Neurocritical care (NCC) research has soldiered on despite disruptions in operational rhythms and intermittent pauses due to COVID19. This article presents advances in NCC pertaining to cerebrovascular disease: bedside physiologic parameters, secondary injury, and neuroprotection (Figure-1). Given the impact of COVID19 on the brain, a brief supplement summarizes recent findings pertaining to virus/vaccine pathologies in NCC-units.
Figure 1:
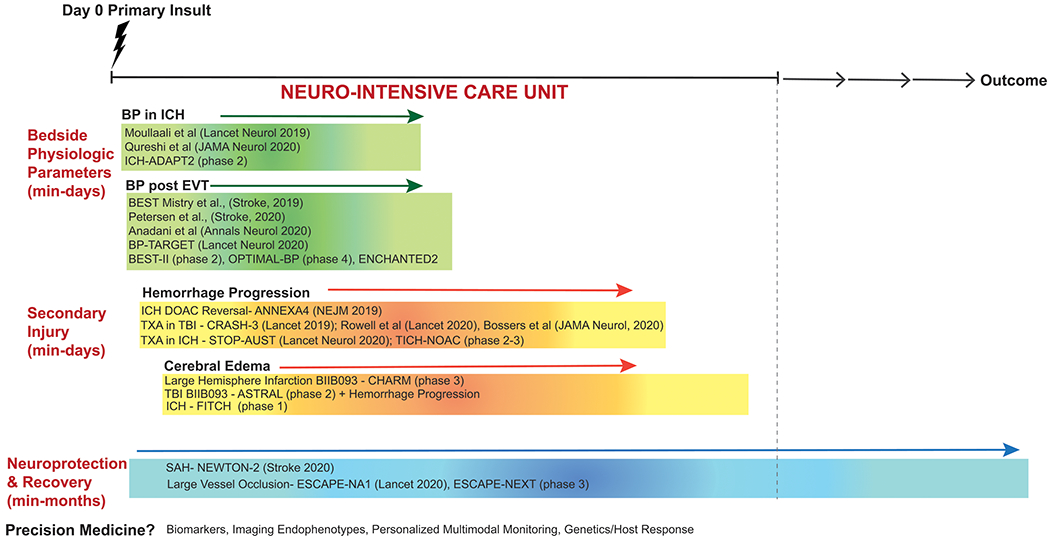
Key neurocritical care updates in cerebrovascular disease categorized by time from injury including bedside physiologic parameters (blood pressure), secondary injury (hemorrhage progression, cerebral edema), and neuroprotection.
Physiologic Parameters:
The quest for the ‘ideal’ BP in different acute neurological pathologies remains elusive. Variation exists even within individuals depending on time, host-response, and spatial location relative to the site/type of primary injury. Precision cerebrovascular health, an emerging field, requires big-data collection, curation and large-scale bioinformatics. Although precision-medicine has tremendous potential to improve management (particularly at extremes of the normal distribution), ‘optimal’ BP targets within a disease process may be similar across a plurality of patients or subgroups.
BP after ICH
Results from two earlier landmark randomized controlled trials (RCTs) were inconsistent: although neither reported a difference between systolic BP (SBP) 110-139 vs 140-179 mmHg, INTERACT2 had a signal of benefit vs one of harm in ATACH2. Key differences in ethnicity, treatment duration, and achieved SBP precluded easy comparisons. The preplanned pooled analysis (n=3829) is informative1. Achieved SBP, variability, and magnitude of reduction were collectively associated with better safety and efficacy including hematoma expansion, neurological deterioration, functional independence, and mortality. Every 10 mmHg reduction in SBP over 24h (to 120-130 mmHg), increased favorable functional recovery odds by 10%. Smooth control was valuable. Rapid large reductions (≥60 mmHg within 1h) were detrimental. Linear associations between SBP reduction and favorable outcome extended beyond 140 mmHg with little harm. Effects of ultra-intensive reduction SBP <120 mmHg, occurring in ~2%, remain unclear. ADAPT-2 (phase-2, adaptive randomization, NCT02281838) comparing <140 vs <180 is recruiting.
