Abstract
Objective:
The aim of the study was to investigate the effects of a novel polymerizable collagen cross-linker methacrylate-functionalized proanthocyanidins (MAPA) on the polymerization, microhardness and leaching of a HEMA-based experimental dental adhesive system.
Methods:
Three MAPAs were synthesized using different methacrylate (MA) to proanthocyanidins (PA) feeding ratios of 1:2, 1:1, and 2:1 to obtain MAPA-1, MAPA-2, and MAPA-3, respectively. The resulting three MAPAs and PA were added to an experimental adhesive formulated with HEMA and a tri-component photoinitiator system (0.5wt% CQ/EDMAB/DPIHP) at 1%, 5% and 10% MAPA or PA concentrations (wt%). The adhesive polymerization kinetics was measured continuously in real-time for 10 min using a Fourier-transform infrared spectroscopy (FTIR) with an attenuated total reflectance (ATR) accessory. Degree of conversion (DC) and Vickers microhardness (MH) of cured adhesives were measured at 72 h post-cure. The leaching of cured adhesives in DI water was monitored using UV-Vis spectrophotometer. Statistical analysis was performed using one-way and two-way ANOVA, Tukey’s (p<0.05).
Results:
The adhesive formulations with 1%, 5% and 10% MAPAs-1, -2, -3 all generated higher rate of polymerization and 10-min DC than the formulations with PA at the same concentrations. At 72 h post-cure, the adhesive formulation with 5% MAPA-2 exhibited significantly higher DC (99.40%) and more than doubled MH (18.93) values than the formulation with 5% PA (DC=89.47%, MH=8.41) and the control (DC=95.46%, MH=9.33). Moreover, the cured adhesive with 5% MAPA-2 demonstrated significantly reduced leaching in HEMA monomers and other components in comparison with cured adhesive with 5% PA and the control.
Significance:
Synthesized MAPA is a novel class of polymerizable collagen cross-linker that not only stabilizes dentin collagen via its PA component, but also improves polymerization, mechanical properties and stability of HEMA-based adhesives via its MA component. By inheriting the benefit while overcoming the drawback of PA, MAPA offers a revolutionary solution for improved bond-strength and longevity of dental restorations.
Keywords: Polymerizable collagen cross-linker, grape seed extract proanthocyanidins (PA), methacrylate functionalized proanthocyanidins (MAPA), dental adhesive, polymerization, kinetics, rate of polymerization, degree of conversion, Vickers microhardness, leaching
1. Introduction
Dental adhesion in modern restorative dentistry is achieved through the dentin-adhesive hybrid layer, in which adhesive resin penetrates into the demineralized dentin surface and forms micro-interlocked entanglement with exposed collagen fibrils [1]. One of the leading causes for the failure of restorations is the lack of integrity in the dentin-adhesive hybrid layer, mainly due to incomplete resin infiltration and degradation of exposed demineralized dentin collagen, which is further aggravated by etching acid activated endogenous matrix metalloproteinases (MMPs) and cysteine cathepsins [2–5].
Collagen crosslinking has been shown as an effective way to stabilize the hybrid layer [6–9]. A group of naturally occurring polyphenolic compounds called proanthocyanidins (PA) is of special interest due to its dual-functionality as a cross-linker and MMP inhibitor as well as its lack of toxicity [10, 11] [12] [13]. Grape seed extract (GSE) is one of the most abundant sources of PA and has been widely studied for its applications in adhesive dentistry. Dentin collagen treated with GSE-PA has exhibited increased ultimate tensile strength, elastic modulus, bond strength and improved resistance to collagenase digestion [14–16]. It was further revealed that the collagen stabilization effect of GSE-PA could be achieved in treatment time of less than 30s, making it clinically feasible [17]. These evidences render GSE-PA a promising agent for improving the durability of adhesive bonding in modern dental restorations. For the rest of the article, GSE-PA is denoted as PA unless otherwise stated.
Generally, there are three ways to integrate PA into an adhesive system: PA-containing primers, PA-containing etchants, and PA-containing adhesives [12]. The use of PA as a primer prior to adhesion has been shown to protect exposed collagen fibrils in the hybrid layer from biodegradation, improve bond strength and decrease interfacial permeability [7, 18, 19]. However, this approach adds an extra step to the procedure, which is not preferred in clinical practice. Alternatively, PA can be added to the etchant of an etch-and-rinse adhesive system. Several studies found that the incorporation of 2% PA to phosphoric acid of <20% concentration protected dentin collagen from enzymatic degradation, produced higher resin-dentin bond strength and less nanoleakage [20–22]. However, this approach does not work for higher acid concentrations (20% and above) due to the breakdown of B-type interflavane links of PA in more acidic solutions [23]. Another drawback of this approach is the poor solubility of PA in acidic solutions, which requires additional solvents such as ethanol or acetone in the formulation and thus limits the shelf life of the etchant.
Another convenient way is to add PA to dentin adhesives. Compared to the other two approaches, one advantage of this approach is the presence of PA in the hybrid layer for an extended period. Research evidence suggested that the presence of 5% PA in dental adhesives inhibited the biodegradation of unprotected collagen fibrils within the hybrid layer when bonded to the acid etched dentin [6]. However, PA is a known radical scavenger and can interfere with adhesive monomer polymerization that relies on the generation and propagation of radical species to make the resin set [24]. Experimentally, PA has been shown to hamper the photopolymerization and reduce the flexural strength, modulus of elasticity and microhardness of dental adhesives [25, 26]. A recent clinical evaluation of PA added to a two-step etch-and-rinse adhesive showed that incorporating 2% and 5% PA to the adhesive impaired the longevity of composite restorations after 24 months [27]. The dentin-adhesive bond strength is determined by the integrity of both the dentin collagen and the adhesive. Therefore, the use of PA together with adhesives faces an obvious dilemma and leads to unsatisfactory long-term performance.
To address the dilemma, we developed methacrylate-functionalized PAs (MAPAs), which contain polymerizable methacryloxy (MA) component and collagen crosslinking PA component, as a novel class of polymerizable collagen cross-linker (PCC) to add to dental adhesives. MAPAs are expected to help with the adhesive polymerization via the co-polymerization function of MA while inheriting the collagen stabilization function of PA. Furthermore, the chemical bonding of MAPA to adhesive monomers via co-polymerization may potentially reduce its leaching from the cured adhesive polymer. Our previous and ongoing research has already shown effective collagen stabilization function of MAPA [28] (Part I study). The current study (Part II study) aimed to evaluate the effects of MAPA on the polymerization and microhardness of an experimental adhesive. Meanwhile, the leaching of compounds from light-cured adhesives was examined. The null hypotheses were (1) MAPA have the same effect on adhesive polymerization as PA; (2) MAPA have the same influence on the microhardness of light-cured adhesive as PA; and (3) light-cured adhesive with MAPA has the same leaching profile as that with PA.
2. Materials & Methods
General
All chemicals were purchased from Sigma-Aldrich (St. Louise, MO, USA) unless otherwise stated. MegaNatural Gold grape seed extract (contains >90% proanthocyanidin) was donated by the manufacturer (Polyphenolics, Madera, CA, USA).
