Abstract
The central nucleus of amygdala (CeA) mediates positively-valenced reward motivation as well as negatively-valenced fear. Optogenetic or neurochemical stimulation of CeA circuitry can generate intense incentive motivation to pursue and consume a paired natural food, sex, or addictive drug reward, and even create maladaptive ‘wanting what hurts’ such as attraction to a shock rod. Evidence indicates CeA stimulations selectively amplify incentive motivation (‘wanting’) but not hedonic impact (‘liking’) of the same reward. Further, valence flips can occur for CeA contributions to motivational salience. That is, CeA stimulation can promote either incentive motivation or fearful motivation, even in the same individual, depending on situation. These findings may carry implications for understanding CeA roles in neuropsychiatric disorders involving aberrant motivational salience, ranging from addiction to paranoia and anxiety disorders.
Keywords: Central amygdala, Reward, Emotion, Motivation, Cues, Incentive salience
1. Introduction
The importance of the amygdala in emotion and motivation has been recognized ever since early observations that amygdala lesions in monkeys produced Kluver-Bucy syndrome [1–6]. Those amygdala lesions reduced the ability of ordinarily-threatening stimuli to evoke fear and aggressive responses, and conversely released increased exploratory sniffing of familiar stimuli as though they were unfamiliar. Notably, amygdala lesions also produced attempts to eat inedible stimuli such as rocks or metal objects, and increased sexual behaviors towards inappropriate partners, such as juvenile monkeys or human experimenters (or, in cats, attempts to copulate with chickens) [2,7]. As Weiskrantz aptly proposed in his early 1950s study of amygdala lesions in monkeys, “It is suggested that the effect of amygdalectomy is to make it difficult for animals to identify reinforcing stimuli”, noting further that “This idea has features in common with the unpublished suggestion of Olds that amygdalectomy produces lack of discrimination of ‘motivationally relevant stimuli’” [3].
Indeed, amygdala neurons are activated by both positively and negatively valenced stimuli [8,9]. It has been suggested that amygdala integrates affective significance with internal physiological state to coordinate actions [10]. Consistent with this view is that both substance use disorder and posttraumatic stress disorder are associated with hyperreactivity of amygdala to relevant cues, either drug-related in the former, or threat-related in the latter [11,12].
The amygdala contains several subregions and nuclei, including on the lateral side the basolateral nucleus (BLA), and central nucleus (CeA) connected in series. The CeA sends outputs to the lateral division of the bed nucleus of the stria terminalis (BNST), among other targets, and together the CeA and BNST compose the lateral extended amygdala complex. On the medial side of amygdala, the cortical nucleus and medial nucleus of amygdala (MeA) are similarly connected in series, and the MeA and its output target, the medial BNST, together constitute the medial extended amygdala complex [13,14].
This review will focus particularly on the ability of CeA, when neurobiologically stimulated, to generate intense increases in incentive motivation. Optogenetic, chemogenetic, and pharmacological manipulations (mostly performed in rats in what follows, unless otherwise noted) have demonstrated that localized neural stimulations in the CeA can dramatically generate increases in incentive motivation for natural rewards and drug rewards, even sometimes to irrational levels. Simultaneously, such opioid and optogenetic excitations of CeA can also narrow the focus of intensified incentive motivation onto particular reward targets, at the expense of pursuing other rewards. In cases of optogenetic stimulation, where the temporal pattern of neuronal activation can be precisely controlled, associative pairing of CeA stimulations with a particular reward can create exclusive pursuit for that paired sugar or cocaine reward, or even maladaptive attraction to a stimulus that delivers aversive outcomes, such as a stationary ‘shock rod’ [15–18].
Neuronal activation in CeA under a range of conditions can interact with associative learning to assign intense incentive value specifically and narrowly to stimuli paired with those neural activations. Several lines of evidence indicate that this pursuit is mediated by the psychological process of mesolimbic incentive salience or ‘wanting’, in which motivation value becomes attributed to Pavlovian conditioned stimuli (CSs) associated with the emotional unconditioned stimulus (UCS), making those cues become strongly attractive and able to trigger motivation to consume associated reward. Further, our studies have shown that even a negatively-valenced painful UCS that is normally avoided (i. e., an electrified shock-rod), can within limits become the target of incentive salience when paired with optogenetic CeA stimulations, producing maladaptive ‘wanting of what hurts’ [15]. Finally, we will discuss how CeA-related circuitry can switch valence ‘modes’ of motivational salience attribution in different situations, switching from positive incentive salience to negative fearful salience to instead magnify cue-elicited defensive reactions.
2. Neurobiological features of central amygdala
2.1. Central amygdala vs. Basolateral amygdala circuitry
Important amygdala subdivisions include the basolateral complex (BLA), central nucleus (CeA), medial nucleus (MeA), as well as other subregions such as superficial cortical nucleus (CoA), and the GABAergic intercalated nuclei (ITc) situated between BLA and CeA [19]. The BLA consists predominantly of glutamatergic neurons (>80 %) while CeA (and MeA) contains mostly GABAergic neurons [20,21]. Classical views of amygdala organization have suggested BLA is a cortical-like ‘input structure’ that receives thalamo-cortical sensory inputs (e.g., auditory) and then provides direct excitatory projections (or feedforward inhibition via ITc) to CeA [22]. In turn, CeA has been viewed as an amygdala ‘output’ that projects to BNST, hypothalamus, brainstem and other structures [23,24]. This serial processing view has often been applied to understanding acquisition and expression of fear memories and other motivated behaviors [25,26].
Beyond simply being an output structure for BLA, CeA also receives its own unique inputs and contains distinct neuronal populations, opening up the possibility that CeA neural events can directly generate motivated behavior. CeA receives direct input from brainstem sensory structures such as nucleus of solitary tract and pontine parabrachial nucleus [28], as well as from insular cortex [29,30] and ventral tegmental area [31–33] in mice and rats. Additionally, BLA has direct output projections that bypass CeA, such as to structures within basal forebrain or hippocampus [34] (Fig. 1a).
Fig. 1. CeA Circuitry and Macrosystem Framework.
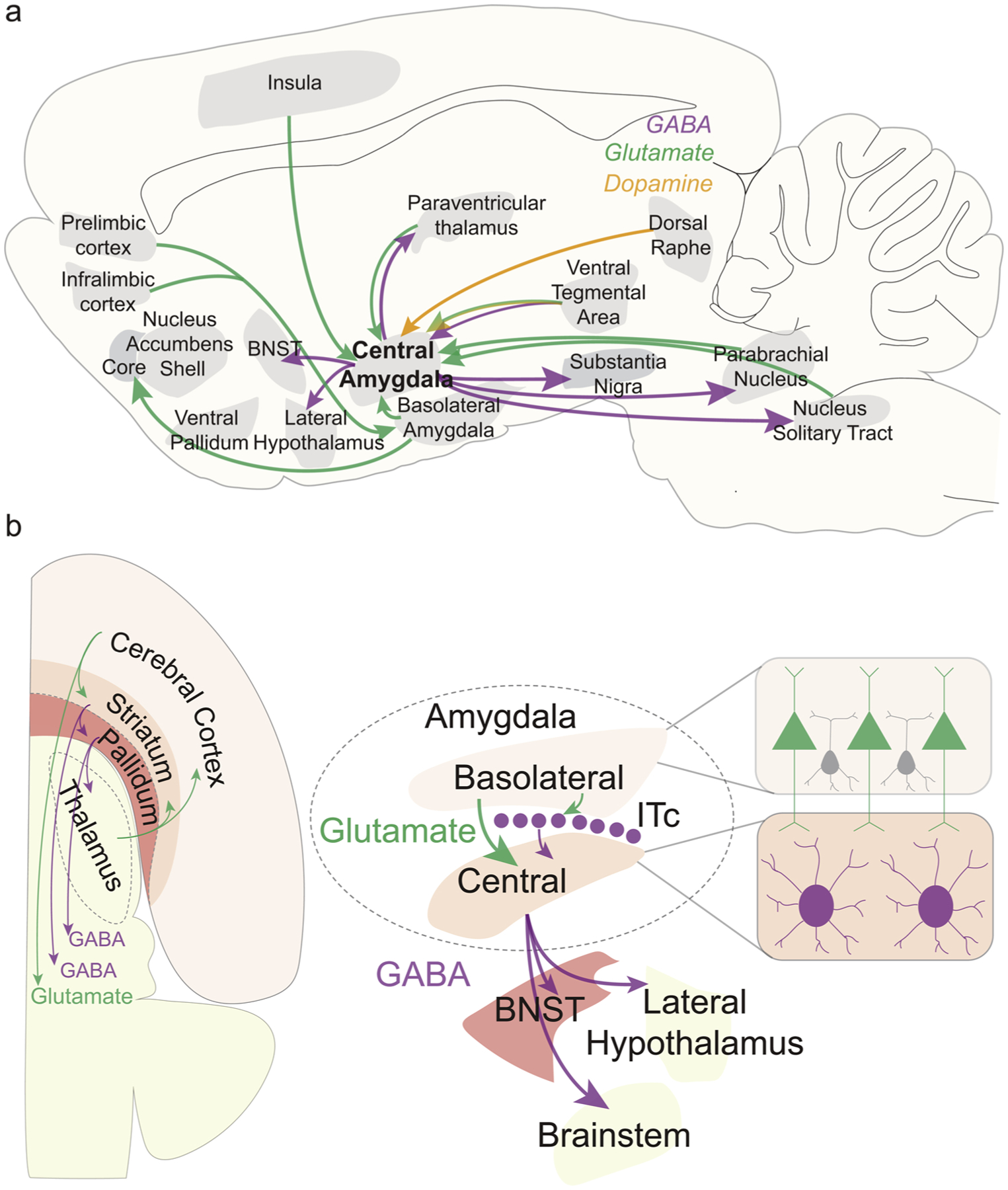
(a) Sagittal brain map shows afferent projections to central amygdala and basolateral amygdala and their efferent connections to other structures. (b) Horizontal brain map on left shows a macrosystem framework that classifies all structures in the telencephalon into either cortical, striatal or pallidal levels, adapted from Larry Swanson’s general organizational model of triple descending projections from cerebral hemisphere [35]. Cortical structures send glutamatergic projections to striatal structures, which send GABAergic projections to pallidal-level structures, which also send largely GABAergic outputs to diencephalic and brainstem targets, along with re-entrant thalamocortical loops. Within this framework, basolateral amygdala (BLA) is cortical-like, in part due to its principal glutamatergic neurons that project to striatal-level central amygdala or to intercalated cell masses (ITc). Central amygdala (CeA) is striatal like, in part due to its largely GABAergic medium spiny neurons that project to bed nucleus stria terminalis (BNST), hypothalamus and other targets. Finally, BNST is pallidal-level in this framework, largely GABAergic, and projects heavily to hypothalamus and brainstem structures.
Within a macrosystem framework that proposes the entire telencephalic forebrain to consist of cortical, striatal, and pallidal level structures [35], BLA is viewed as a cortical-like structure and CeA as a striatal-like structure [24,36,37]. For example, BLA possesses glutamatergic neurons which are cortical-like pyramidal neurons with a minority of parvalbumin-expressing interneurons (proportions similarly found in cortical structures), and has been suggested to share embryological origins with other cortical structures [38]. By contrast, CeA as a striatal-like structure contains GABAergic medium spiny-like neurons [39], and receives rich mesolimbic dopamine projections to largely separate populations of CeA neurons with either D1-type or D2-type dopamine receptors [31,33,40]. CeA projects heavily to the lateral bed nucleus of the stria terminalis (BNST), which has been suggested to be the pallidal-like structure in the macrosystem framework [14,35]. Together, the CeA and lateral BNST also constitute the lateral extended amygdala complex [36,37]. CeA outputs primarily target BNST, lateral hypothalamus, and brainstem structures [41] (Fig. 1b). The fact that CeA possesses striatal-like features may underlie its ability for local CeA stimulations (e.g., opioid; optogenetic) to generate intense eating, reward seeking, or conversely fear-related motivations, similarly to other striatal regions such as nucleus accumbens (NAc) and several regions of neostriatum [42–44].
2.2. Central amygdala neuronal populations
The CeA contains a rich variety of peptide- or receptor- defined neuronal subtypes. For example, the lateral portion of CeA contains a large proportion of neurons expressing corticotropin-releasing hormone (Crh) neurons [45], protein kinase-c delta (PKC-d), somatostatin (SOM), or neurotensin [21,40,46]. Dense expression of receptors for orexin, oxytocin, serotonin 2a receptors as well as dopamine type-1 (D1r) versus type-2 (D2r) are also present on particular neurons in CeA [40,47] (Fig. 2).
Fig. 2. Species-specific CeA Anatomy and Cell-type distribution.
