Abstract
Molecular detection of pathogenic nucleic acids from patient samples requires incubating biochemical reactions at specific temperatures to amplify DNA. This incubation is typically carried out with an electrical heater and a temperature controller. To reduce test cost; eliminate the need to manufacture incubators, which may require significant time; and to enable electricity-free operation, we use an energetic compound such as Mg(Fe) alloy mixed with a phase-change material (PCM) that undergoes phase transformation at the desired incubation temperature. We dub this composite Energetic Phase Change Material (EPCM). When the EPCM is brought into contact with water, the magnesium alloy interacts with the water to produce heat. The EPCM heats up to its phase transition temperature. Any excess heat is absorbed as latent heat and the system is maintained at its desired incubation temperature, independent of ambient temperatures, long enough to facilitate enzymatic amplification. The EPCM together with colorimetric amplicon detection facilitates an inexpensive, disposable point-of-need diagnostic test that does not require any electric power. We demonstrate the feasibility of our approach by detecting SARS-Cov-2 in saliva samples either without any instrumentation or with a palm-size CCD camera that enables us to follow the amplification process in real time.
Graphical Abstract
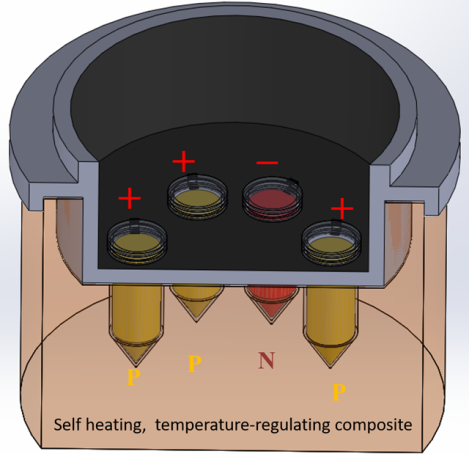
Introduction
Short of widely available vaccines and/or herd immunity, effective control of pandemics such as COVID-19 requires frequent, extensive screening with prompt results. Epidemiology models [1–3] recommend testing individuals as often as 2–3 times a week to inform these individuals whether they should self-quarantine or may go to work or school without endangering others; to enable contact tracing; and to enable policy makers adapt informed, rational, region-specific control measures. Strategies based on self-collection of samples and mailing these samples to centralized laboratories for testing involve significant delays between sample collection and results, defeating one’s ability to take prompt, preventive measures after exposure. Although hand delivery of samples to centralized collection sites eliminates mailing time, the process still takes 1–2 days to obtain results; carries some risk of exposure to the sample provider and others; is inconvenient; and may compromise sample’s integrity. Optimally, tests should be carried out at home, enabling a test and isolate strategy without the need to travel to and queue at clinics or at sample collection sites; thereby minimizing the risk of contracting / spreading the disease as well as reducing inconvenience, lost time from work, and lost productivity.
Currently, ‘molecular’ diagnostics (enzymatic Nucleic Acid Amplification Tests, NAATs) are the most sensitive and specific tests for pathogen detection. The development of portable, easy-to-use, low-cost medical diagnostics technology has been the focus of substantial efforts for three decades and is especially critical during the current COVID-19 pandemic [4–5]. Although the polymerase chain reaction (PCR) is the gold standard of molecular diagnostics, PCR is challenging to implement at the point-of-care (POC) because it requires precise thermal cycling, stringent sample preparation, and either optical or lateral flow-based detection. The advent of isothermal nucleic acid amplification has simplified sample processing and thermal control requirements, making inexpensive point-of-care (POC) and home molecular tests possible.
Reverse Transcription Loop mediated amplification (RT-LAMP) [6] is an isothermal enzymatic amplification method that has gained wide acceptability during the COVID-19 epidemic. RT-LAMP requires a constant incubation temperature, ranging from 60 to 65°C, for about 30 minutes. RT-LAMP is more tolerant of contaminants and requires less stringent sample preparation than PCR. The RT-LAMP process also produces about an order of magnitude more amplicons than PCR. The abundance of RT-LAMP amplicons enables a plethora of detection methods, ranging from fluorescent intercalating dye, fluorescent probes, colorimetric dyes, bioluminescence, and turbidity. Colorimetric dyes can be detected by eye without a need for a reader. Although the temperature control requirements of RT-LAMP are much simpler than that of PCR, the process still needs to be carried out at a fixed temperature, which requires an incubator. Generally, such incubators consist of an electrical heater and a temperature controller; may represent a significant cost for home use; require substantial manufacturing time; and are susceptible to supply line shortages such as the current global shortage in semiconductor chips, which may in turn prevent rapid deployment.
As an alternative to electrically powered incubators, various researchers including ourselves [7–17] have proposed the use of exothermic chemical reactions to generate heat and Phase Change Material (PCM) to regulate temperature. The idea of using exothermic reactions to produce heat is not new. Meals Ready to Eat (MRE) and military rations come with a pouch of an energetic material such as magnesium alloy that produces heat when mixed with water [18]. Since these flameless heaters are widely used, they are manufactured in quantity and at low cost. However, since precise temperature is not critical when warming meals, MREs do not come with means for temperature control. In contrast, temperature control is essential when incubating enzymatic amplification. Among other things, the enzymes are inefficient at too low a temperature (e.g., <55 °C for RT-LAMP) and degrade at too high a temperature (e.g., >70 °C for RT-LAMP). Hence, it is essential to maintain the incubation temperature within acceptable tolerances regardless of ambient temperatures that may vary greatly from one region of the world to another and from one season to another.
