Abstract
The amygdala is critical for emotional processing and motivated behavior. Its role in these functions is due to its processing of the valence of environmental stimuli. The amygdala receives direct sensory input from sensory thalamus and cortical regions to integrate sensory information from the environment with aversive and/or appetitive outcomes. As many reviews have discussed the amygdala’s role in threat processing and fear conditioning, this review will focus on how the amygdala encodes positive valence and the mechanisms that allow it to distinguish between stimuli of positive and negative valence. These findings are also extended to consider how valence encoding populations in the amygdala contribute to local and long-range circuits including those that integrate environmental cues and positive valence. Understanding the complexity of valence encoding in the amygdala is crucial as these mechanisms are implicated in a variety of disease states including anxiety disorders and substance use disorders.
Keywords: valence, amygdala, learning
1. Introduction
The amygdala is a brain region that is critical for emotional processing and motivated behavior[1]. Its role in these functions is linked to its processing of both fearful and rewarding environmental stimuli. Despite this important role, the amygdala is a relatively small region comprised of interconnected nuclei including the basolateral complex of the amygdala (BLA) made up of the lateral (LA), basal (BA), and basomedial (BM) cell groups and the central nucleus (CeA) with lateral (CeL) and medial (CeM) subdivisions[2].
The amygdala receives primary sensory information which allows it to encode and transmit the valence of environmental stimuli to promote adaptive behaviors[3], [4]. For example, the amygdala receives direct input from sensory thalamus and cortical regions to integrate sensory information from the environment with aversive and/or appetitive outcomes [5], [6]. Therefore, motivated behavior can be driven by the valence of the reinforcer and/or the valence of the stimuli paired with that reinforcer. Reward and punishment are reinforcers of opposite valence and these reinforcers typically lead to opposing behaviors (approach versus avoidance)[7].
The amygdala has been classically associated with fear and threat processing and how neutral environmental stimuli become paired with an aversive outcome. As numerous reviews have discussed the role of the amygdala in threat processing and fear conditioning, this review will focus on how the amygdala encodes valence and the mechanisms that allow it to distinguish between stimuli of positive and negative valence. We will examine how valence encoding occurs at the population level and circuit level and how this process is involved in various cognitive processes. We will also consider the integration of environmental cues and positive valence in the amygdala and the ways in which it supports maladaptive behaviors such as drug abuse.
2. Neuron populations for encoding valence
Evidence demonstrating that the amygdala is important for affective processing has led to investigation into how it encodes valence[8]–[10]. Approximately 37% of neurons in the amygdala are selectively responsive to motivationally relevant stimuli[8]. Neurons have been identified that respond to unconditioned stimuli of both positive and negative valence, while other neurons have valence specific responses[11]. In addition, some of these neurons modulate their firing rate following reversal of the expected outcome value, further suggesting that neurons in the amygdala can track valence[12].
In the basolateral complex of the amygdala (BLA), about a fifth of the neurons track valence[11], [13], [14] and evidence shows these positive and negative valence-encoding neurons are intermingled[15], [16]. There is also evidence for inhibitory relationships between the positive and negative encoding neurons such that presentation of a positive valence stimulus results in increased firing rate in positive encoding neurons and decreased firing rate in negative encoding neurons[17]. In monkeys performing a trace-conditioning task, electrophysiological recordings showed that cues of each valence elicited higher levels of activity in separate but anatomically interspersed populations of neurons[9]. Moreover, activity-dependent labeling of BLA neurons using nicotine or an opposite sex conspecific as a positive reinforcer or footshock as a negative reinforcer showed non-overlapping populations in the rat BLA[16], [18].
Furthermore, it has been shown that distinct BLA ensembles mediate responses to appetitive and aversive stimuli and underlie associative learning by assigning valence to sensory stimuli[18]. For example, in one experiment, rats were presented with novel odors that informed the response outcome of entering a fluid port. Specifically, one odor instructed a “go” response of entering the fluid port to obtain sucrose; whereas, a different odor instructed a “no-go” response, such that rats had to learn to withhold a response to avoid receiving quinine. Neural activity was examined during odor evaluation and the activity of BLA neurons specifically reflected either sucrose or quinine by showing increased excitatory responses to the odor paired with sucrose[19]. A similar training paradigm in non-human primates extended this finding to suggest that the signal about cue valence is distinct from the signal that represents cue identity. Subjects were trained on two parallel cue outcome associations with one cue followed by a rewarding outcome and the other followed by an aversive outcome, and then the cues assigned to each outcome were reversed. A significant proportion of cue-selective neurons encoded the outcome with which they were currently paired and not the sensory features of the cue[9].
Recent advances have allowed for more precise identification of amygdala neuron populations based on their function, projection target, and genetic markers[20]–[26]. Interestingly, using a genetic strategy to transcriptionally profile neurons in the BLA, researchers found that negative and positive encoding neurons were spatially segregated into the anterior and posterior portions of the BLA, respectively[26]. Rpos2 (R-spondin 2) expressing BLA neurons located in the anterior BLA were activated by footshock that elicited freezing, while Ppp1r1b (protein phosphatase 1 regulation inhibitory subunit 1B) neurons in the posterior BLA were critical for positive behaviors such as approach to an opposite sex conspecific[26]. These findings suggest that negative and positive neurons are segregated in anterior and posterior regions of the BLA and may only be intermingled at the transition between the two regions[26].
It is likely that Rpos2-expressing neurons and Ppp1r1b expressing neurons each represent a subset of negative and positive valence encoding neurons. This evidence supports the theory in which specific amygdala neuronal subpopulations mediate valence[27]. However, it has also been suggested that BLA neurons that respond to positive valence stimuli may also respond to negative stimuli under different conditions, implying that neuronal populations have different affective modes[27], [28]. Determining if affective valence encoding is the result of neural subsystems or neurons that have dynamic states or a combination of both will improve our understanding of how valence encoding occurs. Moreover, examining how valence encoding is organized will also provide insight into the distribution of amygdala subpopulations and the extent to which valence encoding neurons are intermingled. Future experiments should use a wider range of behavior situations to probe the dynamics and interplay of amygdala neurons in order to better understand how valence encoding influences learning and behavior.