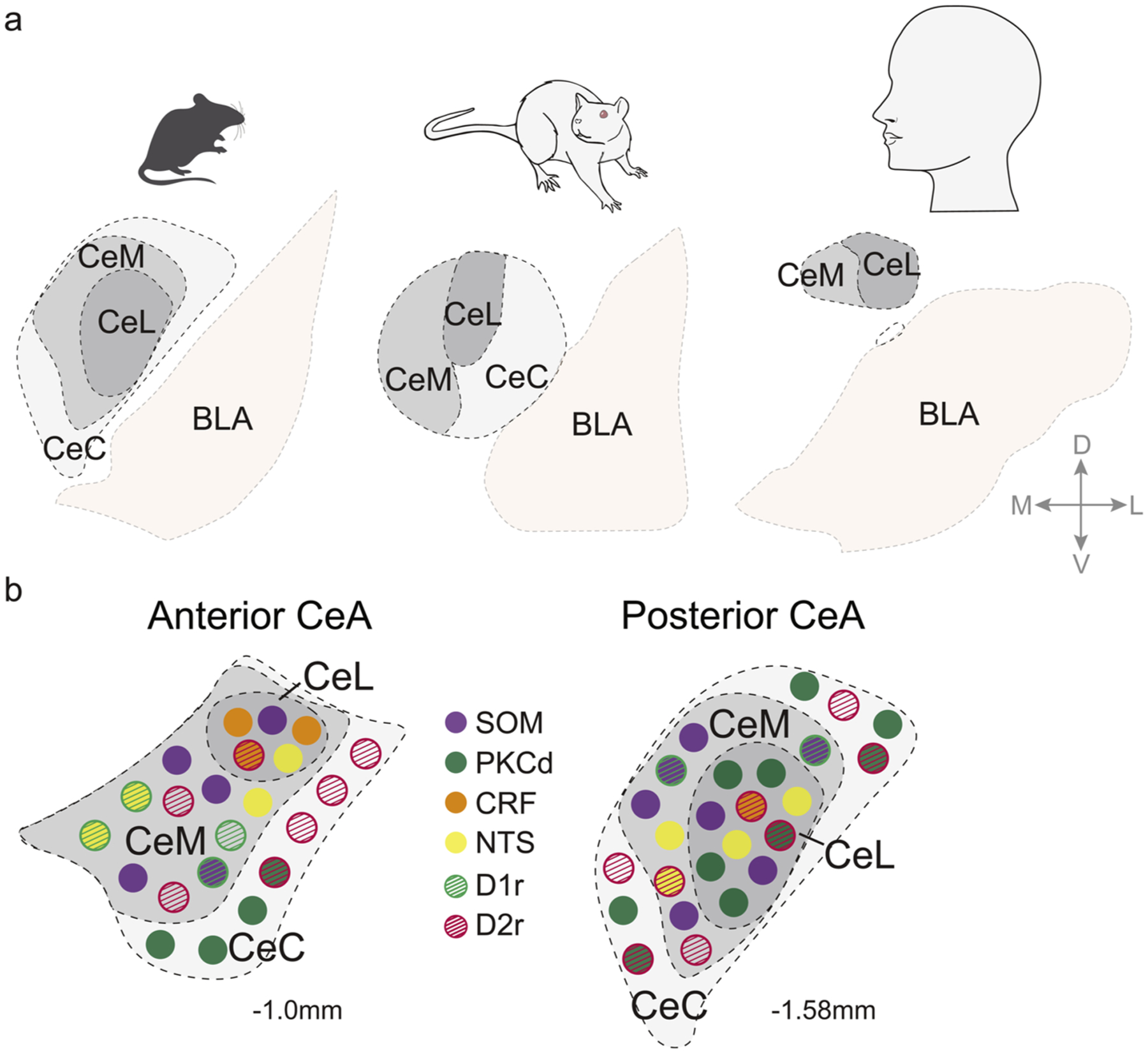
(a) Coronal views show central nucleus of amygdala and basolateral amygdala organization in mouse (left), rat (middle), or human (right) (adapted from [166–168]. (b) Coronal views show CeA neuronal subtypes and their approximate anatomical distribution across sub-regions in anterior CeA and in posterior CeA, based on expression of select mRNA markers in mice [40, 46]. This figure does not reflect the fact that some neuronal subtypes co-express multiple neurotransmitter peptides (e.g., NTS cells also express SOM), nor is it meant to depict exact proportions of specific cell types. Abbreviations: CeC, central CeA; CeM, medial CeA; CeL, lateral CeA; BLA, basolateral amygdala; D, dorsal; V, ventral; M, medial; L, lateral; A, anterior; P, posterior; SOM, somatostatin; PKCd, protein kinase-C delta; CRF, corticotropin releasing factor; NTS, neurotensin; D1r, dopamine receptor type 1; D2r, dopamine receptor type 2.
Studies using Cre-dependent manipulations of particular CeA neurons in transgenic rodents have given rise to suggestions that specific cell types might promote opposing roles in reward and aversion. An example is PKC-d neurons versus SOM neurons in CeA of mice, which are largely non-overlapping populations and which, combined, constitute almost all CeA neurons [40]. In mouse models of pain, PKC-d neurons typically promote pain, while SOM neurons are anti-nociceptive. For example, PKC-d neurons are hyperexcitable after pain-causing injuries and their activity is necessary for nerve injury-induced tactile and thermal hypersensitivity. By contrast, pain-causing injuries reduce spontaneous activity of SOM neurons [48]. Divergent roles of these populations in fear learning and expression are also reported. Optogenetic manipulations show that PKC-d neurons are both necessary for contextual fear conditioning and their activation is sufficient to elicit unconditioned freezing (without any training) [40]. In contrast, SOM neurons may promote positive reinforcement and reward seeking. For example, mice will self-stimulate SOM neurons, but not PKC-d neurons [49]. Additionally, other manipulations that reduce SOM gene expression in the CeA of rats via RNA interference (RNAi) also reduce incubation of methamphetamine seeking after forced abstinence. Conversely, RNA-i mediated gene silencing of CeA PKC-d reverses the decrease in methamphetamine seeking after social choice-induced voluntary abstinence [50]. Thus PKC-d and SOM populations appear to mediate opposing roles at least in several situations. Consistent with this, ex vivo electrophysiological recordings show that PKC-d neurons can inhibit SOM neurons [51], perhaps lending to their opposing roles. However, whether such opposing roles are always fixed and stable is not yet clear [52], and requires more work.
Selective targeting of receptor-defined populations within CeA has also suggested other opposing roles in motivation. For example, optogenetic stimulation of CeA neurons with D1 receptors (D1r) enhances food seeking, and even supports self-stimulation [40]. Incubation of drug seeking is also associated with increased D1r neuron activity in CeA [53]. By contrast, CeA D2r neuronal stimulation has been reported to suppress food seeking, and incubation of drug craving is associated with reduced D2r activity [53]. However, CeA D2r neurons may also support some positive self-stimulation in rats, raising some doubt about whether the putative ‘anti-reward’ function of CeA D2r neurons is reliably stable [54]. Perhaps relevant, most D2 receptors in CeA are localized to PKC-d neurons while D1 receptors are mostly localized to SOM neurons which also express prodynorphin [40,46]. Overall, the high density of receptor populations for hormones and monoamines that are related to emotion and motivation suggests that CeA is a hub for integrating certain motivated states (e.g., orexin in the case of hunger) or affective moods with generating motivations (e.g., drive to eat).
3. CeA and incentive motivation
Incentive motivation for rewards, including food, water, sex, drugs of abuse, etc. often involves a particular psychological process known as incentive salience or ‘wanting’ (in quotation marks to distinguish it from the ordinary sense of wanting as a cognitive goal). ‘Wanting’ is mediated by dopamine-related mesocorticolimbic circuitry, and follows specific rules of operation [55,56].
For example, Rule 1: Incentive salience is typically triggered by learned Pavlovian cues for reward (CS+s or conditioned stimuli), as well as by surprising encounters with rewards themselves (UCSs or unconditioned stimuli) [57,58].
Rule 2: Cue-triggered ‘wanting’ causes phasic surges of motivation to obtain and consume an associated UCS reward, such as when the smell of food evokes a sudden urge to eat. In neuroscience laboratories, cue-triggered ‘wanting’ is often assessed in Pavlovian-Instrumental Transfer (PIT) studies, cue-triggered relapse studies, etc. [59–61]. Cue-triggered ‘wanting’ also contributes to the ability of second-order instrumental schedules in which CS+s accompany rewards to promote the pursuit and consumption of UCS rewards.
Rule 3: Incentive salience also makes Pavlovian cues for rewards become attractive themselves, able to elicit approach as sign-tracking, and often to elicit consummatory actions such as nibbling directed towards a cue, such as when a rat chews a metal lever CS+ even if it is an unconsumable object [57,62,63]. Similarly in humans, cocaine addicts are reported to sometimes scrabble for white specks that resemble cocaine, even if they know the specks are not cocaine, a phenomenon known as ‘chasing ghosts’ [64].
Rule 4: Pavlovian reward cues, even when absent, may still be ‘wanted’ and therefore sought out. This can be observable as instrumental conditioned reinforcement studies, in which an individual works to obtain cue presentations (although there are also alternative psychological possibilities besides incentive salience that may contribute to conditioned reinforcement) [65,66].
Rule 5: The intensity of ‘wanting’ triggered by a reward cue can be modulated by current states of brain mesocorticolimbic circuitry, and so can be influenced by relevant physiological states such as hunger or thirst, by stress, drugs of abuse, etc. [67–69].
3.1. CeA circuitry modulates motivation for food
Localized manipulations of CeA circuitry can powerfully magnify incentive motivation toward food, drugs and other rewards. Pharmacological microinjections into CeA of mu-opioid agonists, such as DAMGO (D-Ala2, N-MePhe4, Gly-ol-enkephalin), can increase food consumption three-times above baseline consumption levels under ad libitum conditions [61,70,71]. By contrast, DAMGO microinjections in BLA cause no changes in food intake. Furthermore, enhancements in food intake caused by DAMGO microinjections in NAc can be suppressed by concurrent microinjections of GABA-A agonist muscimol in CeA [72], implying necessity of CeA in NAc-generated food motivation.
Targeted activation of genetically-defined CeA neuron types in mice have revealed cell-type specific roles in food-directed motivation. For example, optogenetic channelrhodopsin (ChR2) activation of CeA PKC-d neurons robustly suppresses food intake, whereas optogenetic ChR2 activation of CeA PKC-d− neurons (putative SOM+) promotes food intake in the presence of anorexigenic agents such as lithium chloride [73]. PKC-d− neurons tend to inhibit PKC-d+ neurons which in turn inhibit projection neurons in the medial portion of CeA [51]. Thus, increases in food intake likely occur through final disinhibition of CeA medial projection outputs. Disinhibition of CeA output circuitry is similar to the proposed mu-opioid mechanism of DAMGO in CeA [74]. In addition, targeting specific receptor-defined or peptide-defined cell populations within CeA has revealed several more populations that mediate food consumption. For example, optogenetic activation of CeA neurotensin neurons that project to the parabrachial nucleus of the pons enhances consumption of sweet sucrose or saccharin solutions, and chemogenetic stimulation of CeA serotonin 2A receptor neurons promotes palatable food consumption [75,76].
CeA also appears to integrate signals about current physiological state to help modulate incentive motivation. For example, CeA lesions disrupt the enhancement of NaCl seeking and intake normally produced by physiological sodium depletion, but does not prevent salt appetite-induced increases in ‘liking’ reaction to concentrated NaCl taste [77]. Similarly, chemogenetic inhibition of CeA serotonin receptor-expressing neurons reduces chow consumption when in a state of hunger, but not the lesser level of consumption under normal ad libitum conditions [76].
3.2. CeA focusing of incentive motivation
Recent optogenetic studies in our lab indicate that pairing CeA stimulations with particular stimuli can recruit mesocorticolimbic circuitry to amplify incentive motivation, and focus it onto the laser-paired reward that becomes ‘wanted’ above all other rewards in a ‘winner-take-all’ fashion. For example, when two separate rewards are available to be earned, pairing optogenetic CeA excitation with earning just one of them makes that laser-paired reward become exclusively preferred, and enhances the effort to earn it while the other reward becomes relatively ignored (Fig. 3a). For example, in an initial study using a two-choice instrumental sucrose task, rats could press either of two different levers (with two distinct accompanying auditory tones), both of which earned identical sucrose pellets [17]. Pairing CeA excitation with pressing only one of those levers and with its outcome made that lever the exclusively pursued option, even though rats could earn equal sucrose without laser excitation by pressing the alternative lever. Furthermore, CeA-pairing also increased the intensity of motivation to earn that particular sucrose reward, measured by effort breakpoint in a progressive ratio test of incentive motivation: rats reached breakpoints twice as high for sucrose paired with CeA excitation than for sucrose alone. CeA excitation paired with sucrose raises incentive motivation to somewhat compulsive levels, as rats will choose to endure a low-intensity footshock to earn CeA-paired sucrose when they could alternatively earn sucrose alone without a footshock [84] (Fig. 3b). Similar optogenetic enhancements of sucrose ‘wanting’ intensity can also be induced by selective stimulation of CRF-expressing neurons in CeA, using Crh-Cre rats [18].
Fig. 3. CeA focuses incentive motivation for sucrose and cocaine.