Synthesis and characterization of MAPAs
Three MAPAs were synthesized using different MA to PA feeding ratios with MAPA-1, MAPA-2, and MAPA-3 having MA:PA mole ratios of 1:2, 1:1, and 2:1, respectively. The chemical synthesis was accomplished via the coupling between a commercial grape seed extract (GSE, containing >90% PA) and methacryloyl chloride. The actual MA:PA ratios in the resulting MAPAs were estimated using 1H NMR spectra to be 0.6:1, 0.9:1 and 1.5:1 for MAPA-1, MAPA-2, MAPA-3, respectively. Detailed synthetic procedures and structural characterizations of the MAPA compounds were described in Part I study [28]. Fig. 1a shows the schematic diagram of a representative synthesized MAPA compound. The actual chemical structures are different for the three MAPA compounds, with MA to PA ratios characterized by the 1720/1605 peak ratio in Fig. 1b.
Fig. 1.
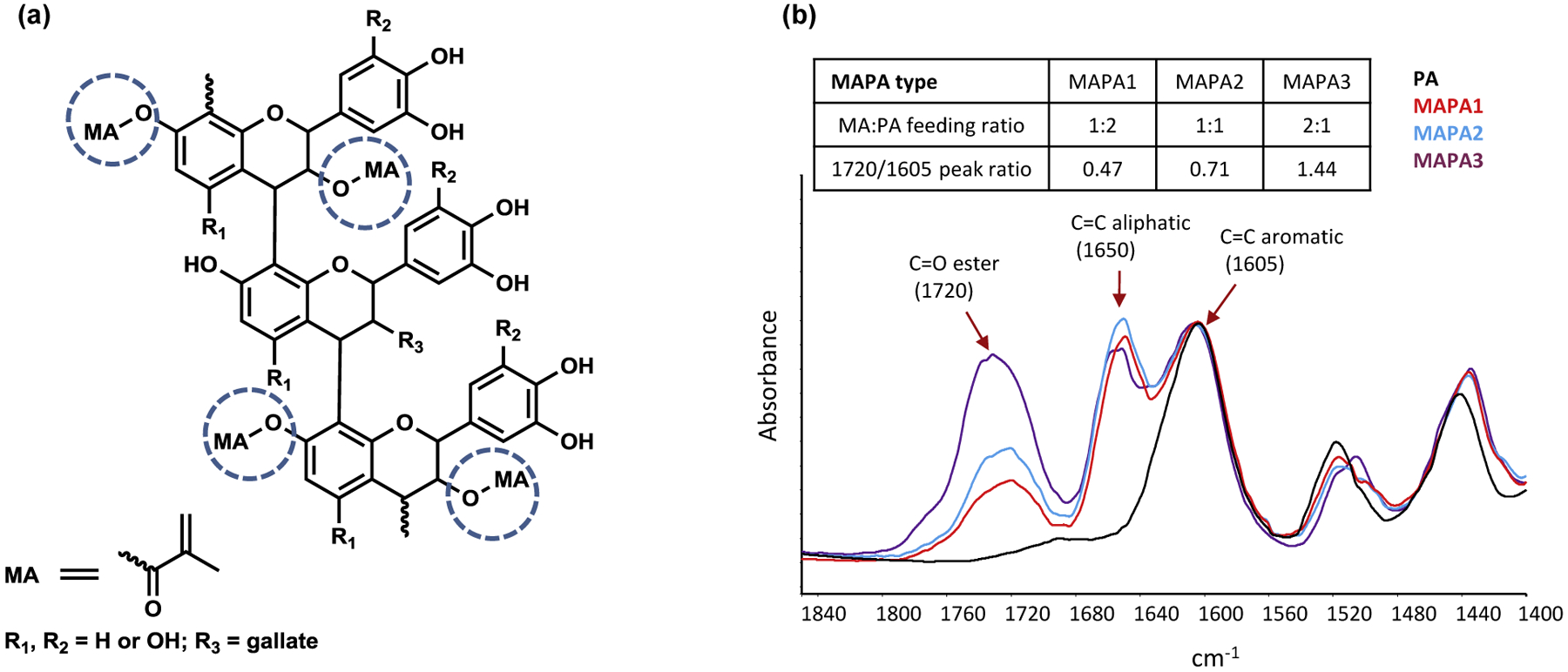
(a) Schematic diagram of a representative MAPA compound, consisting of PA and MA components. (b) FTIR spectra of MAPA1, 2, 3 and PA powder. MAPA1, 2, 3 structures are characterized by 1720/1605 peak ratio, in which the 1720 peak comes from the ester C=O bond in the MA component and the 1605 peak comes from the C=C aromatic bond in the PA component.
Formulation of experimental adhesives
The experimental adhesive was formulated by adding 0.5 wt% of camphorquinone (CQ), ethyl 4-(dimethylamino)benzoate (EDMAB) and diphenyliodonium hexafluorophosphate (DPIHP) to the 2-hydroxyethylmethacrylate (HEMA) monomer (control group). MAPA-1, MAPA-2, MAPA-3 and PA powders were then added to the adhesive solution at concentrations of 1%, 5% and 10% (wt%) to form the test groups. All adhesive solutions were prepared in the dark at room temperature and stored in a refrigerator.
The experimental adhesive formulation in the current study consisted of HEMA only as its net resin instead of a blend of bisphenol A glycidyl dimethacrylate (Bis-GMA) and HEMA as commonly used in commercial adhesives for well-thought-out reasons. HEMA monomers contain one methacrylate group and polymerize at a relatively slower pace in a linear fashion while Bis-GMA monomers contain two methacrylate groups and polymerize much faster in a crosslinking fashion. The fast rate of polymerization and high crosslinking density of Bis-GMA may mask any potential effects of MAPA on polymerization. Therefore, Bis-GMA was purposely excluded from the current adhesive formulation to allow the observation of the potential effects of MAPA and to facilitate the study of basic mechanisms.
Polymerization kinetics study
The same FTIR spectrometer was used for the kinetics study. Polymerization kinetics was monitored real-time using the Spectrum TimeBase software at a resolution of 4 cm−1 in the wavenumber range between 700 and 4000 cm−1. A small volume of adhesive was dropped onto the top plate of the ATR diamond crystal and was immediately covered with a transparent plastic cover slip (Fisher brand, Catalog # 12–547). The adhesive was light-cured with a LED light curing unit (3M ESPE Elipar S10, 1200 mW/cm2, Germany) for 100s. Three samples were tested for each group (n = 3).
To determine the degree of conversion (DC), the commonly used aliphatic C=C stretching band at 1637 cm−1 couldn’t be used for our formulations due to the interference of bands associated with the phenyl groups in PA and MAPAs (Fig. 2a). Fortunately, the =C-H bending band at 815 cm−1 was well separated and therefore used for DC calculation instead (Fig. 2b). The peak at 1716 cm−1 (C=O stretching) was chosen as the internal standard for normalization. Two-point baseline and maximum band height ratio protocol were used to measure the absorption intensity. The DC% was calculated using Equation (1):
| (1) |
Fig. 2.
