaningful consequences. On the other hand, macrophages have an extended presence at implant sites and a more robust and definitive response. As a result, emphasis has been placed on studying macrophage responses to biomaterials. It is important to note that while adaptive immune cells were initially thought to be peripheral to immune response to biomaterials, the role of helper T-cells in determining the fate of tissue regenerative biomaterials has been shown to be essential (117). Surface and physical properties of biomaterials have been described as mediators of macrophage activation. Macrophages respond to physical cues both in vitro and in vivo.
McWhorter, et al. (118) studied macrophage polarization on micropatterned surfaces. They manufactured substrates which had either 20 or 50 μm thick alternating lines of fibronectin and pluronic F127 forming a micropattern. F127 is largely bioinert and deters cell adhesion, facilitating cellular elongation preferentially along the fibronectin components of the micropatterned substrate. The authors compared cell shape and markers associated with M1 and M2 macrophage activation in cells cultured on either patterned or non-patterned substrates. Macrophages cultured on micropatterned substrates displayed a distinct elongated shape, whereas those cultured on non-patterned substrates were more rounded with protrusions. Micropatterned substrates also induced increased expression of Arginase-1, which is an M2 biomarker. Molecules that are associated with inducing M2 polarization acted synergistically with physical cues to enhance Arignase-1 expression by up to 20-fold. In contrast, when macrophages on patterned substrates were exposed to classical activation pathway stimulators LPS and IFN-γ, expression of iNOS which is a known biomarker for the M1 phenotype was only moderately increased suggesting that physical cues can have a protective effect against pro-inflammatory signaling molecules. Looking at actin microstructure, the authors found that inhibition of pathways involved in actin contractility lowered the expression of Arginase-1, but did not affect cell shape or spreading (118). This observation did not hold for cytokine mediated activation, suggesting that macrophages rely on cytoskeletal contractility after sensing physical cues to modulate polarization.
Another recent study performed by Tylek, et al. (119) showed that porosity heavily influenced macrophage polarization. They used a manufacturing technique called melt electrowriting to create three dimensional scaffolds of varying pore sizes (40–100 μm) and geometries (triangular, round, square shaped pores) from PCL. It was observed that a pore size of 40 μm induced definitive M2-like polarization in contrast to 50, 80, and 100 μm pore sizes. Interestingly, round geometry of the pores significantly decreased expression of the M2 macrophage marker CD-163 compared to rectangular, triangular, and disordered pore shapes. Additionally, a decrease in pore size from 100 to 40 μm significantly increased CD-163 expression. Further, the authors measured phagocytic activity of macrophages cultured on porous and 2-dimensional film scaffolds using a fluorescent bead assay, and observed significantly decreased phagocytic activity on all porous scaffolds at day 7 as compared to 2-dimensional films made from the same PCL polymer (119).
Li, et al. (120) studied composite structures made from PCL nanofibers and hyaluronic acid (HA) hydrogel networks as filler materials to restore tissue function and mechanical integrity. The composites had the combined advantages of mechanical stability, injectability and large matrix pore sizes which made the materials durable, easy to administer and capable of modulating immune cell response. In vitro cultures of macrophages in PCL-HA composites displayed a significantly higher degree of M2 polarization, whereas a greater extent of M1 polarization was observed in the HA hydrogels alone. They theorized that the compliant nature combined with the porous framework of the PCL-HA enabled tissue revascularization and cell infiltration in a model of rabbit soft tissue defect repair. Histological analysis showed that angiogenesis and integration with host tissue had occurred (120). Wolf et al. (121) studied the role of ECM in altering macrophage phenotype. In a rat model of abdominal muscle defects, the authors implanted polypropylene meshes coated with a urinary bladder ECM-derived hydrogel matrix. In the animals which received an ECM coated mesh, there was a reduction in the number of M1 macrophages as well as a reduction in the number of foreign body giant cells FBGCs on the surface of the implants at 7, 14 and 35 days compared to uncoated meshes (121). These results offer insights into the significant advantages of device centric approaches to modulate host immune response. Tailoring physical properties of biomaterials such as surface topography, roughness and porosity can induce a favorable immune microenvironment which is critical for the longevity of functional biomaterials.
3.3. Clinical case study: Coronary stents
Cardiovascular disease is the leading cause of death globally, accounting for nearly 18 million deaths globally in 2017 (122). An estimated 12–29% of elderly individuals suffer from peripheral artery disease requiring vascular intervention (123). Vascular intervention technologies have undergone a gradual evolution in parallel with advances made in understanding physiological responses to biomaterials. Table 2 provides an overview of coronary stent technologies that have been studied in clinical settings. Percutaneous coronary intervention is one of the most widely practiced surgical procedures involving the use of biomaterials in the U.S. The first balloon angioplasty was performed in the 1970s (124). Early enthusiasm around this procedure was dampened due to the occurrence of adverse events, such as in stent restenosis (ISR) due to excessive proliferation of smooth muscle cells after injury, elastic recoil, and abrupt closure of blood vessels (125). Patients then needed to undergo coronary artery bypass grafting surgery to restore homeostasis, leading to the development and use of bare metal stents (BMS), which were approved by the FDA in 1993 (126). However, both ISR and thrombosis remained issues with utilization of BMS. BMS therefore needed to be supplemented with systemic pharmacological agents, such as warfarin and aspirin, to avoid thrombosis and restenosis (127). Conventional BMS require placement using a balloon, which dilates the wall of blood vessels by exerting radial mechanical stress. The procedure is also associated with significant de-endothelialization and acute overexertion of pressure on the intimal and medial tissue, leading to smooth muscle cell proliferation and vessel constriction (128). Furthermore, injury to the endothelial cells and intimal tissue results in the secretion of pro-inflammatory signaling molecules, immune cell infiltration, and thickening of the intima (129–131). Attempts have been made to coat BMS with biocompatible polymers, such as polyethyleneterephthalate and polytetrafluoroethylene, though without any impact on restenosis or thrombotic events in large clinical trials (132, 133). Several efforts to modify the surface properties of stent coatings such as the use of nanofibers to enhance surface roughness and mimic ECM features are in preclinical development (134).