The benefit of intensive SBP control may not extend to patients presenting with SBP≥220 mmHg2. In a post-hoc analysis of ATACH2, of the 228 patients with initial SBP>220 mmHg, intensive reduction yielded higher rates of 24h neurological deterioration (p=0.04) without reducing hematoma expansion. No differences were observed in 90d death or severe disability. Although this suggests low long-term risk of intensive reduction in this subgroup, caution is warranted given sample size and the potential for acute decline.
BP after Endovascular Treatment (EVT) for Large Vessel Occlusion (LVO)
Optimal BP targets post EVT are likely critical—but remain unclear. While post-EVT hemorrhagic transformation from reperfusion injury may appear asymptomatic, recent evidence suggests that conventionally-defined ‘mild’ hemorrhagic transformation contributes to disability3.
The 2019 AHA/ASA guideline-updates recommend post-EVT BP≤180/105 mmHg (Class-IIb). However, higher SBP after recanalization is associated with unfavorable outcomes. Institutional practices vary: ~24% adhere to the AHA/ASA threshold4. In a multicenter prospective study (n=484), peak post-EVT SBPs>158 mmHg increased likelihood of unfavorable outcome (not significant in adjusted analyses)5. A retrospective multicenter study (n=1019) compared SBP<140, <160, and <180 mmHg after revascularization4. Both SBP<140 and SBP<160 were preferable to <180 mmHg: SBP<140 had higher odds of favorable functional outcome (OR=1.53, 95% CI=1.07-2.19) and lower odds of hemicraniectomy (OR=0.18, CI=0.16-0.21). SBP<160 decreased 90-day mortality odds (OR=0.41, CI 0.18-0.96). Final infarct volumes were unknown. BP recording methods and management varied. These data identified the need for RCTs.
BP-TARGET randomized 324 patients to intensive (100-129) vs standard (130-185 mmHg) management post-EVT3. 24-36h ICH was no different, nor were secondary outcomes (functional independence, mortality). Achieved BPs were only modestly different between groups: 128±11 vs 138±17 mmHg, limiting true comparisons of intensive vs. liberal control. Another consideration involves the ‘optimal’ SBP threshold post-EVT given potential concerns of targeting SBP~120s towards the nadir of the U-shaped curve associated with unfavorable outcome. Several trials like BEST-II (phase-2, NCT04116112, ≤180 vs <160 vs <140mmHg), OPTIMAL-BP (phase-4, NCT04205305, <180 vs <140), and ENCHANTED-2 (NCT04140110, <120 vs 140-180 mmHg) are ongoing. BP-TARGET highlights challenges of operationalizing treatment targets (even within trials) and the recurring theme that accounting for heterogeneity/patient-specific characteristics may be valuable in future RCTs. This is conceptually supported by a prospective study (n=90) where personalized, autoregulation-based BP targets post-EVT had a larger impact on outcome vs. static thresholds (140 or 160 mmHg)6. Deviation from autoregulation-based targets increased secondary injury and unfavorable outcome.
Secondary Injury:
Hemorrhage Progression (HP)
HP prognosticates unfavorable outcome in ICH and TBI. Therapeutic anticoagulation increases this risk. Andexanet-alfa (AA), FDA approved in 2018, is the only selective agent for reversing life-threatening bleeding from Factor-Xa inhibition. ANNEXA-4 (n=352), demonstrated reduced anti-Xa activity with AA. 64% of these patients had ICH—effective hemostasis was achieved in 80% and anti-Xa activity reduction modestly predicted hemostatic efficacy (AUC=0.64)7. Mortality was 14%, with thrombotic events in 10%. AA is ~4X more expensive than 4-factor prothrombin complex concentrates (4F-PCC). Retrospective work in ICH suggests similar hemostasis (~81.8%) and possibly lower thrombosis (~3.8%) with 4F-PCC. No differences between 4F-PCC and AA in ICH have been demonstrated: a phase-4 study (NCT03661528) is recruiting.