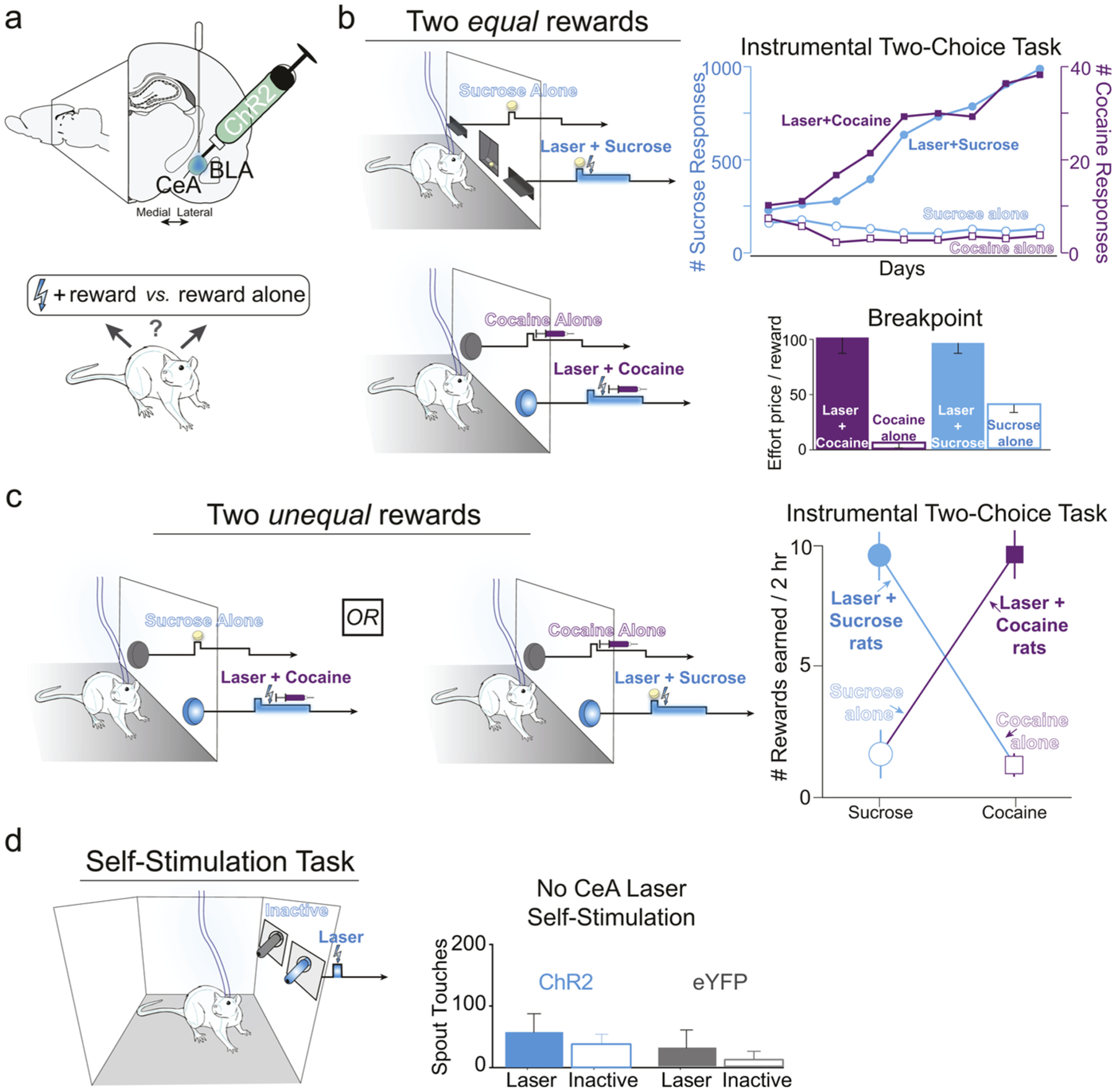
(a) CeA channelrhodopsin (ChR2) stimulation was paired with earning either sucrose or cocaine, when rats could choose between that reward and another identical or different physical reward not paired with CeA laser. (b) CeA ChR2-pairing caused exclusive preference for the paired reward, whether for sucrose or intravenous (i.v.) cocaine (“Laser + Cocaine”; “Laser + Sucrose”) over the alternative reward that lacked CeA laser. Additionally, rats were more intensely motivated to earn that paired reward, showing higher effort breakpoint in a progressive ratio test of motivation. (c) CeA ChR2 stimulation was capable of creating either ‘sucrose addict’ rats that ignored alternative i.v. cocaine, or ‘cocaine addict’ rats that ignored sucrose, when all rats could choose freely between sucrose and cocaine. Rats exclusively preferred to earn sucrose over cocaine when earning sucrose was paired with CeA laser stimulation but cocaine was earned without laser. Alternatively other rats preferred to earn cocaine over sucrose when cocaine was paired with CeA laser and sucrose was not. (d) However, in the same rats, CeA ChR2 stimulation was only weakly reinforcing alone or not reinforcing at all, as measured in a self-stimulation task where touches on a laser spout earned CeA laser, whereas touches on an inactive spout earned nothing.
Subsequent studies in outbred rats showed that similar CeA-induced optogenetic enhancement of incentive motivation using human synapsin promoter (hSyn) also extends to drugs of abuse. For example, when optogenetic CeA excitation is paired with earning one of two available intravenous cocaine options via nose-poking into two different portholes, rats exclusively chose their CeA laser-paired cocaine option, and worked twice as hard to earn that cocaine reward in a breakpoint test, compared to a cocaine reward not paired with CeA excitation [16] (Fig. 3b). By contrast to central amygdala, BLA laser-pairing failed to enhance motivation or alter preference between two sucrose rewards, or between two cocaine rewards. Combined, these findings suggest that CeA excitation can narrow incentive motivation focused onto a particular laser-paired reward (at the expense of alternative rewards), and enhance the intensity of incentive motivation above the levels that are ordinarily evoked by the same sucrose or cocaine rewards.
What about a choice between sucrose reward versus intravenous cocaine reward? In a different study using a two-choice instrumental task, we allowed rats to choose either to earn sucrose pellets by nosepokes into one porthole or to earn intravenous cocaine infusions by nosepokes into a different porthole. For one group of rats, optogenetic CeA excitation was arbitrarily paired with earning sucrose pellets, whereas the same laser excitation was paired with earning cocaine for a separate group of rats. When rats were able to freely choose between either reward, CeA excitation caused exclusive preference of its paired reward. That is, the group of CeA rats that had laser paired arbitrarily with earning sucrose became ‘sucrose addicts’ that exclusively pursued sucrose and ignored the alternative intravenous cocaine reward. Conversely, the second group of CeA rats that had laser paired with cocaine became ‘cocaine addicts’ that pursued only cocaine and ignored sucrose (Fig. 3c). This pattern indicates that strongest incentive value is not inherent in either sucrose nor cocaine as physical stimuli, but rather in the individual brain’s evaluation of those stimuli – which here was hijacked by associative pairing with CeA excitation that recruited mesocorticolimbic activation. In this way, CeA excitation can powerfully hijack motivation and create exclusive preference for particular rewards, regardless of any baseline preference of one over the other [15]. This may have implications for understanding how exclusive pursuit develops in addiction, leading to relative neglect of alternative life rewards [85,86].
3.3. Maladaptively ‘wanting what hurts’
Another finding from our same study showed that optogenetic CeA induction of intense incentive motivation can extend even to seemingly irrational pursuit of painful outcomes, such as attraction to an electrified shock rod [15]. In this experiment, optogenetic CeA excitation was paired with voluntary approaches and touching of an electrified shock rod that delivered brief shocks when touched. After one or two initial touches out of curiosity, normal rats will completely avoid the shock rod and stay as far away from it as possible [87,88]. Normal rats also emit fearful anti-predator defensive burying movements with their forepaws toward the noxious shock rod, which can sometimes bury the rod under sand or bedding.
By contrast, when optogenetic ChR2 activation of CeA was paired with shock-rod encounters, the CeA ChR2 rats repeatedly approached and touched the shock rod with paws or mouth, often displaying consummatory nibbling, sniffing, and biting of the rod even though they received multiple oral shocks on mouth or teeth [15]. Demonstrating that CeA ChR2 rats were motivated to instrumentally reach this shock rod, they were also willing to climb multiple times over a large physical barrier placed between them and the shock rod in order to reach and touch it again, whereas control eYFP rats, with an optically-inactive virus lacking the ChR2 gene, did not. Further, another auditory Pavlovian cue associated with shock rod encounters also became attractive and sought after by CeA ChR2 rats. For example, unlike control eYFP rats, CeA ChR2 rats worked hard on a new instrumental response to hear presentations of a paired auditory cue that was previously paired with rod shocks, even more than they work to hear a sound associated with their home cage [15].
Underlying the maladaptive attraction of CeA ChR2 rats to the painful shock rod, CeA-induced ‘wanting’ recruited activation of mesocorticolimbic incentive circuitry similarly to the ‘sucrose addicted’ and cocaine addicted’ CeA ChR2 rats described above. This was reflected by increased Fos expression in VTA, in NAc medial shell, and orbitofrontal cortex in all these groups. Such evidence supports a role for CeA in recruiting mesolimbic structures to enhance and focus motivation for particular learned incentives, potentially creating addictions to particular rewards or even irrational pursuit of a target that offers only pain.
4. Psychological mechanisms of CeA-mediated incentive motivation
4.1. Does CeA circuitry promote action reinforcement?
The incentive motivation induced by optogenetic CeA stimulations described above involve many different psychological components, each of which in principle could be capable of focusing and increasing incentive motivation. One conceivable explanation for how CeA enhances incentive motivation could have been that CeA ChR2 excitation is highly reinforcing on its own. If so, positive laser reinforcement could cause rats to seek out the sucrose, cocaine, or shock rod paired with CeA stimulation, because they were actually seeking CeA stimulation itself [89]. However, in our hands ChR2 stimulation of CeA neurons by laser is relatively weakly reinforcing for most rats on its own, even in individuals where the laser stimulation exerted strong control of sucrose/cocaine pursuit or induced shock-rod attraction (Fig. 3d). When given the opportunity to self-stimulate CeA laser, either through location-based place preference assays, or through operant nose-poke or spout-touch tasks, most CeA ChR2 rats in our studies did not robustly self-stimulate laser, even when the same stimulation parameters caused enhanced motivation for sucrose, cocaine, or a shock rod [15–17,90]. Indeed, some CeA ChR2 rats completely failed to self-stimulate for laser alone, yet showed as strong attraction to laser-paired sucrose, cocaine or shock rod target as other rats that did self-stimulate. Thus, hSyn ChR2 laser stimulation of multiple CeA neuronal subtypes does not appear to be a very reliable or potent reward on its own, compared to its ability to amplify and focus incentive motivation upon an affective external stimulus that is associatively paired with laser illuminations (e.g., sucrose, cocaine or shock-rod). Although specific neuronal subpopulations or subregions within CeA might eventually prove to support more robust optogenetic self-stimulation [40,91,92], we conclude that stimulation of CeA neurons under these conditions seems to intensely magnify or create the incentive value of its laser-paired sucrose, cocaine, or shock-rod target stimulus, without being an intense reward on its own.
Importantly, global CeA excitation also does not appear to induce an aversive state at the same stimulation parameters that caused enhancement of sucrose, cocaine, or shock-rod motivation [16,92]. Aversive states such as drug withdrawal that precipitate reward seeking behaviors is often interpreted as hedonic self-medication to reduce an aversive state, for example by opponent-process theories [93,94]. However, CeA ChR2 rats do not avoid laser stimulation, which suggests CeA excitation enhances incentive motivations for paired sucrose or cocaine (or even shock-rod) that is not mediated by hedonic self-medication of any negative affective state.
4.2. ‘Wanting’ vs ‘liking’ components of reward
Relative lack of pure reinforcement by CeA, shown by lack of laser self-stimulation, implies that the powerful incentive motivation effects such as sucrose/cocaine pursuit or shock-rod attraction, are facilitated by having an external target stimulus for CeA pairing that itself has affective properties. Thus, an alternative hypothesis is that CeA enhances incentive motivation by transforming the brain’s evaluation of targets themselves and of their associated cues.
Reward contains multiple psychological components: ‘liking’, ‘wanting’, and learning [95,96]. Liking refers to the reward’s hedonic impact or experienced pleasure, while wanting refers to the motivation or desire to earn that reward. Liking and wanting can occur below consciousness under some conditions [97,98], so subjective liking and wanting are distinguished from objective ‘liking’ and ‘wanting’ reactions by quotation marks. Reward ‘liking’ and ‘wanting’ generally co-occur in life, with levels of ‘wanting’ being associated with how much a reward is ‘liked’. However, under certain situations, ‘liking’ and ‘wanting’ can diverge. For example, the incentive-sensitization theory posits that in drug addiction, drug-induced mesolimbic sensitization causes incentive salience or motivation to pursue drugs (drug ‘wanting’) to increase over time even if the pleasure derived from taking drugs stays relatively the same or declines [99,100].
The divergence of reward ‘wanting’ from ‘liking’ likely occurs because these psychological components are mediated by overlapping but separable brain mechanisms. ‘Liking’ can be distinguished from ‘wanting’ for food rewards using the affective taste reactivity test, which measures orofacial ‘liking’ expressions elicited by sweetness vs. ‘disgust’ elicited by bitterness, emitted by species ranging from human infants to rats [101–103]. In the taste reactivity test, sweet or bitter taste solutions are infused directly into the mouth of the experimental rat via previously implanted oral cannulae, and the elicited affective orofacial reactions are video-recorded and assessed. In this way, the taste reactivity test does not rely on the willingness of the experimental subject to approach and consume the rewards, and instead assesses immediate affective reactions elicited by the hedonic properties of a controlled sensory stimulus [101,102]. Sweet sucrose solution elicits midline rhythmic tongue protrusions and lateral tongue protrusions or lip licking, as well as bouts of paw licking. By contrast, bitter solutions such as quinine that are ‘disgusting’ elicit mouth gapes, headshakes, paw flails, etc. Importantly, affective reactions reflect the hedonic impact of a taste rather than its sensory qualities. For example, ‘liking’ reactions to sweetness change to ‘disgust’ when that taste is associatively paired with nausea (such as in a conditioned taste aversion), and decline in normal satiation or sensory satiety [104,105].
Illustrating the difference between brain mechanisms of ‘wanting’ and ‘liking’, for example, pharmacological microinjections of opioid or dopamine agonists, can increase ‘wanting’ nearly anywhere within NAc medial shell [106–108]. By contrast, ‘liking’ enhancement by opioids or endocannabinoids, reflected by increased positive affective reactions to sweet taste, are restricted to a much smaller set of sites within a ‘hedonic hotspot’ contained within the rostrodorsal quadrant of medial NAc shell, and not at other NAc shell sites [106–109]. However, dopamine stimulations anywhere in NAc increase only ‘wanting’ (motivation for reward or reward-related cues) without altering reward ‘liking’, even when the dopamine stimulation is localized within the same rostrodorsal NAc hedonic hotspot. [108,110–112].