To maintain the desired temperature, independent of ambient conditions, passive control strategies rely on PCMs [7–17]. Heat released during the exothermic reaction initially increases the system’s temperature up to the PCM’s phase transition temperature that matches the enzymatic amplification incubation temperature. Additional energy released by the exothermic reaction goes into melting the PCM and is stored as latent heat. So long as the PCM co-exists in two phases (e.g., liquid and solid), the system’s temperature remains fixed at the PCM’s phase transition temperature and independent of the ambient temperature. Once the exothermic reaction has been consumed and the system starts to cool, the PCM solidifies, releasing latent heat to prolong the time interval of fixed temperature. Here, we use the biobased PCM PureTemp [19] that can be synthesized to undergo phase transition at any desired temperature in the range from −37 °C to +151 °C.
In all prior works, the PCM was placed between the RT-LAMP reaction chamber and the energetic (heat producing) material to avoid overheating the RT-LAMP chamber. Unfortunately, PCMs with the desired phase transition temperature have low thermal conductivity. This results in a relatively lengthy temperature ramp up time, which prolongs the test and may impact RT-LAMP specificity even when warm start is used. To reduce PCM thermal resistance, prior investigators blended the PCM with high thermal conductivity particles such as carbon nanotubes [20] and/or placed a thermal spreader (heat sink) into the PCM [14]. Both remedies add cost, while providing less than optimal performance. Here, we propose a simple alternative. We blend the energetic material with the granular PCM to form a homogenous composite, dubbed Energetic Phase Change Material (EPCM) that produces heat while self-regulating its temperature. The intimate contact of the reactant granules with the phase change material pellets assures high heat transfer between the fuel and phase change material. This arrangement enables us to bring the heat source in proximity with the enzymatic amplification chamber, thereby greatly reducing the temperature ramp up time.
We demonstrate the utility of our approach by detecting SARS-Cov-2 in saliva. Saliva is convenient to self-collect and has proven comparable to nasopharyngeal (NP) and nasal swab samples [21–23]. We use RT-LAMP to amplify SARS-Cov-2 RNA in a tube. Since RT-LAMP produces abundance of amplicons, test results can be detected with dyes that change color either in response to the presence of amplicons or polymerase byproducts, enabling instrument-free sample processing and detection. As an alternative, we demonstrate that the amplification process can be monitored in real time with a USB CCD camera. The method described herein can also be used to detect multiple co-endemic pathogens by aliquoting the sample into multiple tubes, each dry- storing reagents for a specific target.
Method
Incubator:
To obtain combined heating and temperature control, we mix for 5 minutes (FISHER brand® Vortex Genie 2™12-812) magnesium-iron (Mg/Fe) powder like the one used in Meals-Ready-to-Eat (flameless rations for military and outdoor recreation food cooking) (MRE, US 1992 06421, 8970-01-321-9153) with PureTemp-63 PCM (PureTemp, Minneapolis) to form a nearly homogeneous mixture, dubbed EPCM (SI Section S1). The bio-based PureTemp™ materials are nontoxic and can be synthesized to provide any desired phase change temperature in the range from −37°C to 151°C. PureTemp 63 undergoes phase transition from solid to liquid in the temperature range between 61°C and 65°C and has latent heat of 208 kJ/kg.
Addition of water to the magnesium alloy triggers the exothermic reaction [18]:
| (1) |
with substantial release of energy. Initially, the released energy increases the system’s temperature until it reaches the phase transition temperature. Thereafter, any further energy release from the reaction (1) effectuates phase transformation and is stored as latent heat in the PCM without further increasing its temperature.
We placed our EPCM mixture in a 35 mm diameter, 25 mm tall, custom made, 3D-printed cup with 2 mm thick wall (Fig. S1). The cup was, printed with photopolymer clear resin on a Form 3 stereolithographic printer (Formlab, Somerville, MA). The cup’s lid (Figure S2 center) includes a few bores to accommodate 200 μL tubes (BIO-RAD, #TFI0201). Three 1-mm wide slots around the lid’s periphery allow the venting of any hydrogen gas produced during the exothermic reaction (equation 1). The cup is placed in a Styrofoam block for thermal insulation. In operation, about 2/3 of each tube’s length is submersed in the EPCM. We coated the top of the lid with black electrical tape or 2-mm thick black foam rubber to suppress background fluorescence reflection / emission (Fig. 1B) and (with foam rubber) provide thermal insulation. Such a coating is not needed for colorimetric detection. To further reduce cost, one can replace the 3D-printed cup with molded Styrofoam.
