Abstract
Objective:
To assess leukemia risks among children with Down syndrome in a large, contemporary cohort.
Study design:
Retrospective cohort study including 3,905,399 children born 1996–2016 in seven U.S. healthcare systems or Ontario, Canada and followed from birth to cancer diagnosis, death, age 15 years, disenrollment, or December 30, 2016. Down syndrome was identified using ICD9/10 diagnosis codes. Cancer diagnoses were identified through linkages to tumor registries. Incidence and hazard ratios (HRs) of leukemia were estimated for children with Down syndrome and other children adjusting for health system, child’s age at diagnosis, birth year, and sex.
Results:
Leukemia was diagnosed in 124 of 4,401 children with Down syndrome and 1,941 of 3,900,998 other children. In children with Down syndrome, the cumulative incidence of acute myeloid leukemia (AML) was 1,405/100,000 [95% confidence interval (CI)=1076–1,806] at age 4 and unchanged at age 14. The cumulative incidence of acute lymphoid leukemia (ALL) in children with Down syndrome was 1,059/100,000 (95%CI=755–1,451) at age 4 and 1,714/100,000 (95%CI=1,264–2,276) at age 14. Children with Down syndrome had higher risk of AML before age 5 than other children (HR=399, 95%CI=281–566). Largest HRs were for megakaryoblastic leukemia before age 5 (HR=1,500, 95%CI=555–4,070). Children with Down syndrome had a higher risk of ALL than other children regardless of age (<5 years: HR=28, 95%CI=20–40, ≥5 years HR=21, 95%CI=12–38).
Conclusions:
Down syndrome remains a strong risk factor for childhood leukemia, and associations with AML are stronger than previously reported.
Keywords: Down syndrome, acute myeloid leukemia, acute lymphoid leukemia, childhood leukemia risk, retrospective cohort
Down syndrome is the most common genetic disease, at 1.4 per 1,000 live births.(1, 2) Children with Down syndrome have substantially increased risk of multiple health conditions(3, 4) including 10- to 20-fold higher leukemia incidence than the general population.(5) Although acute lymphoblastic leukemia (ALL) is more common in children than acute myeloid leukemia (AML), children with Down Syndrome have a particularly elevated risk (estimated 150-fold) of developing AML before age 5.(3, 6, 7) Their increased risk of megakaryoblastic leukemia (AML-M7) is estimated to be 400- to 600-fold.(8–10)
Reported risk estimates for leukemia among children with Down syndrome are mostly based on studies of children born as early as the 1930s.(5, 7, 11, 12) Over time, childhood ALL incidence has increased (13) and AML has mostly remained stable except for increases in some subgroups (14, 15), so it is unknown whether past estimates for leukemia risk among Down syndrome children accurately reflect today’s at-risk population. Medical practice changes such as increases in imaging procedures that expose children to low dose ionizing radiation may have altered cancer risk in children with Down syndrome.(16) A steep rise in medical imaging over the past two decades, particularly in computed tomography (CT) examinations, exposes children to higher levels of radiation than in earlier periods(17, 18) and CT scanning has been shown to increase leukemia risk.(19, 20) Children with Down syndrome may be more likely to have more CT and other low-dose ionizing radiation examinations compared with the general pediatric population due to their many comorbid conditions.(21)
To assess the modern relationship between childhood leukemia risk and Down syndrome status, we estimated risk of childhood leukemia associated with Down syndrome in a contemporary cohort of 3.9 million children born in seven U.S.-based integrated healthcare systems or Ontario, Canada. We evaluated how the association between leukemia and Down syndrome varied by leukemia subtype, age, and birth country.
Methods
This study included all live births in 7 U.S. healthcare systems and Ontario, Canada between January 1, 1996 and December 30, 2016 (N=3,905,399). The U.S. systems all participate in the Health Care Systems Research Network(22): Kaiser Permanente (KP) Hawaii, KP Northern California, KP Northwest (Oregon/Southwest Washington), KP Washington, Geisinger (Pennsylvania), Harvard Pilgrim Health Care (Massachusetts), and Marshfield Clinic Health System (Wisconsin). Institutional review boards of all collaborating institutions approved the study with a waiver of informed consent.
Identifying Down syndrome
Down syndrome was identified using International Classification of Diseases (ICD) diagnosis 9th edition code 758.0 and 10th edition codes Q90.0, Q90.1, Q90.2, or 90.9. For U.S. sites, Down syndrome diagnoses were obtained from sites’ clinical and administrative data including electronic health records through the Virtual Data Warehouse (VDW). Briefly, the VDW is a set of data standards and programs applied at each U.S. site to their raw administrative and billing data, ensuring data compatibility and harmonization for multi-site studies.(22) In Ontario, Down syndrome diagnoses were identified using ICD-10th edition (Canadian modification) from the Canadian Institute for Health Information’s Discharge Abstract Database of all inpatient hospital discharges in the province.
There is currently no universally used or validated definition for Down syndrome identification from U.S. healthcare system electronic records.(23) Most U.S. children with Down syndrome diagnosis codes had these codes on many unique examination days (mean=59.3 days, median=28, standard deviation=84). For children who had Down syndrome diagnosis codes on fewer than 5 unique days (N=257, 16.14%), we reviewed a sample of clinical records (N=48) from two sites to determine the accuracy of identifying Down syndrome when there were fewer than five diagnosis codes. Down syndrome was confirmed through chart review in 2/20 (10%) of children with only one code, 5/14 (35.7%) with codes on two days, 6/6 (100%) with codes on three days, and 7/8 (87.5%) with codes on four days. Based on this review, we considered 1,280 children to have Down syndrome in the U.S. cohort if they had diagnosis codes on at least three unique days or had Down syndrome confirmed during detailed chart review. We classified 990,241 children as not having Down syndrome in the U.S. cohort if they had no Down syndrome diagnostic codes or identified with no Down syndrome during chart review. Children with Down syndrome codes on only one or two days were excluded from analysis (N=224).
In Ontario, we considered children with at least one Down syndrome diagnosis code during a hospital stay to have Down syndrome based on a standard, previously validated definition.(24–27) A total of 31.4% children with Down syndrome only had a single diagnostic code from a hospital admission in this study period. This was different than what we found in the U.S. ascertainment process because Ontario records were counted only during hospital stays. Using these methods, we identified 4,401 children with and 3,900,998 without Down syndrome in our combined U.S. and Ontario cohort.
Primary Outcome and Potential Confounding Variables
The primary outcome was an incident diagnosis of primary leukemia with no previous diagnosis of any cancer. For U.S. sites, cancer diagnoses were obtained through linkage to local, regional and/or state tumor registries. In Ontario, cancer diagnoses were obtained from the Pediatric Oncology Group of Ontario Networked Information System (POGONIS)(28), a registry of all cancer cases diagnosed in the five Ontario pediatric centers. Leukemias were classified following Surveillance Epidemiology and End Results mapping of International Classification of Childhood Cancer-3 categories to the 2008 World Health Organization classification(29, 30) (Table I; available at www.jpeds.com) into: ALL, AML, chronic leukemia and myeloproliferative disorders, myelodysplastic syndrome (MDS), and unspecified and other specified leukemia. AML was subdivided into AML-M7 (more common in children with Down syndrome) and other AML. Transient myeloproliferative disorder, which occurs in about 10% of infants with Down syndrome and is often referred to as “pre-leukemia,”(31) was not considered leukemia.
Table 1.
Classification of leukemia morphology codes.
| Subtype | Morphology codes |
|---|---|
| Acute lymphoid leukemias | 9727–9729, 9811, 9813–9818, 9820, 9821, 9826, 9827, 9832–9837, 9848 |
| Acute myeloid leukemias | 9742, 9840, 9860, 9861, 9865–9867, 9869, 9871–9874, 9891, 9895–9897, 9911, 9920, 9930 |
| Megakaryoblastic leukemia (AML-M7) | 9898, 9910 |
| Chronic myeloproliferative disorders and leukemias | 9803, 9831, 9863, 9868, 9875, 9876, 9940, 9945, 9946, 9950, 9960–9966 |
| Myelodysplastic syndrome and other myeloproliferative disorders | 9975, 9980, 9982–9987, 9989, 9991, 9992 |
| Unspecified and other specified leukemias | 9733, 9751, 9800, 9801, 9805–9809, 9864, 9931 |
Morphology codes obtained from Surveillance, Epidemiology, and End Results Program(29)
Children’s characteristics including birth year, sex, and maternal age at birth; competing events (deaths and other cancer diagnoses); and censoring events (disenrollment dates for U.S. sites and end of eligibility from the Ontario provincial health insurance) were obtained from the VDW for U.S. sites and administrative databases at ICES (including POGONIS) for Ontario. Race and ethnicity were available only for U.S. sites.
Statistical analyses
Children were followed from birth until any cancer diagnosis, death, age 15 years, end of outcome capture due to U.S. healthcare system disenrollment or end of eligibility from the Ontario provincial health insurance plan because they moved out of the province, or end of follow up (December 31, 2016), whichever occurred first. We calculated descriptive characteristics and crude incident rates for the entire cohort and stratified by Down syndrome status and leukemia outcomes. We estimated unadjusted incidence and cumulative incidence of leukemia by age overall and by leukemia subtype in children with and without Down syndrome accounting for competing risks of death or other cancers.