4.3. CeA and hedonic ‘liking’
So, it is natural to ask whether CeA ChR2 pairings that powerfully amplify motivational ‘wanting’ also enhance ‘liking’ for the target stimulus. Anatomically, the CeA has sometimes been suggested to be ideally situated for modulating hedonic ‘liking’ of foods. This is because the CeA contains dense reciprocal connections with brainstem gustatory areas such as the nucleus of the solitary tract (the primary taste relay nucleus in the brainstem) and the pontine parabrachial nucleus (the second taste relay nucleus) [113], and also receives taste information from the gustatory region of the insular cortex [114]. Alternatively, CeA might modulate motivational ‘wanting’ without necessarily changing hedonic ‘liking’. For example, earlier studies assessed the CeA’s role in ‘liking’ using the taste reactivity test after amygdala lesions or drug microinjections, and generally found CeA manipulations not to alter ‘liking’ even when they altered ‘wanting’. Electrolytic lesions of CeA that abolish NaCl intake during sodium appetite states do not affect the increase in positive (‘liking’) reactions to intense NaCl tastes produced by the sodium appetite [77], showing a decoupling of CeA roles in ‘liking’ versus ‘wanting’. Similarly, local CeA opioid-stimulating DAMGO microinjections triple food intake and increase sign-tracking to a sucrose cue, but do not increase ‘liking’ reactions to sucrose [61]. Contrary observations, such as that electrical stimulation of CeA slightly increases aversive reactions to bitter quinine, may be explained because those electrode stimulations also excite fibers of passage to and from the nucleus of the solitary tract and pontine parabrachial area that may more directly mediate taste and ‘liking’ [115,116]. Also, aversive gapes to bitter quinine have been reported to be associated with increased Fos induction in rostral portion of CeA, but although CeA activation may be correlational, there is yet no evidence to our knowledge that CeA activation actually causes aversion [117].
Optogenetic CeA ChR2 excitation would be more selective to CeA neurons themselves than traditional electrode stimulations, which also excite fibers of passage, and so is of special interest regarding CeA neuronal roles in ‘liking’. Results of taste reactivity studies in our lab so far indicate that CeA ChR2 stimulation fails to alter ‘liking’ reactions to sucrose (nor has any effect on ‘disliking’), even in the same rats in which CeA laser enhances pursuit of sucrose and focuses ‘wanting’ exclusively on the laser-paired sucrose in a two-choice task [17,118]. Thus, we conclude that CeA neurons do not mediate pleasure ‘liking’ even when CeA ChR2 stimulation has profound effects on incentive ‘wanting’.
4.4. Incentive salience attribution to reward-related cues
In addition to becoming learned predictors, reward-associated cues that are attributed with incentive salience become attractive motivational magnets (e.g., Pavlovian sign-tracking), and trigger surges of motivation to consume their associated rewards [55,95,119]. In drug addiction, sensitization of cue-triggered ‘wanting’ can cause relapse even after long periods of abstinence [99,100]. In humans, the amygdala is activated by cues for drug rewards [80], palatable food rewards [120, 121], as well as by subliminal sexual and drug cues [79]. In people with substance use disorder, heightened amygdala cue reactivity relates to a subject’s drug of choice, but not to other drug or sexual cues in general, and these cues elicit self-reports of increased craving for their preferred drug of choice [58,122].
In the animal neuroscience laboratory, several assays have been used to assess whether a reward cue has been imbued with incentive salience. Attribution of incentive salience can be measured using a Pavlovian autoshaping task, most clearly revealed as sign-tracking attraction to a reward-predicting cue, which becomes an attractive motivational target itself [57]. Through repeated Pavlovian pairings of a distinct cue or conditioned stimulus (i.e., a discrete light, auditory tone, or insertion of a movable lever) with reward delivery, such cues often come to be approached and investigated, sometimes even with attempts to ingest/consume the inedible cue [123,124]. That is because a cue imbued with incentive salience often takes on motivational features of the associated reward itself [55]. For example, rats will engage in nibbling, sniffing, and biting of a light or lever that predicts food [62,63, 125]. Several sign-tracking studies have demonstrated that CeA is necessary for acquisition of autoshaping behaviors [126,127]. Additionally, excitotoxic lesions of CeA or of CeA connections to substantia nigra disrupt normal acquisition of orienting responses generated by either a visual or auditory conditioned stimulus (CS) predictive of food unconditioned stimulus (UCS) [128–130]. By contrast, local DAMGO stimulations in CeA increase consummatory nibbles and sniffs of either lever CS (in the case of sign-trackers) or food cup CS (in the case of goal-trackers) above the level normally directed at the same lever CS or food cup CS [131,132]. CeA DAMGO microinjections particularly increased nibbles and sniffs (that usually occur early in a rat’s interaction with the metal lever CS or cup CS), at the expense of slower bites (that typically occur towards the end of CS+ presentation immediately prior to sucrose UCS delivery) (Fig. 4a). That suggests that CeA DAMGO stimulation alters the behavioral topography of CS+ directed consummatory responses by extending the initial consummatory phase of nibbling and sniffing. Overall, this pattern demonstrates that opioid stimulation of CeA can amplify incentive salience and focus it specifically on an individual’s own particular prepotent reward cue, as measured by sign-tracking/goal-tracking paradigms.
Fig. 4. Incentive salience enhancements by optogenetic ChR2 stimulation or mu-opioid microinjection in CeA.
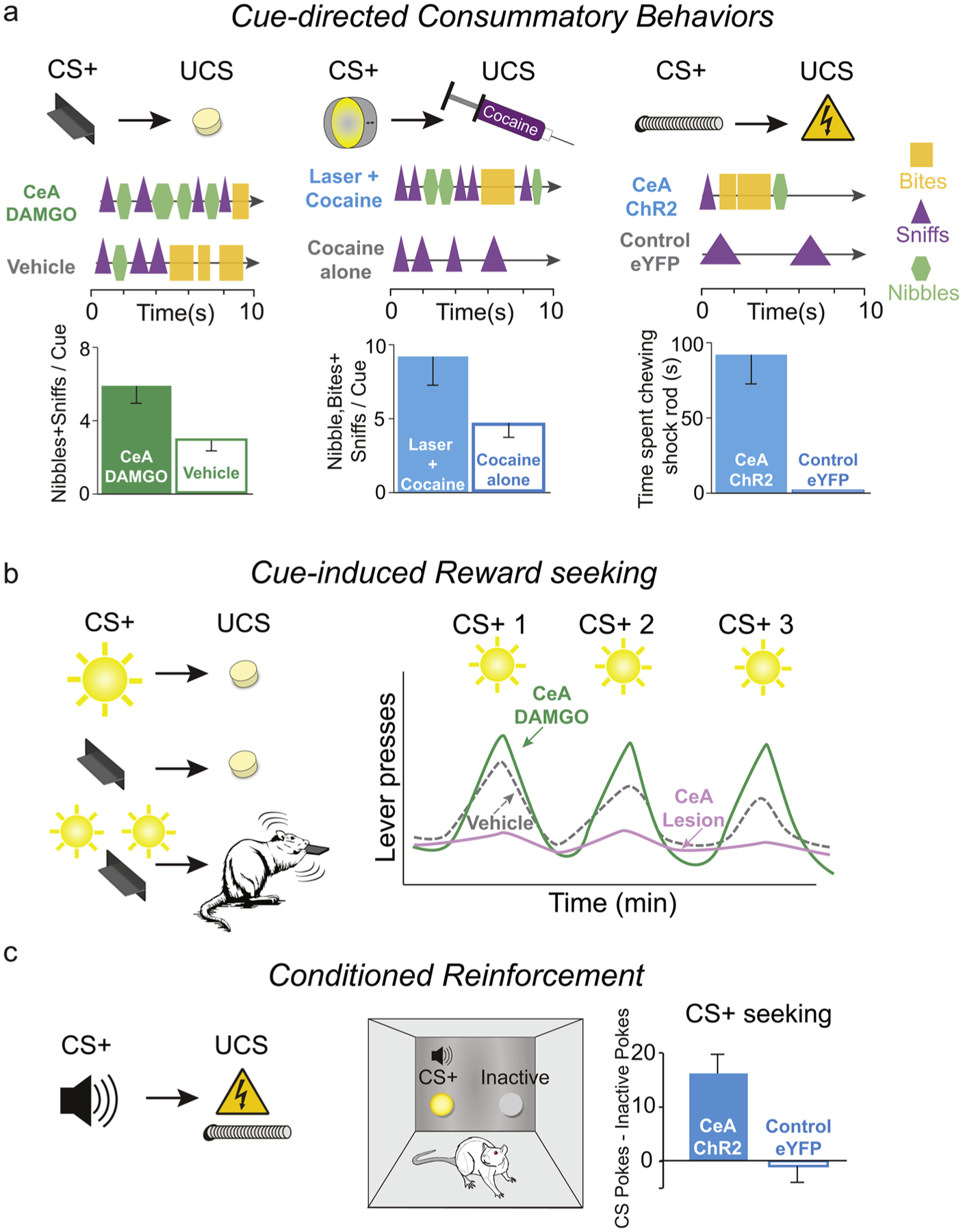
(a) One feature of incentive salience attribution is that consummatory behaviors, such as nibbles or bites, become directed towards a ‘wanted’ Pavlovian cue (CS+; e.g., presentation of a movable metal lever or porthole) that predicts reward (UCS). CeA ChR2-paired stimulation or opioid stimulation (via DAMGO microinjection) enhances CS+ directed consummatory nibbles, sniffs, and bites of the metal CS+ cue when the paired UCS reward is sucrose (left) [131,132], cocaine (middle) [16], or even an aversive shock rod (right) [15]. In a Pavlovian sign-tracking/goal-tracking paradigm, opioid DAMGO microinjection in CeA enhances nibbles and sniffs initially directed toward a lever CS+ in sign-tracking rats, and so reduce slow bites that typically occur near the end of the CS+ immediately prior to UCS sucrose reward delivery [131]. CeA opioid microinjections also produces similar enhancements of nibbling of the goal dish CS in goal-tracking rats [132]. (b) In a Pavlovian-instrumental-transfer (PIT) test of cue-triggered ‘wanting’ to obtain UCS reward, phasic presentation of a previously Pavlovian paired CS+ (sound) invigorates instrumental lever pressing to obtain sucrose under reward extinction conditions. CeA DAMGO microinjections specifically enhance CS+ induced reward seeking above vehicle microinjection levels, increasing the phasic peaks of cue-triggered ‘wanting’, without altering baseline lever pressing in the absence of CS+ [61]. Conversely, CeA lesions abolish cue-triggered peaks of ‘wanting’ [136]. (c) Incentive salience attribution causes reward predictive cues (CS+) to become ‘wanted’ themselves. In an instrumental conditioned reinforcement test, rats will work to earn presentations of a Pavlovian reward CS+ when it is absent. Rats that were maladaptively attracted to approach and nibble on a painful shock-rod due to CeA ChR2 pairing, subsequently worked hard by making nosepokes to hear presentations of auditory CS+ that also had been paired with shock rod encounters (UCS) in a conditioned reinforcement test [15].
Second, the ability of a previously paired cue to elicit temporary surges in ‘wanting’ to obtain its associated unconditioned reward appears to also be mediated by CeA circuitry. Cue-triggered ‘wanting’ for reward can be tested in a Pavlovian-to-Instrumental Transfer (PIT) assay, which measures phasic peaks of increased reward ‘wanting’ caused by presentation of reward-predictive Pavlovian cues as rats instrumentally work for reward during an extinction session [60,61, 133]. Lesions or pharmacological disruptions via muscimol/baclofen GABA agonists disrupt these phasic peaks of cue-induced reward seeking, whereas DAMGO microinjections into CeA oppositely and selectively increase cue-induced seeking in PIT above normal levels (without elevating baselines of instrumental effort in the absence of cues) [61,133–136] (Fig. 4b). Further, in incubation of drug craving, the growth in ability of drug cues (for cocaine, morphine, or methamphetamine) to reinstate drug seeking after protracted withdrawal and drug abstinence, is regulated by time-dependent increases in activity of the extracellular signal-related kinase (ERK) pathway in CeA (but not BLA) [81,83,137]. Such ‘incubation’ of drug craving may reflect mesolimbic sensitization processes, which persist and underlie hyper-reactivity to drug-related cues, processed in part by CeA to precipitate drug reinstatement via excessive cue-triggered ‘wanting’.
Optogenetic CeA ChR2 stimulations that amplify and focus motivation onto sucrose, cocaine, or an aversive shock rod also appear to recruit enhanced incentive salience attribution to the CeA-paired cues. This is reflected in increased neurobiological activation of mesolimbic systems during CeA ChR2-induced attractions to sucrose, cocaine or shock-rod targets. It is also behaviorally manifested as increases in ‘wanting’ to obtain paired Pavlovian cues when they are absent [15]. For example, as described above, CeA ChR2 rats will actively work hard to hear repeated presentations of an auditory CS cue that was previously associated with shock rod encounters, whereas normal rats never do, indicating that shock cues have paradoxically become ‘wanted’ by CeA ChR2 rats (Fig. 4c). Further, in our 2-choice instrumental tasks, where lever presses or nosepokes are required to earn sucrose or cocaine, CeA ChR2 rats direct increased consummatory nibbles, sniffs, and bites selectively at the metal lever or porthole associated with the CeA-paired sucrose or the CeA-paired cocaine, more than to the alternative lever or porthole that earns identical sucrose or cocaine without CeA excitation [15,16] (Fig. 4a). Such consummatory actions directed toward a Pavlovian CS or cue are a signature feature of incentive salience, as the Pavlovian cue takes on some motivational features of its reward UCS. The nibbles, sniffs, and bites of the cue lever/porthole that are enhanced by CeA ChR2 stimulations are not required to instrumentally earn the sucrose, and usually occur after the sucrose has already been earned [17]. The CeA-induced nibbles and bites are especially striking in the case of cocaine, as normal rats typically do not nibble or bite cocaine cues at all, even if the rats are attracted to approach the cues [124,138]. That suggests that CeA ChR2 stimulation heightens the incentive salience of the laser-paired cocaine cue to unusual levels, making the cue sufficiently attractive to engage orally. Similarly, CeA ChR2 rats often direct nibbles, bites and sniffs toward their aversive shock rod, which itself could be viewed as a cue object associated with shock, even though doing so results in receiving orofacial shocks directly to the mouth, teeth and nose [15].