Table 2.
Summary of vascular stent technologies approved for clinical use.
| Device | Implant | Manufacturer | Material | Active agent | Reference(s) |
|---|---|---|---|---|---|
| Bare metal stents | Vision | Abbott | Cobalt-Chromium | None | [126,127] |
| Rebel | Boston scientific | Platinum-Chromium | None | ||
| Integrity | Medtronic | Cobalt-Chromium | None | ||
| Drug eluting stents | TAXUS | Boston scientific | Stainless steel, SIBS. | Paclitaxel | [135.136.137.138] |
| Cypher | J&J | Stainless steel, PBMA/PEVA. | Sirolimus | ||
| Xience | Abbott | Cobalt-Chromium, PBMA/PVDF-HFP. | Everolimus | ||
| Promus | Boston scientific | Platinum-Chromium, PBMA/PVDF-HFP. | Everolimus | ||
| Endeavor | Medtronic | Cobalt-Chromium, Phosphoryl choline. | Zotarolimus | ||
| Resolute | Medtronic | Cobalt-Chromium, biolynx. | Zotarolimus | ||
| JANUS | Sorin biomedica | Cobalt-Chromium-Carbofilm. | Tacrolimus | ||
| Bioabsorbable vascular scaffolds | ABSORB | Abbott | PLLA, PDLLA | Everolimus | [127] |
| Synergy | Boston scientific | Platimun-Chromium, PLGA | Everolimus | ||
| Orsiro | Biotronik | Cobalt-Chromium, PLLA | Sirolimus | ||
| Endothelial cell capture stents | Genous | OrbusNiech medical technologies | Stainless steel, Polysaccharide coating | CD34+ antibody | [139.140] |
| COMBO | Stainless steel, ULG CD34 + antibody coating | Sirolimus, CD34+antibody |
To circumvent the issues of thrombosis and neointimal hyperplasia, stents capable of delivering therapeutics locally have been developed (125). Drug-eluting stents (DES) are composed of a metal backbone to provide structural integrity with drug-loaded polymer coatings to provide local, sustained drug release. The TAXUS stent was the first DES, and delivered the anti-mitotic drug paclitaxel (135). Around the same time, the Cypher DES containing the anti-proliferative and anti-inflammatory drug, sirolimus, was also developed (136). Both stents reduced ISR compared to BMS, supporting the potential for sustained-release technologies (137). However, large trials showed no benefit in reducing stent-associated mortality compared to BMS (138). The second generation of DES (Endeavour, Xience, Resolute, Promus) incorporated either everolimus or zotarolimus, which are structural analogs to sirolimus and show similarities in effective cytostatic activity. These drugs also show potent immunosuppressive function and are used in transplant surgery to prevent pathological rejection. Large clinical trials and subsequent meta-analyses showed that overall, second generation DES were associated with reduced overall mortality, and lower rates of myocardial infarction, late stent thrombosis, and ISR.
There are however, key challenges that remain to be addressed concerning DES. Major among them is delayed re-endothelialization of the injured blood vessel, which is associated with late stage thrombosis events (130). The latest generation of stent technologies aim to promote the in situ cellularization of scaffolds. Endothelial progenitor cell capture stents (EPCCs) work by presenting a CD34 antibody on the luminal side of the stent to encourage EPCs binding and proliferation (139). Early studies of sirolimus-eluting EPCCs show non-inferiority to paclitaxel-eluting stents in mitigating neointimal hyperplasia and in the incidence of adverse cardia events (140).
4. Concluding remarks and future perspectives
Biomaterials have substantially contributed to improving quality of life and extending lifespan of millions of people. Preventing biomaterial-associated infections and controlling host-material interactions will continue to be a critical factor for successful surgical outcomes. As biomaterials continue to evolve, it is becoming increasingly evident that a local, materials-centric approach offers distinct advantages in contrast to systemic therapies. Functionalizing biomaterials as drug depots to deliver high concentrations of antimicrobials locally, as well as employing surface modifications that deter bacteria from adhering and proliferating, are promising strategies to combat the increasing threat of antimicrobial drug resistance and circumvent issues with systemic toxicity and patient compliance. However, long-term surgical outcomes are often still poor due to fibrotic response, which can occur long after drug release has ceased. Strategies for modifying the biomaterial surface, such as patterning, may work to modulate the local immune cell signaling and stromal cell response to successfully integrate biomaterials with host tissue. We posit that the next generation of biomedical implants may incorporate topographical features as well as local drug delivery for both short- and long-term modulation of biological response. Biomaterials engineered to evade microbial colonization and instruct host cellular responses have great therapeutic and translational potential.
Acknowledgements:
BioRender was used to create Figures 1 and 3.
Funding:
This work was supported by the National Institutes of Health (R01HL141612), the Department of Defense Vision Research Program (W81XWH2010922), the Robert H. Smith Family Foundation, the KKESH-WEI Collaborative Research Fund, and an unrestricted grant from Research to Prevent Blindness to the Wilmer Eye Institute.
Footnotes
Ethical Statement:
Ethics approval and consent to participate:
Not applicable.
Consent for publication:
All authors provide their consent for publication.
Availability of data and materials:
Not applicable.
Competing Interests:
K.S.P. and L.M.E. are inventors on patent applications related to electrospinning approaches for producing drug-eluting sutures. All other authors declare no conflict of interest.