Associations between Down syndrome and leukemia (overall and by subtype) were calculated using the Fine and Gray extension of the Cox proportional hazards model to account for competing risks.(32) We stratified models by health system site and adjusted for birth year and sex. We assessed effect modification by age at leukemia diagnosis (ie, non-proportional hazards) by including a time-dependent interaction between Down syndrome and age group (<5 and ≥5 years for leukemia types based on incidence curve peaks, Figure 1). Effect modification of the association between Down syndrome and leukemia risk by birth year was explored by including an interaction term between Down syndrome and birth year grouped into four categories: 1996–2000, 2001–2005, 2006–2010, 2011–2016. For this model, we restricted follow-up to a fixed age (<5 years) to avoid confounding by age, because children born more recently had shorter follow-up. We conducted sensitivity analyses adjusting for maternal age or children’s race among children with non-missing information.
Figure 1. Leukemia incidence (per 100,000 children) by subtype and age for children with Down syndrome (A) and other children (B), and cumulative leukemia incidence (per 100,000) through age 14 overall and by subtype for children with Down syndrome (C) and other children (D).
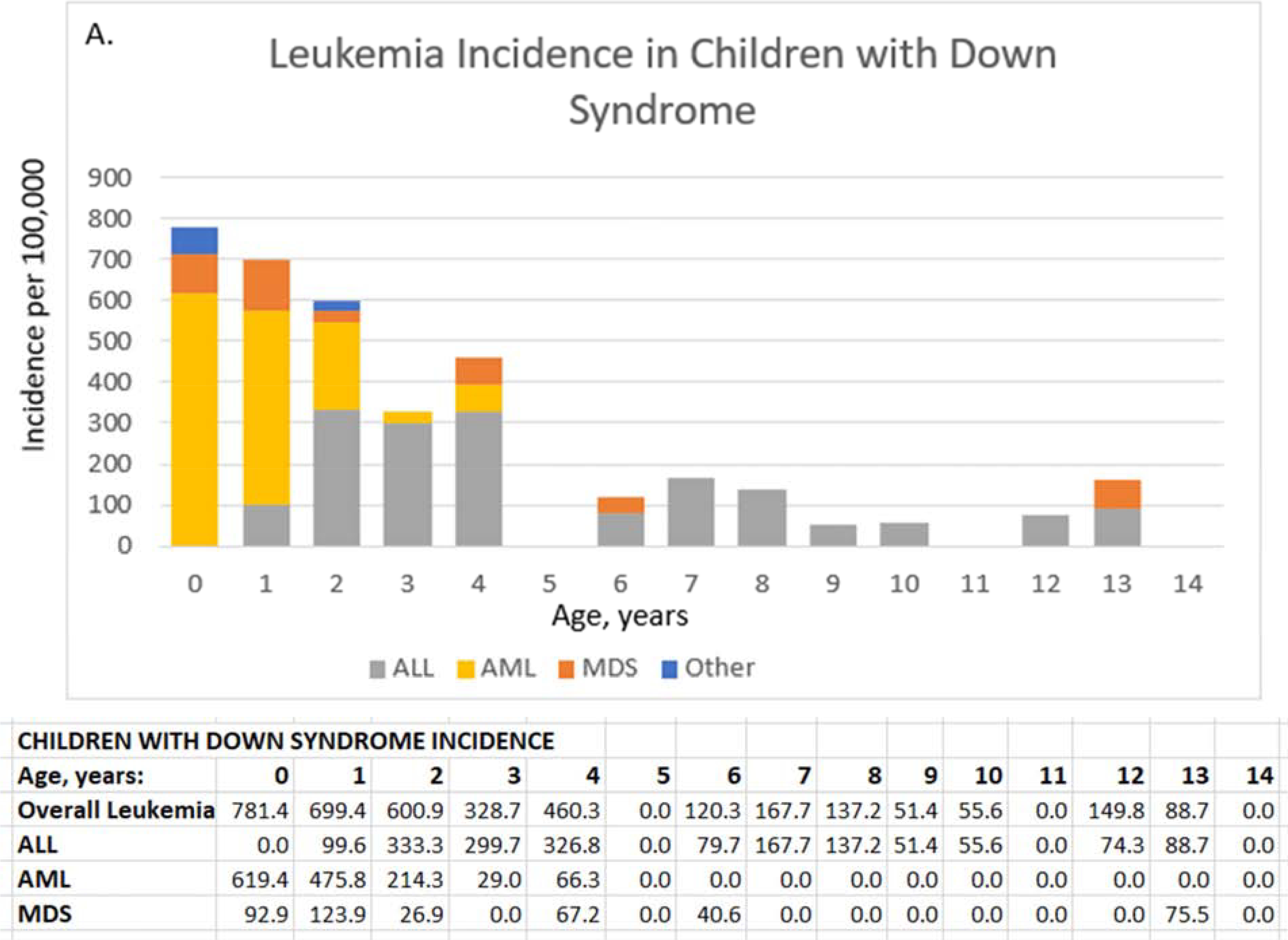
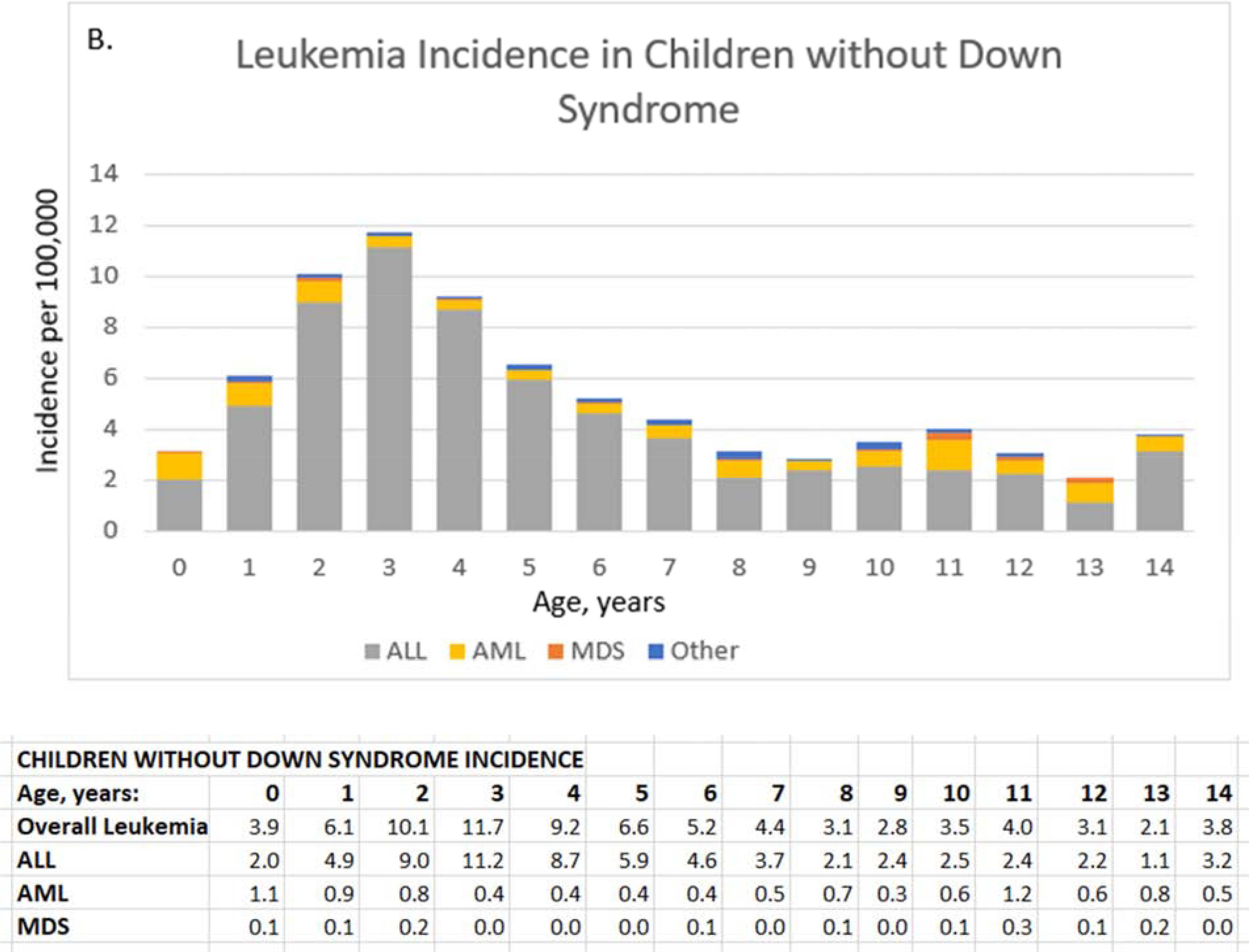
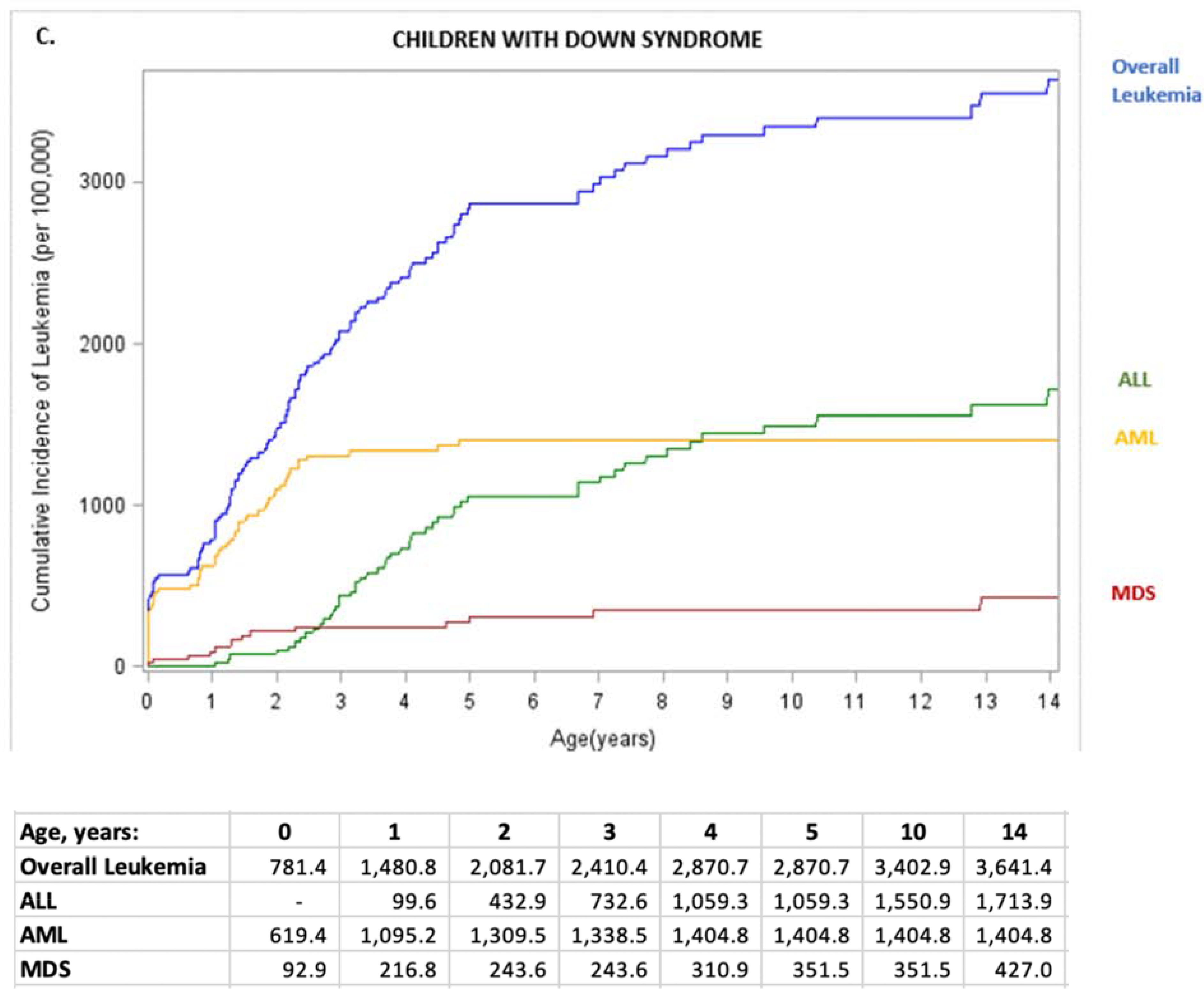
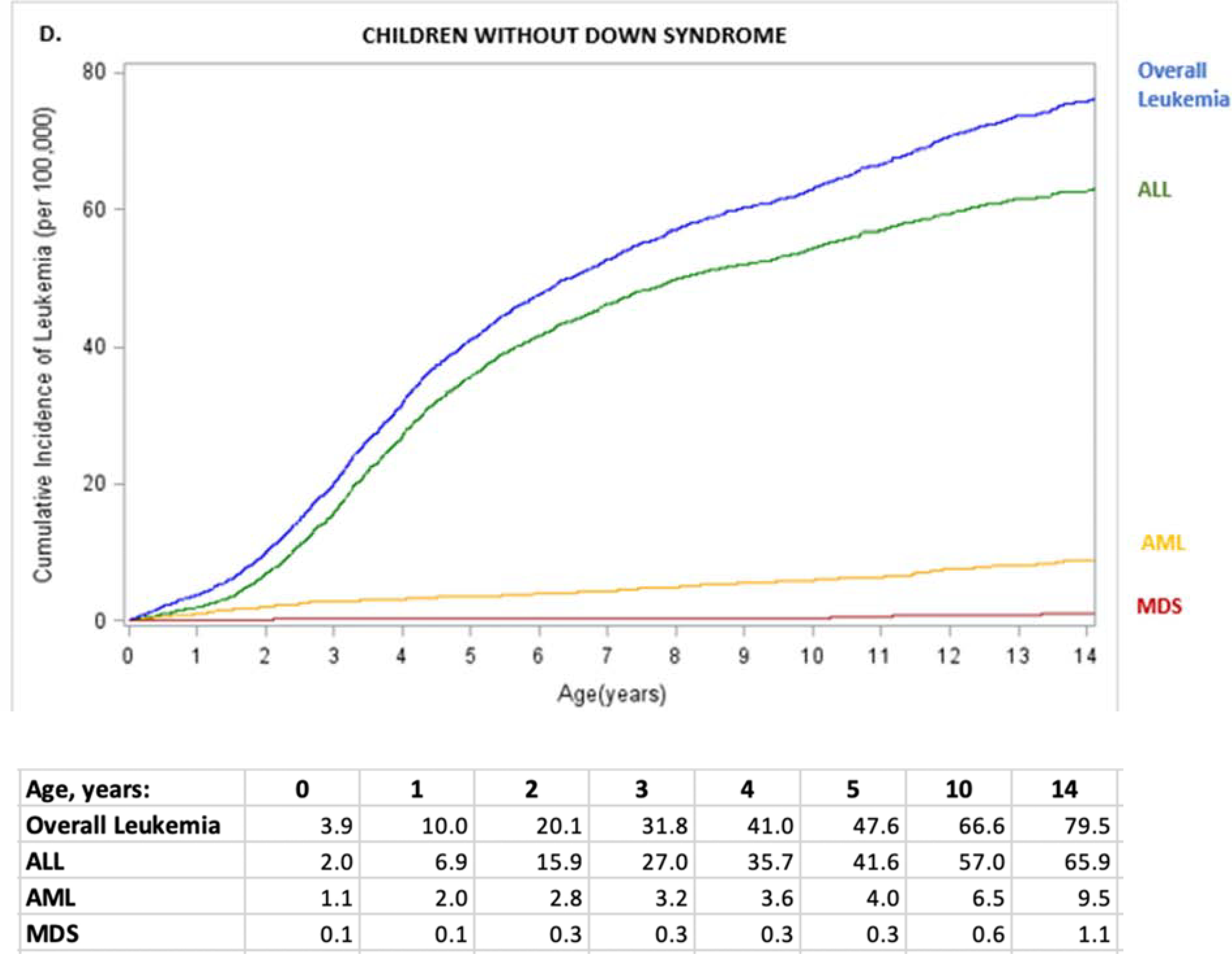
ALL, acute lymphoid leukemia; AML, acute myeloid leukemia; MDS, myelodysplastic syndrome. ALL, acute lymphoid leukemia; AML, acute myeloid leukemia; MDS, myelodysplastic syndrome; Other, chronic and other leukemia; overall leukemia includes ALL, AML, MDS, and Other. Values for other leukemias are small and suppressed from the tables.