Fig. 1:
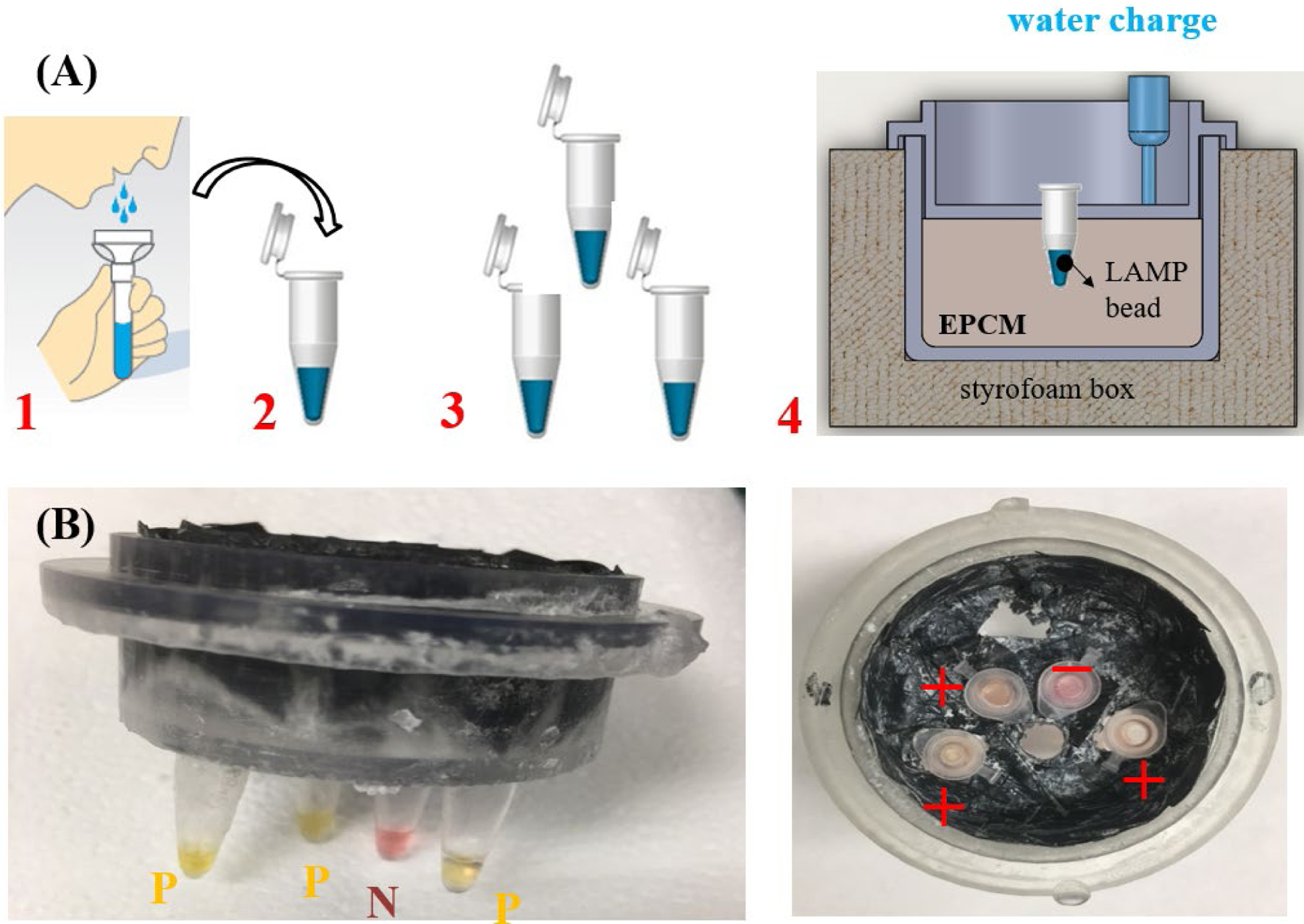
Chemical heater-based, instrumentation-free, molecular detection of SARS-Cov-2 in saliva. (A) Test workflow: (1) collect saliva sample in a tube; (2) mix saliva with lysis buffer; (3) aliquot the sample to individual tubes, each dry-storing RT-LAMP reaction mix specific to the selected target; (4) insert tube(s) in the chemical heater and add water to the EPCM to initiate exothermic reaction and heat the tubes to 60 – 65 °C for ~30 minutes. (B) Color change of the tube indicates whether the test is positive (yellow, three tubes) or negative (red, one tube). Here, the negative test is a negative control to verify that the color change does not occur because of the chemical composition of the sample.
The cup can accommodate multiple tubes. We envision that these tubes will house dry-stored (lyophilized) reaction mixes and primers, with each tube reaction mix formulated to detect a specific target pathogen of interest. One of these tubes serves as a negative control and another tube can be used as a positive control to detect targets that are present in all samples such as beta actin. The positive control verifies the reagent integrity; the absence of inhibitors in the sample; and the appropriateness of the incubation temperature.
After the insertion of the sample into the tubes, the tubes are placed into the cup and water is added to the EPCM from above to initiate the exothermic reaction. Since the water only partially wets the PCM, it takes 2–3 minutes for the water to infiltrate into the porous EPCM. Some of the water may absorb into the PureTemp® material and over time degrade the PCM. This is, however, not a concern here since we use the biodegradable PCM for a relatively short time and dispose of it after use. For more information on materials and methods see supplemental information.