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References:
- 1.Wang X Overview on biocompatibilities of implantable biomaterials. Advances in Biomaterials Science and Biomedical Applications in Biomedicine; Lazinica R, Ed. 2013:111–55. [Google Scholar]
- 2.Kang C-W, Fang F-Z. State of the art of bioimplants manufacturing: part II. Advances in Manufacturing. 2018;6(2):137–54. [Google Scholar]
- 3.Gristina AG. Biomaterial-centered infection: microbial adhesion versus tissue integration. Science. 1987;237(4822):1588–95. [DOI] [PubMed] [Google Scholar]
- 4.Berríos-Torres SI, Umscheid CA, Bratzler DW, Leas B, Stone EC, Kelz RR, et al. Centers for disease control and prevention guideline for the prevention of surgical site infection, 2017. JAMA surgery. 2017;152(8):784–91. [DOI] [PubMed] [Google Scholar]
- 5.Schierholz J, Beuth J. Implant infections: a haven for opportunistic bacteria. Journal of Hospital Infection. 2001;49(2):87–93. [DOI] [PubMed] [Google Scholar]
- 6.Darouiche RO. Treatment of infections associated with surgical implants. New England Journal of Medicine. 2004;350(14):1422–9. [DOI] [PubMed] [Google Scholar]
- 7.Arefian H, Vogel M, Kwetkat A, Hartmann M. Economic evaluation of interventions for prevention of hospital acquired infections: a systematic review. PloS one. 2016;11(1):e0146381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Founou RC, Founou LL, Essack SY. Clinical and economic impact of antibiotic resistance in developing countries: A systematic review and meta-analysis. PloS one. 2017;12(12):e0189621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Giraldi G, Montesano M, Sandorfi F, Iachini M, Orsi G. Excess length of hospital stay due to healthcare acquired infections: methodologies evaluation. Ann Ig. 2019;31(5):507–16. [DOI] [PubMed] [Google Scholar]
- 10.Amin Yavari S, Castenmiller SM, van Strijp JA, Croes M. Combating Implant Infections: Shifting Focus from Bacteria to Host. Advanced Materials. 2020:2002962. [DOI] [PubMed] [Google Scholar]
- 11.VanEpps JS, Younger JG. Implantable device related infection. Shock (Augusta, Ga). 2016;46(6):597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arciola CR, Campoccia D, Speziale P, Montanaro L, Costerton JW. Biofilm formation in Staphylococcus implant infections. A review of molecular mechanisms and implications for biofilm-resistant materials. Biomaterials. 2012;33(26):5967–82. [DOI] [PubMed] [Google Scholar]
- 13.López D, Vlamakis H, Kolter R. Biofilms. Cold Spring Harbor perspectives in biology. 2010;2(7):a000398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mack D Molecular mechanisms of Staphylococcus epidermidis biofilm formation. Journal of Hospital Infection. 1999;43:S113–S25. [DOI] [PubMed] [Google Scholar]
- 15.Prince AS. Biofilms, antimicrobial resistance, and airway infection. New England Journal of Medicine. 2002;347(14):1110–1. [DOI] [PubMed] [Google Scholar]
- 16.Rao RS, Karthika RU, Singh S, Shashikala P, Kanungo R, Jayachandran S, et al. Correlation between biofilm production and multiple drug resistance in imipenem resistant clinical isolates of Acinetobacter baumannii. Indian journal of medical microbiology. 2008;26(4):333. [DOI] [PubMed] [Google Scholar]
- 17.Wiegering A, Sinha B, Spor L, Klinge U, Steger U, Germer C, et al. Gentamicin for prevention of intraoperative mesh contamination: demonstration of high bactericide effect (in vitro) and low systemic bioavailability (in vivo). Hernia. 2014;18(5):691–700. [DOI] [PubMed] [Google Scholar]
- 18.Xiong M-H, Bao Y, Yang X-Z, Zhu Y-H, Wang J. Delivery of antibiotics with polymeric particles. Advanced drug delivery reviews. 2014;78:63–76. [DOI] [PubMed] [Google Scholar]
- 19.Jamaledin R, Yiu CK, Zare EN, Niu LN, Vecchione R, Chen G, et al. Advances in antimicrobial microneedle patches for combating infections. Advanced Materials. 2020;32(33):2002129. [DOI] [PubMed] [Google Scholar]
- 20.Heta S, Robo I. The side effects of the most commonly used group of antibiotics in periodontal treatments. Medical Sciences. 2018;6(1):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Francis NA, Gillespie D, Nuttall J, Hood K, Little P, Verheij T, et al. Antibiotics for acute cough: an international observational study of patient adherence in primary care. British Journal of General Practice. 2012;62(599):e429–e37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Viswanathan M, Golin CE, Jones CD, Ashok M, Blalock SJ, Wines RC, et al. Interventions to improve adherence to self-administered medications for chronic diseases in the United States: a systematic review. Annals of internal medicine. 2012;157(11):785–95. [DOI] [PubMed] [Google Scholar]
- 23.Heuer H, Krögerrecklenfort E, Wellington E, Egan S, Van Elsas J, Van Overbeek L, et al. Gentamicin resistance genes in environmental bacteria: prevalence and transfer. FEMS microbiology ecology. 2002;42(2):289–302. [DOI] [PubMed] [Google Scholar]
- 24.Berger-Bächi B, Rohrer S. Factors influencing methicillin resistance in staphylococci. Archives of microbiology. 2002;178(3):165–71. [DOI] [PubMed] [Google Scholar]