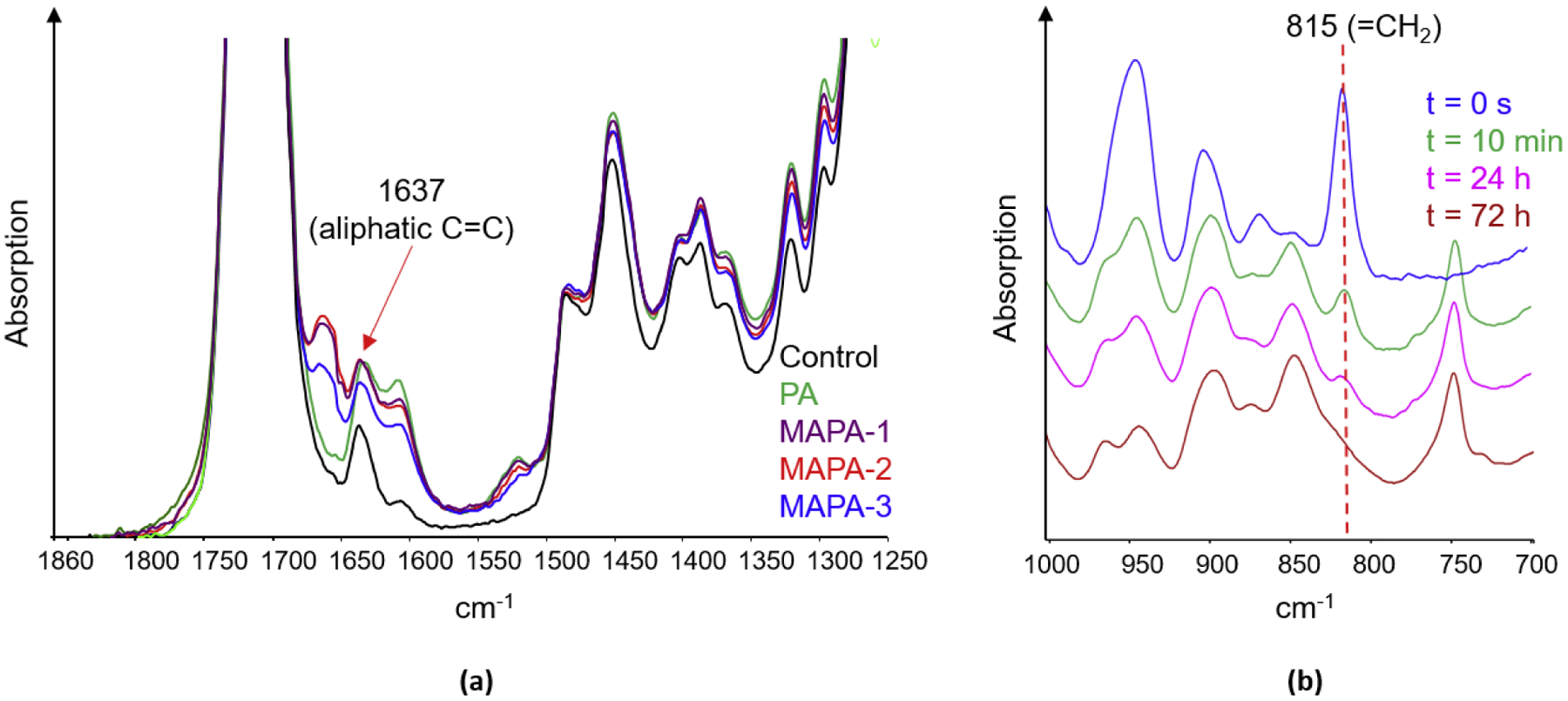
(a) FTIR spectra of control, 10% PA and 10% MAPA-1,-2,-3 adhesives at 10 min of cure, showing interferences at the 1637 cm−1 peak (aliphatic C=C) by nearby peaks.
(b) FTIR spectra of 10% MAPA-2 adhesive at various times of cure, showing decrease of the 815 cm−1 peak (=CH2 vibration) over time.
Polymerization kinetics was continuously collected for 10 min and the DC% at 10 min of cure was calculated. Taking into consideration of both the polymerization kinetic results in the current study and the dentin collagen stabilization results in another study [28], the best candidate among MAPA-1, MAPA-2 and MAPA-3 was selected and subjected to further studies as follows.
Terminal DC and microhardness assessment
Terminal DC and microhardness were assessed for control adhesive and adhesives containing 1%, 5% and 10% of the selected MAPA and PA. Thin adhesive sample disks were made using a 0.5 mm spacer and light-cured using the protocol described earlier. Afterwards, the light-cured adhesive sample disks were carefully removed and stored in the dark at room temperature for the assessments. Preliminary testing using FTIR indicated that it took up to 72 h for the adhesives monomers to undergo complete polymerization (Fig. 2b). After 72 h post-cure, the bottom of the stored sample disks were pressed on the top plate of ATR diamond crystal and IR spectra were acquired using Perkin Elmer’s Spectrum software. Terminal DC% was calculated using Equation (1).
Vickers microhardness was assessed on the bottom surface of the sample disks after 72 h post-cure using a microhardness tester (MO Tukon, Wilson Instrument Division, Bridgeport, CT, USA) at a constant load of 50 gf for 15s. The microhardness values were calculated automatically by the tester. Five random measurements were made for each sample surface. The optimal concentration of the selected MAPA was determined based on the terminal DC and microhardness results.
Leaching study
A leaching study was performed for cured control adhesive, adhesive samples with 5% MAPA and 5% PA in triplicates (n = 3) using UV-visible absorption spectroscopy. During the test, the cured adhesive samples were immersed in 3 mL of distilled water. The UV-Vis absorption spectra of the supernatant covering the cured adhesive samples were measured using ultraviolet–visible 8452A diode array spectrophotometer (Hewlett Packard Corporation, Palo Alto, California, USA). The first measurements were taken immediately after the adhesives were immersed in water on the same day of curing (day 0), and then in the following days: Day 1, 2, 3, 4, 5, 7, 9, 12, 15, 22, and 29. A separate calibration test was performed using PA of different concentrations to obtain the linear response region for the test.
Statistical Analysis
Statistical analyses were performed using Microsoft Excel 2016 and GraphPad Prism 5 (GraphPad Software, Inc., Version 5, San Diego, CA, USA). All DC and MH data were analyzed using one-way and two-way analysis of variance (ANOVA) with Tukey’s post hoc test (α = 0.05). Data were plotted in bar graphs as means ± standard deviation.
3. Results
Polymerization kinetics during 10 min of cure
The representative real-time polymerization kinetic curves during the first 10 min polymerization for all test groups were shown in Fig. 3a. The DC% at 10 min of cure were summarized in Fig. 3b.
Fig. 3.