We fit separate models for the two countries because privacy restrictions prevented sharing individual level data from Ontario to the U.S. authors. Hazard ratios (HRs) were combined by calculating the weighted average on the log-scale, weighting inversely by the standard error.(33)
We used a two-sided alpha 0.05 to evaluate statistical significance. Statistical analyses used SAS software, version 9.4. R software(34) was used to combine U.S. and Ontario HRs.
Results
Down syndrome was diagnosed in 4,401 children (incidence 1.12/1,000 children [95% CI=1.09–1.16], Table 2). Approximately 65% of children were followed for ≥5 years and 40% for ≥10 years. Children with vs. without Down syndrome were more often male (54.7% vs. 51.3%) and more likely to have mothers age 40 years or older at the time of the child’s birth (18.6% vs. 3.7%). Down syndrome incidence varied 10-fold with mother’s age, ranging from 0.56/1,000 for mothers <20 years old to 5.51/1,000 for mothers ≥40 years of age. In the U.S. cohort, compared with other children, children with Down syndrome were more often white (73.1% vs. 65.0%), less often Asian (19.8% vs. 26.8%), and more often ethnically Hispanic (24.8% vs. 19.3%). Children with vs. without Down syndrome were more likely to be diagnosed with leukemia (2.8% vs. 0.05%), another cancer (0.5% vs. 0.1%), or die (4.0% vs. 0.3%) during follow-up. Four children with Down syndrome were diagnosed with a solid cancer: two with yolk sac tumor, one with Myxoid Liposarcoma, and one with retinoblastoma.