3. Connectivity and circuitry involved in encoding valence
3.1. Basolateral Amygdala (BLA)
The amygdala and its subdivisions contribute to circuits that encode valence. Human imaging studies have highlighted the importance of the amygdala in valence circuitry by identifying a “valence-general affective space” consisting of cells in the anterior insula, rostral and dorsal anterior cingulate cortex, ventral striatum, thalamus, occipitotemporal cortex and amygdala[29]. More specifically, some evidence suggests that projections from the BLA to nucleus accumbens (NAc) encode positive valence[20], [30], BLA projectors to the ventral hippocampus (vHPC) respond to valence-related cues[30] and projections from the BLA to portions of the CeA encode negative valence[20], [31]. It is important to note that these findings come from studies in which each conditioned stimulus (CS) could only trigger one conditioned response (CR). As a result, it is unclear if alterations in activity of the BLA and its projections reflect valence of the cue, valence of the conditioned behavior, or a mixture of both[28]. To address this question, researchers used the risk-reward interaction task in which rats were trained to respond to each CS in a different manner depending on where in the apparatus the CS was presented. For example, when the light CS appeared below one of the shock sectors of the chamber it signaled a footshock, while a CS presented behind a wall indicated a water reward would be delivered at that location[28]. This method revealed that the magnitude and time course of activity of BLA cells during the CS depended on the type and timing of the CR. The CS sensory responses of these cells were separate from the activity that drove the CRs. Additionally, it was found that the incidence of valence-coding cells in the BLA did not exceed chance and that valence encoding for CSs and CRs occurred at the population level[28]. Thus, these findings imply that coding dimensions may be represented in the ensemble activity of BLA neurons[28]. Moreover, these results highlight that valence encoding may be only partially correlated with anatomical position and/or projection target rather than specific cells types controlling valenced behaviors.
There are a number of afferent projections to the BLA from regions including the basal forebrain, dorsal raphe nucleus (DRN), and ventral tegmental area (VTA) that play a role in valence processing[7]. The dense cholinergic input from the basal forebrain, especially from the nucleus basalis magnocellularis, responds to both appetitive and aversive stimuli[32]. This cholinergic signaling supports the acquisition of associative learning by affecting the firing of BLA principal neurons through activation of acetylcholine receptors that enhance glutamatergic synaptic transmission and induce long term potentiation at cortical-amygdala circuits[33]. Additionally, the DRN sends a dense projection to the BLA[34], [35] and these serotonergic DRN neurons show phasic firing changes to punishment and reward-predictive cues[36]. While the role of serotonin in the BLA has not been extensively studied, higher levels of 5HT3A in the BLA has been linked to stronger Pavlovian approach behaviors in a task in which cues predicted food delivery[37], indicating that serotonin signaling may be involved in valence encoding that drives adaptive behavior. Likewise, the VTA to BLA connection plays an important role in valence encoding. Populations of dopaminergic VTA neurons are activated by various appetitive and aversive stimuli. For example, two-photon imaging and photometry in behaving mice revealed that VTA dopamine axons innervating the basal portion of the amygdala were activated by both reward and punishment and that they acquired responses to cues predicting these outcomes[38]. Moreover, plasticity of BLA neurons is enhanced by dopamine released during emotionally valenced tasks[39], [40], thus implying that separate VTA inputs could mediate positive versus negative valence encoding in the BLA[7].
Another crucial pathway for valence processing is the link between the BLA and the posterior dorsomedial striatum (pDMS)[41]. After lesioning the BLA and disconnecting it from the pDMS with muscimol infusions, rats were unable to use recently acquired associations to direct new response-outcome associations[41], implying that valence information from the BLA is essential for response-outcome learning mediated by the pDMS.
Recently, studies have used optogenetics to parse out how specific amygdala projection neurons regulate reward-related behaviors. One study showed that BLA neurons projecting to the nucleus accumbens (NAc) or the medial division of the central amygdala (CeM) undergo opposing synaptic changes following reward conditioning[20]. In the first stage of this study, mice were trained in a conditioning paradigm where a tone was paired with sucrose delivery. Following the acquisition of the association between tone and sucrose delivery, synapses on NAc projectors showed an increase in AMPAR/NMDAR ratio, which was indicative of greater glutamatergic synaptic strength relative to controls[20]. Extending this finding by using optogenetic manipulations revealed that photostimulation of NAc projectors supported positive reinforcement and photoinhibition of CeM projectors enhanced reward conditioning[20].
3.2. Central Amygdala (CeA)
While much research has focused on the BLA, additional amygdala subdivisions contribute to circuits that encode valence. Studies have shown the central amygdala (CeA), like the BLA, receives direct sensory input as well as projections from cortical areas[42], which positions it well to encode stimulus valence. The CeA is involved in forming associations between environmental stimuli and reward[43], [44] via its interactions with the substantia nigra pars compacta (SNc) and the ventral tegmental area (VTA)[45], [46]. In the CeA, a molecularly defined population of GABAergic neurons expressing the serotonin receptor 2a participates in circuits that modulate food consumption and promote positive reinforcement[47]. Furthermore, CeA projections to midbrain dopaminergic regions were shown to participate in a task in which rats acquired conditioned orienting responses to a visual cue that signaled food delivery[48] again highlighting a role for this region in driving behavior in response to valence.
As with the BLA, experimenters have turned to optogenetic tools to probe the CeA involvement in valenced behaviors. In a two-choice task, rats had excitatory laser stimulation of the CeA paired with sucrose, cocaine, or a shock-delivering rod during training to determine the motivational effects of CeA stimulation on later behavior[49]. Initially, rats expressing a control virus or channelrhodopsin (ChR2) in the CeA were trained to nosepoke into a single porthole to earn a single daily reward. Training days alternated between each porthole and each reward such that rats earned exclusively sucrose or exclusively cocaine every other day. During the subsequent training, rats were presented with both portholes simultaneously and received bilateral laser stimulation of the CeA paired with the laser-designated reward of either sucrose or cocaine[49]. In the two-choice test session, control rats chose equally between sucrose and cocaine regardless of which had been paired with laser stimulation. Conversely, CeA ChR2 rats that received stimulation paired with sucrose pursued and consumed only sucrose, while ignoring cocaine. Likewise, pairing laser with cocaine in CeA ChR2 rats led to exclusive pursuit of cocaine[49].
In a separate experiment to examine the effects of CeA stimulation linked to a negative valence outcome, encounters with a shock rod were paired with CeA ChR2 stimulation. On the first shock-rod day, rats received a mild shock upon contact with the rod. To pair CeA ChR2 excitation with the entire shock-rod encounter, laser illumination began when any part of the animal was within 2 centimeters of the rod and continued until it withdrew more than 2 centimeters away. While control rats learned to avoid the shock rod, CeA ChR2 animals that received paired stimulation approached and touched the rod multiple times per session and returned to the rod even after receiving a shock. Interestingly, these data revealed that a stimulus with aversive properties could become an incentive target when paired with limbic activation. All rats with CeA stimulation during appetitive and aversive pairings showed recruitment of mesocorticolimbic structures including VTA, NAc, posterior insula, and dorsolateral neostriatum[49]. This result indicates that mesocorticolimbic structures commonly associated with positive valence were also activated to mediate attraction to an aversive stimulus[49].
The CeA has been traditionally considered in fear, anxiety and other aversive contexts, but there is also strong evidence for its role in appetitive situations. Research focusing on valence encoding in the CeA and its associated circuitry provide clues as to how it can support a wide range of behaviors. It is possible that stimulus encoding processes interact with CeA neuronal activity to determine the valence of the outcome. Therefore, the representation of valence may be a combination of the stimulus identity and neural activity, which can be flexibly modulated by situational factors.
