Abstract
Individuals with autism spectrum disorder (ASD) and their first-degree relatives demonstrate automaticity deficits reflected in reduced eye-voice coordination during rapid automatized naming (RAN), suggesting that RAN deficits may be a genetically meaningful marker of ASD language-related impairments. This study investigated whether RAN deficits in ASD extend to a language typologically distinct from English. Participants included 23 Cantonese-speaking individuals with ASD and 39 controls from Hong Kong (HK), and age- and IQ-comparable groups of previously-studied English-speaking individuals with ASD (n = 45) and controls (n = 44) from the US. Participants completed RAN on an eye tracker. Analyses examined naming time, error rate, measures of eye movement reflecting language automaticity, including eye-voice span (EVS; location of eyes versus the named item) and refixations. The HK-ASD group exhibited longer naming times and more refixations than HK-Controls, in a pattern similar to that observed in the US-ASD group. Cultural effects revealed that both HK groups showed longer EVS and more fixations than US groups. Naming time and refixation differences may be ASD-specific impairments spanning cultures/languages, whereas EVS and fixation frequency may be more variably impacted. A potential underlying mechanism of visual “stickiness” may be contributing to this breakdown in language automaticity in ASD.
Subject terms: Autism spectrum disorders, Biomarkers, Cognitive neuroscience, Language
Introduction
Rapid automatized naming (RAN) involves serial naming of arrays composed of common symbols (numbers, letters) and non-symbols (colors, objects), with the objective of completing the task as quickly and accurately as possible. This simplicity of the RAN task belies the complexity of the different component skills underlying RAN, including core executive (e.g., working memory, processing speed, inhibitory control of attention)1, and linguistic skills (e.g., retrieval of lexical information, phonological processing and memory)2, as well as the ability to coordinate these processes. RAN is commonly used as an index of automaticity for reading, and has been identified as one of the strongest predictors of successful reading across multiple languages3. An extensive network of underlying neural correlates have been implicated in RAN ability (e.g., frontal cortex, temporo-parietal areas, dorsal posterior regions, inferior frontal cortex, and the ventral-visual pathway and cerebellum)4–7, which highly overlaps with the language-reading network. Not surprisingly, then, RAN has been studied as a measure of language fluency that taps basic executive and language skills underpinning higher-level language skills, such as reading3 and narrative8. Moreover, impairments in RAN performance have been documented in many language-related disorders, including autism spectrum disorder (ASD)8–10. Given that reading deficits are not typically observed in ASD (with subgroups of individuals actually exhibiting outstanding word reading ability11,12, repeated observations of RAN deficits in ASD appear to reflect differences in skills tapped by RAN but not specific to reading. Similar RAN-related differences have also been documented among unaffected relatives of individuals with ASD8,10, who too show subtle differences in complex communication (e.g., social language)13–16. For example, early family studies of individuals with ASD have shown links between literacy and language-based skills in their siblings and parents17. A recent study similarly revealed protracted development and lower performance in early literacy and language-based skills during childhood among parents who then went on to have a child (or children) with ASD as adults18. Twin studies have also demonstrated that RAN abilities are highly heritable19, highlighting RAN as a neurobiological marker of genetically influenced language and executive abilities20–24, that might be fruitfully studied to understand the basis of language-related impairments across different neurodevelopmental disorders.
Inefficient RAN performance, specifically, longer naming time and greater error rates, has been documented in individuals with ASD relative to typical controls8–10. RAN naming was also found to be associated with social communication skills and repetitive behaviors (RRBs) in ASD8,9, suggesting that the skills tapped by RAN performance (naming and errors) may importantly relate to clinical symptoms of ASD, including global executive and social-communicative impairments. Slower naming and greater error rates have also been observed among clinically unaffected first-degree relatives (both siblings and parents) of individuals with ASD8–10, pointing towards RAN as a marker of language-related mechanisms possibly influenced by ASD genetic liability.