- 25.Price LB, Stegger M, Hasman H, Aziz M, Larsen J, Andersen PS, et al. Staphylococcus aureus CC398: host adaptation and emergence of methicillin resistance in livestock. MBio. 2012;3(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yazdankhah SP, Scheie AA, Høiby EA, Lunestad B-T, Heir E, Fotland TØ, et al. Triclosan and antimicrobial resistance in bacteria: an overview. Microbial drug resistance. 2006;12(2):83–90. [DOI] [PubMed] [Google Scholar]
- 27.Costerton JW, Cheng K, Geesey GG, Ladd TI, Nickel JC, Dasgupta M, et al. Bacterial biofilms in nature and disease. Annual Reviews in Microbiology. 1987;41(1):435–64. [DOI] [PubMed] [Google Scholar]
- 28.An YH, Dickinson RB, Doyle RJ. Mechanisms of bacterial adhesion and pathogenesis of implant and tissue infections. Handbook of Bacterial Adhesion: Springer; 2000. p. 1–27. [Google Scholar]
- 29.Fletcher M, Savage DC. Bacterial adhesion: mechanisms and physiological significance: Springer Science & Business Media; 2013. [Google Scholar]
- 30.Dunne WM. Bacterial adhesion: seen any good biofilms lately? Clinical microbiology reviews. 2002;15(2):155–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Costerton JW, Lappin-Scott HM. Introduction to microbial biofilms. Microbial biofilms. 1995:1–11. [Google Scholar]
- 32.Vega NM, Gore J. Collective antibiotic resistance: mechanisms and implications. Current opinion in microbiology. 2014;21:28–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lewis K Multidrug tolerance of biofilms and persister cells. Bacterial biofilms: Springer; 2008. p. 107–31. [DOI] [PubMed] [Google Scholar]
- 34.Cai Z, Wang Y, Zhu L-J, Liu Z-Q. Nanocarriers: a general strategy for enhancement of oral bioavailability of poorly absorbed or pre-systemically metabolized drugs. Current Drug Metabolism. 2010;11(2):197–207. [DOI] [PubMed] [Google Scholar]
- 35.Lee JW, Prausnitz MR. Drug delivery using microneedle patches: not just for skin. Taylor & Francis; 2018. [DOI] [PubMed] [Google Scholar]
- 36.Teo AJ, Mishra A, Park I, Kim Y-J, Park W-T, Yoon Y-J. Polymeric biomaterials for medical implants and devices. ACS Biomaterials Science & Engineering. 2016;2(4):454–72. [DOI] [PubMed] [Google Scholar]
- 37.Balaji AB, Pakalapati H, Khalid M, Walvekar R, Siddiqui H. Natural and synthetic biocompatible and biodegradable polymers. Biodegradable and biocompatible polymer composites: processing, properties and applications Woodhead Publishing series in composites science and engineering Duxford: Woodhead Publishing. 2017:3–32. [Google Scholar]
- 38.Pappalardo D, Mathisen Tr, Finne-Wistrand A Biocompatibility of resorbable polymers: a historical perspective and framework for the future. Biomacromolecules. 2019;20(4):1465–77. [DOI] [PubMed] [Google Scholar]
- 39.Rebelo R, Fernandes M, Fangueiro R. Biopolymers in medical implants: a brief review. Procedia engineering. 2017;200:236–43. [Google Scholar]
- 40.Sionkowska A Current research on the blends of natural and synthetic polymers as new biomaterials. Progress in polymer science. 2011;36(9):1254–76. [Google Scholar]
- 41.Suhardi V, Bichara D, Kwok S, Freiberg A, Rubash H, Malchau H, et al. A fully functional drug-eluting joint implant. Nature biomedical engineering. 2017;1(6):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Association AO. Hip and Knee Arthroplasty. Annual report 2013. Adelaide: AOA; 2015. [Google Scholar]
- 43.Burnett RSJ, Kelly MA, Hanssen AD, Barrack RL. Technique and timing of two-stage exchange for infection in TKA. Clinical Orthopaedics and Related Research (1976–2007). 2007;464:164–78. [DOI] [PubMed] [Google Scholar]
- 44.Ashbaugh AG, Jiang X, Zheng J, Tsai AS, Kim W-S, Thompson JM, et al. Polymeric nanofiber coating with tunable combinatorial antibiotic delivery prevents biofilm-associated infection in vivo. Proceedings of the National Academy of Sciences. 2016;113(45):E6919–E28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kehinde EO, Rotimi VO, Al-Awadi KA, Abdul-Halim H, Boland F, Al-Hunayan A, et al. Factors predisposing to urinary tract infection after J ureteral stent insertion. The Journal of urology. 2002;167(3):1334–7. [PubMed] [Google Scholar]
- 46.Chew BH, Lange D. Ureteral stent symptoms and associated infections: a biomaterials perspective. Nature Reviews Urology. 2009;6(8):440. [DOI] [PubMed] [Google Scholar]
- 47.Cadieux PA, Chew BH, Knudsen BE, DeJong K, Rowe E, Reid G, et al. Triclosan loaded ureteral stents decrease proteus mirabilis 296 infection in a rabbit urinary tract infection model. The Journal of urology. 2006;175(6):2331–5. [DOI] [PubMed] [Google Scholar]
- 48.Chuanchuen R, Karkhoff-Schweizer RR, Schweizer HP. High-level triclosan resistance in Pseudomonas aeruginosa is solely a result of efflux. American journal of infection control. 2003;31(2):124–7. [DOI] [PubMed] [Google Scholar]
- 49.Fan F, Yan K, Wallis NG, Reed S, Moore TD, Rittenhouse SF, et al. Defining and combating the mechanisms of triclosan resistance in clinical isolates of Staphylococcus aureus. Antimicrobial agents and chemotherapy. 2002;46(11):3343–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mcmurry LM, Oethinger M, Levy SB. Overexpression of marA, soxS, or acrAB produces resistance to triclosan in laboratory and clinical strains of Escherichia coli. FEMS microbiology letters. 1998;166(2):305–9. [DOI] [PubMed] [Google Scholar]