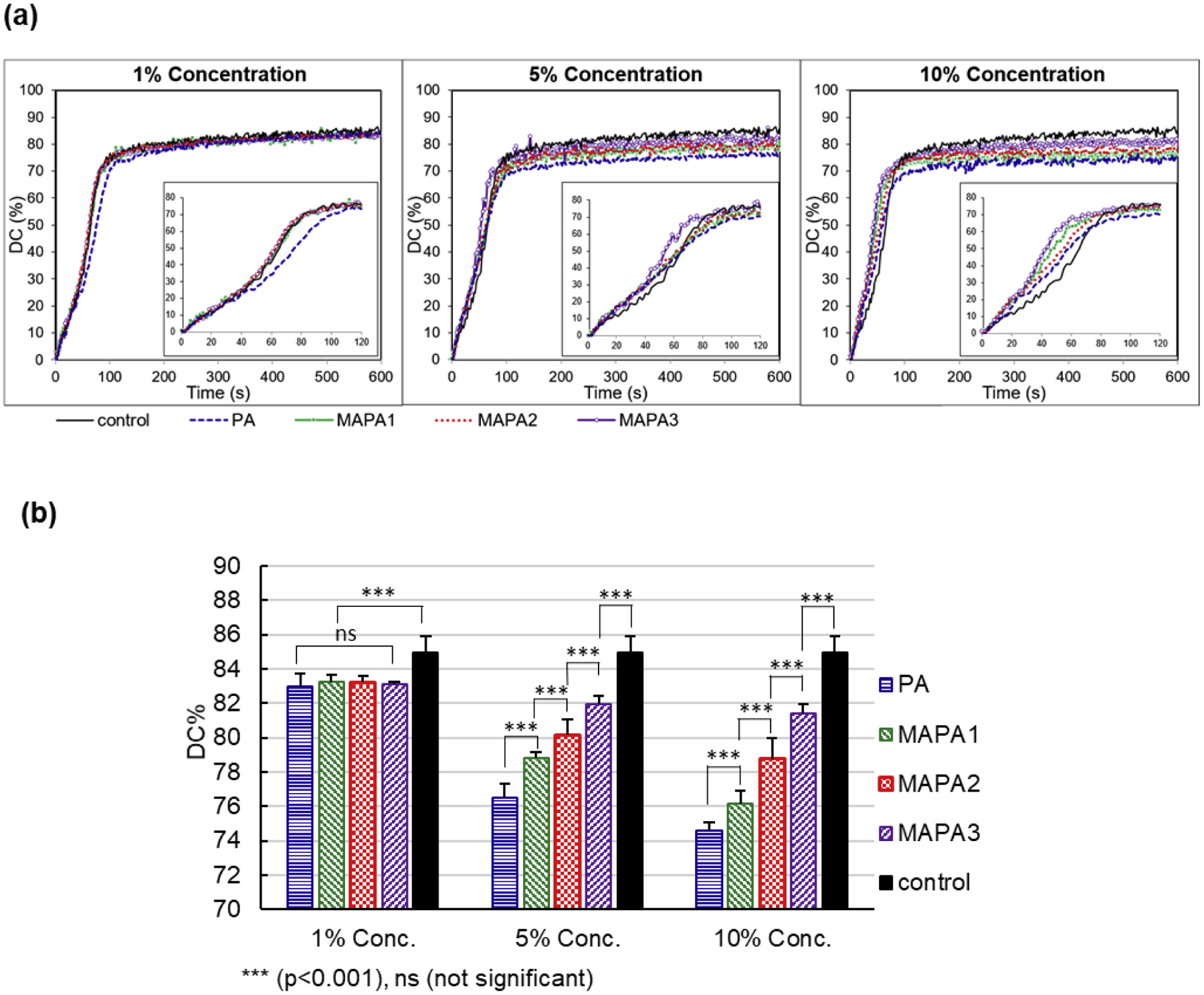
(a) Representative photopolymerization kinetic curves during first 10 min of cure. (b) DC% at 10 min of cure, for all tested adhesive groups (n = 3).
During the first 10 min of polymerization, the rate of polymerization (RoP) changed over time in different patterns for different adhesives. The control adhesive showed three phases in its polymerization kinetics: in the first phase (0–50 s), it showed a steady polymerization rate with an average RoP of ~0.6 %/s; in the second phase (50–85 s), its RoP increased to ~1.2 %/s; and in the third phase (after 85 s), its kinetic curve reached a plateau with a dramatically reduced RoP (~0.03 %/s during 85–600 s). At 1% concentration, all test adhesives showed similar kinetics to control except for the PA adhesive, which showed a relatively lower RoP of ~ 0.63 %/s after 40 s until reaching a plateau at about 120 s. At 5% concentration, the MAPA-1, MAPA-2 and PA adhesives showed similar kinetics, with a RoP of 0.7–0.8 %/s before reaching a plateau at about 90 s. Their RoPs were higher than control before 60 s and lower than control between 60 and 90s. The MAPA-3 adhesive showed the highest RoP of ~1.3%/s during 40–70 s until reaching a plateau at about 70 s. At 10% concentration, the MAPA-2 and PA adhesives showed similar kinetics, with a RoP of ~1.0 %/s between 30–60 s. the MAPA-1 adhesive and MAPA-3 adhesive showed higher RoPs at ~1.3 %/s and ~1.5%/s respectively between 30–60 s. All test groups showed higher RoPs than control before 60 s. Their RoPs became lower than control after 60 s and reached plateaus sooner than control.
The DC results at 10 min of cure for all adhesive groups were illustrated as bar chart in Fig. 3b. For all test adhesives, increasing concentration from 1% to 5% to 10% significantly reduced the DC values. At 1% concentration, all test adhesives showed similar DC values, which were significantly lower than the control. At 5% and 10% concentrations, the DC values showed a significant descending trend in the order of control > MAPA-3 > MAPA-2 > MAPA-1 > PA.
Meanwhile, a parallel study showed that MAPA-1 and MAPA-2 exhibited significantly better collagen stabilization effects than MAPA-3 [28]. In other words, in terms of collagen stability, the order is PA ~ MAPA-1 ~ MAPA-2 > MAPA-3 (~ means similar). Taking both the adhesive polymerization results and collagen stabilization results into consideration, MAPA-2 was deemed the best candidate among the three MAPAs to achieve a balanced function between collagen stabilization and adhesive polymerization and was therefore chosen for further terminal DC and microhardness studies.
Terminal DC and Microhardness at 72 h post-cure
Fig. 4 summarizes terminal DC (a) and microhardness (b) results at 72 h post-cure for the control, MAPA-2 and PA adhesives at three concentrations (1%, 5% and 10%). At 1% concentration, the terminal DC and microhardness values were similar for all three formulations. At 5% concentration, the MAPA-2 adhesive showed significantly higher DC and microhardness values than both the control and PA adhesive. At 10% concentration, the terminal DC of the MAPA-2 adhesive was similar to that of the control, but significantly higher than that of the PA adhesive; the microhardness of the MAPA-2 adhesive was also significantly higher than both control and the PA adhesive, with the latter two showing similar values. Clearly, adhesive formulation with 5% MAPA-2 showed the highest terminal DC and the highest microhardness. It is worth noting that the microhardness of that formulation is more than twice as high as that of the control.
Fig. 4.