Studies examining eye fixation patterns during RAN have begun to delineate more specific breakdowns along the processing stages involved in RAN, among both individuals with ASD and their first-degree relatives. For instance, eye voice span (EVS; the number of items ahead the eyes are in comparison to the voice at the onset of each vocal response) is thought to index the fluent coordination of pre-processing abilities (visual input in working memory) and the conversion of this visual input to phonological code and articulatory commands3,25,26, encompassing the influences of basic language processes (e.g., syntactic and semantic processing)27 and working memory28. Fixation frequency and the type of fixation (e.g., perseverations and regressions) also index important skills underlying language processing fluency, namely, local processing/attention. For example, regressions, or, the backward movement of the eyes to prior items during RAN, can indicate a failure to plan and organize in advance, resulting in need for self-corrections29–32. On the other hand, a greater number of perseverations is an indication of a perseverative or local attentional style (i.e., “sticky attention”)26,30. Prior work reported shorter EVS and a greater number of fixations and refixations (perseverations and regressions) among individuals with ASD and their first degree relatives8,9, providing an important indication of key mechanistic disruptions during RAN processing that can inform the language-related impairments in ASD, and that are likely influenced by ASD genetic risk. Additionally, perseverative and regressive eye movement patterns have been shown to relate to poorer narrative quality and increased sensorimotor behaviors in those with ASD8, suggesting a connection to ASD-related clinical symptoms. Together, findings demonstrate how careful examination of eye movement and speech during RAN might reveal inefficiencies or impairments in the basic mechanisms that provide the foundation for more complex, downstream language abilities such as narrative skill.
Previous studies exploring RAN performance in ASD have been restricted to English-speaking individuals in the United States (US)8–10, leaving unclear whether RAN differences may be present in speakers of languages that are typologically distinct from English language and culture, and whether RAN deficits represent a robust marker of impacted skills underlying the ASD language phenotype. For example, prior work in English- and Italian-speaking children with specific language impairments, showed that Italian speakers had greater control of phonological aspects of speech than did English speakers, highlighting the impact of phoneme-grapheme mapping differences that are more inherent to some languages than others33. Similarly, a review paper highlighted phonological processing and awareness differences in languages that differed in orthographical transparency (e.g., Greek/German versus English/French)34. Differences in linguistic features across languages can also result in deficits that are specific to speakers of those languages. For instance, Cantonese-speaking children with specific language impairment35 and Mandarin-speaking children with ASD36 display impairments in lexical tone perception that could not manifest in the same way in non-tone languages. In a more direct cross-language comparison, English-speaking children with dyslexia are known to have marked deficits in phonological awareness37 but morphological, rather than phonological awareness is a primary feature of dyslexia of Chinese38,39. Cross-linguistic comparisons are therefore critical in understanding potential language-specific profiles in ASD and other neurodevelopmental disorders, particularly across languages that are typologically distinct, and potentially imposing unique structural challenges.
Prior studies exploring RAN performance in typical development and dyslexia have demonstrated strongly consistent findings across languages. For instance, in both typically developing Chinese and English speakers, quicker RAN naming and longer EVS were found in symbolic (letters and digits) versus non-symbolic (colors and objects) conditions40–42. Additionally, children with dyslexia in both East Asian and Western cultures were shown to demonstrate similar patterns of differences from their respective control groups, with slower RAN naming and shorter EVS, and more extensive impairment emerging in the symbolic conditions42–44. RAN has also been widely studied across languages as a longitudinal and concurrent predictor of reading ability (particularly for predicting reading accuracy and reading fluency)45,46, with RAN-reading ability correlations of similar strength reported across languages that differ in orthography (e.g., English, Greek, Chinese)45.
Several studies have now demonstrated impairments in aspects of RAN performance—from slower naming to decreased eye-voice coordination and efficiency during naming—in ASD and among clinically unaffected relatives who are at increased genetic liability8–10, as well as in groups who carry a high confidence ASD risk gene47. An important next step in understanding the role of these language-related impairments in ASD risk is to document RAN performance in languages other than English. Although prior work has not investigated RAN in Chinese, a language that is inherently distinct from English, eye-tracking studies conducted in East Asian populations have tapped into similar deficits in RAN-related skills in ASD, with mixed evidence for a linguistic or cultural effect. For example, similar to patterns demonstrated in ASD groups from Western cultures, Chinese children with ASD demonstrated a preference of looking at repetitive movements versus random movements in a preferential looking paradigm48, which may derive from slower attentional disengagement or “sticky” or local attention49,50. Additionally, impaired cognitive flexibility in Chinese children with ASD was also found and was reflected in their visual scanning patterns 51. In contrast, some key differences in eye-movement patterns have emerged across East Asian and Western cultures in the general population, implicating an effect of culture. For instance, findings from Lee et al. (2016)33 revealed a wider distribution of fixations (i.e., global processing/attention) across scenes depicting faces in Taiwanese participants, compared to more centrally-focused fixations (i.e., local processing/attention) in American participants.