4. Integration of environmental cues and positive valence
4.1. Valence encoding and cognitive processes
Studies have examined pathways from the amygdala to cortical areas to determine how cognitive processes are related to valence encoding. Projections to cortical areas from the amygdala allow attentional signals to modulate stimulus processing[50]. Importantly, evidence indicates that attention modulates amygdala valence signals[51]. For example, in a paradigm where spatial location provided information about reward predictive stimuli, amygdala neural activity correlated with spatial attention[50]. Specifically, as monkeys directed attention toward stimuli predicting reward, amygdala responses reflected how spatial attention was allocated, indicating that the amygdala is capable of enhancing attention to stimuli associated with positively valenced experiences[50].
While whole amygdala lesions or inactivation in rats and monkeys do not affect the effort to earn reward[52]–[55], impairments are found when tasks incorporate choice paradigms because the BLA is necessary for representing motivationally-significant outcomes. As a result, BLA disconnection from cortical circuits biases choice in favor of low effort, small rewards[56], [57]. Furthermore, animal models of cognitive effort such as a modified 5-choice serial reaction time task can reflect diverse valence-related cognitive processes as they involve cortical, limbic, and striatal connectivity. In these experiments, rats can choose to sustain greater attentional effort to obtain a large reward or choose a less difficult trial with a lower attentional cost and lower magnitude reward[58]. In the low effort/low reward condition, the port is illuminated for 1 second to allow the rat to attend to the cue and respond, whereas in the high effort/high reward condition the port is only illuminated for 0.2 seconds[58]. When the BLA is inactivated under these task conditions, rats that were previously classified as hard-working tended to opt for low effort/low reward and the “slacker” rats tended to engage in more attentional effort, implying that attentional effort is integrated with reward and value in the BLA[58]. Overall, this study demonstrated that BLA circuits may be involved in cost-benefit choice calculations by coding the subjective value of options.
These conclusions are reflected in human imaging studies. For example, a multivariate fMRI approach was used to extract valence-specific patterns during evaluation of food-liking such that the valence localizers could then be used to identify hedonic valuation processes as participants performed a separate willingness-to-eat task. The valence specific patterns of amygdala signaling strongly predicted decisions on food consumption[59]. Measuring single neuronal activity in the amygdala as humans performed a novel bidding task helped to further clarify its role in encoding and computing stimulus values at the time of choice[60]. In this study, 16 of the 51 recorded amygdala units exhibited responses that were linearly associated with behavioral bid choice and 11 of those 16 neurons were located in or bordered the BLA[60]. The proportion of neurons with positive associations was similar to the proportion exhibiting negative associations, again indicating that the amygdala contains detectors for both appetitive and aversive stimuli[60]. Thus, human studies highlight the role for the amygdala in the computation of stimulus value across a range of decision-making and cognitive tasks.
4.2. Encoding valence of reward-related environmental cues
The amygdala is an important region for cue-related learning processes and is involved in assigning valence to environmental stimuli. Amygdala neurons concurrently encode multiple task dimensions such as the sensory properties of conditioned cues, the behaviors they elicit, and their valence[61]. Lesion studies have supported these findings as amygdala lesions impair the ability to respond to cues in the face of changing reward value[1]. In the amygdala, distinct neurons are excited by and respond to cues and reward-related stimuli. For example, BLA-vHPC projectors respond to cues of both positive and negative valence, while BLA-NAc projections are excited by reward predictive cues[30]. On a smaller scale, activity of single BLA neurons can track both positive and negative reward-prediction errors, which allows the BLA to use this information to support effective cue-reward learning[62].
Pavlovian learning tasks provide the opportunity to further investigate valence encoding and cognition. For one, neurotoxic damage to the CeA produces deficits in the acquisition of learned orienting behavior to visual or auditory cues, thus revealing a role of the CeA in the allocation of attention[43], [63]. The contribution of distinct amygdala nuclei to instrumental learning and performance has also been examined using forms of Pavlovian-instrumental transfer (PIT) in which conditioned stimuli (CS) can modulate an animal’s instrumental performance[64]. In this paradigm, Pavlovian cues and instrumental actions are trained in separate phases. In the Pavlovian phase, one or more stimuli are paired with the delivery of reward such as food pellets or sucrose. During instrumental training, a contingency is established between lever pressing and one or more outcomes, typically food delivery. In the test phase, the instrumental actions are performed again, but the conditioned stimuli (CS) trained in the Pavlovian phase are present. The effect of CS presentation on instrumental responding (transfer effect) is determined by comparing responding when the CS is absent or present and it is generally found that a CS paired with an appetitive outcome enhances responding[65]. Using a PIT design, experimenters could dissociate the general motivational and specific excitatory effects of the reward-related cue in the same animal by using two cues that predicted the outcomes earned by the actions in training and a third cue that predicted an outcome that was not earned by those actions. In sham animals, the first two cues resulted in outcome-selective PIT, while the third cue more generally increased the performance of both actions. BLA lesions in these animals abolished outcome specific effects of the cue while sparing its general motivational effects. On the other hand, lesions of the CeA eliminated the general motivational effects of the cues, but did not impact the specific effects[66].
In another Pavlovian conditioning study, rats were exposed to the association of a visual stimulus with a food reward. They were also exposed to a second stimulus that was never paired with reward. Across training, control animals came to approach the food-paired cue with increasing regularity while reducing their approach to the neutral cue. Conversely, CeA lesions lead to impaired approach whereas lesions to other areas including the BLA had no effect[67]. A large body of research concerning conditioned reward-approach responses has revealed that the BLA plays a more precise role in representing the affective value of the conditioned stimuli and this information is used to support the translation of conditioned associations into instrumental action[68]–[70] while the CeA appears to more generally support Pavlovian conditioned responses[67]. Taken together, these findings indicate that the BLA and CeA have distinct contributions to reward-related processes and the ways in which they control instrumental performance.
Despite the clear link between the amygdala and cue-reward learning, the neural mechanisms underlying how the valence of cues is encoded are not well understood. To address this, some studies have examined the involvement of neuromodulators in the encoding process. For example, transient glutamate release in the BLA has been found to promote encoding of outcome-specific motivational information provided by reward-predictive cues [71]. Another study focused on the role of corticotropin releasing hormone (CRH) and found that changes in CRH in the CeA are elicited by positive stimuli[72]. Increases in CRH immunoreactivity are as pronounced following exposure to appetitive stimuli as they are following aversive stimuli. Thus, it seems the CRH system in the CeA serves to bring attention to events associated with food and reward availability in addition to its well-established role of signaling threat[72].