Table 2.
Characteristics of children without and with Down syndrome in the study cohort.
| Children with Down Syndrome | Children without Down Syndrome | Down Syndrome Prevalence per 1,000 children* | ||||
|---|---|---|---|---|---|---|
| N | Column %† | N | Column %† | (95% CI) | ||
| Total Site | 4,401 | 3,900,998 | 1.12 | (1.09–1.16) | ||
| U.S. Site 1 | 42 | 1.0 | 49,446 | 1.3 | 0.84 | (0.59–1.10) |
| U.S. Site 2 | 53 | 1.2 | 38,147 | 1.0 | 1.39 | (1.01–1.76) |
| U.S. Site 3 | 71 | 1.6 | 34,586 | 0.9 | 2.05 | (1.57–2.53) |
| U.S. Site 4 | 127 | 2.9 | 92,799 | 2.4 | 1.37 | (1.13–1.60) |
| U.S. Site 5 | 131 | 3.0 | 92,291 | 2.4 | 1.42 | (1.17–1.66) |
| U.S. Site 6 | 171 | 3.9 | 133,671 | 3.4 | 1.28 | (1.09–1.47) |
| U.S. Site 7 | 685 | 15.6 | 549,301 | 14.1 | 1.25 | (1.15–1.34) |
| Ontario | 3,121 | 70.9 | 2,910,757 | 74.6 | 1.07 | (1.03–1.11) |
| Age at End of Follow Up in Years | ||||||
| <1 | 416 | 9.5 | 281,534 | 7.2 | 1.48 | (1.33–1.62) |
| 1 | 376 | 8.5 | 335,564 | 8.6 | 1.12 | (1.01–1.23) |
| 2 | 332 | 7.5 | 279,480 | 7.2 | 1.19 | (1.06–1.31) |
| 3 | 309 | 7.0 | 244,026 | 6.3 | 1.26 | (1.12–1.41) |
| 4 | 265 | 6.0 | 223,216 | 5.7 | 1.19 | (1.04–1.33) |
| 5 – 9 | 990 | 22.5 | 936,017 | 24.0 | 1.06 | (0.99–1.12) |
| 10 – 14 | 1,713 | 38.9 | 1,601,161 | 41.0 | 1.07 | (1.02–1.12) |
| Sex | ||||||
| Male | 2,407 | 54.7 | 2,000,932 | 51.3 | 1.20 | (1.15–1.25) |
| Female | 1,994 | 45.3 | 1,900,066 | 48.7 | 1.05 | (1.00–1.09) |
| Maternal Age in Years at Child’s Birth | ||||||
| 10–19 | 74 | 1.9 | 131,238 | 3.7 | 0.56 | (0.44–0.69) |
| 20–29 | 826 | 21.2 | 1,456,990 | 41.4 | 0.57 | (0.53–0.61) |
| 30–39 | 2,272 | 58.3 | 1,798,159 | 51.1 | 1.26 | (1.21–1.31) |
| 40+ | 723 | 18.6 | 130,542 | 3.7 | 5.51 | (5.11–5.91) |
| Unknown | 506 | (11.5) | 384,069 | (9.8) | 1.32 | (1.20–1.43) |
| Race‡ | ||||||
| White | 681 | 73.1 | 428,038 | 65.0 | 1.59 | (1.47–1.71) |
| Black | 66 | 7.1 | 54,354 | 8.3 | 1.21 | (0.92–1.51) |
| Asian/Pacific Islander | 185 | 19.8 | 176,186 | 26.8 | 1.05 | (0.90–1.20) |
| Other/Unknown | 348 | (27.2) | 331,663 | (33.5) | 1.05 | (0.94–1.16) |
| Ethnicity‡ | ||||||
| Hispanic | 274 | 24.8 | 155,848 | 19.3 | 1.8 | (1.55–1.96) |
| Non-Hispanic | 831 | 75.2 | 650,435 | 80.7 | 1.3 | (1.19–1.36) |
| Unknown | 175 | (13.7) | 183,958 | (18.6) | 1.0 | (0.81–1.09) |
| Leukemia | ||||||
| Acute Lymphoid Leukemia (ALL) | 49 | 39.5 | 1,629 | 83.9 | 29 | (21.1–37.3) |
| Acute Myeloid Leukemia (AML) | 57 | 46.0 | 213 | 11.0 | 211 | (162.4–259.8) |
| AML-M7, % of AMLs | 29 | (50.9) | 24 | (11.3) | 547 | (413.2–681.2) |
| Other AML, % of AMLs | 28 | (49.1) | 189 | (88.7) | 129 | (84.4–173.6) |
| Chronic | 1 | 0.8 | 35 | 1.8 | 28 | (0.00–81.46) |
| MDS | 14 | 11.3 | 22 | 1.1 | 389 | (230.0–548.1) |
| Other Leukemias | 3 | 2.4 | 42 | 2.2 | 67 | (0.00–139.5) |
| Reason for end of follow-up | ||||||
| Leukemia | 124 | 2.8 | 1,941 | 0.05 | 60.0 | (49.8–70.3) |
| Other Cancer | 4 | 0.5 | 4,193 | 0.1 | 1.0 | (0.02–1.89) |
| Death | 178 | 4.0 | 12,110 | 0.3 | 14.5 | (12.37–16.60) |
| Disenrollment from Health System | 710 | 16.1 | 541,808 | 13.9 | 1.3 | (1.21–1.40) |
| Age 15 | 893 | 20.3 | 846,615 | 21.7 | 1.1 | (0.98–1.12) |
| End of Study | 2,492 | 56.6 | 2,494,331 | 63.9 | 1.0 | (0.96–1.04) |
Column percentages exclude “Unknown” unless specified by (%).
Race and ethnicity available only from U.S. sites.
Prevalence = Row percentage × 10
AML-M7, megakaryoblastic leukemia; MDS, myelodysplastic syndrome
A total of 2,065 children were diagnosed with leukemia, and Down syndrome was more prevalent among children with vs. without leukemia (5.7% vs. 0.1%; Table 3). Most (67%) leukemia diagnoses occurred before age 5. Compared with children without leukemia, children with leukemia were more often male (57% vs. 51%), white (73% vs. 65%), and Hispanic (23% vs. 19%).
Table 3.
Characteristics of children with and without leukemia diagnosis.
| Leukemia | Column n%† | No Leukemia | Column n%† | |
|---|---|---|---|---|
| Total Status | 2,065 | 3,903,334 | ||
| Down Syndrome | 124 | 5.7 | 4,277 | 0.1 |
| No Down Syndrome | 1,941 | 94.3 | 3,899,057 | 99.9 |
| Age at Diagnosis or End of Follow-up in years | ||||
| <1 | 180 | 9 | 281,770 | 7 |
| 1 | 237 | 11 | 335,703 | 9 |
| 2 | 342 | 17 | 279,470 | 7 |
| 3 | 352 | 17 | 243,983 | 6 |
| 4 | 259 | 13 | 223,222 | 6 |
| 5 – 9 | 488 | 24 | 936,519 | 24 |
| 10 – 14 | 207 | 10 | 1,602,667 | 41 |
| Birth Year | ||||
| 1996–2000 | 583 | 28 | 881,268 | 23 |
| 2001–2005 | 620 | 30 | 929,245 | 24 |
| 2006–2010 | 577 | 28 | 961,798 | 25 |
| 2011–2016 | 285 | 14 | 1,131,023 | 29 |
| Sex | ||||
| Male | 1,167 | 57 | 2,002,172 | 51 |
| Female | 898 | 43 | 1,901,162 | 49 |
| Maternal Age in Years at Child’s Birth | ||||
| 10–19 | 68 | 4 | 131,244 | 4 |
| 20–29 | 787 | 41 | 1,457,029 | 41 |
| 30–39 | 955 | 50 | 1,799,476 | 51 |
| 40+ | 93 | 5 | 131,172 | 4 |
| Unknown | 162 | (7.8) | 384,413 | (9.8) |
| Race‡ | ||||
| White | 278 | 73 | 428,441 | 65 |
| Black | 22 | 6 | 54,398 | 8 |
| Asian | 83 | 22 | 176,288 | 27 |
| Other/Unknown | 24 | (5.9) | 331,987 | (33.5) |
| Ethnicity‡ | ||||
| Hispanic | 89 | 23 | 156,033 | 19 |
| Non-Hispanic | 302 | 77 | 650,964 | 81 |
| Unknown | 16 | (3.9) | 184,117 | (18.6) |
Column percentages exclude “Unknown” unless specified by (%).
Race and ethnicity available only from U.S. sites.
Incidence
Leukemia incidence by age differed by whether child had Down syndrome (Figure 1). In children with Down syndrome, ALL was more common between ages 2–4 years (333.3 to 326.8/100,000), and AML was more common at younger ages, with highest incidence during the first year of life (619.4/100,000) (Figure 1, A). For other children, AML incidence remained very low (≤1.1/100,000 person-years) through age 14 years whereas ALL peaked at age 3 years (11.2/100,000) and steadily declined to 2.1/100,000 at age 8 years with small fluctuations between ages 9 and 14 years (1.1 to 3.2/100,000) (Figure 1, B).