This study builds on these prior observations of RAN impairments in ASD and first-degree relatives in Western populations, by investigating RAN performance and gaze patterns in children and adults with ASD in an East Asian culture (i.e., Hong Kong; HK). Previously published data from English-speaking individuals with ASD and controls were included for comparison. We predicted that individuals with ASD from HK would show similar RAN performance and fixation patterns as the US ASD group8–10, and show slower naming speed, greater error rates, shorter EVS, and higher fixation and refixation frequencies than their age-matched controls. Finally, we also predicted that impaired RAN performance would correlate with greater ASD symptom severity. As noted, data from the US sample were reported previously8 and were included for comparison with new data obtained from HK participants, in order to examine potential cross-cultural differences in RAN. Data from the US sample have not been re-analyzed independently, nor have they been re-produced here as standalone findings.
Methods
Participants
Participants included 23 individuals with ASD and 39 controls from Hong Kong (HK) and age- and IQ-comparable samples from the US (n = 45 individuals with ASD and 44 controls) who have been previously studied8. All participants from HK spoke Cantonese as their first language, as well as English. Participants from the US spoke English as their primary language. Every effort was made to ensure that samples were comparable, while also ensuring maximum sample sizes. Specifically, a minimum age of 10 years was set to mitigate the impact of developing rapid naming automaticity53, and age and IQ were included as covariates for most statistical analyses (see below). Participants from HK were recruited using advertisements on social media platforms (e.g., Facebook) and flyers that were distributed to schools and organizations. Participants from the US, who were included in a prior publication8, were recruited through study advertisements distributed to ASD clinics and advocacy organizations, participant registries, and word of mouth.
All participants were screened for family history of genetic disorders related to ASD (e.g., fragile X syndrome), dyslexia, or brain injury (based on parent report). Additionally, all participants had normal or corrected-to-normal visual acuity, IQ ≥ 80, and chronological age ≥ 10 years (see Table 1 for sample characteristics). ASD diagnostic status was confirmed using the Autism Diagnostic Observation Schedule-Second Edition (ADOS-2)54 modules 3 or 4 and/or the Autism Diagnostic Interview-Revised (ADI-R)55 for US participants. For the HK-ASD group, ASD status was reliant upon participant’s report, and/or report from the clinics/schools from where the participants were recruited. HK-ASD participants also received the ADOS-2 modules 3 or 4 upon study enrollment to examine ASD symptomatology as a correlate. Of the HK-ASD participants, 15 participants met criteria for ASD based on the ADOS. For quality control purposes, exploratory analyses were conducted to compare RAN performance between those HK-ASD participants who met ADOS ASD criteria and those who did not. Findings did not differ between groups (ps > 0.081), suggesting that overall findings were not likely driven by one HK-ASD subgroup versus the other.
Table 1.
Sample characteristics—RAN.
| Hong Kong | United States | Culture effect | Diagnosis effect | Diagnosis X culture interaction | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Control group | ASD group | Control group | ASD group | |||||||
| M (SD) [range] | M (SD) [range] | M (SD) [range] | M (SD) [range] | F | p | F | p | F | p | |
| n (M/F)* | 39 (20:19) | 23 (18:5) | 44 (21:23) | 45 (36:9) | – | |||||
| Age (years) | 20.5 (6.2) | 17.9 (8.9) | 19.5 (5.4) | 17.9 (6.1) | 0.24 | 0.627 | 3.84 | 0.052 | 0.18 | 0.671 |
| [10.2–31.9] | [10.1–32.9] | [10.5–33.3] | [10.0–35.1] | |||||||
| PIQ | 110.8 (13.5) | 106.4 (11.8) | 112.9 (14.4) | 104.1 (14.9) | 0.006 | 0.936 | 9.42 | 0.003 | 0.83 | 0.364 |
| [82–139] | [84–126] | [79–143] | [68–131] | |||||||
| ADOS total severity score^ | – | 4.9 (2.5) | – | 6.6 (2.3) | 0.04 | 0.848 | – | |||
| [1–10] | [1–10] | |||||||||
Bold indicates significance p < .05.