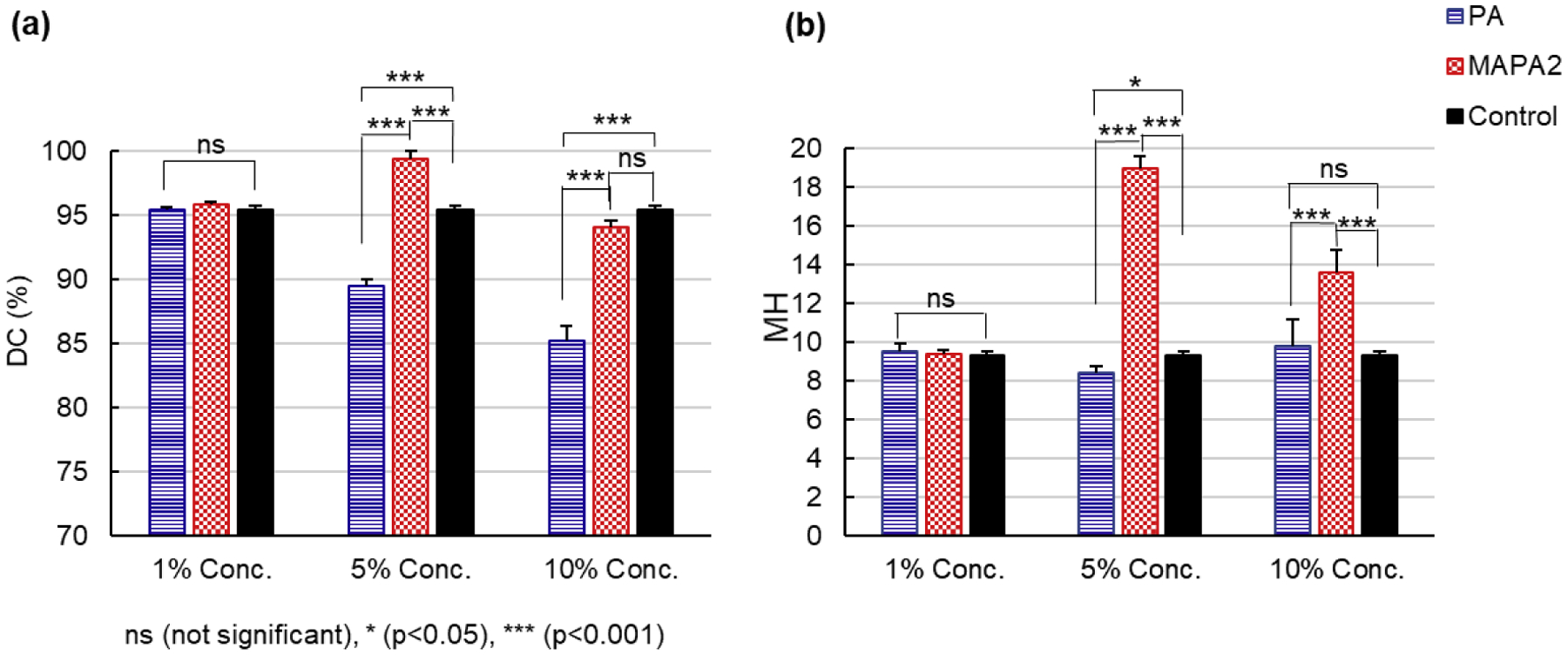
(a) Degree of conversion. (b) Vicker’s Microhardness at 72 h post-cure for control, PA and MAPA-2 adhesives (n = 3).
Leaching of cured adhesives
The UV-Vis absorption spectra of the supernatant covering the cured control, 5% MAPA-2 and 5% PA adhesives were taken on day 0, 1, 2, 3, 4, 5, 7, 9, 12, 15, 22, and 29. The reference UV-Vis spectra of aqueous solutions of HEMA, EDMAB, DPIHP and PA were shown in Fig. 5a. The reference spectra of MAPA-2 and CQ were not measured due to their poor water solubility. To avoid complications of the absorbance below 250 nm, only absorbance peaks above 250 nm were used for characterization purposes. Representative absorbance curves for days 0, 1, 5, 12, and 29 were plotted for Fig. 5 (b), (c), and (d) to avoid curve crowdedness. According to Fig. 5a, PA exhibits a characteristic peak near 282 nm;and EDMAB exhibits one major characteristic peak near 320 nm. Fig. 5b shows the leaching results of the control adhesive. EDMAB leaching was detected starting day 1 and continued to increase at a slow rate throughout day 29. Fig. 5c shows the leaching results of the 5% PA adhesive. Both PA and EDMAB leaching was detected starting day 1. PA leaching continued to increase throughout day 29. Fig. 5d shows the leaching results of the MAPA-2 adhesive. EDMAB leaching was detected starting day 1 and stayed about the same throughout day 29. Minimal PA leaching was detected starting day 9, and continued to increase slowly throughout day 29. For qualitative comparison purposes, the absorbance for all the adhesive samples was normalized to 1mg adhesive in 3 mL of water. Note that PA follows the Beer-Lambert law with a linear relationship between the concentration and the absorbance of its aqueous solution up to ~1.6 absorbance at 282 nm, which is way higher than the maximum absorbance of all the samples of study at 282 nm. The absorbance values at 282 nm for all testing days were plotted in the inserts of Fig. 5 (c) and (d) to illustrate different PA leaching trends semi-quantitatively. The absorbance value at 282 nm includes contributions from both PA and EDMAB. From the control data and the shape of the curves, the EDMAB contribution stabilized after day 5. Most absorbance changes after day 5 came from the PA contribution. In comparison, the 5% PA adhesive showed significantly more PA leaching than the 5% MAPA-2 adhesive throughout day 29.
Fig. 5.