Cumulative Incidence
The cumulative incidence of leukemia up through age 14 years differed for children with Down syndrome vs. other children for each subtype (Figure 1, C and D). In children with Down syndrome, AML cumulative incidence increased sharply through age 2 years (1,309.5/100,000, 95% CI=996–1,694) with no increase between ages 4 and 14 years (Figure 1, C). ALL cumulative incidence increased with age, surpassing the incidence of AML at age 8 years with 1,404.8/100,000 (95% CI= 1,076–1,806) cases. By 14 years, 3,641.4/100,000 (95% CI=3,015–4,353) children with Down syndrome had a diagnosis of any leukemia.
In children without Down syndrome, AML cumulative incidence gradually increased with age: 3.6/100,000 at age 4 years (95% CI=3–4), 5.9/100,000 at age 9 years (95% CI=5–7), and 9.5/100,000 age 14 years (95% CI=8–11) (Figure 1, D). In contrast, ALL cumulative incidence increased more rapidly with age to 35.7/100,000 at age 4 years (95% CI=34–38), 54.4/100,000 at age 9 years (95% CI=52–57), and 65.9/100,000 at age 14 years (95% CI=63–69). By age 14 years, 79.5/100,000 (95% CI=76–83) children without Down syndrome had a diagnosis of any leukemia.
Hazard Ratios
HRs for leukemia risk associated with Down syndrome were similar for the U.S. and Ontario across leukemia subtypes (Figure 2 and Table 4 [available at www.jpeds.com]). For combined estimates, the risk of any leukemia diagnosis associated with Down syndrome was greatest in children age <5 years (HR=75, 95% CI=62–91) compared with children age ≥5 years (HR=18, 95% CI=8–43). The strongest association was observed in AML-M7 for ages <5 years (HR=1,500, 95% CI=555–4,070); the strong positive association was still present with AML even after excluding AML-M7 cases at ages <5 years (HR=197, 95% CI=70–553). The risk of ALL associated with Down syndrome for children <5 years (HR=28, 95% CI=20–40). HRs were also large for MDS leukemias at age <5 years (HR=645, 95% CI=186–2240). HRs were inestimable or lower for older groups than younger groups but still large for ALL age ≥5 (HR=21, 95% CI=12–38). In sensitivity analyses, HRs were similar or slightly larger after additional adjustment for maternal age and/or race (Table 5; available at www.jpeds.com).
Figure 2. Forest plot comparing hazard ratios from U.S. sites, Ontario, and pooled analyses for association of Down syndrome vs. no Down syndrome with leukemia risk by age, overall and by leukemia subtype.
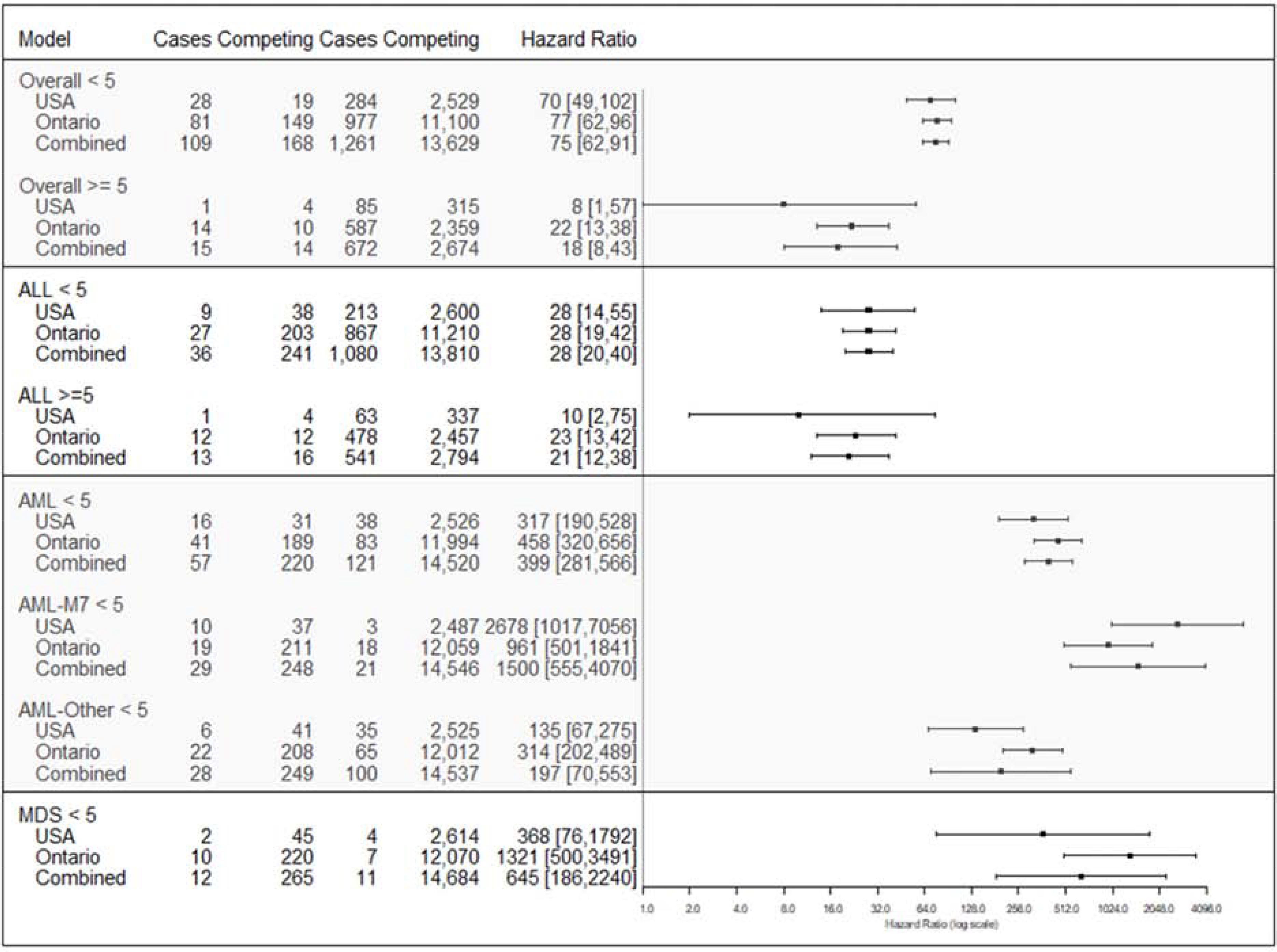
ALL, acute lymphoid leukemia; AML, acute myeloid leukemia; AML-M7, megakaryoblastic leukemia; MDS, myelodysplastic syndrome; HR, hazard ratio; CI, confidence interval. We could not calculate AML ≥ 5 due to small sample size. Adjusted by sex for both U.S. and Ontario estimates and for site for U.S. estimates. X-axis is on log scale.
Table 5.
Sensitivity analysis comparing hazard ratios adjusted for maternal age (combined United States and Ontario) and race and ethnicity (United States only).
| Maternal Age Adjusted U.S. Ontario | Unadjusted Comparison U.S. Ontario | Maternal Age, Race, Ethnicity Adjusted U.S. | Unadjusted Comparison U.S. | |||||
|---|---|---|---|---|---|---|---|---|
| Leukemia | Hazard Ratio | 95% Confidence Interval | Hazard Ratio | 95% Confidence Interval | Hazard Ratio | 95% Confidence Interval | Hazard Ratio | 95% Confidence Interval |
| Overall | ||||||||
| age < 5y | 76 | (62, 93) | 75 | (62, 91) | 73 | (48, 111) | 70 | (49, 102) |
| age ≥ 5y | 21 | (13, 36) | 18 | (8, 43) | 8 | (1, 56) | 8 | (1, 57) |
| ALL | ||||||||
| age < 5y | 31 | (22, 43) | 28 | (20, 40) | 33 | (17, 66) | 28 | (14, 55) |
| age ≥ 5y | 22 | (12, 39) | 21 | (12, 38) | 10 | (1, 72) | 10 | (2, 75) |
| AML | ||||||||
| age < 5y | 401 | (287, 560) | 399 | (281, 566) | 289 | (153, 545) | 317 | (190, 528) |
| AML - M7 | ||||||||
| age < 5y | 939 | (411, 2150) | 1,500 | (555, 4070) | 2,854 | (833, 9776) | 2,678 | (1017, 7056) |
| AML - Other | ||||||||
| age < 5y | 188 | (54, 656) | 197 | (70, 553) | 86 | (34, 218) | 135 | (67, 275) |
ALL, acute lymphoid leukemia; AML, acute myeloid leukemia; AML7, megakaryoblastic leukemia; MDS, myelodysplastic syndrome; y, years; CI, confidence interval
Discussion
In a large, contemporary cohort of 3.9 million children, we found that the leukemia risks were greatly elevated in children with Down syndrome, and for some leukemia subtypes the risks were greater than previously reported.(3–5, 7, 35) Before age 15 years, 36 in 1,000 children with Down syndrome were diagnosed with a leukemia compared with 0.8 in 1,000 other children. The associations between Down syndrome and leukemia risk were strongest for AML, especially AML-M7, and in children <5 years.