*Sample size reflects image with maximum number of participants.
^ADOS total comparison severity score labels are as follows: 0–2 = “minimal-to-no evidence”, 3–4 = “low”, 5–7 = “moderate”, 8–10 = “high”. ADOS modules administered across samples included modules 3 and 4.
IQ was measured using the non-verbally administered Test for Nonverbal Intelligence—Fourth Edition (TONI-4) for HK participants and Wechsler Intelligence Scale for Children—Third Edition (WISC-III) or the Wechsler Abbreviated Scale of Intelligence (WASI) for US participants. The nonverbal standard score derived from the TONI-4 has been shown to be highly correlated with the Perceptual Reasoning Index from the WISC-III56, and the instruments are comparable in their measured constructs. As such, nonverbal IQ from the TONI-4 for HK participants and Performance IQ (PIQ) from the Wechsler scales for US participants were considered to represent “IQ” henceforth.
RAN procedures
Participants completed a RAN task (both non-symbolic or colors/objects and symbolic or numbers/letters) from the Comprehensive Test of Phonological Processing (CTOPP)57. The four stimulus types included two runs each and procedures were identical to Nayar et al. (2018)8. In short, each run contained an array of 36 items, and participants were instructed to name as accurately and rapidly as possible from left to right in each row, and from top to bottom for all rows. Before each stimulus type, a practice row of nine symbols was completed by all participants to ensure comprehension of task instructions. Color runs depicted colored circles and object runs depicted simple pictures of objects that participants were required to label. Participants’ gaze was tracked during RAN using a Tobii TX300 (sampled at 60 HZ) series eye tracker for HK participants and a Tobii T60 series eye tracker for US participants. Participants were seated approximately 50–60 cm from the screen. Gaze was calibrated prior to the task using a standard 5-point grid built in Tobii Studio. Participants were re-calibrated following any large movements; accuracy of calibration was 0.5°. An eye-tracking/calibration window was monitored for calibration deviance during task administration. Finally, calibration checks were embedded in the task (e.g., center cross-hair in between runs) to ensure tracking accuracy across runs. Stimuli were presented on a 21’’ TFT monitor in HK and a 17’’ TFT monitor in the US, with a resolution of 1280 × 1024 pixels. Digit and number runs were displayed in black on a gray background, while color and object runs were colored on a gray background. Voice responses were recorded using an external USB microphone.
Participants were tested in a quiet laboratory space or at the participant’s residence, with comparable administration procedures and environments across groups. All study procedures were approved by respective University Institutional Review Boards (Northwestern University and The Chinese University of Hong Kong) and all methods were performed in accordance with the relevant guidelines and regulations. Written informed consents and assents when applicable were obtained from all participants and/or their parent/legal guardian for study participation.
Data processing
RAN variables of interest (see below) were averaged across both runs for each stimuli type. They were then further averaged into non-symbolic (colors, objects) and symbolic (numbers, letters) conditions, which were used in final analyses. Research has previously demonstrated significant differences between symbolic and non-symbolic performance in RAN, such that symbolic conditions are thought to be more automatically processed after 6 years of age8,44,58, and have been shown to be more impaired in ASD8,9.
Vocal responses
HK participants completed most conditions (non-symbolic conditions and the number runs) in Cantonese. Although English was their second language, and because Cantonese is a non-alphanumeric system with no direct translation of the English alphabet to the Cantonese language, HK participants completed the letter runs in English. It is also noteworthy that English language instruction is the standard in schools in HK, starting as early as 2 years, 8 months. As such, HK participants had many years of practice with the English alphabet system, and so these stimuli were highly familiar to both groups of participants, permitting stronger experimental control than using different stimuli across cultures. Importantly, there were no significant differences in RAN naming time between letter and number runs within HK participants (condition effect; F(1, 52) = 0.12, p = 0.74) and group X condition effect; F(1, 52) = 0.00, p = 0.98)), indicating comparability between the two symbolic conditions.
The onset and offset of the articulation of each item was marked using the Penn Phonetics Lab Forced Aligner, an automatic and forced phonetic alignment toolkit that synchronizes phonetic transcriptions with speech signals59. The onset and offset boundaries were further examined by trained coders who were blind to participant diagnosis, marking errors or deviations reflective of incorrect naming8,9,60. Based on the beginning and ending time of each trial, eye movement and vocal responses were aligned, following methods outlined in Nayar et al. (2018)8.