Another approach for investigating the mechanisms of valence encoding has been to use tasks in which animals use associative learning strategies. For example, employing an associative learning procedure revealed how specific glutamate receptors contribute to valence encoding during appetitive conditioning[73]. After training rats to associate a discrete auditory cue with entering a nose port for a fructose-glucose solution, animals were tested for the expression of conditioned responding without reward delivery. During these tests, auditory cue presentation led to increased fluid port entries in the original sugar paired context relative to a neutral context[73]. Experimenters then inhibited mGluR5 receptors in the anterior BLA, which led to enhanced contextual discrimination of the conditioned stimulus port, thus highlighting the role of BLA mGluR5 in limiting context-dependent expression of appetitive conditioned responding[73].
Additional associative learning studies have elucidated how amygdala neurons are involved in encoding cue properties. Two separate groups of rats were trained to respond to a sucrose reward. The paired group was trained with a reward-predictive cue and the unpaired group was trained with a randomly presented cue. These animals also underwent extinction and reinstatement procedures following training. In the paired group relative to the unpaired group, the proportion of neurons that were phasically responsive during reinstatement was significantly higher[74]. Interestingly, these experiments also revealed two distinct populations of neurons that responded to cues in the trials when the cue was used as an incentive or as a reinforcer. These results imply that separate populations of BLA cue responsive neurons encode the motivating properties or reinforcing properties of a cue previously associated with reward[74].
Our lab and others have examined how neurons in the lateral portion of the amygdala (LA) develop and maintain neural responses to a conditioned stimulus (CS) that has been paired with a reinforcing unconditioned stimulus (US). The traditional model for this process is that an initially weak afferent pathway carrying sensory information about the cue and strong afferents carrying US information converge in the LA. Through Hebbian plasticity mechanisms, there is enhancement at the excitatory synapses carrying CS information[75]–[77]. Tye et al. have shown that acquisition of cue-directed reward-seeking requires neuronal plasticity in the LA[78]. In these experiments, beam breaks at a nose-poke response port were reinforced with a cue and sucrose reward in about 50% of trials. For rats that acquired the task, half of the recorded neurons that did not respond to the cue before acquisition developed a phasic response to cue onset following acquisition. Cue encoding increased across sessions and this increase was predictive of behavior as greater proportions of neurons were recruited to encode the cue as performance improved. Furthermore, rats that learned the cue-reward association had larger AMPAR/NMDAR ratio at thalamo-amygdala synapses relative to non-learners, indicative of strengthened glutamatergic synapses. Therefore, these findings demonstrated that during cue-reward learning, cue-responsive neurons were rapidly recruited and thalamo-amygdalar synapses were selectively strengthened[78].
4.3. Valence, associative learning, and drugs of abuse
Environmental cues can also become associated with the reinforcing properties of drugs of abuse, gaining positive valence that strongly drives drug seeking behaviors. It is thought that these cue-drug associations are powerfully involved in eliciting craving and relapse. As expected, the amygdala is critical for the cue learning that drives drug seeking behavior[79]. The CeA exerts influence over ascending arousal systems that leads to increased salience, attractiveness, and motivational properties of drug-associated cues[79]. As part of the extended amygdala (bed nucleus stria terminalis, transition zone in medial nucleus accumbens, and CeA), the CeA is critical for integrating changes in reward associated with drug dependence[80].
Additional work focusing on the BLA has shown that it mediates the learning processes that allow drug paired stimuli to acquire incentive value and control over drug-seeking behavior[81]. For example, inhibition of BLA function via lesions or reversible inactivation impairs the acquisition of self-administration on a second order schedule of reinforcement[70]. Similarly, these types of manipulations attenuate reinstatement following exposure to cocaine paired cues[82]–[84]. These studies highlight that while the BLA is not responsible for the primary reinforcing effects of drug, it is essential for a cue to elicit the affective representation of the drug reinforcer[70]. Likewise, functional integrity of the BLA has been shown to be necessary for the reinstatement of cocaine seeking behavior elicited by cocaine cues, but not by cocaine itself[85], [86].
As with non-drug reward, it is crucial to determine how neurons in the amygdala develop and maintain responses to drug-paired cues. Prior cue-reward learning experiments[74] highlighted the contribution of thalamo-amygdala synapses and our lab has found similar findings in the context of drugs of abuse. Our lab has found that the strength of a drug cue association corresponds to the strength of synapses between the medial geniculate nucleus (MGN) of the thalamus and the LA[87]. In these studies, rats were trained to self-administer cocaine or saline paired with an audiovisual cue daily for 14 days. Animals that self-administered cocaine displayed potentiation of excitatory postsynaptic currents at MGN-LA synapses relative to the saline self-administration group[87]. These findings suggest that MGN-LA synapses serve to encode and pair the drug predictive cue with the cocaine experience, giving the cue positive valence. Conversely, cue extinction, or repeated presentation of the cue without any cocaine, reversed the potentiation of synaptic strength at MGN-LA synapses and reduced cue-induced reinstatement (a model of drug relapse)[87]. Moreover, optogenetic induction of long-term depression (LTD) of MGN-LA synapses, which recapitulated the reversal of cocaine-induced potentiation induced by extinction, was sufficient to reduce subsequent cue-induced relapse-like behavior[87], thus implying that manipulation of these synapses can alter the valence attributed to environmental stimuli.
Identifying projections such as the MGN-LA pathway that develop and maintain responses to cues greatly improves our knowledge of valence encoding and transmission in the amygdala. Further support comes from studies showing that neurons in the sweet-responsive and bitter-responsive mouse cortex project to distinct areas of the amygdala and optogenetically manipulating selective taste inputs imposed positive or negative valence on a neutral tasting stimulus. Likewise, manipulating these pathways was also able to reverse the hedonic value of the tastant[88]. This bidirectional effect likely extends to other appetitive stimuli and behaviors and provides a framework with which to consider valence encoding in the amygdala.
5. Conclusions
As detailed in this review, the amygdala is an important node in valence encoding circuits. Currently, the anatomical distribution of valence encoding neurons within the BLA is unclear, but there is strong evidence for both positive and negative detecting cells that participate in valence-related tasks. More precise identification of amygdala neuron populations based on function, target, and genetic identity in combination with a wider range of behavioral situations will better address these remaining questions. Although their organization within the amygdala is not well characterized, these neurons participate in many valence encoding circuits that are critical for reinforcement, decision-making based on motivationally relevant outcomes, and cue-reward learning. Understanding how the amygdala encodes valence at the population level, circuit level, and in various behavior tasks is especially important given that the amygdala is implicated in many disease states including substance use disorders and anxiety disorders. Therefore, further investigation of valence encoding mechanisms may provide insight into viable interventions for these disorders.
Figure 1. BLA Circuit Model.