Tranexamic acid (TXA) is of interest given its inhibition of fibrinolysis. In TBI, the multicenter RCT CRASH-3 (n=12737) reported a small mortality benefit (absolute risk-reduction=1.7%) limited to mild-moderate TBI8. Eligibility vs enrollment data were not presented. Heterogeneity in local practices affect global generalizability (~66% from Pakistan, Malaysia). A multicenter RCT of moderate-severe TBI (US/Canada, n=1063) confirmed no improvement in HP or outcome9. A comparative-effectiveness trial (n=1827) suggested increased mortality in severe-TBI10. Results are similarly disappointing in ICH. In TICH-2 (n=2325), TXA within 8h minimally decreased ICH growth (1ml, p=0.0432) without improving outcomes11. The multicenter phase-2 STOP-AUST RCT (n=100) evaluated TXA within 4.5h using the spot-sign to select patients—again, there were no differences in ICH growth, mortality, or complications12. The imaging-biomarker possibly selected a more responsive population (8% difference in ICH growth vs 4% from TICH-2, non-significant). Earlier treatment may be beneficial (trend at ≤3h). Although these studies represent much-needed progress informing patient selection and timing for future trials, the current impact of TXA in the NICU seems limited.
Cerebral Edema
Cerebral edema causes acute neurological deterioration across a wide range of pathologies; insight into its biological underpinnings continues to exponentially increase. Although the classic taxonomy of cytotoxic/cellular vs vasogenic edema vs HP remains clinically informative, it is increasingly recognized that these processes represent a spectrum of edema evolution that may be molecularly related. Several promising targets have emerged including sulfonylurea receptor 1—transient receptor potential Melastatin-4 (SUR1-TRPM4), Sphingosine-1-phosphate (S1P), Aquaporin-4 (AQP4), Arginine vasopressin(AVP), Sodium-Hydrogen exchanger, Na-K-Cl-cotransporter, matrix-metalloproteinase-9(MMP9). Anti-Vascular Endothelial Growth Factor agents have long demonstrated anti-edema benefit in glioblastoma. SUR1-TRPM4, S1P and AVP inhibitors are currently in clinical trials.
SUR1-TRPM4, a cation channel uniquely upregulated after injury in major cell-types of the neurovascular unit, results in sodium influx and oncotic edema. It overlaps with other molecular contributors to edema (AQP4, MMP9). Preclinical inhibition with glibenclamide reduces secondary injury in several models. Earlier clinical trials in large hemispheric infarction (LHI) and TBI have demonstrated promising reduction in cerebral edema and HP. An intravenous formulation (BIIB093) is under investigation in LHI (phase-3, CHARM, NCT02864953), and contusional-TBI (phase-2, ASTRAL, NCT03954041). Precision-medicine based selection of high-risk patients (biomarkers, imaging, genetics) may inform future trial design. S1P-subtype expression on endothelial cells and adherens junctions regulate BBB permeability via the cytoskeleton and endothelial morphology. Small studies of inhibition (fingolimod) suggest perihematomal edema reduction with ongoing evaluation in ICH (phase-1, FITCH, NCT04088630).
Neuroprotection:
In SAH, NEWTON2 revealed no improvement in 90-day outcome with 600 mg intraventricular EG-1962 (sustained-release nimodipine) vs oral nimodipine13. A non-significant trend towards favorable outcome was seen in severe/high-grade cases. EG-1962 reduced angiographic-vasospasm vs oral nimodipine (50% vs 63%, p=0.025) and hypotension (7% vs 10%). Given absence of safety concerns, EG-1962 may have a role in severe cases/those on vasopressor agents.