Results
Thermal Performance
Once water is added to the cup, the water seeps through the EPCM and reacts with the Mg(Fe) alloy. The, hydrogen gas produced during this reaction percolates up through the powder mix. After the EPCM has reached the PCM’s phase change temperature, any additional energy produced by the reaction is consumed as latent heat – transforming the PCM from a solid into a liquid - instead of increasing the system’s temperature; thereby, our system maintains its desired incubation temperature independent of the ambient temperature within a broad range of ambient temperatures. Once the chemical reaction ran its course, thermal losses to the environment are mitigated by the release of latent heat as the PCM transforms back from a liquid into a solid, enabling the reaction chamber to maintain a nearly uniform temperature for time intervals well beyond what is needed for the amplification process.
To characterize our system’s thermal performance, we placed a thermocouple (OMEGA, K type, # TFIR-24S-50) into one of the tubes with 20 μL of water and monitored the tube’s temperature as a function of time. These experiments were carried out in an environmental oven with the oven temperature ranging from 10°C to 40°C (Fig. 2A) to mimic operation under various ambient conditions. Despite the significant variations in the ambient temperature, our EPCM maintains a reasonably stable incubation temperature as needed for the RT-LAMP process. We carried out all experiments in triplicate with good reproducibility.
Fig 2:
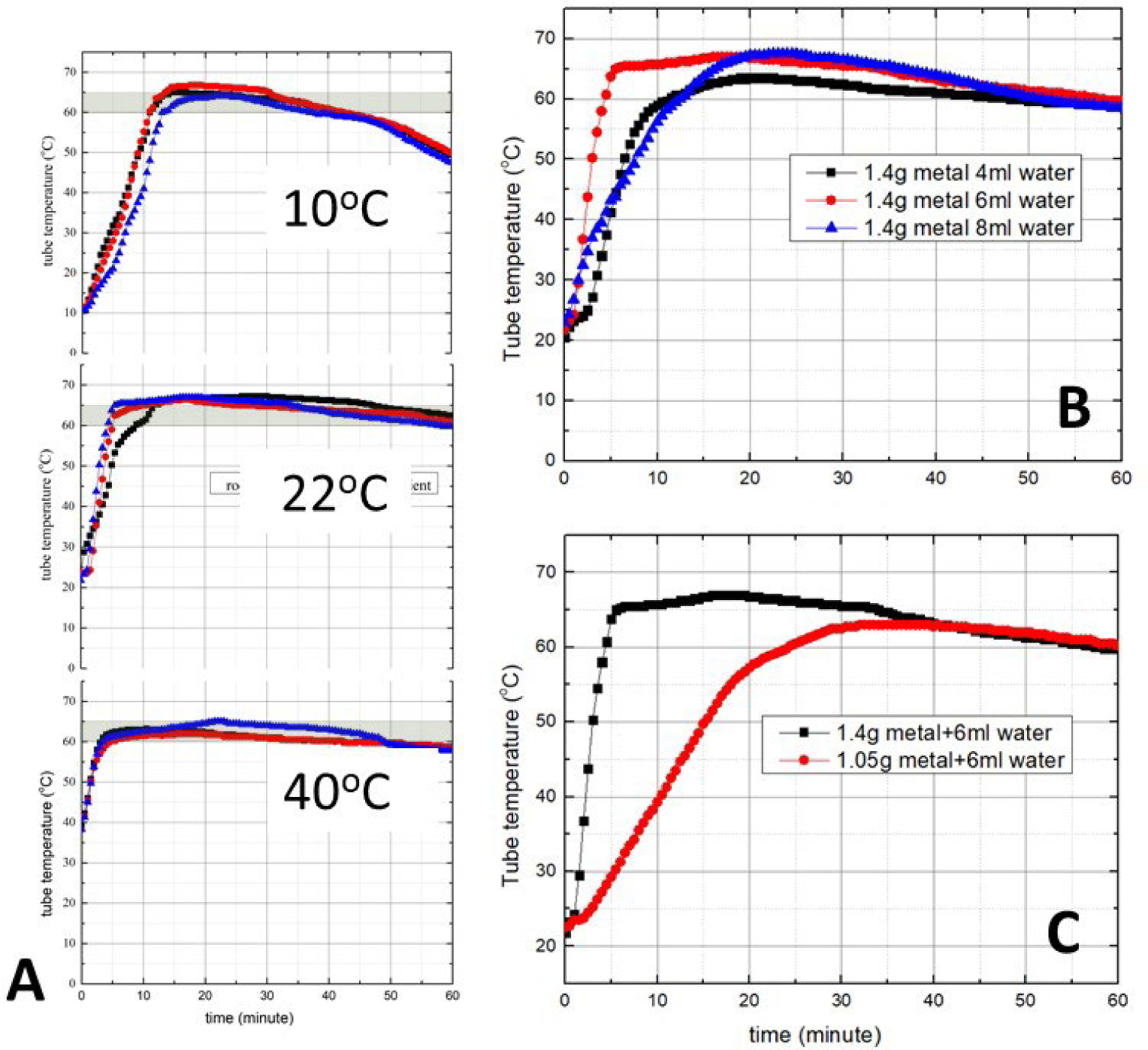
The tube temperature as a function of time (A) when the ambient temperature is 10°C, 22°C, and 40 °C (1.4 g Mg(Fe), 6 mL water, all experiments in triplicate); (B) when the water volume is 4, 6, and 8 mL (1.4 g Mg(Fe), ambient temperature 22°C); and (C) when the Mg(Fe) mass is 1.05 g and 1.4 g (6 mL water, ambient temperature 22°C). In all cases, the mass of the PCM (PureTemp 63) is 10.5 g.