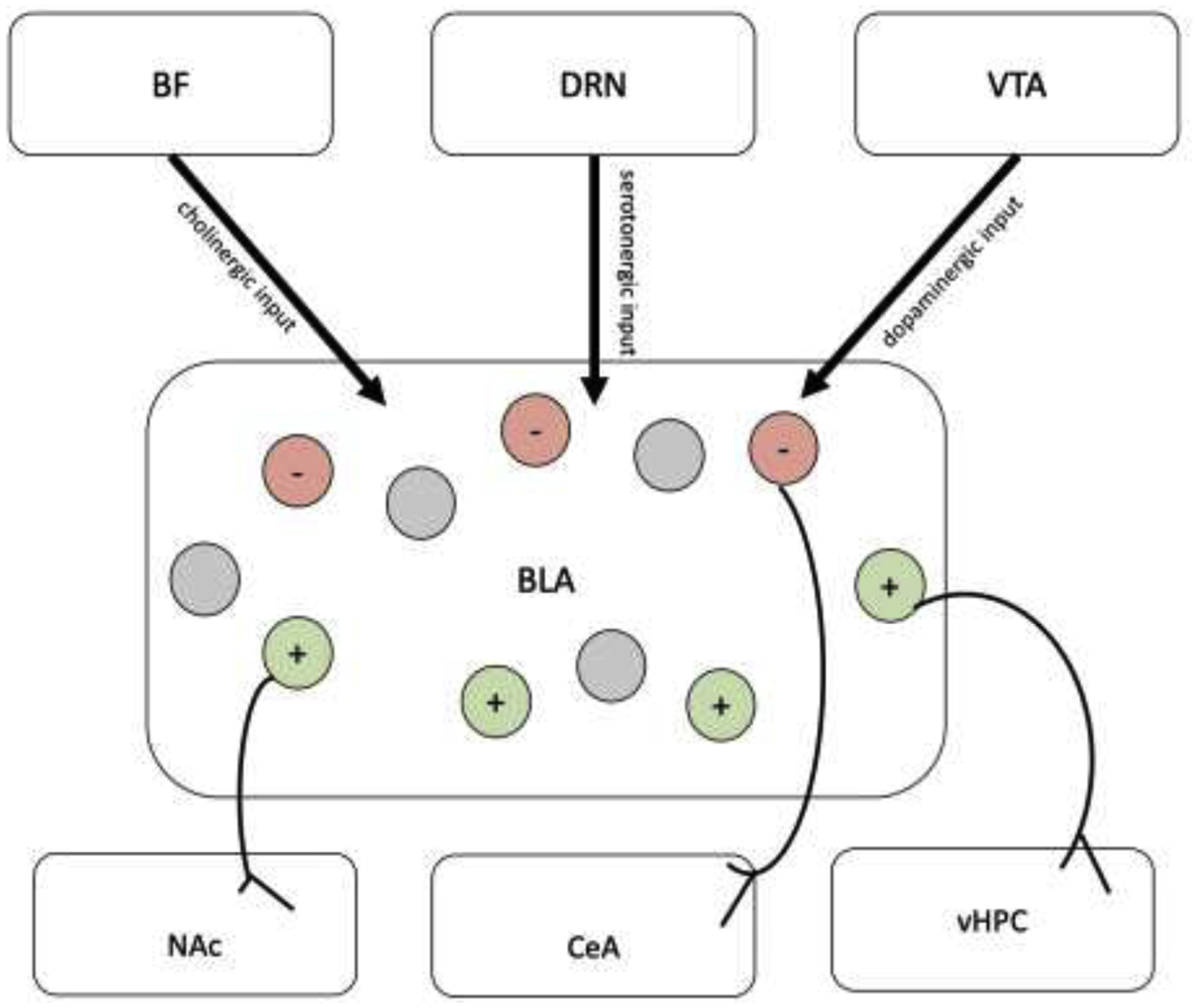
Both basolateral amygdala (BLA) afferent and efferent projections contribute to valence encoding. The BLA receives cholinergic input from the basal forebrain (BF), serotonergic input from the dorsal raphe nucleus (DRN), and dopaminergic input from the ventral tegmental area (VTA). These projections respond to appetitive and aversive stimuli, which allows them to modulate valence encoding. Within the BLA, neuron ensembles encode positive versus negative valence and project to various downstream targets. For example, BLA projectors to the nucleus accumbens (NAc) encode positive valence, BLA projectors to portions of the central amygdala (CeA) encode negative valence, and projections from the BLA to the ventral hippocampus (vHPC) respond to valence-related cues.
Figure 2. Cue-reward learning at thalamo-amygdala synapses.
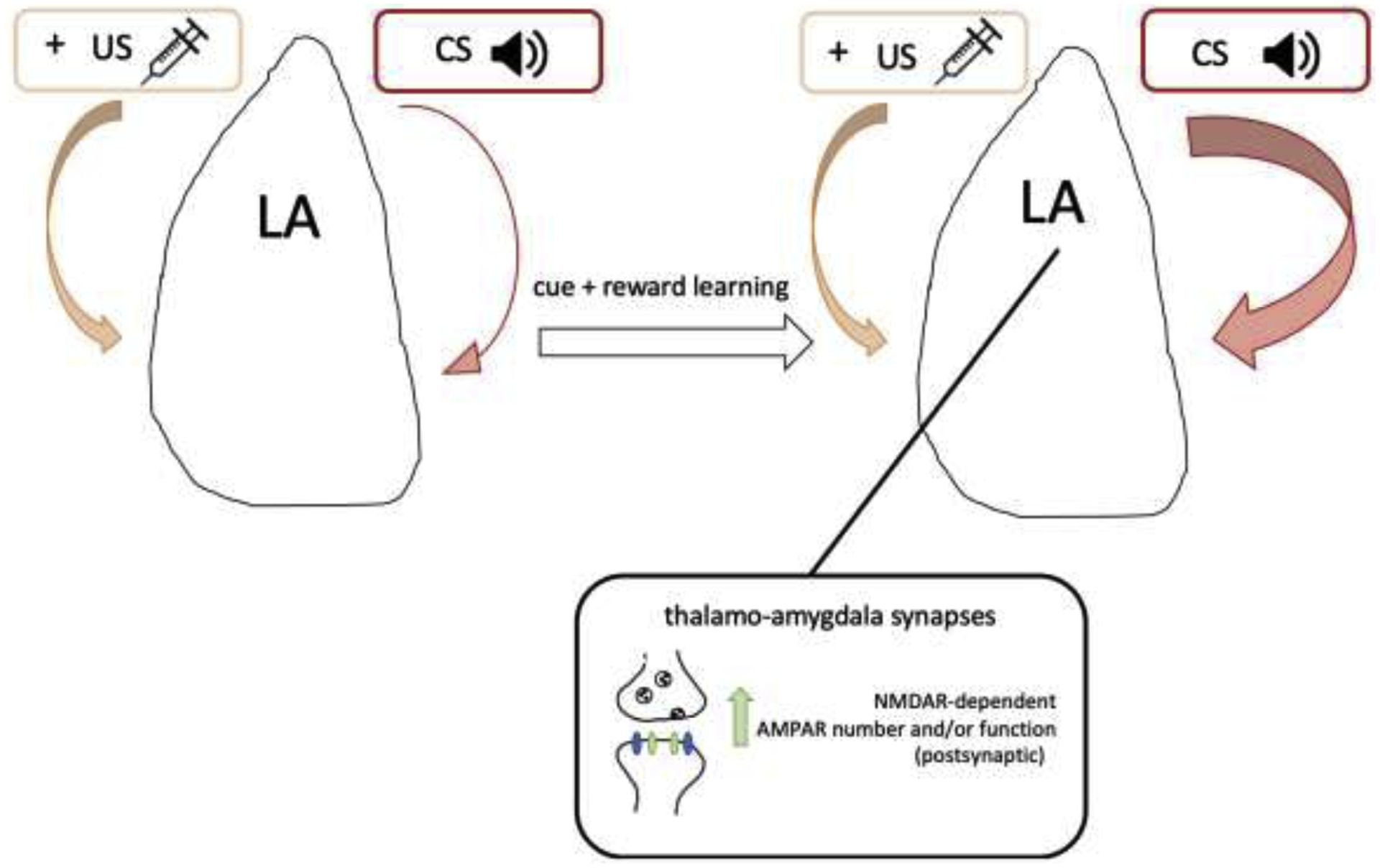
Neurons in the lateral portion of the amygdala (LA) develop and maintain neural responses to a conditioned stimulus (CS) that has been paired with a reinforcing or aversive unconditioned stimulus (US). An initially weak pathway carrying sensory information about the CS (i.e. auditory cue) and strong afferents carrying US (i.e. sucrose, cocaine, footshock) information converge in the LA. Through Hebbian plasticity mechanisms, there is enhancement at the excitatory synapses carrying CS information. Specifically, animals that learn cue-reward associations have a larger AMPAR/NMDAR ratio at thalamo-amygdala synapses, indicative of increased glutamatergic synaptic strength. This strengthening is due to NMDAR-dependent increases in postsynaptic AMPAR number and/or function. In the case of positive valence, thalamo-amygdala synapses serve to encode and pair reward predictive cues with the reinforcing experience, thus giving the cue positive valence.