Down syndrome-associated risks were higher and occurred earlier for AML compared with ALL. Through age 4 years, 14 in 1,000 children with Down syndrome were diagnosed with AML, with no diagnoses after that age. Through age 4 years, almost 11 in 1,000 children with Down syndrome were diagnosed with ALL, and incidence continued to increase after that age. ALL also gradually increased with age in other children. Our findings are similar to those reported using data from 1997–2002 by the Children’s Oncology Group that showed the age distribution for ALL in children with Down syndrome was similar to the general population with peak incidence at age 5 years.(3) Our results are also consistent with the Danish Cytogenetic Registry(7) evaluation of 2,814 children born with Down syndrome in 1968–1995, in which all AML cases occurred in children under age 3 years, though our HR for overall leukemia risk in children with Down Syndrome age <5 years of 75 (95% CI=62, 91) is higher than their standardized incidence ratio of 56 (95% CI=38, 81), possibly due to the more recent years of our cohort.
Our study of 124 leukemia cases in 4,401 children with Down syndrome is one of the largest studies to date on this topic. A larger linkage study of children born with birth defects including Down syndrome(35) also utilized a contemporary cohort of children born from 1992–2013; however, they did not assess leukemia risks by age, nor did they distinguish AML-M7 separately from other AML cases. We found stronger associations between Down syndrome and any leukemia in children aged <5 years (HR=75 [95% CI=62–91]) than the previously reported 10- to 20-fold increases.(3) In our study, children <5 years with Down syndrome had nearly 400-times higher risk of AML than other children. Previous reports cited AML relative risks as high as 150 for children age <5 years(4) and 125 in children age <18 years.(35) We also found a much higher AML-M7 hazard ratios of 1,500 (95% CI=555–4,070) than previous estimates of a 500–600 fold increase in children age <5 years with Down Syndrome.(3, 7, 36) Our estimate of approximately 28-times higher ALL risk for children age <5 years with Down syndrome was similar to reports of 28–40 in earlier studies.(4, 35)
Although the mechanism explaining why children with Down syndrome are more likely to get leukemia is unknown, several theories may at least partially explain their higher risk. In cell-line and gene expression studies, trisomy 21 directly causes genetic defects that are associated with development of abnormalities in lymphopoiesis and would be associated with leukemia.(37) Expression of a range of functions coded on chromosome 21 can cause aberrations of cell function that make the cells susceptible to transformation, and along with associated abnormalities of DNA repair, increase susceptibility to leukemia.(4, 38–42) Prior studies have also suggested that increased susceptibility of childhood infections in early life, maternal and paternal exposures such as alcohol consumption and pesticide exposure, administration and/or high frequency of medical imaging, or vitamin and mineral deficiencies may contribute to increased risk of childhood leukemia in children with Down syndrome.(3, 43–46) Increased risk for AML in children with Down syndrome has also been suggested to be linked with maternal reproductive history, infertility, and alcohol consumption.(3, 47)
Ionizing radiation exposure from in utero(48) and childhood imaging has increased over the past two decades,(18, 49, 50) and studies suggest an association between low-dose imaging radiation and pediatric leukemia risk. However these studies did not consistently evaluate exposures in children with Down syndrome.(20, 51–54) Studying whether increased exposure to low-dose ionizing radiation from imaging contributes to leukemia risk in children with Down syndrome is important, because imaging with ionizing radiation is often used to diagnose and manage their early-life health issues.(55–57) If exposure to ionizing radiation is shown to be associated with leukemia risk in this children, other modes of imaging that utilizing non ionizing radiation, such as ultrasound and MRI, would be used instead as the first line image tests.
Few known potential confounders are associated with both Down syndrome and childhood leukemia. Maternal age is related to both(58–64); however, in sensitivity analysis adjusting for maternal age, results did not substantially change. White children have a higher incidence rate of ALL than black children that is not explained by socioeconomic status,(65) and white children in our study were more likely to have Down syndrome (1.59/1,000) than Black children (1.21/1,000); however, our hazard ratios were similar after adjusting for race in sensitivity analysis. In our cohort, we also found that males and Hispanic children were more likely to be diagnosed with Down syndrome and more likely to develop leukemia than their counterparts. We adjusted for sex in our main models and did not observe meaningful changes after adjusting for ethnicity in our sensitivity analysis. It is possible that unmeasured confounding factors may explain the increased leukemia risk in Down syndrome children; however, we believe that this is unlikely given the very strong associations we found.(66)
A strength of our study is the large cohort with more Down syndrome leukemia cases than most previous studies. This allowed more precise risk estimation, especially for rare AML-7, which was previously estimated from small case reports.(12) Following children since birth also allowed for complete capture of leukemia and other cancer diagnoses and deaths from other causes.
A potential limitation of the study was the use of different definitions for identifying children with Down syndrome at U.S. sites and in Ontario. For U.S. sites, we ascertained Down syndrome diagnosis codes from all healthcare encounters and specifically required codes from at least three unique days unless chart reviewed. In Ontario, Down syndrome diagnosis codes were available only from hospital visits; we followed a standard validated definition of requiring only one Down syndrome code from an inpatient setting.(24–27) Thus, we may have misclassified some children in Ontario as not having Down syndrome if they were never hospitalized or some children as having Down syndrome if they only had a single diagnosis code at a hospital visit. Children with leukemia were also probably more likely to be hospitalized so Down syndrome diagnoses may be coded in these children more often than children without leukemia. Misclassification would bias Ontario results to the null for weaker associations compared with the U.S. sites.
The two countries had different Down syndrome prevalence rates for children under age 15 (Table 2). The Down syndrome prevalence in Ontario was 1.07/1,000 (95% CI 1.03/1,000 – 1.11/1,000), which was lower than 6 of the 7 U.S. sites (range 1.25–2.05/1000). This difference in prevalence rates might suggest the results from the two countries should not be combined. However, the two countries’ widely overlapping hazard ratio confidence intervals in Figure 2 suggests that the small differences in Down syndrome prevalence rates did not result in meaningfully different associations between Down syndrome status and childhood leukemia. This finding supported our decision to pool results from these two countries together for this study.
Examining Down syndrome in children born into U.S. healthcare systems may also limit the generalizability of this study to children with health insurance; however, six of the seven U.S. sites included data on children with Medicaid. This study design also has an advantage because it ensured complete capture of the participant’s medical history. For example, this enabled us to exclude children with a prior cancer diagnosis, which is important because some chemotherapy drugs increase leukemia risk. Furthermore, the Ontario cohort makes up nearly 75% of our data and consisted of all children born in the province with complete capture of medical history. Therefore, we believe our results are generalizable because findings from these two cohorts are consistent (Figure 2).
Overall and despite these possible limitations, our estimated age-specific incidence of ALL in children <15 years without Down syndrome is consistent with estimates for the general population (3–4/100,000 with peak incidence at age 2–5 years).(67) In addition, our finding that ALL was more common in children without Down syndrome is consistent with general population incidence rates showing ALL is five times more frequent than AML under age 15 years(67) with peak incidence under age 1 year.(68) Thus, the higher rates of leukemia in our cohort appear valid and not due to potential artifact such as diagnostic coding errors.
In a large, contemporary cohort of children from the U.S. and Ontario, Canada, we found a stronger association between Down syndrome and AML risk than previously reported. The effect of Down syndrome on leukemia risk was largest for AML-M7 and for AML diagnoses in children under age 5 years. Associations between Down syndrome and ALL were consistent with previous reports. Further research is warranted to investigate why our study’s AML rates for children with Down syndrome were much higher than previous reports and whether they are related to identifiable exposures such as ionizing radiation from medical imaging.
Acknowledgments
We thank Chris Tachibana (KP Washington) who provided scientific editing. We acknowledge the contributions of the study team analysts: Glen Buth, BAAS (Marshfield Clinic Health System), Melanie Francisco, PhD (KP Northwest), Matthew Lakoma, MPH (Harvard Pilgrim Health Care), Dustin W. Ballard, BS (Geisinger), Joanne M. Mor, MS (KP Hawaii), M. Kay Theis, MS (KP Washington), and Kamala A. Deosaransingh, MPH (KP Northern California). We acknowledge the contributions of the project coordinators/managers: Prachi Chavan, MPH (University of California, San Francisco), Charisma L. Jenkins, PSM (KP Northwest), Yolanda Prado, BS (KP Northwest), Casey Luce, MSPH (KP Washington), Deborah Multerer, BA (Marshfield Clinic Health System), Deanna Jarrett, MS (Geisinger), Mallory Snyder, BA (Geisinger), Yannica S. Martinez, MS (KP Hawaii), Giancarlo Di Giuseppe, MPH (Pediatric Oncology Group of Ontario), Cindy Fong, BSc, CCRP (ICES), Julie R. Munneke, BA (KP Northern California), Lisa M. Moy, MPH (KP Northern California), and Diana Ly (University of California, San Francisco). The abovenamed contributors did not receive compensation outside regular employment pay.