To minimize the temperature ramp up time, we used 6 mL of water (4.3 mL water per gram of Mg(Fe)), which exceeds the stoichiometric water mass (2.1 mL). The use of excess water increases the rate of water infiltration into the EPCM, reducing the temperature ramp up time, and making up for any water absorbed by the PCM and for water evaporation. Smaller (4 mL) and greater (8 mL) volumes of water increased the temperature ramp up time from approximately 3 min to nearly 10 min (Fig. 2B) without a significant change in the incubation temperature. Hence, it appears that 6 mL water is nearly optimal for our set-up. The ramp up time also depends on the mass ratio of the Mg(Fe) fuel and the PCM. A reduction in the Mg(Fe) mass results in an increase in the ramp up time (Fig. 2C).
Molecular diagnostics
To assess the diagnostic capabilities of our system, we used contrived samples of SARS-Cov-2 virions isolated from a cell line and suspended in saliva donated by healthy individuals. We incubated our samples at 65°C for 15 min to lyse and inactivate the virus. The samples were then diluted 10, 100, and 1000-fold. The saliva samples were aliquoted into three 200 μL tubes together with the LAMP reaction mix (WarmStart® Colorimetric LAMP 2X Master Mix, New England Biolabs, USA augmented with EvaGreen® fluorescent dye, SI section S3). In each case, we added a set of six primers targeting the N3 gene of SARS-Cov-2 [25] to a total reaction volume of 20 μL per tube. We triplicated our test to examine reproducibility. In applications, each tube may contain a reaction mix for a different target. The fourth tube was used as a negative (no primer) control to examine for possible color change resulting from sample composition. Once the tubes were sealed, they were placed into our heating cup (Fig. 1) and water was added to the EPCM to initiate the exothermic reaction. We used two methods to detect the presence of amplicons: colorimetric dye (Fig. 1B) and fluorescent intercalating dye (Fig. 3). Concurrently, the same samples were processed with a benchtop (BIO-RAD CFX96™ Real-Time System, C1000 Touch Thermal Cycler) thermal cycler programmed to operate at a fixed temperature (65°C) (SI Section S5).
Fig. 3:
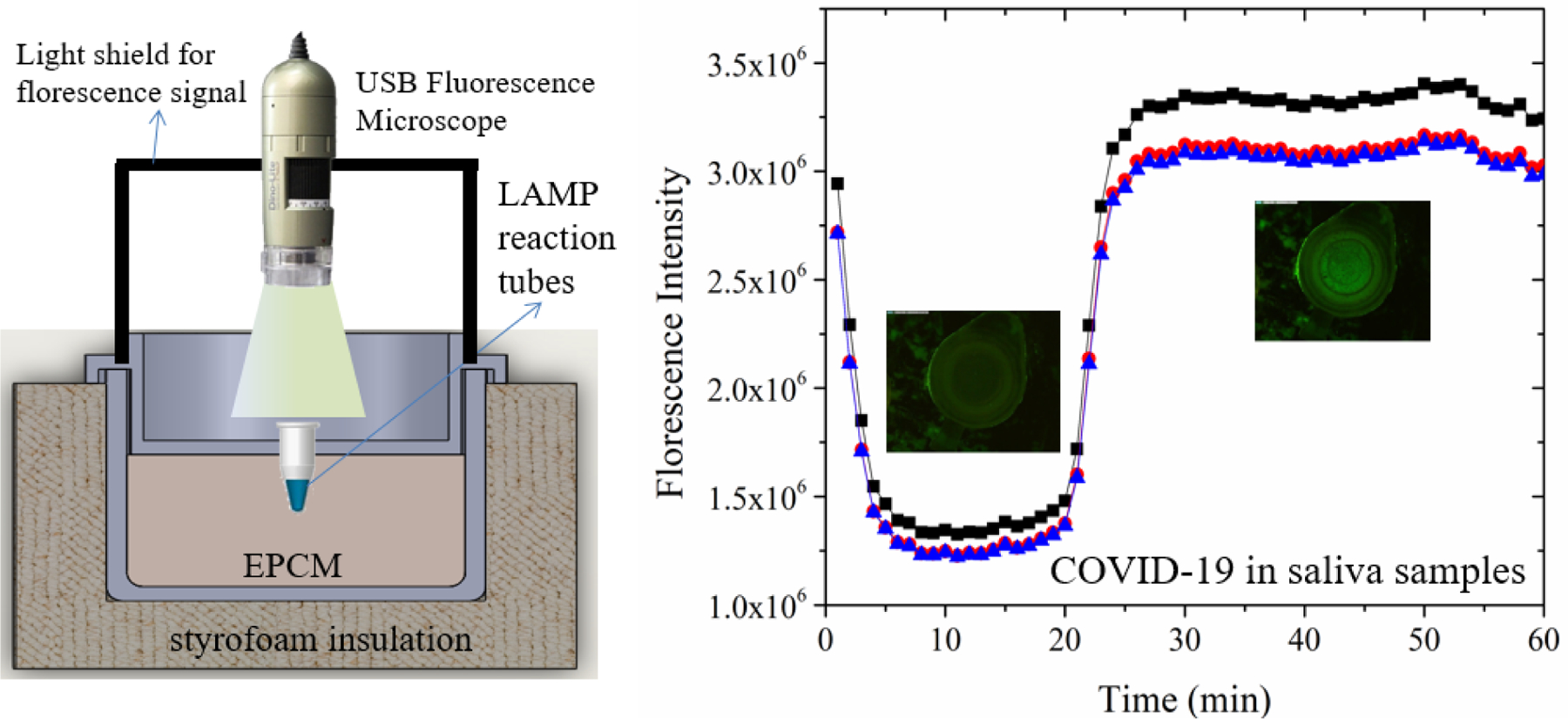
Real-time monitoring of RT-LAMP incubated with a chemical heater. (Left) schematics of experimental set up to monitor fluorescence intensity of the RT-LAMP reaction, amplifying COVID-19 RNA in diluted saliva. (Right) Fluorescence (EvaGreen™ intercalating dye) emission captured with our USB microscope (see Fig. S2 for a photograph) as a function of time from positive samples. The insets are photographs of the fluorescent signal detected with the USB microscope through the lid of the test tube.
The colorimetric detection in Fig. 1 uses the pH indicator phenol red [25]. Since one of the polymerase reaction byproducts is protons, the amplification process leads to a decrease in the pH of the reaction mix. This change in pH can be readily observed with pH indicators. In our case, the phenol dye changes color from red to yellow when polymerase takes place (Fig. 1B). The pH indicator is not optimal for testing saliva because the saliva’s pH may vary among individuals and may depend on diet. In our assay, the negative control guards against false positives such as change of color resulting from sample acidity. A color change of the negative control would invalidate our test. Alternatively, we can use other colorimetric dyes that are insensitive to pH such as