Highlights.
Neuron populations in the amygdala encode positive versus negative valence.
Amygdala nuclei are part of a variety of circuits that encode valence and support motivated behavior.
Valence encoding of reward associated cues that drive behavior requires plasticity in the amygdala.
Cues that acquire valence via amygdala circuits can promote maladaptive behavior including drug abuse
Funding source
This work was supported by the National Institutes of Health [R01DA042029, R01AA028215].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Competing Interest
None
References
- [1].Murray EA, “The amygdala, reward and emotion,” Trends Cogn. Sci, vol. 11, no. 11, pp. 489–497, 2007. [DOI] [PubMed] [Google Scholar]
- [2].Janak PH and Tye KM, “From circuits to behaviour in the amygdala,” Nature, vol. 517, no. 7534, pp. 284–292, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Stefanacci L and Amaral DG, “Some observations on cortical inputs to the macaque monkey amygdala: An anterograde tracing study,” J. Comp. Neurol, 2002. [DOI] [PubMed] [Google Scholar]
- [4].Mcdonald AJ, “Cortical pathways to the mammalian amygdala,” Prog. Neurobiol, 1998. [DOI] [PubMed] [Google Scholar]
- [5].Morrison SE and Salzman CD, “Re-valuing the amygdala,” Curr. Opin. Neurobiol, vol. 20, no. 2, pp. 221–230, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Barberini CL, Morrison SE, Saez A, Lau B, and Salzman CD, “Complexity and competition in appetitive and aversive neural circuits,” Front. Neurosci, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Correia SS and Goosens KA, “Input-specific contributions to valence processing in the amygdala,” Learn. Mem, vol. 23, no. 10, pp. 534–543, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Fuster JM and Uyeda AA, “Reactivity of limbic neurons of the monkey to appetitive and aversive signals,” Electroencephalogr. Clin. Neurophysiol, 1971. [DOI] [PubMed] [Google Scholar]
- [9].Paton JJ, Belova MA, Morrison SE, and Salzman CD, “The primate amygdala represents the positive and negative value of visual stimuli during learning,” Nature, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Shabel SJ and Janak PH, “Substantial similarity in amygdala neuronal activity during conditioned appetitive and aversive emotional arousal,” Proc. Natl. Acad. Sci. U. S. A, vol. 106, no. 35, pp. 15031–15036, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Belova MA, Paton JJ, Morrison SE, and Salzman CD, “Expectation Modulates Neural Responses to Pleasant and Aversive Stimuli in Primate Amygdala,” Neuron, vol. 55, no. 6, pp. 970–984, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Nishijo H, Ono T, and Nishino H, “Topographic distribution of modality-specific amygdalar neurons in alert monkey,” J. Neurosci, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Schoenbaum G, Chiba AA, and Gallagher M, “Neural encoding in orbitofrontal cortex and basolateral amygdala during olfactory discrimination learning,” J. Neurosci, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Namburi P, Al-Hasani R, Calhoon GG, Bruchas MR, and Tye KM, “Architectural Representation of Valence in the Limbic System,” Neuropsychopharmacology, vol. 41, no. 7, pp. 1697–1715, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Beyeler A et al. , “Organization of Valence-Encoding and Projection-Defined Neurons in the Basolateral Amygdala,” Cell Rep., vol. 22, no. 4, pp. 905–918, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Redondo RL, Kim J, Arons AL, Ramirez S, Liu X, and Tonegawa S, “Bidirectional switch of the valence associated with a hippocampal contextual memory engram,” Nature, vol. 513, no. 7518, pp. 426–430, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Belova MA, Paton JJ, and Salzman CD, “Moment-to-moment tracking of state value in the amygdala,” J. Neurosci, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Gore F et al. , “Neural Representations of Unconditioned Stimuli in Basolateral Amygdala Mediate Innate and Learned Responses,” Cell, vol. 162, no. 1, pp. 134–145, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Schoenbaum G, Chiba AA, and Gallagher M, “Orbitofrontal cortex and basolateral amygdala encode expected outcomes during learning,” Nat. Neurosci, 1998. [DOI] [PubMed] [Google Scholar]
- [20].Namburi P et al. , “A circuit mechanism for differentiating positive and negative associations,” Nature, vol. 520, no. 7549, pp. 675–678, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Felix-Ortiz AC and Tye KM, “Amygdala inputs to the ventral hippocampus bidirectionally modulate social behavior,” J. Neurosci, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Felix-Ortiz AC, Beyeler A, Seo C, Leppla CA, Wildes CP, and Tye KM, “BLA to vHPC inputs modulate anxiety-related behaviors,” Neuron, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Senn V et al. , “Long-range connectivity defines behavioral specificity of amygdala neurons,” Neuron, 2014. [DOI] [PubMed] [Google Scholar]
- [24].Stuber GD et al. , “Excitatory transmission from the amygdala to nucleus accumbens facilitates reward seeking,” Nature, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Wolff SBE et al. , “Amygdala interneuron subtypes control fear learning through disinhibition,” Nature, 2014. [DOI] [PubMed] [Google Scholar]
- [26].Kim J, Pignatelli M, Xu S, Itohara S, and Tonegawa S, “Antagonistic negative and positive neurons of the basolateral amygdala,” Nat. Neurosci, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Berridge KC, “Affective valence in the brain: modules or modes?,” Nat. Rev. Neurosci, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Kyriazi P, Headley DB, and Pare D, “Multi-dimensional Coding by Basolateral Amygdala Neurons,” Neuron, vol. 99, no. 6, pp. 1315–1328.e5, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Barrett LF and Bliss-Moreau E, “She’s Emotional. He’s Having a Bad Day: Attributional Explanations for Emotion Stereotypes,” Emotion, 2009. [DOI] [PubMed] [Google Scholar]