- 51.Minardi D, Ghiselli R, Cirioni O, Giacometti A, Kamysz W, Orlando F, et al. The antimicrobial peptide Tachyplesin III coated alone and in combination with intraperitoneal piperacillin-tazobactam prevents ureteral stent Pseudomonas infection in a rat subcutaneous pouch model. Peptides. 2007;28(12):2293–8. [DOI] [PubMed] [Google Scholar]
- 52.Alves P, Gomes L, Vorobii M, Rodríguez-Emmenegger C, Mergulhão F. The potential advantages of using a poly (HPMA) brush in urinary catheters: Effects on biofilm cells and architecture. Colloids and Surfaces B: Biointerfaces. 2020;191:110976. [DOI] [PubMed] [Google Scholar]
- 53.Orlando F, Ghiselli R, Cirioni O, Minardi D, Tomasinsig L, Mocchegiani F, et al. BMAP-28 improves the efficacy of vancomycin in rat models of gram-positive cocci ureteral stent infection. Peptides. 2008;29(7):1118–23. [DOI] [PubMed] [Google Scholar]
- 54.Cirioni O, Ghiselli R, Silvestri C, Minardi D, Gabrielli E, Orlando F, et al. Effect of the combination of clarithromycin and amikacin on Pseudomonas aeruginosa biofilm in an animal model of ureteral stent infection. Journal of antimicrobial chemotherapy. 2011;66(6):1318–23. [DOI] [PubMed] [Google Scholar]
- 55.Apisarnthanarak A, Singh N, Bandong AN, Madriaga G. Triclosan-coated sutures reduce the risk of surgical site infections: a systematic review and meta-analysis. infection control & hospital epidemiology. 2015;36(2):169–79. [DOI] [PubMed] [Google Scholar]
- 56.Leaper D, Wilson P, Assadian O, Edmiston C, Kiernan M, Miller A, et al. The role of antimicrobial sutures in preventing surgical site infection. The Annals of The Royal College of Surgeons of England. 2017;99(6):439–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rothenburger S, Spangler D, Bhende S, Burkley D. In vitro antimicrobial evaluation of Coated VICRYL* Plus Antibacterial Suture (coated polyglactin 910 with triclosan) using zone of inhibition assays. Surgical Infections. 2002;3(S1):s79–s87. [DOI] [PubMed] [Google Scholar]
- 58.Chen X, Hou D, Wang L, Zhang Q, Zou J, Sun G. Antibacterial surgical silk sutures using a high-performance slow-release carrier coating system. ACS applied materials & interfaces. 2015;7(40):22394–403. [DOI] [PubMed] [Google Scholar]
- 59.Kashiwabuchi F, Parikh KS, Omiadze R, Zhang S, Luo L, Patel HV, et al. Development of absorbable, antibiotic-eluting sutures for ophthalmic surgery. Translational vision science & technology. 2017;6(1):1-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bae S, DiBalsi MJ, Meilinger N, Zhang C, Beal E, Korneva G, et al. Heparin-eluting electrospun nanofiber yarns for antithrombotic vascular sutures. ACS applied materials & interfaces. 2018;10(10):8426–35. [DOI] [PubMed] [Google Scholar]
- 61.Chen S, Ge L, Mueller A, Carlson MA, Teusink MJ, Shuler FD, et al. Twisting electrospun nanofiber fine strips into functional sutures for sustained co-delivery of gentamicin and silver. Nanomedicine: Nanotechnology, Biology and Medicine. 2017;13(4):1435–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.He CL, Huang ZM, Han XJ. Fabrication of drug-loaded electrospun aligned fibrous threads for suture applications. Journal of Biomedical Materials Research Part A: An Official Journal of The Society for Biomaterials, The Japanese Society for Biomaterials, and The Australian Society for Biomaterials and the Korean Society for Biomaterials. 2009;89(1):80–95. [DOI] [PubMed] [Google Scholar]
- 63.Padmakumar S, Joseph J, Neppalli MH, Mathew SE, Nair SV, Shankarappa SA, et al. Electrospun polymeric core–sheath yarns as drug eluting surgical sutures. ACS applied materials & interfaces. 2016;8(11):6925–34. [DOI] [PubMed] [Google Scholar]
- 64.Weldon CB, Tsui JH, Shankarappa SA, Nguyen VT, Ma M, Anderson DG, et al. Electrospun drug-eluting sutures for local anesthesia. Journal of controlled release. 2012;161(3):903–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Parikh KS, Omiadze R, Josyula A, Shi R, Anders NM, He P, et al. Ultra-thin, High Strength, Antibiotic-eluting Sutures for Prevention of Ophthalmic Infection. Bioengineering & Translational Medicine. 2020:e10204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hizal F, Rungraeng N, Lee J, Jun S, Busscher HJ, van der Mei HC, et al. Nanoengineered superhydrophobic surfaces of aluminum with extremely low bacterial adhesivity. ACS applied materials & interfaces. 2017;9(13):12118–29. [DOI] [PubMed] [Google Scholar]
- 67.Zhang X, Wang L, Levänen E. Superhydrophobic surfaces for the reduction of bacterial adhesion. Rsc Advances. 2013;3(30):12003–20. [Google Scholar]
- 68.Song F, Koo H, Ren D. Effects of material properties on bacterial adhesion and biofilm formation. Journal of dental research. 2015;94(8):1027–34. [DOI] [PubMed] [Google Scholar]
- 69.Quirynen Mv, Van Der Mei H, Bollen C, Schotte A, Marechal M, Doornbusch G, et al. An in vivo study of the influence of the surface roughness of implants on the microbiology of supra-and subgingival plaque. Journal of dental research. 1993;72(9):1304–9. [DOI] [PubMed] [Google Scholar]
- 70.Hsu LC, Fang J, Borca-Tasciuc DA, Worobo RW, Moraru CI. Effect of micro-and nanoscale topography on the adhesion of bacterial cells to solid surfaces. Applied and environmental microbiology. 2013;79(8):2703–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Puckett SD, Taylor E, Raimondo T, Webster TJ. The relationship between the nanostructure of titanium surfaces and bacterial attachment. Biomaterials. 2010;31(4):706–13. [DOI] [PubMed] [Google Scholar]