the intercalating dye Luco Crystal Violet (LCV) that changes from colorless to violet in the presence of dsDNA as we have previously described [26]. See Supplemental Fig. S3. The color change can be observed either through the tube lid (Fig. 1B right)) or by removing the cup’s lid at a prescribed time (e.g., 30 min) after the start of the heating process (Fig. 1B, left). In our experiments, we detected no false positives. All the tubes containing templates changed color, indicating a reproducible positive test while the negative control did not change color (Fig. 1B). Both our tests with the EPCM and the benchtop (SI Fig. S5) yielded comparable results. We obtained positive results with ×10 and ×100-fold diluted samples but failed to produce a positive signal with a ×1000-fold dilution. Our instrumentation-free home test appears to have a similar sensitivity to that of the benchtop RT-LAMP.
We can also monitor the amplification reaction in real time with intercalating dye (EvaGreen™). This dye is quenched while in solution emitting relatively low intensity fluorescence. In the presence of dsDNA, the dye binds to the dsDNA and its emission intensity increases greatly. We excited and detected the fluorescent dye with a portable CCD camera (Dino-Lite Edge AM4115T.GFBW-R9) (Fig. 3). At low temperatures (prior to amplification), we observe significant emission from the dye possibly due to the presence of primer target complexes. As the temperature increases, the emission intensity decreases both due to reduced emission efficiency with temperature (as is common to most fluorescent dyes) and the dissociation of short double stranded fragments. Once amplificons are produced, the emission intensity increases. Consistent with the benchtop experiments and the colorimetric detection, tests with ×10 and ×100-fold sample dilutions yielded positive results while the ×1000 dilution did not yield a positive signal.
Discussion
We have demonstrated the feasibility of incubating LAMP or RT-LAMP reaction instrumentation and electricity - free with the reaction temperature being self-regulated through the use of the energetic composite comprised of the PCM PureTemp63 and the reactive material Mg(Fe). Our incubator is made of readily available, inexpensive materials, enabling fast deployment. Since we use just a few grams of Mg(Fe) and PureTemp, the materials cost just a few pennies. Our approach has a few distinguishing features compared to previous implementations of chemical heaters [7–17]. By mixing the reactants and the PCM, we minimize heat transfer resistance between the exothermic reaction and the reaction tube without a need for thermal waveguides or enhancement of PCM conductivity with dispersed high conductivity particles as was previously done. Our EPCM enables our system to go from room temperature to the RT-LAMP operating temperature within 3 minutes. The reaction rate in our system is tempered because of the slow infiltration of the water into the powder mix, which prolongs the reaction and prevents an initial significant temperature overshoot that may denature LAMP enzymes. The porous nature of the EPCM also allows for the escape of hydrogen gas product without forming hydrogen bubbles and/or splattering of reactants. Since PCMs are available with a wide range of phase transition temperatures (e.g., ranging from −37 to +151 °C), the method described herein could be applied to various incubation reactions including other isothermal amplifications, ligations, and restrictions.