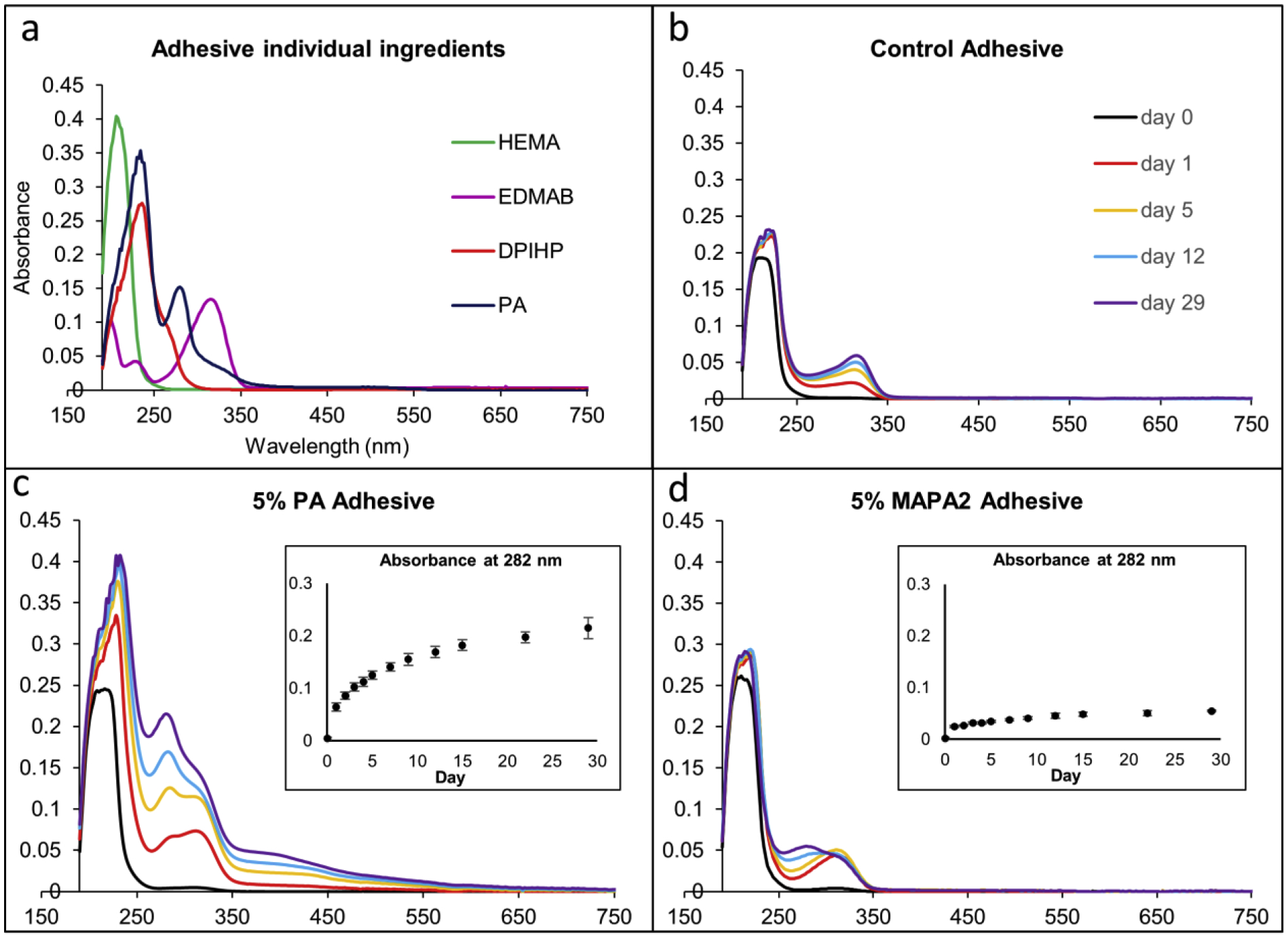
UV-Vis absorbance results of (a) HEMA, EDMAB, DPIHP, and PA solutes in water (b) cured control adhesive (c) cured 5% PA adhesive, and (d) cured 5% MAPA-2 adhesive. The absorption curves in (b), (c), and (d) are normalized to 1 mg adhesive in 3 mL of water. Representative absorbance curves are plotted for (b), (c), and (d) to avoid figure crowdedness. Complete absorbance data at 282 nm from day 0 to day 29 (average and standard deviation) are included in the inserts of (c) and (d).
4. Discussion
PA from grape seed extract was found to be a promising collagen cross-linker and MMP inhibitor, with great potential in enhancing mechanical strength and stability of dentin collagen matrix and improving the longevity of dental restoratives [12, 14]. Researchers have been exploring practical ways of integrating PA to adhesive systems in the past decade. Green et al. first reported that the presence of 5% PA in dental adhesives inhibited the biodegradation of unprotected collagen fibrils within the hybrid layer in 2010 [6]. However, a follow-up study in 2013 by Liu et al. showed that the incorporation of PA to adhesives hampered the photopolymerization of adhesive monomers [25]. The authors further revealed the excellent capability of PA in stabilizing dentin collagens in clinical relevant time of less than 30s when applying PA to etched dentin as a primer in 2013 and reported that adding PA to phosphoric acid (<20%) etchant protected collagen from enzymatic degradation in experimental settings in 2014 [17, 20]. Since then, researchers have been continuously searching for more convenient and effective ways of improving dental adhesive systems with PA and PA related compounds. Recently, we synthesized MAPA by coupling methacrylate to PA to produce a novel class of polymerizable collagen cross-linker.
Binding methacrylate to other molecules or species produces bi-functional or multifunctional adducts that could polymerize or co-polymerize with other monomers to form a polymer matrix, while maintaining the functions inherited from original molecules or species. Methacrylate functionalized molecules have been previously used in dental adhesives in the form of polymerizable initiators/co-initiators [29–31] and antibacterial monomers [32]. The novel MAPA is expected to inherit PA’s collagen crosslinking and MMP inhibiting capabilities while helping with adhesive polymerization via MA’s co-polymerization capability with adhesive monomers.
Since the functionalities of MAPAs depend on their unique chemical structures, we were interested in understanding how different ratios of MA and PA in the MAPA affect collagen protection and adhesive polymerization. To this end, three MAPAs were synthesized using different MA to PA feeding ratios in the chemical synthesis process. The final products were characterized by 1H NMR and FTIR spectroscopic techniques. 1H NMR measurements, as discussed in [28], showed that the MA:PA ratios for MAPA-1, MAPA-2, and MAPA-3 were 0.6:1, 09:1, and 1.5:1, respectively, in the final products. The MA:PA ratios were also analyzed qualitatively from their IR spectra using the ratio of the 1720 cm−1 peak height to the 1608 cm−1 peak height. The 1720 cm−1 peak intensity represents the amount of C=O ester bonds in the MA component of the structure while the 1608 cm−1 peak intensity reflects the amount of aromatic rings in the PA component of the structure. The 1720/1608 ratios of MAPAs-1,-2,-3 were measured to be 0.47, 0.71 and 1.44 respectively, evidencing an increasing amount of MA linkage to PA in the final products.
The first task of the current study was to investigate the impact of the three synthesized MAPAs on the polymerization kinetics of the experimental adhesives in comparison to PA and control during 10 min of cure. At 1% concentration, the three MAPA adhesives showed similar polymerization kinetics to that of the control, which all showed auto-acceleration (stage II) behavior due to the so-called gel effect. The 1% PA adhesive, however, showed much smaller gel effect (Trommsdorff effect) with a lower polymerization rate than other groups, presumably due to the radical scavenging effect of PA. At 5% and 10% concentrations, the addition of MAPAs or PA compound all significantly affected the adhesive polymerization kinetics. Specifically, all three MAPA and PA compounds increased the initial RoP (0 – 40 s) of the experimental adhesive compared to control. Since PA is a known radical scavenger that was found to impede adhesive polymerization [33] [25], the observation that PA increased the initial RoP of the adhesive is a little surprising but can be possibly explained by the following: PA are oligomeric and polymeric flavonoids with high molecular weight and the addition of PA increases the viscosity of the HEMA (molecular weight = 130.14 g/mol) experimental adhesive system. The increased viscosity will reduce the diffusion rate of propagating radicals in the system, leading to less radical-radical encountering and therefore lower termination rates, which is reflected as higher initial RoP. All three MAPA compounds increased the initial RoP more than the PA compound because of the co-polymerization effect of their MA components in addition to their higher viscosity. At 10-min of cure, all MAPA and PA adhesives showed significantly lower DC than control. Among them, PA reduced the 10-min DC the most followed by MAPA-1, MAPA-2 and MAPA-3 at each concentration, which suggests that the co-polymerization function of the MA component in the MAPA compound indeed compensates for the adverse effect of the PA component on adhesive polymerization to various extents. Although a higher ratio of MA to PA in the MAPA compound seemed to better help with the adhesive polymerization, the results from the collagen protection study [28] showed good collagen protection from MAPA-1 and MAPA-2 (both similar to PA), but not from MAPA-3. The combined results led to a logical selection of MAPA-2 among the three MAPAs as the best candidate for further investigation due to its balanced effects on both collagen protection and adhesive polymerization.