In these cases, attempts by CeA ChR2 rats to ‘ingest’ or orally explore a cocaine cue or shock rod cue paired with CeA excitation may reflect extremely high levels of incentive salience attributed to the cue. How this intense motivation becomes behaviorally expressed is partly shaped by physical properties of the CS cues themselves (e.g., metal nose port or shock rod, as discrete physical objects that can be readily nibbled). Indeed, if another rat is presented as a Pavlovian CS that predicts a subsequent food UCS, conditioned behaviors of the recipient rat towards its CS+ rat are social in nature (anogenital sniffing and playful behaviors) rather than ingestive [139]. Therefore, CeA ChR2 control of motivation likely involves facilitation of incentive salience attribution towards laser-paired cues as an important psychological component, interacting with stimulus features of targets, to potentially enhance the attractiveness of both UCS and CS cue.
5. Motivational salience: incentive vs. Fear
How can CeA ChR2 enhancement of appetitive motivation and incentive salience align with the many demonstrations that CeA circuitry helps mediate oppositely-valenced conditioned responses to threat (such as freezing) in Pavlovian fear conditioning situations [140–142]? In fact, similar to those studies, our study also found that pairing CeA ChR2 stimulation with footshock in a traditional Pavlovian fear conditioning paradigm potentiated fearful conditioned freezing to a footshock-predictive auditory cue, and increased avoidance of a contextual odor cue associated with footshock, sometimes in the same rats that displayed CeA enhancements of incentive motivation for sucrose or cocaine rewards, or even attraction to the shock rod [15,92].
An explanation may be that motivational salience can occur with either positive valence as incentive salience or negative valence as fearful salience, and it is possible to flip the valence between the two forms under some conditions [143]. Despite being affectively opposite, the two may still share some neural and psychological features [144, 145]. Neurally, both may engage mesolimbic systems, including dopamine signals in NAc [43,146]. Psychologically, just as incentive salience makes reward cues attention-riveting and attractive, calling forth approach and consumption, fearful salience makes threat cues attention-riveting and threatening, often calling forth active defensive reactions [147]. Regarding CeA ChR2 flips of valence between intensifying ‘wanting’ versus ‘fear’, we suggest the Pavlovian fear conditioning situation may possibly promote fearful reactions more than the shock rod situation because the Pavlovian footshock UCS is unavoidable, inescapable, and unlocalizable [148]. By contrast, shock rods are discrete and localizable stimuli and interaction with them is controllable [149]. This difference might be important in determining the valence of motivational salience induced by CeA ChR2 stimulation.
Fearful salience, in part also involving mesolimbic dopamine [43], has been suggested to underscore certain positive symptoms of paranoid schizophrenia, where fearful salience is aberrantly assigned to innocuous stimuli that become perceived as threatening [150,151]. Consistent with an amygdala role in bivalent motivational salience, some CeA neurons can respond to both aversive outcomes (e.g., footshock) and rewarding outcomes (e.g., sucrose) [92], or to their predictive cues [152]. CeA processing of both rewarding and threatening stimuli suggests that, rather than strictly encoding only reward or only fear per se, some neurons in CeA might integrate sensory information, context, and motivational state to assign motivational salience with positive or negative valence to particular targets, making them either ‘wanted’ or ‘feared’.
This hypothesis helps explain several conflicting findings. First, that in our studies CeA ChR2 stimulations more powerfully confer incentive value on external affective targets (sucrose, cocaine, or shock rod) to create intense and focused attraction, rather than promoting reinforcement alone. The bias toward amplifying the motivational salience of external affective stimuli may be why CeA ChR2 stimulation was relatively weak at supporting laser self-stimulation, and completely failed in some rats, even when in the same rat laser produced strong attraction to a paired sucrose, cocaine or shock rod stimulus. Lack of pure reinforcement despite strong enhancement of the motivational value of those physical sensory stimuli, together with CeA ChR2 magnification of ‘fearful’ conditioned reactions to an auditory CS and contextual cue for footshock in the Pavlovian fear conditioning paradigm, implies that CeA ChR2 neurons stimulated by laser do not generate positive valence in a dedicated fashion. Rather, the valence of salience they produce (rewarding or fearful) is influenced by the externally-paired affective target and situation. This hypothesis would be consistent with the classical interpretations proposed by Weiskrantz, namely that amygdala is needed to recognize the motivational value of stimuli, as well as more recent ones that propose the amygdala assigns motivational significance to stimuli in a bivalent manner [3,153,154].
5.1. Multiple valence ‘modes’ of CeA motivation
Within a motivational salience framework, cognitive appraisal of one’s environment or situational factors can influence the subsequent valence mode of motivation, making targets either approached or avoided. Switching between these multiple motivational modes may also involve neural mechanisms in striatal regions such as NAc medial shell. For example, under baseline conditions, NAc microinjections that pharmacologically block glutamate AMPA receptors can generate either feeding/reward motivation or negative/fear motivations depending partly on anatomical site, with more rostral NAc shell injection sites generating increases in food intake (‘wanting’) or establish conditioned place preference but caudal NAc shell microinjections generating defensive treading (‘fear’) and conditioned place avoidance [155–157]. The positive valence motivation to consume reward requires only endogenous dopamine D1 receptor signaling at the microinjection site. By contrast, the negative valence fearful motivation requires endogenous D2 dopamine signals as well as D1 [43]. However, a stressful environment with bright lights and loud music can expand the ‘fear’ generating zones of NAc medial shell, allowing even rostral microinjection sites to generate fearful behaviors and simultaneously switch to additionally require D2 dopamine signals to do so [43,158]. By contrast, a more comfortable, home-like environment can expand the reward-generating sites, allowing even caudal sites to elicit increases in eating and simultaneously to drop the need for D2 signals at those caudal sites to generate the positive motivation [43]. This suggests that neural sites in NAc do not have a dedicated labeled-line modular function, but rather possess different ‘modes’ of motivational function [52].
Indeed, CeA circuitry also demonstrates sensitivity to situational or environmental factors in switching motivational valence evoked by changes in CeA activity. Environment can flexibly switch the activity of mutually inhibitory CeA neurons to produce conditioned flight or passive freezing [159,160]. Furthermore, our CeA ChR2 results show that the same CeA excitation can transform the valence of motivational salience, flipping from positively intensifying incentive motivation for cocaine or sucrose or even creating maladaptive positive attraction to an aversive shock-rod to negatively potentiating fearful conditioned reactions in a Pavlovian fear learning situation [15]. Flips between positive and negative motivational valence may involve external factors such as stimulus and situational properties (controllable shock; localizable vs. unlocalizable shock source; presence of a safe zone, etc.) that can flexibly gate CeA’s appetitive vs. fearful modes. This is in contrast to proposals that most specific CeA cell types/circuits are dedicated to mediate only positively-valenced motivation or instead dedicated to negatively-valenced motivation, but not both [26,48]. In any case, consideration of the influence of environmental factors is important when determining the effectiveness of treatments in neuropsychiatric disorders such as addiction or schizophrenia.
5.2. Translational relevance to neuropsychiatric disorders?
Addiction involves several characteristic features, including excessive pursuit, escalation of drug intake and relapse, often triggered by drug-related cues especially if encountered in states of stress or emotional excitement, while relatively neglecting other life rewards [59, 161]. Animal studies of the neural bases of incentive salience attribution described above can prove useful in identifying how brain circuitry heightens the motivational attraction triggered by reward-related cues, and focus that intensified incentive motivation onto a particular target. The results discussed here help show how CeA-related circuitry can powerfully amplify the motivational value of an incentive, such as cocaine or sucrose, and narrowly focus the motivation on that specific target at the expense of other rewards. The CeA’s ability to recruit incentive salience circuitry is so powerful it can even create maladaptive addictive-like ‘wanting’ for a painful shock-rod and its cues, in the absence of any ‘liking’. Eventually treatments that target such brain circuitry may aid in preventing drug relapse, or escalation of drug intake [162,163].
Converging evidence has demonstrated that CeA excitation may not necessarily in itself be rewarding or aversive, but rather may help assign motivational significance to brain representations of particular external stimuli, able to amplify either their incentive or aversive motivational value. Involvement of CeA in aberrant fear conditioning and other animal models of human post-traumatic stress disorder is well established. A better understanding of how CeA-related circuitry applies aberrant motivational salience to cues, including negatively-valenced fearful salience as well as positively-valenced incentive salience, may also help lead to insights into anxiety and paranoia in human neuropsychiatric disorders such as bipolar disorder or schizophrenia [164,165].
6. Future directions
The ability for CeA paired ChR2 stimulations to amplify ‘wanting’ focused onto paired incentive targets (such as sucrose or cocaine) has mostly been demonstrated with global optogenetic CeA excitations, while rarely targeting specific cell types or discrete CeA output projections. An important future direction will be to distinguish what cell types within lateral, medial, or central portions of CeA mediate this focused ‘wanting’ for incentives and aversive shock rod. The recent increase in availability of a variety of transgenic rats has allowed for probing of different CeA cell types in incentive motivation assays. Indeed, recent work shows that optogenetic excitation of CRF expressing neurons within CeA of rats can amplify ‘wanting’ for sucrose, and promotes self-stimulation similarly to the hSyn stimulations described above (whereas excitation of CRF neurons in BNST suppresses sucrose motivation, and produces avoidance) [18].
Future work may also investigate the role of D1 receptor- vs. D2 receptor-expressing neurons of CeA in incentive motivation. Preliminary work has demonstrated that rats will self-stimulate D1r- neurons of CeA and also self-stimulate CeA D2r-neurons at moderate levels [54], but future work will be needed to investigate their role in motivation for sucrose or cocaine. Examining discrete output projections from CeA, especially arising from specific cell types, that mediate amplified ‘wanting’, such as to lateral hypothalamus or ventral pallidum, is also of importance.
Finally, while CeA may mediate both positively-valenced and negatively-valenced motivation, few studies have explored how CeA circuitry might actively switch between motivational valence modes. Future work that examines mechanisms of valence plasticity or bivalent modes of CeA would be especially useful in elucidating how specific conditions (environmental or psychological factors) precipitate a switch between CeA neuronal stimulation promoting incentive motivation versus promoting fearful motivation, possibly involving some of the same CeA cell types or projections in both forms of motivational salience.
7. Conclusion
Although central amygdala may be most studied for its role in generating fear-related responses, CeA circuitry also controls incentive motivation. CeA-related circuitry can both amplify ‘wanting’ by recruiting larger mesocorticolimbic circuitry, and narrowly focus it onto a particular target associated with CeA stimulation. Optogenetic and pharmacological stimulations of CeA appear to enhance UCS and CS+ directed ‘wanting’, without altering reward ‘liking’. CeA stimulation itself is not strongly reinforcing on its own, and instead most powerfully modulates the motivational value of perceived external affective stimuli. Evidence suggests that the attribution of incentive salience to cues associated with the paired affective stimulus is one psychological component of CeA-induced appetitive motivation. However, rather than mediating only positive or negatively-valenced motivations, some CeA neuronal systems may have multiple valence modes, able to participate in bivalent motivation, controlling both which targets should be approached and which should be avoided. CeA-related circuitry can elevate ‘wanting’ to irrational and dangerous levels and focus it even onto maladaptive targets. This highlights a potentially crucial role for amygdala-related circuitry in irrational pursuits occurring in addiction or other compulsive disorders.
Funding
This work was supported by the National Institutes of Health (F32 MH122192 to SMW and R01 MH063649 and R01 DA015188 to KCB).
Footnotes
Declaration of Competing Interest
The authors declare no competing interest.