- 72.Neoh KG, Hu X, Zheng D, Kang ET. Balancing osteoblast functions and bacterial adhesion on functionalized titanium surfaces. Biomaterials. 2012;33(10):2813–22. [DOI] [PubMed] [Google Scholar]
- 73.Linklater DP, Baulin VA, Juodkazis S, Crawford RJ, Stoodley P, Ivanova EP. Mechano-bactericidal actions of nanostructured surfaces. Nature Reviews Microbiology. 2020:1–15. [DOI] [PubMed] [Google Scholar]
- 74.Román-Kustas J, Hoffman JB, Reed JH, Gonsalves AE, Oh J, Li L, et al. Molecular and Topographical Organization: Influence on Cicada Wing Wettability and Bactericidal Properties. Advanced Materials Interfaces. 2020;7(10):2000112. [Google Scholar]
- 75.Bhadra CM, Truong VK, Pham VT, Al Kobaisi M, Seniutinas G, Wang JY, et al. Antibacterial titanium nano-patterned arrays inspired by dragonfly wings. Scientific reports. 2015;5(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Linklater DP, Nguyen HKD, Bhadra CM, Juodkazis S, Ivanova EP. Influence of nanoscale topology on bactericidal efficiency of black silicon surfaces. Nanotechnology. 2017;28(24):245301. [DOI] [PubMed] [Google Scholar]
- 77.Kim W, Ng JK, Kunitake ME, Conklin BR, Yang P. Interfacing silicon nanowires with mammalian cells. Journal of the American Chemical Society. 2007;129(23):7228–9. [DOI] [PubMed] [Google Scholar]
- 78.Shalek AK, Robinson JT, Karp ES, Lee JS, Ahn D-R, Yoon M-H, et al. Vertical silicon nanowires as a universal platform for delivering biomolecules into living cells. Proceedings of the National Academy of Sciences. 2010;107(5):1870–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Garnett E, Yang P. Light trapping in silicon nanowire solar cells. Nano letters. 2010;10(3):1082–7. [DOI] [PubMed] [Google Scholar]
- 80.Anderson JM, Rodriguez A, Chang DT, editors. Foreign body reaction to biomaterials. Seminars in immunology; 2008: Elsevier. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zimmerli W, Sendi P, editors. Pathogenesis of implant-associated infection: the role of the host. Seminars in immunopathology; 2011: Springer. [DOI] [PubMed] [Google Scholar]
- 82.Kristian SA, Birkenstock TA, Sauder U, Mack D, Götz F, Landmann R. Biofilm formation induces C3a release and protects Staphylococcus epidermidis from IgG and complement deposition and from neutrophil-dependent killing. The Journal of infectious diseases. 2008;197(7):1028–35. [DOI] [PubMed] [Google Scholar]
- 83.Zimmerli W, Waldvogel FA, Vaudaux P, Nydegger UE. Pathogenesis of foreign body infection: description and characteristics of an animal model. Journal of infectious diseases. 1982;146(4):487–97. [DOI] [PubMed] [Google Scholar]
- 84.Selders GS, Fetz AE, Radic MZ, Bowlin GL. An overview of the role of neutrophils in innate immunity, inflammation and host-biomaterial integration. Regenerative biomaterials. 2017;4(1):55–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sekizuka E, Grisham MB, Li M, Deitch EA, Granger DN. Inflammation-induced intestinal hyperemia in the rat: role of neutrophils. Gastroenterology. 1988;95(6):1528–34. [DOI] [PubMed] [Google Scholar]
- 86.Lawrence DW, Pryzwansky KB. The vasodilator-stimulated phosphoprotein is regulated by cyclic GMP-dependent protein kinase during neutrophil spreading. The Journal of Immunology. 2001;166(9):5550–6. [DOI] [PubMed] [Google Scholar]
- 87.Weber M, Steinle H, Golombek S, Hann L, Schlensak C, Wendel HP, et al. Blood-contacting biomaterials: in vitro evaluation of the hemocompatibility. Frontiers in bioengineering and biotechnology. 2018;6:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mariani E, Lisignoli G, Borzì RM, Pulsatelli L. Biomaterials: foreign bodies or tuners for the immune response? International journal of molecular sciences. 2019;20(3):636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jhunjhunwala S, Aresta-DaSilva S, Tang K, Alvarez D, Webber MJ, Tang BC, et al. Neutrophil responses to sterile implant materials. PloS one. 2015;10(9):e0137550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cohen HC, Lieberthal TJ, Kao WJ. Poly (ethylene glycol)-containing hydrogels promote the release of primary granules from human blood-derived polymorphonuclear leukocytes. Journal of Biomedical Materials Research Part A. 2014;102(12):4252–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Novak ML, Koh TJ. Phenotypic transitions of macrophages orchestrate tissue repair. The American journal of pathology. 2013;183(5):1352–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wynn TA, Vannella KM. Macrophages in tissue repair, regeneration, and fibrosis. Immunity. 2016;44(3):450–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gonzalez-Simon AL, Eniola-Adefeso O. Host response to biomaterials. Engineering Biomaterials for Regenerative Medicine: Springer; 2012. p. 143–59. [Google Scholar]
- 94.Badolato R, Ponzi AN, Millesimo M, Notarangelo LD, Musso T. Interleukin-15 (IL-15) induces IL-8 and monocyte chemotactic protein 1 production in human monocytes. Blood, The Journal of the American Society of Hematology. 1997;90(7):2804–9. [PubMed] [Google Scholar]