Our ability to directly insert test tubes directly into the EPCM simplifies hardware design and manufacturing. In our experiments, we packaged the EPCM in a 3D-printed plastic cup, which is inserted into a Styrofoam block for thermal insulation. The plastic cup can be replaced, however, with other readily available materials such as Styrofoam to further reduce cost.
Since the RT-LAMP process produces a great number of amplicons, it provides diverse opportunities for detecting amplification results, ranging from fluorescent dyes like in PCR to colorimetric dyes. Here, we show results obtained with pH indicator that changes color from red to yellow in positive tests. Alternatively, we can use, among other things, LCV dye [25]. The ability to incubate the reaction without an electrical system and to observe test results without a reader, provides us with a very inexpensive system that may rival in cost lateral flow-based rapid tests while maintaining the advantages of molecular tests such as high specificity and sensitivity and rapid adaptability to new targets.
Our EPCM incubator provides nearly equivalent performance to that of state-of-the-art benchtop machines. Samples that were positive in the benchtop test were also positive when incubated with our EPCM, and samples that were negative on the benchtop were also negative with our disposable test. Since we do not know the viral load of our samples, we cannot report on limits of detection. However, the limit of detection depends not only on incubation conditions, but to a greater degree on the assay, the development of which is not part of this project. Based on prior work [14–15], we anticipate that a limit of detection of 10 target copies per reaction is achievable.
When it is desirable to monitor the amplification reaction in real time, one can use a USB- CCD camera placed a few centimeters above the tube(s) lid to monitor fluorescence emission from DNA-intercalating dye or molecular probes included in the reaction mix. Instead of a dedicated camera, one can use a smartphone, wherein the fluorescence is excited with the smartphone flash and detected with the smartphone camera as we have previously demonstrated [14].
Here, we have demonstrated our system’s capability when operating with saliva samples and testing for COVID-19. Our system can operate, however, with other types of samples such as urine, blood, and food. While the sample preparation workflow depends on the sample type and the assay, the incubation process is generic.
Supplementary Material
Acknowledgments
This work was supported, in part, by a gift from Mr. Jeff Horing, and NIH grant 1 R21 AI134594-01 to the University of Pennsylvania.
Footnotes
CONFLICT OF INTEREST
The authors filed an invention disclosure on the Energetic Phase Change Material with the University of Pennsylvania.
REFERENCES
- 1.Lanièce Delaunay C, Saeed S, Nguyen QD, “Evaluation of testing frequency and sampling for severe acute respiratory syndrome Corona virus 2 surveillance strategies for long-term care facilities” Research Letter / JAMDA 21 (2020) 1568–1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chin ET, Huynh BQ, Chapman LAC, Murrill M, Baus S, and Lo NC, “Frequency of routine testing for COVID-19 in high-risk healthcare environments to reduce workplace outbreaks” medRxiv 10.1101/2020.04.30.20087015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Larremore DB, Wilder B, Lester E, Shehata S, Burke JM, Hay JA, Tambe M, Mina MJ, Parker R, “Test sensitivity is secondary to frequency and turnaround time for COVID-19 surveillance” medRxiv 10.1101/2020.06.22.20136309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Almassian DR, Cockrell LM and Nelson WM, “Portable nucleic acid thermal cyclers” Chem Soc Rev (2013), 42, 8769–8798, 10.1039/C3CS60144G [DOI] [PubMed] [Google Scholar]
- 5.Ravi N, Cortrade DL, Ng E, Wang SX, “Diagnostics for SARS-CoV-2 detection: A comprehensive review of the FDA-EUA COVID-19 testing landscape” Biosensors and Bioelectronics 165 (2020) 11254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Notomi Tsugunori, et al. “Loop-mediated isothermal amplification of DNA.” Nucleic acids research 28.12 (2000): e63–e63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weigl Bernhard H., Domingo Gonzalo, Gerlach Jay, Tang Dennis, Harvey Darrel, Talwar Nick, Fichtenholz Alex, van Lew Bill, and LaBarre Paul, “Noninstrumented nucleic acid amplification assay”, Proc. SPIE 6886, Microfluidics, BioMEMS, and Medical Microsystems VI, 688604 (12 February 2008); 10.1117/12.763650 [DOI] [Google Scholar]