Funded by the National Cancer Institute (R01CA185687). E.B.’s time was funded by the National Cancer Institute (R50CA211115). This study was partially supported by ICES, which is funded by an annual grant from the Ontario Ministry of Health and Long-Term Care (MOHLTC). Parts of this material are based on data and/or information compiled and provided by the Canadian Institute for Health Information (CIHI). This research was facilitated by the Pediatric Oncology Group of Ontario’s Networked Information System, financially supported by Ontario’s Ministry of Health and Long-Term Care. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, ICES, the Ontario MOHLTLC, or CIHI. The authors declare no conflicts of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Parker SE, Mai CT, Canfield MA, Rickard R, Wang Y, Meyer RE, et al. Updated National Birth Prevalence estimates for selected birth defects in the United States, 2004–2006. Birth defects research Part A, Clinical and molecular teratology. 2010;88(12):1008–16. [DOI] [PubMed] [Google Scholar]
- 2.Canada S. Canada [Country] and Canada [Country] (table) Ottawa: Statistics Canada Catalogue 2017. [November 29, 2017]. Available from: https://www12.statcan.gc.ca/census-recensement/2016/dp-pd/prof/index.cfm?Lang=E.
- 3.Ross JA, Spector LG, Robison LL, Olshan AF. Epidemiology of leukemia in children with Down syndrome. Pediatr Blood Cancer. 2005;44(1):8–12. [DOI] [PubMed] [Google Scholar]
- 4.Webb D, Roberts I, Vyas P. Haematology of Down syndrome. Archives of disease in childhood Fetal and neonatal edition. 2007;92(6):F503–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fong CT, Brodeur GM. Down’s syndrome and leukemia: epidemiology, genetics, cytogenetics and mechanisms of leukemogenesis. Cancer genetics and cytogenetics. 1987;28(1):55–76. [DOI] [PubMed] [Google Scholar]
- 6.Ran YN, Yu J, Xian Y, Wen XH, Guo YX, Guan XM, et al. [Acute leukemia associated with Down syndrome: clinical analysis of 21 cases]. Nan fang yi ke da xue xue bao = Journal of Southern Medical University. 2016;36(3):433–6. [PubMed] [Google Scholar]
- 7.Hasle H, Clemmensen IH, Mikkelsen M. Risks of leukaemia and solid tumours in individuals with Down’s syndrome. Lancet. 2000;355(9199):165–9. [DOI] [PubMed] [Google Scholar]
- 8.Robison LL, Nesbit ME Jr., Sather HN, Level C, Shahidi N, Kennedy A, et al. Down syndrome and acute leukemia in children: a 10-year retrospective survey from Childrens Cancer Study Group. The Journal of pediatrics. 1984;105(2):235–42. [DOI] [PubMed] [Google Scholar]
- 9.Kojima S, Matsuyama T, Sato T, Horibe K, Konishi S, Tsuchida M, et al. Down’s syndrome and acute leukemia in children: an analysis of phenotype by use of monoclonal antibodies and electron microscopic platelet peroxidase reaction. Blood. 1990;76(11 ):2348–53. [PubMed] [Google Scholar]
- 10.Creutzig U, Reinhardt D, Diekamp S, Dworzak M, Stary J, Zimmermann M. AML patients with Down syndrome have a high cure rate with AML-BFM therapy with reduced dose intensity. Leukemia. 2005;19(8):1355–60. [DOI] [PubMed] [Google Scholar]
- 11.Zipursky A, Peeters M, Poon A. Megakaryoblastic leukemia and Down’s syndrome: a review. Pediatric hematology and oncology. 1987;4(3):211–30. [DOI] [PubMed] [Google Scholar]
- 12.Zipursky A, Thorner P, De Harven E, Christensen H, Doyle J. Myelodysplasia and acute megakaryoblastic leukemia in Down’s syndrome. Leukemia research. 1994;18(3):163–71. [DOI] [PubMed] [Google Scholar]
- 13.Feng Q, de Smith AJ, Vergara-Lluri M, Muskens IS, McKean-Cowdin R, Kogan S, et al. Trends in Acute Lymphoblastic Leukemia Incidence in the US from 2000–2016: an Increased Risk in Latinos Across All Age Groups. American journal of epidemiology. 2020. [DOI] [PubMed] [Google Scholar]
- 14.Barrington-Trimis JL, Cockburn M, Metayer C, Gauderman WJ, Wiemels J, McKean-Cowdin R. Trends in childhood leukemia incidence over two decades from 1992 to 2013. International journal of cancer. 2017;140(5):1000–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xie Y, Davies SM, Xiang Y, Robison LL, Ross JA. Trends in leukemia incidence and survival in the United States (1973–1998). Cancer. 2003;97(9):2229–35. [DOI] [PubMed] [Google Scholar]
- 16.Smith-Bindman R, Kwan ML, Marlow EC, Theis MK, Bolch W, Cheng SY, et al. Trends in Use of Medical Imaging in US Health Care Systems and in Ontario, Canada, 2000–2016. Jama. 2019;322(9):843–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith-Bindman R, Kwan ML, Marlow EC, Theis MK, Bolch W, Cheng SY, et al. Trends in Use of Medical Imaging in US Health Care Systems and in Ontario, Canada, 2000–2016. (1538–3598 (Electronic)). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miglioretti DL, Johnson E, Williams A, Greenlee RT, Weinmann S, Solberg LI, et al. The use of computed tomography in pediatrics and the associated radiation exposure and estimated cancer risk. JAMA Pediatr. 2013;167(8):700–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hong J-Y, Han K, Jung J-H, Kim JS. Association of Exposure to Diagnostic Low-Dose Ionizing Radiation With Risk of Cancer Among Youths in South Korea. JAMA Network Open. 2019;2(9):e1910584–e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Berrington de Gonzalez A, Salotti JA, McHugh K, Little MP, Harbron RW, Lee C, et al. Relationship between paediatric CT scans and subsequent risk of leukaemia and brain tumours: assessment of the impact of underlying conditions. Br J Cancer. 2016;114(4):388–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meulepas JM, Ronckers CM, Merks J, Weijerman ME, Lubin JH, Hauptmann M. Confounding of the association between radiation exposure from CT scans and risk of leukemia and brain tumors by cancer susceptibility syndromes. Journal of radiological protection : official journal of the Society for Radiological Protection. 2016;36(4):953–74. [DOI] [PubMed] [Google Scholar]
- 22.Ross TR, Ng D, Brown JS, Pardee R, Hornbrook MC, Hart G, et al. The HMO Research Network Virtual Data Warehouse: A Public Data Model to Support Collaboration. EGEMS (Washington, DC). 2014;2(1):1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jensen KM, Cooke CR, Davis MM. Fidelity of Administrative Data When Researching Down Syndrome. Medical care. 2014;52(8):E52–E7. [DOI] [PubMed] [Google Scholar]
- 24.Wen SW, Rouleau J, Lowry RB, Kinakin B, Anderson-Redick S, Sibbald B, et al. Congenital anomalies ascertained by two record systems run in parallel in the Canadian Province of Alberta. Canadian journal of public health = Revue canadienne de sante publique. 2000;91(3):193–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Irvine B, Luo W, León JA. [Google Scholar]
- 26.Agha MM, Glazier RH, Moineddin R, Moore AM, Guttmann A. Socioeconomic status and prevalence of congenital heart defects: does universal access to health care system eliminate the gap? Birth defects research Part A, Clinical and molecular teratology. 2011;91(12):1011–8. [DOI] [PubMed] [Google Scholar]
- 27.Agha MM, Glazier RH, Moineddin R, Booth G. Congenital abnormalities in newborns of women with pregestational diabetes: A time-trend analysis, 1994 to 2009. Birth defects research Part A, Clinical and molecular teratology. 2016;106(10):831–9. [DOI] [PubMed] [Google Scholar]
- 28.POGO. POGONIS - Childhood Cancer Database 2018. [Available from: https://www.pogo.ca/research-data/pogonis-childhood-cancer-database/.