- [30].Beyeler A et al. , “Divergent Routing of Positive and Negative Information from the Amygdala during Memory Retrieval,” Neuron, vol. 90, no. 2, pp. 348–361, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Tye KM, “Neural Circuit Motifs in Valence Processing,” Neuron, vol. 100, no. 2, pp. 436–452, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Hangya B, Ranade SP, Lorenc M, and Kepecs A, “Central Cholinergic Neurons Are Rapidly Recruited by Reinforcement Feedback,” Cell, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Jiang L et al. , “Cholinergic Signaling Controls Conditioned Fear Behaviors and Enhances Plasticity of Cortical-Amygdala Circuits,” Neuron, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Vertes RP, “A PHA-L analysis of ascending projections of the dorsal raphe nucleus in the rat,” J. Comp. Neurol, 1991. [DOI] [PubMed] [Google Scholar]
- [35].Burghardt NS and Bauer EP, “Acute and chronic effects of selective serotonin reuptake inhibitor treatment on fear conditioning: Implications for underlying fear circuits,” Neuroscience. 2013. [DOI] [PubMed] [Google Scholar]
- [36].Cohen JY, Amoroso MW, and Uchida N, “Serotonergic neurons signal reward and punishment on multiple timescales,” Elife, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Barker JM, Zhang H, Villafane JJ, Wang TL, Torregrossa MM, and Taylor JR, “Epigenetic and pharmacological regulation of 5HT3 receptors controls compulsive ethanol seeking in mice,” Eur. J. Neurosci, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Lutas A et al. , “State-specific gating of salient cues by midbrain dopaminergic input to basal amygdala,” Nat. Neurosci, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Muller JF, Mascagni F, and McDonald AJ, “Pyramidal cells of the rat basolateral amygdala: Synaptology and innervation by parvalbumin-immunoreactive interneurons,” J. Comp. Neurol, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Bissière S, Humeau Y, and Lüthi A, “Dopamine gates LTP induction in lateral amygdala by suppressing feedforward inhibition,” Nat. Neurosci, vol. 6, no. 6, pp. 587–592, 2003. [DOI] [PubMed] [Google Scholar]
- [41].Corbit LH, Leung BK, and Balleine BW, “The role of the amygdala-striatal pathway in the acquisition and performance of goal-directed instrumental actions,” J. Neurosci, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Everitt BJ, Cardinal RN, Parkinson JA, and Robbins TW, “Appetitive behavior: Impact of amygdala-dependent mechanisms of emotional learning,” in Annals of the New York Academy of Sciences, 2003. [PubMed] [Google Scholar]
- [43].Gallagher M, Graham PW, and Holland PC, “The amygdala central nucleus and appetitive pavlovian conditioning: Lesions impair one class of conditioned behavior,” J. Neurosci, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].McDannald M, Kerfoot E, Gallagher M, and Holland PC, “Amygdala central nucleus function is necessary for learning but not expression of conditioned visual orienting,” Eur. J. Neurosci, 2004. [DOI] [PubMed] [Google Scholar]
- [45].Lee HJ, Groshek F, Petrovich GD, Cantalini JP, Gallagher M, and Holland PC, “Role of amygdalo-nigral circuitry in conditioning of a visual stimulus paired with food,” J. Neurosci, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Lee HJ, Wheeler DS, and Holland PC, “Interactions between amygdala central nucleus and the ventral tegmental area in the acquisition of conditioned cue-directed behavior in rats,” Eur. J. Neurosci, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Douglass AM et al. , “Central amygdala circuits modulate food consumption through a positive-valence mechanism,” Nat. Neurosci, vol. 20, no. 10, pp. 1384–1394, 2017. [DOI] [PubMed] [Google Scholar]
- [48].Han JS, McMahan RW, Holland P, and Gallagher M, “The role of an amygdalo-nigrostriatal pathway in associative learning,” J. Neurosci, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Warlow SM, Naffziger EE, and Berridge KC, “The central amygdala recruits mesocorticolimbic circuitry for pursuit of reward or pain,” Nat. Commun, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Vuilleumier P, Richardson MP, Armony JL, Driver J, and Dolan RJ, “Distant influences of amygdala lesion on visual cortical activation during emotional face processing,” Nat. Neurosci, 2004. [DOI] [PubMed] [Google Scholar]
- [51].Peck CJ and Salzman CD, “Amygdala neural activity reflects spatial attention towards stimuli promising reward or threatening punishment,” Elife, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Aggleton JP and Passingham RE, “Syndrome produced by lesions of the amygdala in monkeys (Macaca mulatta),” J. Comp. Physiol. Psychol, 1981. [DOI] [PubMed] [Google Scholar]
- [53].Baxter MG, Parker A, Lindner CCC, Izquierdo AD, and Murray EA, “Control of response selection by reinforcer value requires interaction of amygdala and orbital prefrontal cortex,” J. Neurosci, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].McGregor A and Roberts DCS, “Dopaminergic antagonism within the nucleus accumbens or the amygdala produces differential effects on intravenous cocaine self-administration under fixed and progressive ratio schedules of reinforcement,” Brain Res, 1993. [DOI] [PubMed] [Google Scholar]
- [55].McGregor A, Baker G, and Roberts DCS, “Effect of 6-Hydroxydopamine lesions of the medial prefrontal cortex on intravenous cocaine self-administration under a progressive ratio schedule of reinforcement,” Pharmacol. Biochem. Behav, 1996. [DOI] [PubMed] [Google Scholar]
- [56].Floresco SB and Ghods-Sharifi S, “Amygdala-prefrontal cortical circuitry regulates effort-based decision making,” Cereb. Cortex, 2007. [DOI] [PubMed] [Google Scholar]
- [57].Ostrander S, Cazares VA, Kim C, Cheung S, Gonzalez I, and Izquierdo A, “Orbitofrontal cortex and basolateral amygdala lesions result in suboptimal and dissociable reward choices on cue-guided effort in rats,” Behav. Neurosci, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Hosking JG, Cocker PJ, and Winstanley CA, “Dissociable contributions of anterior cingulate cortex and basolateral amygdala on a rodent cost/benefit decision-making task of cognitive effort,” Neuropsychopharmacology, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Tiedemann LJ, Alink A, Beck J, Büchel C, and Brassen S, “Valence Encoding Signals in the Human Amygdala and the Willingness to Eat,” J. Neurosci, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Jenison RL, Rangel A, Oya H, Kawasaki H, and Howard MA, “Value encoding in single neurons in the human amygdala during decision making,” J. Neurosci, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Kyriazi P, Headley DB, and Pare D, “Multi-dimensional Coding by Basolateral Amygdala Neurons,” Neuron, pp. 1–14, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Esber GR and Holland PC, “The basolateral amygdala is necessary for negative prediction errors to enhance cue salience, but not to produce conditioned inhibition,” Eur. J. Neurosci, 2014. [DOI] [PubMed] [Google Scholar]