- 8.LaBarre P, Boyle D, Hawkins K, and Weigl B, “Instrument-free nucleic acid amplification assays for global health settings” Proc. SPIE (2011) 8029. DOI: 10.1371/journal.pone.0019738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.LaBarre P, Hawkins KR, Gerlach J, Wilmoth J, Beddoe A, Singleton J, Boyle D, and Weigl B, “A simple, inexpensive device for nucleic acid amplification without electricity- Toward instrument-free molecular diagnostics in low-resource settings” PLOS One (2011) 6, 5 e19738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu C, Mauk M, Hart R, Qiu X, and Bau H,H, 2011, Self Heating Cartridge for Molecular Diagnostics, Lab on Chip 11, 2686–2692. DOI: 10.1039/C1LC20345B. [DOI] [PubMed] [Google Scholar]
- 11.Curtis KA, Rudolph DL, Nejad I, Singleton J, Wiegl B, LaBarre P, and Owen SM, “Isothermal amplification using a chemical heating device for point-of-care detection of HIV-1” PLoS ONE (2012) 7, 2 e31432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Buser JR, Diesburg S, Singleton J, Guelig D, Bishop JD, Zenter C, Burton R, LaBarre P, Yager P, and Weigl BH, “Precision chemical heating for diagnostic devices” Lab Chip (2015) 15 4423–4432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Curtis KA, Rudolph DL, Morrison D, Guelig D, Diesburg S, McAdams DA,Burton RA, LaBarre P, and Owen M, “Single-use, electricity-free amplifications device for detection of HIV-1” J Virol Methods (2016) 237 132–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liao S-C, Mauk MG, Awashti S, Song J, Friedman H, Bau HH, and Liu C “Smart-Cup: A minimally-instrumented, Smartphone-based point-of-care diagnostic device” Sensors and Actuators B (2016) 229 232–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Song J, Mauk M,G, Hackett B, Cherry S, Bau HH, Liu C, 2016, Instrument-Free Point-of-Care Molecular Detection of Zika Virus, Analytical Chemistry 88 (14), 7289–7294, DOI: 10.1021/acs.analchem.6b01632 . 10.1021/acs.analchem.6b01632http://pubs.acs.org/doi/pdf/10.1021/acs.analchem.6b01632. http://pubs.acs.org/doi/pdf/10.1021/acs.analchem.6b01632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Song J; Liu C; Mauk M; Peng J; Schoenfeld T; Bau HH, 2018, “A Multifunctional Reactor with Dry-Stored Reagents for Enzymatic Amplification of Nucleic Acids”. Analytical Chemistry 90 (2), 1209–1216, DOI 10.1021/acs.analchem.7b03834 . 10.1021/acs.analchem.7b03834http://pubs.acs.org/doi/pdf/10.1021/acs.analchem.7b03834. http://pubs.acs.org/doi/pdf/10.1021/acs.analchem.7b03834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Song J, Pandian V, Mauk MG, Bau HH, Cherry S, Tisi LC, and Liu C, 2018, Smartphone-based Mobile Detection Platform for Rapid Molecular Diagnostics and Spatiotemporal Disease Mapping, Analytical Chemistry 90 (7) 4823–4831. doi: 10.1021/acs.analchem.8b00283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuhn WE, Hu KH, Black A. Flexible Electrochemical Heater. 4,522,190. 1985. June 11
- 19. https://www.puretemp.com/ (viewed Feb 14, 2021)
- 20.Yavari F, Fard HR, Pashayi K, Rafiee MA, Zamiri A, Yu ZZ, Ozisik R, Borca-Tasciuc T, Koratkar N. Enhanced thermal conductivity in a nanostructured phase change composite due to low concentration graphene additives. J Phys Chem. 2011;115(17):8753–8758. [Google Scholar]
- 21.Azzi L, Carcano G, Gianfagna F, Grossi P, Dalla Gasperina D, Genoni A, Fasano M, Sessa F, Tettamanti L, Carini F, Maurino V, Rossi A, Tagliabue A, and Baj A, “Saliva is a reliable too to detect SARS-CoV-2” J. Infection 81 (2020) e45–e50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Takeuchi Y, Furuchi M, Kamimoto A, Honda K, Matsumura H, and Kobayashi R, “Saliva-based PCR tests for SARS-CoV-2 detection” J Oral Sciences (2020) 62, 3, 350–351. [DOI] [PubMed] [Google Scholar]
- 23.Williams E, Bond K, Zhang B, Putland M, Williamson DA, “Saliva as a noninvasive specimen for detection of SARS-CoV-2” J Clinical Microbiology (2020) 58, 8 e00776–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang Yinhua, et al. “Rapid molecular detection of SARS-CoV-2 (COVID-19) virus RNA using colorimetric LAMP.” MedRxiv (2020) [Google Scholar]
- 25.Zhang Yinhua, Odiwuor Nelson, Xiong Jin, Sun Luo, Raphael Ohuru Nyaruaba, Wei Hongping, Tanner Nathan A., 2020, Rapid Molecular Detection of SARS-CoV-2 (COVID-19) Virus RNA Using Colorimetric LAMP, medRxiv 2020.02.26.20028373; doi: [Google Scholar]
- 26.Mohamed El-Tholoth; Bau Haim H.; Jinzhao Song (2020): A Single and Two-Stage, Closed-Tube, Molecular Test for the 2019 Novel Coronavirus (COVID-19) at Home, Clinic, and Points of Entry. ChemRxiv. Preprint. 10.26434/chemrxiv.11860137.v1 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