EVT may transform neuroprotection in LVO by facilitating drug delivery to newly reperfused tissue. Although the multicenter ESCAPE-NA1 RCT evaluating the neuroprotectant nerinetide after EVT was neutral, a prespecified post-hoc analysis in alteplase-ineligible patients demonstrated improved outcome with treatment14. Lower drug levels were observed in alteplase-treated patients. This is biologically plausible given preclinical data that plasmin, generated by alteplase, cleaves/inactivates nerinetide. ESCAPE-NEXT (phase-3, NCT04462536) is evaluating nerinetide in alteplase-ineligible LVO patients undergoing EVT. Finally, novel forms of acellular therapies are being developed in preclinical models15. Neuroprotection thus remains our Everest, with recent valiant efforts falling short but imparting valuable lessons.
Supplementary Material
Acknowledgments
Funding Sources:
KNS—NIH (U24NS107136, U24NS107215, R01NR018335, R01NS107215, U01NS106513, R03NS112859); AHA (18TPA34170180, 17CSA33550004).
RMJ—NIH (K23NS101036, R01NS115815).
Abbreviations:
- 4F-PCC
4-factor prothrombin complex concentrates
- AA
andexanet alpha
- AHA
American heart association
- AQP4
Aquaporin-4
- ASA
American stroke association
- AVP
Arginine vasopressin
- BP
blood pressure
- EVT
endovascular therapy
- HP
hemorrhage progression
- ICH
intracerebral hemorrhage
- LHI
large hemispheric infarction
- LVO
large vessel occlusion
- MMP9
matrix-metalloproteinase-9
- NCC
neurocritical care
- NICU
neurointensive care unit
- OR
odds ratio
- RCT
randomized controlled trial
- S1P
Sphingosine-1-phosphate (S1P)
- SBP
systolic blood pressure
- SUR1-TRPM4
sulfonylurea receptor 1—transient receptor potential Melastatin-4
- TBI
traumatic brain injury
- TXA
tranexamic acid
Footnotes
Disclosures:
KNS—Bard, Hyperfine, Biogen, Novartis; personal fees from Zoll, Ceribell, NControl, Alva.
RMJ—Barrow Neurological Foundation, Biogen (Consultant, Advisory Board).
Bibliography
- 1.Moullaali TJ, Wang X, Martin RH, Shipes VB, Robinson TG, Chalmers J, Suarez JI, Qureshi AI, Palesch YY, Anderson CS. Blood pressure control and clinical outcomes in acute intracerebral haemorrhage: a preplanned pooled analysis of individual participant data. Lancet Neurol. 2019;18:857–864. [DOI] [PubMed] [Google Scholar]
- 2.Qureshi AI, Huang W, Lobanova I, Barsan WG, Hanley DF, Hsu CY, Lin C-L, Silbergleit R, Steiner T, Suarez JI, et al. Outcomes of intensive systolic blood pressure reduction in patients with intracerebral hemorrhage and excessively high initial systolic blood pressure: post hoc analysis of a randomized clinical trial. JAMA Neurol. 2020; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mazighi M, Richard S, Lapergue B, Sibon I, Gory B, Berge J, Consoli A, Labreuche J, Olivot J-M, Broderick J, et al. BP-TARGET investigators. Safety and efficacy of intensive blood pressure lowering after successful endovascular therapy in acute ischaemic stroke (BP-TARGET):a multicentre, open-label, randomised controlled trial. Lancet Neurol. 20:265–274. [DOI] [PubMed] [Google Scholar]
- 4.Anadani M, Arthur AS, Tsivgoulis G, Simpson KN, Alawieh A, Orabi Y, Goyal N, Alexandrov AV, Maier IL, Psychogios M-N, et al. , Blood Pressure Goals and Clinical Outcomes after Successful Endovascular Therapy: A Multicenter Study. Ann. Neurol 2020;87:830–839. [DOI] [PubMed] [Google Scholar]