- 95.Hazuda D, Lee J, Young P. The kinetics of interleukin 1 secretion from activated monocytes. Differences between interleukin 1 alpha and interleukin 1 beta. Journal of Biological Chemistry. 1988;263(17):8473–9. [PubMed] [Google Scholar]
- 96.Krumbholz M, Theil D, Cepok S, Hemmer B, Kivisäkk P, Ransohoff RM, et al. Chemokines in multiple sclerosis: CXCL12 and CXCL13 up-regulation is differentially linked to CNS immune cell recruitment. Brain. 2006;129(1):200–11. [DOI] [PubMed] [Google Scholar]
- 97.Müller E, Christopoulos PF, Halder S, Lunde A, Beraki K, Speth M, et al. Toll-like receptor ligands and interferon-γ synergize for induction of antitumor M1 macrophages. Frontiers in immunology. 2017;8:1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Martinez FO, Sica A, Mantovani A, Locati M. Macrophage activation and polarization. Front biosci. 2008;13(1):453–61. [DOI] [PubMed] [Google Scholar]
- 99.Murray PJ, Allen JE, Biswas SK, Fisher EA, Gilroy DW, Goerdt S, et al. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity. 2014;41(1):14–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Rodell CB, Arlauckas SP, Cuccarese MF, Garris CS, Li R, Ahmed MS, et al. TLR7/8-agonist-loaded nanoparticles promote the polarization of tumour-associated macrophages to enhance cancer immunotherapy. Nature biomedical engineering. 2018;2(8):578–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Vinod N, Hwang D, Azam SH, Van Swearingen AE, Wayne E, Fussell SC, et al. High-capacity poly (2-oxazoline) formulation of TLR 7/8 agonist extends survival in a chemo-insensitive, metastatic model of lung adenocarcinoma. Science advances. 2020;6(25):eaba5542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Keeler GD, Durdik JM, Stenken JA. Localized delivery of dexamethasone-21-phosphate via microdialysis implants in rat induces M (GC) macrophage polarization and alters CCL2 concentrations. Acta biomaterialia. 2015;12:11–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Doloff JC, Veiseh O, Vegas AJ, Tam HH, Farah S, Ma M, et al. Colony stimulating factor-1 receptor is a central component of the foreign body response to biomaterial implants in rodents and non-human primates. Nat Mater. 2017;16(6):671–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chung L, Maestas D, Lebid A, Mageau A, Rosson GD, Wu X, et al. Interleukin-17 and senescence regulate the foreign body response. bioRxiv. 2019:583757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sommerfeld SD, Cherry C, Schwab RM, Chung L, Maestas DR, Laffont P, et al. Interleukin-36γ–producing macrophages drive IL-17–mediated fibrosis. Science immunology. 2019;4(40):eaax4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wolfram D, Rabensteiner E, Grundtman C, Böck G, Mayerl C, Parson W, et al. T regulatory cells and TH17 cells in peri–silicone implant capsular fibrosis. Plastic and Reconstructive Surgery. 2012;129(2):327e–37e. [DOI] [PubMed] [Google Scholar]
- 107.Barin JG, Baldeviano GC, Talor MV, Wu L, Ong S, Quader F, et al. Macrophages participate in IL-17-mediated inflammation. European journal of immunology. 2012;42(3):726–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chung L, Maestas DR, Lebid A, Mageau A, Rosson GD, Wu X, et al. Interleukin 17 and senescent cells regulate the foreign body response to synthetic material implants in mice and humans. Science translational medicine. 2020;12(539). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Darby IA, Laverdet B, Bonté F, Desmoulière A. Fibroblasts and myofibroblasts in wound healing. Clinical, cosmetic and investigational dermatology. 2014;7:301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hannan RT, Peirce SM, Barker TH. Fibroblasts: diverse cells critical to biomaterials integration. ACS biomaterials science & engineering. 2017;4(4):1223–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Rognoni E, Pisco AO, Hiratsuka T, Sipilä KH, Belmonte JM, Mobasseri SA, et al. Fibroblast state switching orchestrates dermal maturation and wound healing. Molecular systems biology. 2018;14(8):e8174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Van Linthout S, Miteva K, Tschöpe C. Crosstalk between fibroblasts and inflammatory cells. Cardiovascular research. 2014;102(2):258–69. [DOI] [PubMed] [Google Scholar]
- 113.Klingberg F, Hinz B, White ES. The myofibroblast matrix: implications for tissue repair and fibrosis. The Journal of pathology. 2013;229(2):298–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Phan SH. The myofibroblast in pulmonary fibrosis. Chest. 2002;122(6):286S–9S. [DOI] [PubMed] [Google Scholar]
- 115.Pitha I, Oglesby E, Chow A, Kimball E, Pease ME, Schaub J, et al. Rho-kinase inhibition reduces myofibroblast differentiation and proliferation of scleral fibroblasts induced by transforming growth factor β and experimental glaucoma. Translational vision science & technology. 2018;7(6):6-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Veiseh O, Doloff JC, Ma M, Vegas AJ, Tam HH, Bader AR, et al. Size-and shape-dependent foreign body immune response to materials implanted in rodents and non-human primates. Nature materials. 2015;14(6):643–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sadtler K, Estrellas K, Allen BW, Wolf MT, Fan H, Tam AJ, et al. Developing a pro-regenerative biomaterial scaffold microenvironment requires T helper 2 cells. Science. 2016;352(6283):366–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.McWhorter FY, Wang T, Nguyen P, Chung T, Liu WF. Modulation of macrophage phenotype by cell shape. Proceedings of the National Academy of Sciences. 2013;110(43):17253–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Tylek T, Blum C, Hrynevich A, Schlegelmilch K, Schilling T, Dalton PD, et al. Precisely defined fiber scaffolds with 40 μm porosity induce elongation driven M2-like polarization of human macrophages. Biofabrication. 2020;12(2):025007. [DOI] [PubMed] [Google Scholar]