References
- [1].Lanska DJ, The klüver-bucy syndrome, Front. Neurol. Neurosci 41 (2018) 77–89, 10.1159/000475721. [DOI] [PubMed] [Google Scholar]
- [2].Devinsky J, Sacks O, Devinsky O, Kluver-Bucy syndrome, hypersexuality, and the law, Neurocase 16 (2010) 140–145, 10.1080/13554790903329182. [DOI] [PubMed] [Google Scholar]
- [3].Weiskrantz L, Behavioral changes associated with ablation of the amygdaloid complex in monkeys, J. Comp. Physiol. Psychol 49 (1956) 381–391, 10.1037/h0088009. [DOI] [PubMed] [Google Scholar]
- [4].LeDoux J, The emotional brain, fear, and the amygdala, Cell. Mol. Neurobiol 23 (2003) 727–738, 10.1023/a:1025048802629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Maclean PD, The limbic system (“visceral brain”) and emotional behavior, AMA Arch. Neurol. Psychiatry 73 (1955) 130–134, 10.1001/archneurpsyc.1955.02330080008004. [DOI] [PubMed] [Google Scholar]
- [6].Klüver H, Bucy PC, Preliminary analysis of functions of the temporal lobes in monkeys, J. Neuropsychiatry Clin. Neurosci 1997 (9) (1939) 606–620, 10.1176/jnp.9.4.606. [DOI] [PubMed] [Google Scholar]
- [7].Green JD, Clemente CD, De Groot J, Rhinencephalic lesions and behavior in cats: an analysis of the Kluver-Bucy syndrome with particular reference to normal and abnormal sexual behavior, J. Comp. Neurol 108 (1957) 505–545, 10.1002/cne.901080308. [DOI] [PubMed] [Google Scholar]
- [8].Belova MA, Paton JJ, Morrison SE, Salzman CD, Expectation modulates neural responses to pleasant and aversive stimuli in primate amygdala, Neuron 55 (2007) 970–984, 10.1016/j.neuron.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Belova MA, Paton JJ, Salzman CD, Moment-to-moment tracking of state value in the amygdala, J. Neurosci 28 (2008) 10023–10030, 10.1523/JNEUROSCI.1400-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Morrison SE, Salzman CD, Re-valuing the amygdala, Curr. Opin. Neurobiol 20 (2010) 221–230, 10.1016/j.conb.2010.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Nutt DJ, Malizia AL, Structural and functional brain changes in posttraumatic stress disorder, J. Clin. Psychiatry 65 (Suppl 1) (2004) 11–17. [PubMed] [Google Scholar]
- [12].Goudriaan AE, de Ruiter MB, van den Brink W, Oosterlaan J, Veltman DJ, Brain activation patterns associated with cue reactivity and craving in abstinent problem gamblers, heavy smokers and healthy controls: an fMRI study, Addict. Biol 15 (2010) 491–503, 10.1111/j.1369-1600.2010.00242.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Alheid GF, Extended amygdala and basal forebrain, Ann. N. Y. Acad. Sci 985 (2003) 185–205, 10.1111/j.1749-6632.2003.tb07082.x. [DOI] [PubMed] [Google Scholar]
- [14].Fudge JL, Kelly EA, Pal R, Bedont JL, Park L, Ho B, Beyond the classic VTA: extended amygdala projections to DA-Striatal paths in the primate, Neuropsychopharmacology 42 (2017) 1563–1576, 10.1038/npp.2017.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Warlow SM, Naffziger EE, Berridge KC, The central amygdala recruits mesocorticolimbic circuitry for pursuit of reward or pain, Nat. Commun 11 (2020) 2716, 10.1038/s41467-020-16407-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Warlow SM, Robinson MJF, Berridge KC, Optogenetic central amygdala stimulation intensifies and narrows motivation for cocaine, J. Neurosci 37 (2017) 8330–8348, 10.1523/JNEUROSCI.3141-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Robinson MJF, Warlow SM, Berridge KC, Optogenetic excitation of central amygdala amplifies and narrows incentive motivation to pursue one reward above another, J. Neurosci 34 (2014) 16567–16580, 10.1523/JNEUROSCI.2013-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Baumgartner HM, Schulkin J, Berridge KC, Activating Corticotropin-Releasing Factor Systems in the Nucleus Accumbens, Amygdala, and Bed Nucleus of Stria Terminalis: Incentive Motivation or Aversive Motivation? Biol. Psychiatry (2021) 10.1016/j.biopsych.2021.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Sah P, Faber ESL, Lopez De Armentia M, Power J, The amygdaloid complex: anatomy and physiology, Physiol. Rev 83 (2003) 803–834, 10.1152/physrev.00002.2003. [DOI] [PubMed] [Google Scholar]
- [20].McDonald AJ, Neurons of the lateral and basolateral amygdaloid nuclei: a Golgi study in the rat, J. Comp. Neurol 212 (1982) 293–312, 10.1002/cne.902120307. [DOI] [PubMed] [Google Scholar]
- [21].Cassell MD, Gray TS, Kiss JZ, Neuronal architecture in the rat central nucleus of the amygdala: a cytological, hodological, and immunocytochemical study, J. Comp. Neurol 246 (1986) 478–499, 10.1002/cne.902460406. [DOI] [PubMed] [Google Scholar]
- [22].Millhouse OE, The intercalated cells of the amygdala, J. Comp. Neurol 247 (1986) 246–271, 10.1002/cne.902470209. [DOI] [PubMed] [Google Scholar]
- [23].Jolkkonen E, Pitk A änen, Intrinsic connections of the rat amygdaloid complex: projections originating in the central nucleus, J. Comp. Neurol 395 (1998) 53–72, . [DOI] [PubMed] [Google Scholar]
- [24].Pape H-C, Pare D, Plastic synaptic networks of the amygdala for the acquisition, expression, and extinction of conditioned fear, Physiol. Rev 90 (2010) 419–463, 10.1152/physrev.00037.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Paŕe D, Quirk GJ, Ledoux JE, New vistas on amygdala networks in conditioned fear, J. Neurophysiol 92 (2004) 1–9, 10.1152/jn.00153.2004. [DOI] [PubMed] [Google Scholar]
- [26].Namburi P, Beyeler A, Yorozu S, Calhoon GG, Halbert SA, Wichmann R, et al. , A circuit mechanism for differentiating positive and negative associations, Nature 520 (2015) 675–678, 10.1038/nature14366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Bernard JF, Alden M, Besson JM, The organization of the efferent projections from the pontine parabrachial area to the amygdaloid complex: a Phaseolus vulgaris leucoagglutinin (PHA-L) study in the rat, J. Comp. Neurol 329 (1993) 201–229, 10.1002/cne.903290205. [DOI] [PubMed] [Google Scholar]
- [29].Allen GV, Saper CB, Hurley KM, Cechetto DF, Organization of visceral and limbic connections in the insular cortex of the rat, J. Comp. Neurol 311 (1991) 1–16, 10.1002/cne.903110102. [DOI] [PubMed] [Google Scholar]
- [30].Ju A, Fernandez-Arroyo B, Wu Y, Jacky D, Beyeler A, Expression of serotonin 1A and 2A receptors in molecular- and projection-defined neurons of the mouse insular cortex, Mol. Brain 13 (2020) 99, 10.1186/s13041-020-00605-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Taylor SR, Badurek S, Dileone RJ, Nashmi R, Minichiello L, Picciotto MR, GABAergic and glutamatergic efferents of the mouse ventral tegmental area, J. Comp. Neurol 522 (2014) 3308–3334, 10.1002/cne.23603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Zhou Z, Liu X, Chen S, Zhang Z, Liu Y, Montardy Q, et al. , A VTA gabaergic neural circuit mediates visually evoked innate defensive responses, Neuron 103 (2019) 473–488, 10.1016/j.neuron.2019.05.027,e6. [DOI] [PubMed] [Google Scholar]
- [33].Avegno EM, Kasten CR, Snyder WB, Kelley LK, Lobell TD, Templeton TJ, et al. , Alcohol dependence activates ventral tegmental area projections to central amygdala in male mice and rats, Addict. Biol (2020), e12990, 10.1111/adb.12990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Krettek JE, Price JL, Amygdaloid projections to subcortical structures within the basal forebrain and brainstem in the rat and cat, J. Comp. Neurol 178 (1978) 225–254, 10.1002/cne.901780204. [DOI] [PubMed] [Google Scholar]
- [35].Swanson LW, Anatomy of the soul as reflected in the cerebral hemispheres: neural circuits underlying voluntary control of basic motivated behaviors, J. Comp. Neurol 493 (2005) 122–131, 10.1002/cne.20733. [DOI] [PubMed] [Google Scholar]
- [36].Alheid GF, Heimer L, New perspectives in basal forebrain organization of special relevance for neuropsychiatric disorders: the striatopallidal, amygdaloid, and corticopetal components of substantia innominata, Neuroscience 27 (1988) 1–39, 10.1016/0306-4522(88)90217-5. [DOI] [PubMed] [Google Scholar]
- [37].Zahm DS, The evolving theory of basal forebrain functional-anatomical “macrosystems”, Neurosci. Biobehav. Rev 30 (2006) 148–172, 10.1016/j.neubiorev.2005.06.003. [DOI] [PubMed] [Google Scholar]
- [38].Puelles L, Thoughts on the development, structure and evolution of the mammalian and avian telencephalic pallium, Philos. Trans. R. Soc. Lond., B, Biol. Sci 356 (2001) 1583–1598, 10.1098/rstb.2001.0973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Swanson LW, Petrovich GD, What is the amygdala? Trends Neurosci. 21 (1998) 323–331, 10.1016/s0166-2236(98)01265-x. [DOI] [PubMed] [Google Scholar]
- [40].Kim J, Zhang X, Muralidhar S, LeBlanc SA, Tonegawa S, Basolateral to central amygdala neural circuits for appetitive behaviors, Neuron 93 (2017) 1464–1479, 10.1016/j.neuron.2017.02.034,e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].van der Kooy D, Koda LY, McGinty JF, Gerfen CR, Bloom FE, The organization of projections from the cortex, amygdala, and hypothalamus to the nucleus of the solitary tract in rat, J. Comp. Neurol 224 (1984) 1–24, 10.1002/cne.902240102. [DOI] [PubMed] [Google Scholar]
- [42].Baumgartner HM, Cole SL, Olney JJ, Berridge KC, Desire or Dread from Nucleus Accumbens Inhibitions: Reversed by Same-Site Optogenetic Excitations, J. Neurosci 40 (2020) 2737–2752, 10.1523/JNEUROSCI.2902-19.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Richard JM, Berridge KC, Nucleus accumbens dopamine/glutamate interaction switches modes to generate desire versus dread: d(1) alone for appetitive eating but D(1) and D(2) together for fear, J. Neurosci 31 (2011) 12866–12879, 10.1523/JNEUROSCI.1339-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].DiFeliceantonio AG, Berridge KC, Dorsolateral neostriatum contribution to incentive salience: opioid or dopamine stimulation makes one reward cue more motivationally attractive than another, Eur. J. Neurosci 43 (2016) 1203–1218, 10.1111/ejn.13220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Pomrenze MB, Millan EZ, Hopf FW, Keiflin R, Maiya R, Blasio A, et al. , A transgenic rat for investigating the anatomy and function of corticotrophin releasing factor circuits, Front. Neurosci 9 (2015) 487, 10.3389/fnins.2015.00487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].McCullough KM, Morrison FG, Hartmann J, Carlezon WA, Ressler KJ, Quantified coexpression analysis of central amygdala subpopulations, Eneuro (2018) 5, 10.1523/ENEURO.0010-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Hu B, Boyle CA, Lei S, Oxytocin receptors excite lateral nucleus of central amygdala by phospholipase Cβ- and protein kinase C-dependent depression of inwardly rectifying K+ channels, J Physiol (Lond) 598 (2020) 3501–3520, 10.1113/JP279457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Wilson TD, Valdivia S, Khan A, Ahn H-S, Adke AP, Martinez Gonzalez S, et al. , Dual and opposing functions of the central amygdala in the modulation of pain, Cell Rep. 29 (2019) 332–346, 10.1016/j.celrep.2019.09.011,e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Kim JY, Yang SH, Kwon J, Lee HW, Kim H, Mice subjected to uncontrollable electric shocks show depression-like behaviors irrespective of their state of helplessness, Behav. Brain Res 322 (2017) 138–144, 10.1016/j.bbr.2017.01.008. [DOI] [PubMed] [Google Scholar]
- [50].Venniro M, Russell TI, Ramsey LA, Richie CT, Lesscher HMB, Giovanetti SM, et al. , Abstinence-dependent dissociable central amygdala microcircuits control drug craving, Proc. Natl. Acad. Sci. U.S.A 117 (2020) 8126–8134, 10.1073/pnas.2001615117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Haubensak W, Kunwar PS, Cai H, Ciocchi S, Wall NR, Ponnusamy R, et al. , Genetic dissection of an amygdala microcircuit that gates conditioned fear, Nature 468 (2010) 270–276, 10.1038/nature09553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Berridge KC, Affective valence in the brain: modules or modes? Nat. Rev. Neurosci 20 (2019) 225–234, 10.1038/s41583-019-0122-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Venniro M, Caprioli D, Zhang M, Whitaker LR, Zhang S, Warren BL, et al. , The anterior insular cortex→Central amygdala glutamatergic pathway is critical to relapse after contingency management, Neuron 96 (2017) 414–427, 10.1016/j.neuron.2017.09.024,e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Abtahi S, Rodberg EM, Berridge KC, Comparing Reward Roles of D1 Neurons Vs D2 Neurons in Accumbens and Amygdala, 2019. [Google Scholar]
- [55].Bindra D, How adaptive behavior is produced: a perceptual-motivational alternative to response reinforcements, Behav. Brain Sci 1 (1978) 41, 10.1017/S0140525X00059380. [DOI] [Google Scholar]