- 5.Mistry EA, Sucharew H, Mistry AM, Mehta T, Arora N, Starosciak AK, De-Los-Rios-La-Rosa F, Siegler JE, Barnhill NR, Patel K, et al. Blood Pressure after Endovascular Therapy for Ischemic Stroke (BEST):A Multicenter Prospective Cohort Study. Stroke. 2019;50:3449–3455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Petersen NH, Silverman A, Strander SM, Kodali S, Wang A, Sansing LH, Schindler JL, Falcone GJ, Gilmore EJ, Jasne AS, et al. , Fixed Compared With Autoregulation-Oriented Blood Pressure Thresholds After Mechanical Thrombectomy for Ischemic Stroke. Stroke. 2020;51:914–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Connolly SJ, Crowther M, Eikelboom JW, Gibson CM, Curnutte JT, Lawrence JH, Yue P, Bronson MD, Lu G, Conley PB, et al. ANNEXA-4 Investigators. Full Study Report of Andexanet Alfa for Bleeding Associated with Factor Xa Inhibitors. N. Engl. J. Med 2019;380:1326–1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.CRASH-3 trial collaborators. Effects of tranexamic acid on death, disability, vascular occlusive events and other morbidities in patients with acute traumatic brain injury (CRASH-3):a randomised, placebo-controlled trial. Lancet. 2019;394:1713–1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rowell SE, Meier EN, McKnight B, Kannas D, May S, Sheehan K, Bulger EM, Idris AH, Christenson J, Morrison LJ, et al. Effect of Out-of-Hospital Tranexamic Acid vs Placebo on 6-Month Functional Neurologic Outcomes in Patients With Moderate or Severe Traumatic Brain Injury. JAMA. 2020;324:961–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bossers SM, Loer SA, Bloemers FW, Den-Hartog D, Van-Lieshout EMM, Hoogerwerf N, van-der-Naalt J, Absalom AR, Peerdeman SM, Schwarte LA, et al. Association Between Prehospital Tranexamic Acid Administration and Outcomes of Severe Traumatic Brain Injury. JAMA Neurology. 2020; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sprigg N, Flaherty K, Appleton JP, Al-Shahi-Salman R, Bereczki D, Beridze M, Christensen H, Ciccone A, Collins R, Czlonkowska A, et al. , TICH-2 Investigators. Tranexamic acid for hyperacute primary IntraCerebral Haemorrhage (TICH-2):an international randomised, placebo-controlled, phase 3 superiority trial. Lancet. 2018;391:2107–2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meretoja A, Yassi N, Wu TY, Churilov L, Sibolt G, Jeng J-S, Kleinig T, Spratt NJ, Thijs V, Wijeratne T, et al. Tranexamic acid in patients with intracerebral haemorrhage (STOP-AUST):a multicentre, randomised, placebo-controlled, phase 2 trial. Lancet Neurol. 2020;19:980–987. [DOI] [PubMed] [Google Scholar]
- 13.Carlson AP, Hänggi D, Wong GK, Etminan N, Mayer SA, Aldrich F, Diringer MN, Schmutzhard E, Faleck HJ, Ng D, et al. NEWTON Investigators. Single-Dose Intraventricular Nimodipine Microparticles Versus Oral Nimodipine for Aneurysmal Subarachnoid Hemorrhage. Stroke. 2020;51:1142–1149. [DOI] [PubMed] [Google Scholar]
- 14.Hill MD, Goyal M, Menon BK, Nogueira RG, McTaggart RA, Demchuk AM, Poppe AY, Buck BH, Field TS, Dowlatshahi D, et al. ESCAPE-NA1 Investigators. Efficacy and safety of nerinetide for the treatment of acute ischaemic stroke (ESCAPE-NA1):a multicentre, double-blind, randomised controlled trial. Lancet. 2020;395:878–887. [DOI] [PubMed] [Google Scholar]
- 15.Vrselja Z, Daniele SG, Silbereis J, Talpo F, Morozov YM, Sousa AMM, Tanaka BS Skarica M, Pletikos M, et al. Restoration of brain circulation and cellular functions hours post-mortem. Nature. 2019;568:336–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