- 120.Li X, Cho B, Martin R, Seu M, Zhang C, Zhou Z, et al. Nanofiber-hydrogel composite–mediated angiogenesis for soft tissue reconstruction. Science translational medicine. 2019;11(490). [DOI] [PubMed] [Google Scholar]
- 121.Wolf MT, Daly KA, Brennan-Pierce EP, Johnson SA, Carruthers CA, D’Amore A, et al. A hydrogel derived from decellularized dermal extracellular matrix. Biomaterials. 2012;33(29):7028–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Jagannathan R, Patel SA, Ali MK, Narayan KV. Global updates on cardiovascular disease mortality trends and attribution of traditional risk factors. Current diabetes reports. 2019;19(7):44. [DOI] [PubMed] [Google Scholar]
- 123.Secretariat MA. Stenting for peripheral artery disease of the lower extremities: an evidence-based analysis. Ontario health technology assessment series. 2010;10(18):1. [PMC free article] [PubMed] [Google Scholar]
- 124.Chen D, Jepson N. Coronary stent technology: a narrative review. The Medical journal of Australia. 2016;205(6):277–81. [DOI] [PubMed] [Google Scholar]
- 125.Simard T, Hibbert B, Ramirez FD, Froeschl M, Chen Y-X, O’Brien ER. The evolution of coronary stents: a brief review. Canadian Journal of Cardiology. 2014;30(1):35–45. [DOI] [PubMed] [Google Scholar]
- 126.Meraj PM, Jauhar R, Singh A. Bare metal stents versus drug eluting stents: where do we stand in 2015? Current treatment options in cardiovascular medicine. 2015;17(8):39. [DOI] [PubMed] [Google Scholar]
- 127.Iqbal J, Gunn J, Serruys PW. Coronary stents: historical development, current status and future directions. British medical bulletin. 2013;106(1). [DOI] [PubMed] [Google Scholar]
- 128.Hu Y, Böck G, Wick G, Xu Q. Activation of PDGF receptor α in vascular smooth muscle cells by mechanical stress. The FASEB Journal. 1998;12(12):1135–42. [DOI] [PubMed] [Google Scholar]
- 129.Scott NA. Restenosis following implantation of bare metal coronary stents: pathophysiology and pathways involved in the vascular response to injury. Advanced drug delivery reviews. 2006;58(3):358–76. [DOI] [PubMed] [Google Scholar]
- 130.Inoue T, Croce K, Morooka T, Sakuma M, Node K, Simon DI. Vascular inflammation and repair: implications for re-endothelialization, restenosis, and stent thrombosis. JACC: Cardiovascular Interventions. 2011;4(10):1057–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Shen H, Dai Z, Wang M, Gu S, Xu W, Xu G, et al. Preprocedural Neutrophil to Albumin Ratio Predicts In-Stent Restenosis Following Carotid Angioplasty and Stenting. Journal of Stroke and Cerebrovascular Diseases. 2019;28(9):2442–7. [DOI] [PubMed] [Google Scholar]
- 132.Jamshidi P, Mahmoody K, Erne P. Covered stents: a review. International journal of cardiology. 2008;130(3):310–8. [DOI] [PubMed] [Google Scholar]
- 133.Stankovic G, Colombo A, Presbitero P, Van den Branden F, Inglese L, Cernigliaro C, et al. Randomized evaluation of polytetrafluoroethylene-covered stent in saphenous vein grafts: the Randomized Evaluation of polytetrafluoroethylene COVERed stent in Saphenous vein grafts (RECOVERS) Trial. Circulation. 2003;108(1):37–42. [DOI] [PubMed] [Google Scholar]
- 134.Oh B, Lee CH. Advanced cardiovascular stent coated with nanofiber. Molecular Pharmaceutics. 2013;10(12):4432–42. [DOI] [PubMed] [Google Scholar]
- 135.Halkin A, Stone GW. Polymer-Based Paclitaxel-Eluting Stents in Percutaneous Coronary Intervention: A Review of the TAXUS Trials. Journal of interventional cardiology. 2004;17(5):271–82. [DOI] [PubMed] [Google Scholar]
- 136.Kereiakes DJ, Choo JK, Young JJ, Broderick TM. Thrombosis and drug-eluting stents: a critical appraisal. Reviews in cardiovascular medicine. 2004;5(1):9–15. [PubMed] [Google Scholar]
- 137.Bharadwaj P, Chadha D. Drug eluting stents: To evolve or dissolve? medical journal armed forces india. 2016;72(4):367–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Maeng M, Jensen LO, Kaltoft A, Hansen HH, Bøttcher M, Lassen JF, et al. Comparison of stent thrombosis, myocardial infarction, and mortality following drug-eluting versus bare-metal stent coronary intervention in patients with diabetes mellitus. The American journal of cardiology. 2008;102(2):165–72. [DOI] [PubMed] [Google Scholar]
- 139.Wendel HP, Avci-Adali M, Ziemer G. Endothelial progenitor cell capture stents—hype or hope? International journal of cardiology. 2010;145(1):115–7. [DOI] [PubMed] [Google Scholar]
- 140.Haude M, Lee SW, Worthley SG, Silber S, Verheye S, Erbs S, et al. The REMEDEE trial: a randomized comparison of a combination sirolimus-eluting endothelial progenitor cell capture stent with a paclitaxel-eluting stent. JACC: Cardiovascular Interventions. 2013;6(4):334–43. [DOI] [PubMed] [Google Scholar]