- [56].Berridge KC, Evolving concepts of emotion and motivation, Front. Psychol 9 (2018) 1647, 10.3389/fpsyg.2018.01647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Robinson TE, Flagel SB, Dissociating the predictive and incentive motivational properties of reward-related cues through the study of individual differences, Biol. Psychiatry 65 (2009) 869–873, 10.1016/j.biopsych.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Metrik J, Aston ER, Kahler CW, Rohsenow DJ, McGeary JE, Knopik VS, et al. , Cue-elicited increases in incentive salience for marijuana: craving, demand, and attentional bias, Drug Alcohol Depend. 167 (2016) 82–88, 10.1016/j.drugalcdep.2016.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Venniro M, Caprioli D, Shaham Y, Animal models of drug relapse and craving: from drug priming-induced reinstatement to incubation of craving after voluntary abstinence, Prog. Brain Res 224 (2016) 25–52, 10.1016/bs.pbr.2015.08.004. [DOI] [PubMed] [Google Scholar]
- [60].LeBlanc KH, Ostlund SB, Maidment NT, Pavlovian-to-instrumental transfer in cocaine seeking rats, Behav. Neurosci 126 (2012) 681–689, 10.1037/a0029534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Mahler SV, Berridge KC, What and when to “want”? Amygdala-based focusing of incentive salience upon sugar and sex, Psychopharmacology 221 (2012) 407–426, 10.1007/s00213-011-2588-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Jenkins HM, Moore BR, The form of the auto-shaped response with food or water reinforcers, J. Exp. Anal. Behav 20 (1973) 163–181, 10.1901/jeab.1973.20-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Kearns DN, Weiss SJ, Sign-tracking (autoshaping) in rats: a comparison of cocaine and food as unconditioned stimuli, Learn. Behav 32 (2004) 463–476. [DOI] [PubMed] [Google Scholar]
- [64].Rosse RB, Fay-McCarthy M, Collins JP, Risher-Flowers D, Alim TN, Deutsch SI, Transient compulsive foraging behavior associated with crack cocaine use, Am. J. Psychiatry 150 (1993) 155–156, 10.1176/ajp.150.1.155. [DOI] [PubMed] [Google Scholar]
- [65].Parkinson JA, Olmstead MC, Burns LH, Robbins TW, Everitt BJ, Dissociation in effects of lesions of the nucleus accumbens core and shell on appetitive pavlovian approach behavior and the potentiation of conditioned reinforcement and locomotor activity by D-amphetamine, J. Neurosci 19 (1999) 2401–2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Wolterink G, Phillips G, Cador M, Donselaar-Wolterink I, Robbins TW, Everitt BJ, Relative roles of ventral striatal D1 and D2 dopamine receptors in responding with conditioned reinforcement, Psychopharmacology 110 (1993) 355–364, 10.1007/BF02251293. [DOI] [PubMed] [Google Scholar]
- [67].Cabanac M, Pleasure: the common currency, J. Theor. Biol 155 (1992) 173–200, 10.1016/s0022-5193(05)80594-6. [DOI] [PubMed] [Google Scholar]
- [68].Berridge KC, From prediction error to incentive salience: mesolimbic computation of reward motivation, Eur. J. Neurosci 35 (2012) 1124–1143, 10.1111/j.1460-9568.2012.07990.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Zhang J, Berridge KC, Tindell AJ, Smith KS, Aldridge JW, A neural computational model of incentive salience, PLoS Comput. Biol 5 (2009), e1000437, 10.1371/journal.pcbi.1000437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Kim E-M, Quinn JG, Levine AS, O’Hare E, A bi-directional mu-opioid-opioid connection between the nucleus of the accumbens shell and the central nucleus of the amygdala in the rat, Brain Res. 1029 (2004) 135–139, 10.1016/j.brainres.2004.10.001. [DOI] [PubMed] [Google Scholar]
- [71].Gosnell BA, Involvement of mu opioid receptors in the amygdala in the control of feeding, Neuropharmacology 27 (1988) 319–326, 10.1016/0028-3908(88)90050-0. [DOI] [PubMed] [Google Scholar]
- [72].Will MJ, Franzblau EB, Kelley AE, The amygdala is critical for opioid-mediated binge eating of fat, Neuroreport 15 (2004) 1857–1860, 10.1097/00001756-200408260-00004. [DOI] [PubMed] [Google Scholar]
- [73].Cai H, Haubensak W, Anthony TE, Anderson DJ, Central amygdala PKC-δ(+) neurons mediate the influence of multiple anorexigenic signals, Nat. Neurosci 17 (2014) 1240–1248, 10.1038/nn.3767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Blaesse P, Goedecke L, Bazelot M, Capogna M, Pape H-C, Jüngling K, μ-Opioid Receptor-Mediated Inhibition of Intercalated Neurons and Effect on Synaptic Transmission to the Central Amygdala, J. Neurosci 35 (2015) 7317–7325, 10.1523/JNEUROSCI.0204-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Torruella-Súarez ML, Vandenberg JR, Cogan ES, Tipton GJ, Teklezghi A, Dange K, et al. , Manipulations of central amygdala neurotensin neurons alter the consumption of ethanol and sweet fluids in mice, J. Neurosci 40 (2020) 632–647, 10.1523/JNEUROSCI.1466-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Douglass AM, Kucukdereli H, Ponserre M, Markovic M, Gründemann J, Strobel C, et al. , Central amygdala circuits modulate food consumption through a positive-valence mechanism, Nat. Neurosci 20 (2017) 1384–1394, 10.1038/nn.4623. [DOI] [PubMed] [Google Scholar]
- [77].Galaverna OG, Seeley RJ, Berridge KC, Grill HJ, Epstein AN, Schulkin J, Lesions of the central nucleus of the amygdala. I: Effects on taste reactivity, taste aversion learning and sodium appetite, Behav. Brain Res 59 (1993) 11–17, 10.1016/0166-4328(93)90146-h. [DOI] [PubMed] [Google Scholar]
- [79].Childress AR, Ehrman RN, Wang Z, Li Y, Sciortino N, Hakun J, et al. , Prelude to passion: limbic activation by “unseen” drug and sexual cues, PLoS One 3 (2008) e1506, 10.1371/journal.pone.0001506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Tang DW, Fellows LK, Small DM, Dagher A, Food and drug cues activate similar brain regions: a meta-analysis of functional MRI studies, Physiol. Behav 106 (2012) 317–324, 10.1016/j.physbeh.2012.03.009. [DOI] [PubMed] [Google Scholar]
- [81].Li X, Zeric T, Kambhampati S, Bossert JM, Shaham Y, The central amygdala nucleus is critical for incubation of methamphetamine craving, Neuropsychopharmacology 40 (2015) 1297–1306, 10.1038/npp.2014.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Lu L, Hope BT, Dempsey J, Liu SY, Bossert JM, Shaham Y, Central amygdala ERK signaling pathway is critical to incubation of cocaine craving, Nat. Neurosci 8 (2005) 212–219, 10.1038/nn1383. [DOI] [PubMed] [Google Scholar]
- [84].Tom RL, Ahuja A, Maniates H, Freeland CM, Robinson MJF, Optogenetic activation of the central amygdala generates addiction-like preference for reward, Eur. J. Neurosci 50 (2019) 2086–2100, 10.1111/ejn.13967. [DOI] [PubMed] [Google Scholar]
- [85].Ahmed SH, Lenoir M, Guillem K, Neurobiology of addiction versus drug use driven by lack of choice, Curr. Opin. Neurobiol 23 (2013) 581–587, 10.1016/j.conb.2013.01.028. [DOI] [PubMed] [Google Scholar]
- [86].Vanderschuren LJMJ, Everitt BJ, Behavioral and neural mechanisms of compulsive drug seeking, Eur. J. Pharmacol 526 (2005) 77–88, 10.1016/j.ejphar.2005.09.037. [DOI] [PubMed] [Google Scholar]
- [87].De Boer SF, Koolhaas JM, Defensive burying in rodents: ethology, neurobiology and psychopharmacology, Eur. J. Pharmacol 463 (2003) 145–161, 10.1016/s0014-2999(03)01278-0. [DOI] [PubMed] [Google Scholar]
- [88].Treit D, Pesold C, Rotzinger S, Noninteractive effects of diazepam and amygdaloid lesions in two animal models of anxiety, Behav. Neurosci 107 (1993) 1099–1105. [DOI] [PubMed] [Google Scholar]
- [89].Pascoli V, Terrier J, Hiver A, Lüscher C, Sufficiency of mesolimbic dopamine neuron stimulation for the progression to addiction, Neuron 88 (2015) 1054–1066, 10.1016/j.neuron.2015.10.017. [DOI] [PubMed] [Google Scholar]
- [90].Seo D-O, Funderburk SC, Bhatti DL, Motard LE, Newbold D, Girven KS, et al. , A GABAergic projection from the centromedial nuclei of the amygdala to ventromedial prefrontal cortex modulates reward behavior, J. Neurosci 36 (2016) 10831–10842, 10.1523/JNEUROSCI.1164-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Servonnet A, Hernandez G, Hage CE, Rompŕe P-P, Samaha A-N, Optogenetic activation of the basolateral amygdala promotes both appetitive conditioning and the instrumental pursuit of reward cues, J. Neurosci (2020), 10.1523/JNEUROSCI.2196-19.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Steinberg EE, Gore F, Heifets BD, Taylor MD, Norville ZC, Beier KT, et al. , Amygdala-midbrain connections modulate appetitive and aversive learning, Neuron 106 (2020) 1026–1043, 10.1016/j.neuron.2020.03.016,e9. [DOI] [PubMed] [Google Scholar]
- [93].Koob GF, Addiction is a reward deficit and stress surfeit disorder, Front. Psychiatry 4 (2013) 72, 10.3389/fpsyt.2013.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Solomon RL, Corbit JD, An opponent-process theory of motivation. I. Temporal dynamics of affect, Psychol. Rev 81 (1974) 119–145, 10.1037/h0036128. [DOI] [PubMed] [Google Scholar]
- [95].Berridge KC, Robinson TE, Aldridge JW, Dissecting components of reward:’ liking’,’ wanting’, and learning, Curr. Opin. Pharmacol 9 (2009) 65–73, 10.1016/j.coph.2008.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Berridge KC, Robinson TE, Parsing reward, Trends Neurosci 26 (2003) 507–513, 10.1016/S0166-2236(03)00233-9. [DOI] [PubMed] [Google Scholar]
- [97].Fischman MW, Foltin RW, Self-administration of cocaine by humans: a laboratory perspective, Ciba Found. Symp 166 (1992) 165–173, discussion 173. [DOI] [PubMed] [Google Scholar]
- [98].Winkielman P, Gogolushko Y, Influence of suboptimally and optimally presented affective pictures and words on consumption-related behavior, Front. Psychol 8 (2017) 2261, 10.3389/fpsyg.2017.02261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Robinson TE, Berridge KC, The neural basis of drug craving: an incentive-sensitization theory of addiction, Brain Res. Brain Res. Rev 18 (1993) 247–291, 10.1016/0165-0173(93)90013-P. [DOI] [PubMed] [Google Scholar]
- [100].Berridge KC, Robinson TE, Liking, wanting, and the incentive-sensitization theory of addiction, Am. Psychol 71 (2016) 670–679, 10.1037/amp0000059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Berridge KC, Measuring hedonic impact in animals and infants: microstructure of affective taste reactivity patterns, Neurosci. Biobehav. Rev 24 (2000) 173–198, 10.1016/s0149-7634(99)00072-x. [DOI] [PubMed] [Google Scholar]
- [102].Grill HJ, Norgren R, The taste reactivity test. I. Mimetic responses to gustatory stimuli in neurologically normal rats, Brain Res. 143 (1978) 263–279, 10.1016/0006-8993(78)90568-1. [DOI] [PubMed] [Google Scholar]
- [103].Steiner JE, The gustofacial response: observation on normal and anencephalic newborn infants, Symp. Oral Sens. Percept (1973) 254–278. [PubMed] [Google Scholar]
- [104].Cabanac M, Physiological role of pleasure, Science 173 (1971) 1103–1107, 10.1126/science.173.4002.1103. [DOI] [PubMed] [Google Scholar]
- [105].Garcia J, Lasiter PS, Bermudez-Rattoni F, Deems DA, A general theory of aversion learning, Ann. N. Y. Acad. Sci 443 (1985) 8–21, 10.1111/j.1749-6632.1985.tb27060.x. [DOI] [PubMed] [Google Scholar]
- [106].Castro DC, Berridge KC, Opioid hedonic hotspot in nucleus accumbens shell: mu, delta, and kappa maps for enhancement of sweetness “liking” and “wanting”, J. Neurosci 34 (2014) 4239–4250, 10.1523/JNEUROSCI.4458-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Peciña S, Berridge KC, Hedonic hot spot in nucleus accumbens shell: where do mu-opioids cause increased hedonic impact of sweetness? J. Neurosci 25 (2005) 11777–11786, 10.1523/JNEUROSCI.2329-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Wyvell CL, Berridge KC, Intra-accumbens amphetamine increases the conditioned incentive salience of sucrose reward: enhancement of reward “wanting” without enhanced “liking” or response reinforcement, J. Neurosci 20 (2000) 8122–8130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Mahler SV, Smith KS, Berridge KC, Endocannabinoid hedonic hotspot for sensory pleasure: anandamide in nucleus accumbens shell enhances “liking” of a sweet reward, Neuropsychopharmacology 32 (2007) 2267–2278, 10.1038/sj.npp.1301376. [DOI] [PubMed] [Google Scholar]
- [110].Berridge KC, Robinson TE, What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res. Brain Res. Rev 28 (1998) 309–369, 10.1016/S0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- [111].Peciña S, Cagniard B, Berridge KC, Aldridge JW, Zhuang X, Hyperdopaminergic mutant mice have higher “wanting” but not “liking” for sweet rewards, J. Neurosci 23 (2003) 9395–9402, 10.1523/JNEUROSCI.23-28-09395.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Berridge KC, Venier IL, Robinson TE, Taste reactivity analysis of 6-hydroxy-dopamine-induced aphagia: implications for arousal and anhedonia hypotheses of dopamine function, Behav. Neurosci 103 (1989) 36–45, 10.1037/0735-7044.103.1.36. [DOI] [PubMed] [Google Scholar]
- [113].Norgren R, Taste pathways to hypothalamus and amygdala, J. Comp. Neurol 166 (1976) 17–30, 10.1002/cne.901660103. [DOI] [PubMed] [Google Scholar]