Then we moved on to investigate the effects of MAPA-2 on terminal DC and microhardness of the experimental adhesive at 72 h post-cure in comparison with PA and control at three concentrations. The DC of all adhesives exhibited significant increases from 10 min of cure to 72 h post-cure due to post-polymerization effect, with the control adhesive experiencing the least increase while the MAPA-2 adhesive experiencing the most increase. The terminal DC of the PA adhesives reduced from 95% to 90% to 85% as the PA concentration went up from 1% to 5% and to 10%, suggesting increasingly negative effect of PA on adhesive polymerization as increasing concentration. The negative effect of PA not only comes from its radical scavenging ability but also from its reddish brown color, which could interfere with the curing-light penetration and reduce degree of conversion on the bottom testing surface of the adhesives. The terminal DC of the MAPA-2 adhesives increased from 96% at 1% concentration to >99% at 5% concentration but decreased to 94% at 10% concentration. The increase of DC from 1% to 5% concentration is most likely due to the positive effect of MA component on adhesive polymerization. The decrease of DC from 5% to 10% concentration is possibly due to the radical scavenging ability of the PA component and the dark color of the MAPA compound. When the negative effects of the PA component and the dark color of the adhesive exceed the positive effect of its MA component, an overall reduction in DC can be resulted. Overall, the 5% MAPA-2 adhesive exhibited the highest terminal DC among all tested adhesives.
The 5% MAPA-2 adhesive also exhibited the highest microhardness (MH = 18.9), a dramatic 100% increase from that of the control (MH = 9.3) and 125% increase from that of the 5% PA adhesive (MH = 8.4). The microhardness of the MAPA-2 adhesive decreased to 13.6 at 10% concentration due to decreased DC. However, the microhardness of the adhesives did not have a very strong correlation with their DC (ρ = 0.55). It is because that the DC calculated based on Equation (1) only reflects methacrylate polymerization via carbon double bond (C=C) to single bond (C-C) conversion while microhardness depends on the whole adhesive matrix which includes both MA polymers and PA polymers. PA polymers are formed via oxidative polymerization process [34]. This explains why the microhardness of the 5% and 10% PA adhesives were similar to that of control even though their DC values were significantly lower than the control, and the microhardness of the 10% MAPA-2 adhesive was much higher than that of control even through their DC values showed no significant difference.
In short, the incorporation of MAPA to the experimental adhesive showed better results than PA in terms of adhesive polymerization and microhardness. The first and second null hypotheses that MAPA have the same effects on photopolymerization and microhardness of the adhesive as PA are rejected.
Finally, we conducted a leaching study for cured control, 5% MAPA-2 and 5% PA adhesives. The results show significantly reduced HEMA monomers leaching from the 5% MAPA-2 adhesive than from the 5% PA and control adhesives, thanks to the higher degree of conversion and therefore less leftover HEMA monomers in the cured MAPA-2 adhesive. Continuous PA leaching was observed from the 5% PA adhesive throughout the entire test period (day 0 – day 37). In comparison, only a small amount of transient PA leaching was observed from the 5% MAPA-2 adhesive. The PA leaching difference can be explained by different statuses of PA component in the cured adhesives. For the 5% PA adhesive, the PA component is physically embedded in the adhesive polymer matrix and therefore can migrate and leach out over time; for the 5% MAPA-2 adhesive, the PA component is covalently bonded to the adhesive polymer matrix via the polymerizable MA groups and thus cannot leach out without first breaking the covalent linkage. The water-exposed ester bond between MA and PA in MAPA-2 on the surface of the adhesive, however, may undergo slow hydrolysis which likely accounts for the small amount of PA leaching from the MAPA-2 adhesive. The control adhesive leached slightly less EDMAB than the 5% PA and 5% MAPA-2 adhesives. A possible explanation is that the PA component may potentially serve as the electron donors during photopolymerization similar to the role of EDMAB and therefore less amount of EDMAB is consumed during the photopolymerization in the 5% PA and 5% MAPA-2 adhesives, with the leftover EDMAB leaching out. Overall, the 5% MAPA-2 adhesive experienced much less leaching than the 5% PA and control adhesives. Hence, the third null hypothesis that cured adhesive with MAPA has the same leaching profile as that with PA is also rejected.
In the current study, the effects of MAPA were investigated in a HEMA-based experimental adhesive formulation. For clinical applications, similar benefits should be expected from the incorporation of MAPA to HEMA-based commercial adhesives, although the specific effects might vary depending on the specific adhesive formulation. In addition to be added in adhesives, MAPA can also be used as a primer. When used as a primer, MAPA is expected to provide collagen stabilization effect just like PA. However, unlike a PA primer which has to be rinsed after application to avoid its detrimental effect on adhesive polymerization, a MAPA primer does not need to be rinsed off due to its ability to co-polymerize with the adhesive. Further research is needed to evaluate the effects of MAPA in commercial adhesive formulations and as a primer in clinical settings. These studies include polymerization evaluation, leaching test, chemo-mechanical assessments (e.g. ultimate tensile strength and modulus of elasticity), and interface studies.
5. Conclusions
The incorporation of MAPA to HEMA-based adhesives improves adhesive polymerization, increases microhardness, and reduces leaching of cured adhesives. The combined results from the current study and from Part I study which shows excellent dentin collagen protection by MAPA render MAPA a potentially revolutionary class of polymerizable collagen cross-linker for enhancing the bond-strength and the longevity of dental restorations.