- 29.SEER. Site Recode ICD-O-3/WHO 2008 Definition 2018. [Available from: https://seer.cancer.gov/siterecode/icdo3_dwhoheme/index.html. [Google Scholar]
- 30.Hasle H, Niemeyer CM, Chessells JM, Baumann I, Bennett JM, Kerndrup G, et al. A pediatric approach to the WHO classification of myelodysplastic and myeloproliferative diseases. Leukemia. 2003;17(2):277–82. [DOI] [PubMed] [Google Scholar]
- 31.Hitzler JK, Zipursky A. Origins of leukaemia in children with Down syndrome. Nat Rev Cancer. 2005;5(1):11–20. [DOI] [PubMed] [Google Scholar]
- 32.Fine JP, Gray RJ. A Proportional Hazards Model for the Subdistribution of a Competing Risk. Journal of the American Statistical Association. 1999;94(446):496–509. [Google Scholar]
- 33.Lumley T. rmeta: Meta-Analysis. 2018. [Google Scholar]
- 34.Team RC. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistial Computing; 2019. [Google Scholar]
- 35.Lupo PJ, Schraw JM, Desrosiers TA, Nembhard WN, Langlois PH, Canfield MA, et al. Association Between Birth Defects and Cancer Risk Among Children and Adolescents in a Population-Based Assessment of 10 Million Live Births. LID - 10.1001/jamaoncol.2019.1215 [doi]. (2374–2445 (Electronic)). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Crispino JD. Up and down in Down syndrome: AMKL. Blood. 2006;107(4):1251. [Google Scholar]
- 37.Roy A, Cowan G, Mead AJ, Filippi S, Bohn G, Chaidos A, et al. Perturbation of fetal liver hematopoietic stem and progenitor cell development by trisomy 21. Proc Natl Acad Sci U S A. 2012;109(43):17579–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Laurent AP, Kotecha RS, Malinge S. Gain of chromosome 21 in hematological malignancies: lessons from studying leukemia in children with Down syndrome. Leukemia. 2020;34(8):1984–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morawiec Z, Janik K, Kowalski M, Stetkiewicz T, Szaflik J, Morawiec-Bajda A, et al. DNA damage and repair in children with Down’s syndrome. Mutation research. 2008;637(1–2):118–23. [DOI] [PubMed] [Google Scholar]
- 40.Hannan MA, Waghray M, Sigut D, Ozand PT. Increased radiosensitivity of cell lines derived from a Down’s syndrome patient with ocular telangiectasia. Journal of child neurology. 1992;7 Suppl:S83–7. [DOI] [PubMed] [Google Scholar]
- 41.Barenfeld LS, Pleskach NM, Bildin VN, Prokofjeva VV, Mikhelson VM. Radioresistant DNA synthesis in cells of patients showing increased chromosomal sensitivity to ionizing radiation. Mutation research. 1986;165(3):159–64. [DOI] [PubMed] [Google Scholar]
- 42.Shafik HM, Au WW, Legator MS. Chromosomal radiosensitivity of Down syndrome lymphocytes at different stages of the cell cycle. Human genetics. 1988;78(1):71–5. [DOI] [PubMed] [Google Scholar]
- 43.Greaves M A causal mechanism for childhood acute lymphoblastic leukaemia. Nature reviews Cancer. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Canfield KN, Spector LG, Robison LL, Lazovich D, Roesler M, Olshan AF, et al. Childhood and maternal infections and risk of acute leukaemia in children with Down syndrome: a report from the Children’s Oncology Group. British journal of cancer. 2004;91(11):1866–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hwee J, Tait C, Sung L, Kwong JC, Sutradhar R, Pole JD. A systematic review and meta-analysis of the association between childhood infections and the risk of childhood acute lymphoblastic leukaemia. British journal of cancer. 2018; 118(1): 127–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mejia-Arangure Jm Fau - Fajardo-Gutierrez A, Fajardo-Gutierrez A Fau - Flores-Aguilar H, Flores-Aguilar H Fau - Martinez-Garcia MC, Martinez-Garcia Mc Fau - Salamanca-Gomez F, Salamanca-Gomez F Fau - Palma-Padilla V, Palma-Padilla V Fau - Paredes-Aguilera R, et al. Environmental factors contributing to the development of childhood leukemia in children with Down’s syndrome. (0887–6924 (Print)). [DOI] [PubMed] [Google Scholar]
- 47.Puumala SE, Ross JA, Olshan AF, Robison LL, Smith FO, Spector LG. Reproductive history, infertility treatment, and the risk of acute leukemia in children with down syndrome: a report from the Children’s Oncology Group. Cancer. 2007;110(9):2067–74. [DOI] [PubMed] [Google Scholar]
- 48.Kwan ML, Miglioretti DL, Marlow EC, Aiello Bowles EJ, Weinmann S, Cheng SY, et al. Trends in Medical Imaging During Pregnancy in the United States and Ontario, Canada, 1996 to 2016. JAMA Netw Open. 2019;2(7):e197249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Smith-Bindman R, Miglioretti DL, Johnson E, Lee C, Feigelson HS, Flynn M, et al. Use of diagnostic imaging studies and associated radiation exposure for patients enrolled in large integrated health care systems, 1996–2010. JAMA. 2012;307(22):2400–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Smith-Bindman RMK, Marlow EC, Theis MK, WE Bolch, Cheng SY, Bowles EJA, Duncan JR, Greenlee RT, Kushi LH, Pole JD, Rahm AK, Stout NK, Weinmann S, Miglioretti DL. Trends in Use of Medical Imaging in U.S. Healthcare Systems and in Ontario, Canada, 2000–2016. JAMA In Press. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pearce MS, Salotti JA, Little MP, McHugh K, Lee C, Kim KP, et al. Radiation exposure from CT scans in childhood and subsequent risk of leukaemia and brain tumours: a retrospective cohort study. Lancet. 2012;380(9840):499–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Journy N, Rehel JL, Ducou Le Pointe H, Lee C, Brisse H, Chateil JF, et al. Are the studies on cancer risk from CT scans biased by indication? Elements of answer from a large-scale cohort study in France. Br J Cancer. 2015;112(1):185–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Meulepas JM, Ronckers CM, Smets A, Nievelstein RAJ, Gradowska P, Lee C, et al. Radiation Exposure From Pediatric CT Scans and Subsequent Cancer Risk in the Netherlands. J Natl Cancer Inst 2019;111(3):256–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nikkila A, Raitanen J, Lohi O, Auvinen A. Radiation exposure from computerized tomography and risk of childhood leukemia: Finnish register-based case-control study of childhood leukemia (FRECCLE). Haematologica. 2018;103(11):1873–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bull MJ. Health Supervision for Children With Down Syndrome. Pediatrics. 2011;128(2):393. [DOI] [PubMed] [Google Scholar]
- 56.Radhakrishnan R, Towbin AJ. Imaging findings in Down syndrome. Pediatric radiology. 2014;44(5):506–21. [DOI] [PubMed] [Google Scholar]
- 57.McDowell KM, Craven DI. Pulmonary Complications of Down Syndrome during Childhood. The Journal of pediatrics. 2011;158(2):319–25. [DOI] [PubMed] [Google Scholar]
- 58.Loane M, Morris JK, Addor MC, Arriola L, Budd J, Doray B, et al. Twenty-year trends in the prevalence of Down syndrome and other trisomies in Europe: impact of maternal age and prenatal screening. European journal of human genetics : EJHG. 2013;21(1):27–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Allen EG, Freeman SB, Druschel C, Hobbs CA, O’Leary LA, Romitti PA, et al. Maternal age and risk for trisomy 21 assessed by the origin of chromosome nondisjunction: a report from the Atlanta and National Down Syndrome Projects. Human genetics. 2009;125(1):41–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ghosh S, Feingold E, Dey SK. Etiology of Down syndrome: Evidence for consistent association among altered meiotic recombination, nondisjunction, and maternal age across populations. American journal of medical genetics Part A. 2009; 149a(7):1415–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sherman SL, Allen EG, Bean LH, Freeman SB. Epidemiology of Down syndrome. Mental retardation and developmental disabilities research reviews. 2007;13(3):221–7. [DOI] [PubMed] [Google Scholar]
- 62.Petridou ET, Georgakis MK, Erdmann F, Ma X, Heck JE, Auvinen A, et al. Advanced parental age as risk factor for childhood acute lymphoblastic leukemia: results from studies of the Childhood Leukemia International Consortium. European journal of epidemiology. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sergentanis TN, Thomopoulos TP, Gialamas SP, Karalexi MA, Biniaris-Georgallis SI, Kontogeorgi E, et al. Risk for childhood leukemia associated with maternal and paternal age. European journal of epidemiology. 2015;30(12):1229–61. [DOI] [PubMed] [Google Scholar]
- 64.Marcotte EL, Druley TE, Johnson KJ, Richardson M, von Behren J, Mueller BA, et al. Parental Age and Risk of Infant Leukaemia: A Pooled Analysis. Paediatric and perinatal epidemiology. 2017;31(6):563–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Swensen AR, Ross JA, Severson RK, Pollock BH, Robison LL. The age peak in childhood acute lymphoblastic leukemia: exploring the potential relationship with socioeconomic status. Cancer. 1997;79(10):2045–51. [DOI] [PubMed] [Google Scholar]
- 66.Haneuse S, VanderWeele TJ, Arterburn D. Using the E-Value to Assess the Potential Effect of Unmeasured Confounding in Observational StudiesUsing the E-Value to Assess the Potential Effect of Unmeasured Confounding in Observational StudiesUsing the E-Value to Assess the Potential Effect of Unmeasured Confounding in Observational Studies. Jama. 2019;321(6):602–3. [DOI] [PubMed] [Google Scholar]
- 67.Belson M, Kingsley B, Holmes A. Risk factors for acute leukemia in children: a review. Environmental health perspectives. 2007;115(1):138–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gurney JG, Severson RK, Davis S, Robison LL. Incidence of cancer in children in the United States. Sex-, race-, and 1-year age-specific rates by histologic type. Cancer. 1995;75(8):2186–95. [DOI] [PubMed] [Google Scholar]