- [114].Schiff HC, Bouhuis AL, Yu K, Penzo MA, Li H, He M, et al. , An insula-central amygdala circuit for guiding tastant-reinforced choice behavior, J. Neurosci 38 (2018) 1418–1429, 10.1523/JNEUROSCI.1773-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Riley CA, King MS, Differential effects of electrical stimulation of the central amygdala and lateral hypothalamus on fos-immunoreactive neurons in the gustatory brainstem and taste reactivity behaviors in conscious rats, Chem. Senses 38 (2013) 705–717, 10.1093/chemse/bjt039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Ross SE, Lehmann Levin E, Itoga CA, Schoen CB, Selmane R, Aldridge JW, Deep brain stimulation in the central nucleus of the amygdala decreases “wanting” and “liking” of food rewards, Eur. J. Neurosci 44 (2016) 2431–2445, 10.1111/ejn.13342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].King CT, Garcea M, Spector AC, Restoration of quinine-stimulated Fos-immunoreactive neurons in the central nucleus of the amygdala and gustatory cortex following reinnervation or cross-reinnervation of the lingual taste nerves in rats, J. Comp. Neurol 522 (2014) 2498–2517, 10.1002/cne.23546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Warlow SM, Castro D, Naffziger E, Berridge KC, Central Amygdala Controls Choice and Amplifies “wanting” for Sucrose and Cocaine Without Altering ‘liking’, 2016. [Google Scholar]
- [119].Hickey C, Peelen MV, Neural mechanisms of incentive salience in naturalistic human vision, Neuron 85 (2015) 512–518, 10.1016/j.neuron.2014.12.049. [DOI] [PubMed] [Google Scholar]
- [120].Nummenmaa L, Saanijoki T, Tuominen L, Hirvonen J, Tuulari JJ, Nuutila P, et al. , μ-opioid receptor system mediates reward processing in humans, Nat. Commun 9 (2018) 1500, 10.1038/s41467-018-03848-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Gottfried JA, O’Doherty J, Dolan RJ, Encoding predictive reward value in human amygdala and orbitofrontal cortex, Science 301 (2003) 1104–1107, 10.1126/science.1087919. [DOI] [PubMed] [Google Scholar]
- [122].Kühn S, Gallinat J, Common biology of craving across legal and illegal drugs - a quantitative meta-analysis of cue-reactivity brain response, Eur. J. Neurosci 33 (2011) 1318–1326, 10.1111/j.1460-9568.2010.07590.x. [DOI] [PubMed] [Google Scholar]
- [123].Brown PL, Jenkins HM, Auto-shaping of the pigeon’s key-peck, J. Exp. Anal. Behav 11 (1968) 1–8, 10.1901/jeab.1968.11-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Uslaner JM, Acerbo MJ, Jones SA, Robinson TE, The attribution of incentive salience to a stimulus that signals an intravenous injection of cocaine, Behav. Brain Res 169 (2006) 320–324, 10.1016/j.bbr.2006.02.001. [DOI] [PubMed] [Google Scholar]
- [125].Tomie A, Grimes KL, Pohorecky LA, Behavioral characteristics and neurobiological substrates shared by Pavlovian sign-tracking and drug abuse, Brain Res. Rev 58 (2008) 121–135, 10.1016/j.brainresrev.2007.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Parkinson JA, Robbins TW, Everitt BJ, Dissociable roles of the central and basolateral amygdala in appetitive emotional learning, Eur. J. Neurosci 12 (2000) 405–413, 10.1046/j.1460-9568.2000.00960.x. [DOI] [PubMed] [Google Scholar]
- [127].Cardinal RN, Parkinson JA, Lachenal G, Halkerston KM, Rudarakanchana N, Hall J, et al. , Effects of selective excitotoxic lesions of the nucleus accumbens core, anterior cingulate cortex, and central nucleus of the amygdala on autoshaping performance in rats, Behav. Neurosci 116 (2002) 553–567, 10.1037/0735-7044.116.4.553. [DOI] [PubMed] [Google Scholar]
- [128].Gallagher M, Graham PW, Holland PC, The amygdala central nucleus and appetitive Pavlovian conditioning: lesions impair one class of conditioned behavior, J. Neurosci 10 (1990) 1906–1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].El-Amamy H, Holland PC, Dissociable effects of disconnecting amygdala central nucleus from the ventral tegmental area or substantia nigra on learned orienting and incentive motivation, Eur. J. Neurosci 25 (2007) 1557–1567, 10.1111/j.1460-9568.2007.05402.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Lee HJ, Wheeler DS, Holland PC, Interactions between amygdala central nucleus and the ventral tegmental area in the acquisition of conditioned cue-directed behavior in rats, Eur. J. Neurosci 33 (2011) 1876–1884, 10.1111/j.1460-9568.2011.07680.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Mahler SV, Berridge KC, Which cue to “want?” Central amygdala opioid activation enhances and focuses incentive salience on a prepotent reward cue, J. Neurosci 29 (2009) 6500–6513, 10.1523/JNEUROSCI.3875-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].DiFeliceantonio AG, Berridge KC, Which cue to “want”? Opioid stimulation of central amygdala makes goal-trackers show stronger goal-tracking, just as sign-trackers show stronger sign-tracking, Behav. Brain Res 230 (2012) 399–408, 10.1016/j.bbr.2012.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Corbit LH, Balleine BW, Double dissociation of basolateral and central amygdala lesions on the general and outcome-specific forms of pavlovian-instrumental transfer, J. Neurosci 25 (2005) 962–970, 10.1523/JNEUROSCI.4507-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Chang SE, Wheeler DS, Holland PC, Effects of lesions of the amygdala central nucleus on autoshaped lever pressing, Brain Res. 1450 (2012) 49–56, 10.1016/j.brainres.2012.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Holland PC, Gallagher M, Double dissociation of the effects of lesions of basolateral and central amygdala on conditioned stimulus-potentiated feeding and Pavlovian-instrumental transfer, Eur. J. Neurosci 17 (2003) 1680–1694, 10.1046/j.1460-9568.2003.02585.x. [DOI] [PubMed] [Google Scholar]
- [136].Hall J, Parkinson JA, Connor TM, Dickinson A, Everitt BJ, Involvement of the central nucleus of the amygdala and nucleus accumbens core in mediating Pavlovian influences on instrumental behaviour, Eur. J. Neurosci 13 (2001) 1984–1992, 10.1046/j.0953-816x.2001.01577.x. [DOI] [PubMed] [Google Scholar]
- [137].Li Y-Q, Li Y-Q, Wang X-Y, Wu P, Zhao M, Xu C-M, et al. , Central amygdala extracellular signal-regulated kinase signaling pathway is critical to incubation of opiate craving, J. Neurosci 28 (2008) 13248–13257, 10.1523/JNEUROSCI.3027-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Robinson TE, Yager LM, Cogan ES, Saunders BT, On the motivational properties of reward cues: individual differences, Neuropharmacology 76 (Pt B) (2014) 450–459, 10.1016/j.neuropharm.2013.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Timberlake W, Grant DL, Auto-shaping in rats to the presentation of another rat predicting food, Science 190 (1975) 690–692, 10.1126/science.190.4215.690. [DOI] [Google Scholar]
- [140].Ciocchi S, Herry C, Grenier F, Wolff SBE, Letzkus JJ, Vlachos I, et al. , Encoding of conditioned fear in central amygdala inhibitory circuits, Nature 468 (2010) 277–282, 10.1038/nature09559. [DOI] [PubMed] [Google Scholar]
- [141].Zimmerman JM, Rabinak CA, McLachlan IG, Maren S, The central nucleus of the amygdala is essential for acquiring and expressing conditional fear after overtraining, Learn. Mem 14 (2007) 634–644, 10.1101/lm.607207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].LeDoux J, The amygdala, Curr. Biol 17 (2007) R868–74, 10.1016/j.cub.2007.08.005. [DOI] [PubMed] [Google Scholar]
- [143].Berridge KC, Kringelbach ML, Pleasure systems in the brain, Neuron 86 (2015) 646–664, 10.1016/j.neuron.2015.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Olney JJ, Warlow SM, Naffziger EE, Berridge KC, Current perspectives on incentive salience and applications to clinical disorders, Curr. Opin. Behav. Sci 22 (2018) 59–69, 10.1016/j.cobeha.2018.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Morrow JD, Saunders BT, Maren S, Robinson TE, Sign-tracking to an appetitive cue predicts incubation of conditioned fear in rats, Behav. Brain Res 276 (2015) 59–66, 10.1016/j.bbr.2014.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Richard JM, Berridge KC, Metabotropic glutamate receptor blockade in nucleus accumbens shell shifts affective valence towards fear and disgust, Eur. J. Neurosci 33 (2011) 736–747, 10.1111/j.1460-9568.2010.07553.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Richard JM, Plawecki AM, Berridge KC, Nucleus accumbens GABAergic inhibition generates intense eating and fear that resists environmental retuning and needs no local dopamine, Eur. J. Neurosci 37 (2013) 1789–1802, 10.1111/ejn.12194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Fanselow MS, Conditioned and unconditional components of post-shock freezing, Pavlov. J. Biol. Sci 15 (1980) 177–182, 10.1007/BF03001163. [DOI] [PubMed] [Google Scholar]
- [149].Blanchard DC, Griebel G, Nutt DJ (Eds.), Handbook of Anxiety and Fear, Elsevier, 2011. [Google Scholar]
- [150].Nagy H, Levy-Gigi E, Somlai Z, Takáts A, Bereczki D, Kéri S, The effect of dopamine agonists on adaptive and aberrant salience in Parkinson’s disease, Neuropsychopharmacology 37 (2012) 950–958, 10.1038/npp.2011.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Kapur S, Psychosis as a state of aberrant salience: a framework linking biology, phenomenology, and pharmacology in schizophrenia, Am. J. Psychiatry 160 (2003) 13–23, 10.1176/appi.ajp.160.1.13. [DOI] [PubMed] [Google Scholar]
- [152].Shabel SJ, Janak PH, Substantial similarity in amygdala neuronal activity during conditioned appetitive and aversive emotional arousal, Proc. Natl. Acad. Sci. U.S.A 106 (2009) 15031–15036, 10.1073/pnas.0905580106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Peck CJ, Lau B, Salzman CD, The primate amygdala combines information about space and value, Nat. Neurosci 16 (2013) 340–348, 10.1038/nn.3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Fernando ABP, Murray JE, Milton AL, The amygdala: securing pleasure and avoiding pain, Front. Behav. Neurosci 7 (2013) 190, 10.3389/fnbeh.2013.00190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Faure A, Reynolds SM, Richard JM, Berridge KC, Mesolimbic dopamine in desire and dread: enabling motivation to be generated by localized glutamate disruptions in nucleus accumbens, J. Neurosci 28 (2008) 7184–7192, 10.1523/JNEUROSCI.4961-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Reynolds SM, Berridge KC, Fear and feeding in the nucleus accumbens shell: rostrocaudal segregation of GABA-elicited defensive behavior versus eating behavior, J. Neurosci 21 (2001) 3261–3270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Richard JM, Berridge KC, Prefrontal cortex modulates desire and dread generated by nucleus accumbens glutamate disruption, Biol. Psychiatry 73 (2013) 360–370, 10.1016/j.biopsych.2012.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Reynolds SM, Berridge KC, Emotional environments retune the valence of appetitive versus fearful functions in nucleus accumbens, Nat. Neurosci 11 (2008) 423–425, 10.1038/nn2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Fadok JP, Krabbe S, Markovic M, Courtin J, Xu C, Massi L, et al. , A competitive inhibitory circuit for selection of active and passive fear responses, Nature 542 (2017) 96–100, 10.1038/nature21047. [DOI] [PubMed] [Google Scholar]
- [160].Fadok JP, Markovic M, Tovote P, Lüthi A, New perspectives on central amygdala function, Curr. Opin. Neurobiol 49 (2018) 141–147, 10.1016/j.conb.2018.02.009. [DOI] [PubMed] [Google Scholar]
- [161].Sinha R, The clinical neurobiology of drug craving, Curr. Opin. Neurobiol 23 (2013) 649–654, 10.1016/j.conb.2013.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Murphy A, Lubman DI, McKie S, Bijral PS, Peters LA, Faiz Q, et al. , Time-dependent neuronal changes associated with craving in opioid dependence: an fMRI study, Addict. Biol 23 (2018) 1168–1178, 10.1111/adb.12554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Logge WB, Baillie AJ, Haber PS, Morley KC, Baclofen modulates cardiovascular responses to appetitive cues in treatment-seeking alcohol use disorder individuals, Hum. Psychopharmacol 35 (2020) e2722, 10.1002/hup.2722. [DOI] [PubMed] [Google Scholar]
- [164].Machielsen MWJ, Veltman DJ, van den Brink W, de Haan L, Comparing the effect of clozapine and risperidone on cue reactivity in male patients with schizophrenia and a cannabis use disorder: A randomized fMRI study, Schizophr. Res 194 (2018) 32–38, 10.1016/j.schres.2017.03.030. [DOI] [PubMed] [Google Scholar]
- [165].Berghorst LH, Kumar P, Greve DN, Deckersbach T, Ongur D, Dutra SJ, et al. , Stress and reward processing in bipolar disorder: a functional magnetic resonance imaging study, Bipolar Disord. 18 (2016) 602–611, 10.1111/bdi.12444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Wang Q, Ding S-L, Li Y, Royall J, Feng D, Lesnar P, et al. , The allen mouse brain common coordinate framework: a 3D reference atlas, Cell 181 (2020) 936–953, 10.1016/j.cell.2020.04.007e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Ding S-L, Royall JJ, Sunkin SM, Ng L, Facer BAC, Lesnar P, et al. , Comprehensive cellular-resolution atlas of the adult human brain, J. Comp. Neurol 524 (2016) 3127–3481, 10.1002/cne.24080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Paxinos G, Watson C, The Rat Brain in Stereotaxic Coordinates, 7th edition, Academic Press, 2013, 10.1016/C2009-0-63235-9. [DOI] [Google Scholar]