- [63].Gallagher M and Holland PC, “The amygdala complex: Multiple roles in associative learning and attention,” Proceedings of the National Academy of Sciences of the United States of America. 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Cardinal RN, Parkinson JA, Hall J, and Everitt BJ, “Emotion and motivation: The role of the amygdala, ventral striatum, and prefrontal cortex,” Neuroscience and Biobehavioral Reviews. 2002. [DOI] [PubMed] [Google Scholar]
- [65].Cartoni E, Balleine B, and Baldassarre G, “Appetitive Pavlovian-instrumental Transfer: A review,” Neuroscience and Biobehavioral Reviews. 2016. [DOI] [PubMed] [Google Scholar]
- [66].Corbit LH and Balleine BW, “Double dissociation of basolateral and central amygdala lesions on the general and outcome-specific forms of pavlovian-instrumental transfer,” J. Neurosci, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Parkinson JA, Robbins TW, and Everitt BJ, “Dissociable roles of the central and basolateral amygdala in appetitive emotional learning,” Eur. J. Neurosci, 2000. [DOI] [PubMed] [Google Scholar]
- [68].Cador M, Robbins TW, and Everitt BJ, “Involvement of the amygdala in stimulus-reward associations: Interaction with the ventral striatum,” Neuroscience, 1989. [DOI] [PubMed] [Google Scholar]
- [69].Burns LH, Robbins TW, and Everitt BJ, “Differential effects of excitotoxic lesions of the basolateral amygdala, ventral subiculum and medial prefrontal cortex on responding with conditioned reinforcement and locomotor activity potentiated by intra-accumbens infusions of d-amphetamine,” Behav. Brain Res, 1993. [DOI] [PubMed] [Google Scholar]
- [70].Whitelaw RB, Markou A, Robbins TW, and Everitt BJ, “Excitotoxic lesions of the basolateral amygdala impair the acquisition of cocaine-seeking behaviour under a second-order schedule of reinforcememt,” Psychopharmacology (Berl)., 1996. [PubMed] [Google Scholar]
- [71].Malvaez M et al. , “Basolateral amygdala rapid glutamate release encodes an outcome-specific representation vital for reward-predictive cues to selectively invigorate reward-seeking actions,” Sci. Rep, vol. 5, no. July, pp. 1–17, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Merali Z, Michaud D, McIntosh J, Kent P, and Anisman H, “Differential involvement of amygdaloid CRH system(s) in the salience and valence of the stimuli,” Prog. Neuro-Psychopharmacology Biol. Psychiatry, vol. 27, no. 8, pp. 1201–1212, 2003. [DOI] [PubMed] [Google Scholar]
- [73].Khoo SYS, LeCocq MR, Deyab GE, and Chaudhri N, “Context and topography determine the role of basolateral amygdala metabotropic glutamate receptor 5 in appetitive Pavlovian responding,” Neuropsychopharmacology, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Tye KM and Janak PH, “Amygdala neurons differentially encode motivation and reinforcement,” J. Neurosci, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Maren S, “Auditory fear conditioning increases CS-elicited spike firing in lateral amygdala neurons even after extensive overtraining,” Eur. J. Neurosci, 2000. [DOI] [PubMed] [Google Scholar]
- [76].Rogan MT, Staubli UV, and LeDoux JE, “Fear conditioning induces associative long-term potentiation in the amygdala,” Nature, vol. 390, no. 6660, pp. 604–607, 1997. [DOI] [PubMed] [Google Scholar]
- [77].McKernan MG and Shinnick-Gallagher P, “Fear conditioning induces a lasting potentiation of synaptic currents in vitro,” Nature, 1997. [DOI] [PubMed] [Google Scholar]
- [78].Tye KM, Stuber GD, De Ridder B, Bonci A, and Janak PH, “Rapid strengthening of thalamo-amygdala synapses mediates cue-reward learning,” Nature, vol. 453, no. 7199, pp. 1253–1257, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Everitt BJ, Cardinal RN, Hall J, Parkinson J. a., and Robbins TW, “Differential involvement of amygdala subsystems in appetitive conditioning and drug addiction.,” amygdala a Funct. Anal, 2009. [Google Scholar]
- [80].Koob GF, “Neuroadaptive mechanisms of addiction: Studies on the extended amygdala,” Eur. Neuropsychopharmacol, 2003. [DOI] [PubMed] [Google Scholar]
- [81].Everitt BJ, Parkinson JA, Olmstead MC, Arroyo M, Robledo P, and Robbins TW, “Associative processes in addiction and reward. The role of amygdala-ventral striatal subsystems,” in Annals of the New York Academy of Sciences, 1999. [DOI] [PubMed] [Google Scholar]
- [82].Meil WM and See RE, “Lesions of the basolateral amygdala abolish the ability of drug associated cues to reinstate responding during withdrawal from self-administered cocaine,” Behav. Brain Res, vol. 87, no. 2, pp. 139–148, 1997. [DOI] [PubMed] [Google Scholar]
- [83].Grimm JW and See RE, “Dissociation of primary and secondary reward-relevant limbic nuclei in an animal model of relapse,” Neuropsychopharmacology, vol. 22, no. 5, pp. 473–479, 2000. [DOI] [PubMed] [Google Scholar]
- [84].Kruzich PJ and See RE, “Differential contributions of the basolateral and central amygdala in the acquisition and expression of conditioned relapse to cocaine-seeking behavior.,” J. Neurosci, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Fuchs RA and See RE, “Basolateral amygdala inactivation abolishes conditioned stimulus- and heroin-induced reinstatement of extinguished heroin-seeking behavior in rats,” Psychopharmacology (Berl)., 2002. [DOI] [PubMed] [Google Scholar]
- [86].Fuchs RA, Feltenstein MW, and See RE, “The role of the basolateral amygdala in stimulus-reward memory and extinction memory consolidation and in subsequent conditioned cued reinstatement of cocaine seeking,” Eur. J. Neurosci, 2006. [DOI] [PubMed] [Google Scholar]
- [87].Rich MT, Huang YH, and Torregrossa MM, “Plasticity at Thalamo-amygdala Synapses Regulates Cocaine-Cue Memory Formation and Extinction,” Cell Rep, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Wang L et al. , “The coding of valence and identity in the mammalian taste system,” Nature, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]