ACKNOWLEDGMENTS
This study was supported by Research Grant R01-DE027049 from the National Institute of Dental and Craniofacial Research, National Institutes of Health, Bethesda, MD 20892, USA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hashimoto M, Ohno H, Endo K, Kaga M, Sano H, Oguchi H. The effect of hybrid layer thickness on bond strength: demineralized dentin zone of the hybrid layer. Dent Mater 2000;16:406–11. [DOI] [PubMed] [Google Scholar]
- 2.Wang Y, Spencer P, Yao X. Micro-Raman imaging analysis of monomer/mineral distribution in intertubular region of adhesive/dentin interfaces. J Biomed Opt 2006;11:024005. [DOI] [PubMed] [Google Scholar]
- 3.Breschi L, Martin P, Mazzoni A, Nato F, Carrilho M, Tjäderhane L, et al. Use of a specific MMP-inhibitor (galardin) for preservation of hybrid layer. Dent Mater 2010;26:571–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang W, Yang W, Wu S, Zheng K, Liao W, Chen B, et al. Effects of acid etching and adhesive treatments on host-derived cysteine cathepsin activity in dentin. J Adhes Dent 2014;16:415–20. [DOI] [PubMed] [Google Scholar]
- 5.Mahalaxmi S, Madhubala MM, Jayaraman M, Sathyakumar S. Evaluation of matrix metalloproteinase and cysteine cathepsin activity in dentin hybrid layer by gelatin zymography. Indian J Dent Res 2016;27:652–6. [DOI] [PubMed] [Google Scholar]
- 6.Green B, Yao X, Ganguly A, Xu C, Dusevich V, Walker MP, et al. Grape seed proanthocyanidins increase collagen biodegradation resistance in the dentin/adhesive interface when included in an adhesive. J Dent 2010;38:908–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Castellan CS, Bedran-Russo AK, Karol S, Pereira PN. Long-term stability of dentin matrix following treatment with various natural collagen cross-linkers. J Mech Behav Biomed Mater 2011;4:1343–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu C, Wang Y. Cross-linked demineralized dentin maintains its mechanical stability when challenged by bacterial collagenase. J Biomed Mater Res Part B, Appl Biomater 2011;96:242–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu C, Wang Y. Collagen cross linking increases its biodegradation resistance in wet dentin bonding. J Adhes Dent 2012;14:11–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Han B, Jaurequi J, Tang BW, Nimni ME. Proanthocyanidin: a natural crosslinking reagent for stabilizing collagen matrices. J Biomed Mater Res Part A 2003;65:118–24. [DOI] [PubMed] [Google Scholar]
- 11.Epasinghe DJ, Yiu CK, Burrow MF, Hiraishi N, Tay FR. The inhibitory effect of proanthocyanidin on soluble and collagen-bound proteases. J Dent 2013;41:832–9. [DOI] [PubMed] [Google Scholar]
- 12.Balalaie A, Rezvani MB, Mohammadi Basir M. Dual function of proanthocyanidins as both MMP inhibitor and crosslinker in dentin biomodification: A literature review. Dent Mater J 2018;37:173–82. [DOI] [PubMed] [Google Scholar]
- 13.Hass V, Luque-Martinez IV, Gutierrez MF, Moreira CG, Gotti VB, Feitosa VP, et al. Collagen cross-linkers on dentin bonding: Stability of the adhesive interfaces, degree of conversion of the adhesive, cytotoxicity and in situ MMP inhibition. Dent Mater 2016;32:732–41. [DOI] [PubMed] [Google Scholar]
- 14.Bedran-Russo AK, Pereira PN, Duarte WR, Drummond JL, Yamauchi M. Application of crosslinkers to dentin collagen enhances the ultimate tensile strength. J Biomed Mater Res Part B, Appl Biomater 2007;80:268–72. [DOI] [PubMed] [Google Scholar]
- 15.Liu R, Fang M, Xiao Y, Li F, Yu L, Zhao S, et al. The effect of transient proanthocyanidins preconditioning on the cross-linking and mechanical properties of demineralized dentin. J Mater Sci: Mater in Med 2011;22:2403–11. [DOI] [PubMed] [Google Scholar]
- 16.Castellan CS, Pereira PN, Grande RH, Bedran-Russo AK. Mechanical characterization of proanthocyanidin-dentin matrix interaction. Dent Mater 2010;26:968–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu Y, Chen M, Yao X, Xu C, Zhang Y, Wang Y. Enhancement in dentin collagen’s biological stability after proanthocyanidins treatment in clinically relevant time periods. Dent Mater 2013;29:485–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fang M, Liu R, Xiao Y, Li F, Wang D, Hou R, et al. Biomodification to dentin by a natural crosslinker improved the resin-dentin bonds. J Dent 2012;40:458–66. [DOI] [PubMed] [Google Scholar]
- 19.Al-Ammar A, Drummond JL, Bedran-Russo AK. The use of collagen cross-linking agents to enhance dentin bond strength. J Biomed Mater Res Part B, Appl Biomater 2009;91:419–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu Y, Dusevich V, Wang Y. Addition of Grape Seed Extract Renders Phosphoric Acid a Collagen-stabilizing Etchant. J Dent Res 2014;93:821–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hass V, Luque-Martinez I, Munoz MA, Reyes MF, Abuna G, Sinhoreti MA, et al. The effect of proanthocyanidin-containing 10% phosphoric acid on bonding properties and MMP inhibition. Dent Mater 2016;32:468–75. [DOI] [PubMed] [Google Scholar]
- 22.Paludo T, Marcondes ML, Souto AA, Lopes GC, Loguercio AD, Spohr AM. Effect of grape seed extract-containing phosphoric acid formulations on bonding to enamel and dentin. Braz Oral Res 2019;33:e098. [DOI] [PubMed] [Google Scholar]
- 23.De-Paula DM, Lomonaco D, Ponte AMP, Cordeiro KE, Moreira MM, Mazzetto SE, et al. Influence of collagen cross-linkers addition in phosphoric acid on dentin biomodification and bonding of an etch-and-rinse adhesive. Dent Mater 2020;36:e1–e8. [DOI] [PubMed] [Google Scholar]
- 24.Quideau S, Deffieux D, Douat-Casassus C, Pouysegu L. Plant polyphenols: chemical properties, biological activities, and synthesis. Angewandte Chemie (International ed in English), 2011;50:586–621. [DOI] [PubMed] [Google Scholar]
- 25.Liu Y, Wang Y. Effect of proanthocyanidins and photo-initiators on photo-polymerization of a dental adhesive. J Dent 2013;41:71–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Epasinghe DJ, Yiu CKY, Burrow MF. Mechanical properties, water sorption characteristics, and compound release of grape seed extract-incorporated resins. J Appl Oral Sci: revista FOB, 2017;25:412–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Souza LC, Rodrigues NS, Cunha DA, Feitosa VP, Santiago SL, Reis A, et al. Two-year clinical evaluation of proanthocyanidins added to a two-step etch-and-rinse adhesive. J Dent 2019;81:7–16. [DOI] [PubMed] [Google Scholar]
- 28.Hass V, Li Y, Wang R, Nguyen D, Peng Z, Wang Y. Methacrylate-functionalized proanthocyanidins as a multifunctional additive to dental adhesive - Part I: collagen crosslinking and enhanced stability. Dent Mater 2020. [Google Scholar]
- 29.Ge X, Ye Q, Song L, Laurence JS, Spencer P. Synthesis and evaluation of a novel co-initiator for dentin adhesives: polymerization kinetics and leachables study. JOM (Warrendale, Pa : 1989), 2015;67:796–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moreira AG, Cuevas-Suarez CE, da Rosa WLO, Ogliari AO, Petzhold CL, Piva E, et al. Piperonyl methacrylate: Copolymerizable coinitiator for adhesive compositions. J Dent 2018;79:31–8. [DOI] [PubMed] [Google Scholar]
- 31.Song L, Sarikaya R, Ye Q, Misra A, Tamerler C, Spencer P. Multifunctional monomer acts as co-initiator and crosslinker to provide autonomous strengthening with enhanced hydrolytic stability in dental adhesives. Dent Mater 2019;36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fujimura Y, Weerasinghe D, Kawashima M. Development of an antibacterial bioactive dental adhesive: Simplicity and innovation. Am J Dent 2018;31:13b–6b. [PubMed] [Google Scholar]
- 33.Wright JS, Johnson ER, DiLabio GA. Predicting the Activity of Phenolic Antioxidants: Theoretical Method, Analysis of Substituent Effects, and Application to Major Families of Antioxidants. J Am Chem Soc 2001;123:1173–83. [DOI] [PubMed] [Google Scholar]
- 34.Patil N, Jérôme C, Detrembleur C. Recent advances in the synthesis of catechol-derived (bio)polymers for applications in energy storage and environment. Prog Polym Sci 2018;82:34–91. [Google Scholar]