Gaze
For each RAN item, the AOI was defined as a region extending vertically and horizontally from the center of each item to the midpoint between adjacent items see for details 8,9. Fixations were assigned to an AOI based on their spatial coordinates, and consecutive fixations within the same AOI were pooled and counted as ‘perseveration’. Quality control procedures followed prior work8, and included the following steps: (1) Parameters before exporting gaze data were set according to published standards61, to account for potential data loss in working with neurodevelopmental populations. These included usage of the built-in I-VT fixation filter in Tobii Studio set to a strict average across both eyes, a velocity threshold of 35°/s, 100 ms duration gap and 0.5° between each new fixation; (2) a minimum fixation duration for unique fixations was set at 100 ms; (3) runs with track loss of > 35% of total fixation duration were excluded; (4) runs with no fixation duration for > 10 s were excluded; (5) consistent with prior work8,60, eye movements associated with the first two and last four items of each array were excluded from analyses (due to increased eye-tracking variability and subsequent challenges inherent to interpreting such eye movements, including mistargeting and long saccades back to the beginning of a row); and (6) minimum and maximum number of fixations per run were established based on outliers (> 2.5 SD above mean) and data distributions. For all samples, this was set at 15 and 50 for letter/number trials, and 20 and 55 for color/object trials, respectively.
Overall, 11%, 1%, 7% and 12% of individual runs were excluded for the HK-control, US-control, HK-ASD, and US-ASD groups, respectively. 2X2 chi-squared revealed significant differences in the proportion of trials excluded by group (Χ2 (2, N = 1400) = 9.1, p < 0.01); the control group from the US demonstrated significantly fewer excluded runs compared to the other three groups. The total number of runs included in the final analyses, however, did not differ significantly by group (Χ2 (2, N = 1208) = 2.0, p = 0.16).
RAN naming performance variables
Naming time was calculated as the time used to articulate all 36 items on each run, including errors.
Frequency of errors was calculated by summing all errors and self-corrections during naming, including repetitions, omissions, or substitutions.
RAN eye movement variables
Eye-voice span (EVS) was defined as the lead in the eyes compared to the item being spoken. EVS parameters were identical to those reported in Nayar et al. (2018)8.
Number of fixations was defined as the total number of fixations regardless of type (see below) made during the run.
Refixations were defined to capture the type of fixation occurring. These included perseverations (the number of consecutively repeated fixations within the same item) and regressions (the number of times there were backward eye movements towards previously-visited items) that occurred during the run.
Statistical analyses
Data were examined to determine appropriate statistical models (parametric versus non-parametric), revealing normal distributions (via visual inspection of histograms and Q–Q plots due to relatively small sample sizes) across conditions, cultures, and diagnoses for all but one variable. Unsurprisingly, errors rates were positively skewed, primarily due to their low occurrence. As such, analyses involving error rates were re-analyzed using a series of Mann–Whitney U non-parametric tests.
For all variables, a series of linear mixed effects regression models were conducted separately for the symbolic and non-symbolic conditions using the lmer package62 for R63. Fixed effects included diagnosis (ASD versus TD), culture (HK versus US), and their interaction term (culture X diagnosis interaction), with sex, and age added as control variables. IQ was also included as a covariate for RAN naming performance variables (naming time and errors), which is consistent with previous studies8. Random effects included the random-by-participant intercept. Model comparisons were conducted between models with and without the interaction term to determine model fit. Stepwise model comparisons revealed no significant differences between models, and as such, all subsequent analyses included the interaction term to address the current study’s primary goals. P-values for linear mixed effects models were adjusted using the Benjamini–Hochberg method64 using a false discovery rate (FDR) of 0.10. FDR level of 0.10 was selected to adjust for multiple tests without potentially missing important effects65 due to relatively smaller sample sizes. Least square means (LSM) post-hoc pairwise comparisons were additionally performed for models showing a significant main effect or interaction effect using the lsmeans package66 (p-values adjusted using Tukey adjustments). As noted previously, the US-ASD and US-control participants were all included in prior work8, and are reported here to provide a basis for comparison with HK groups. Readers are referred to this prior study for pairwise comparisons examining effects between US groups, which are not repeated here. Finally, partial Pearson correlations with age and/or IQ covariates were conducted to assess how RAN performance variables may relate to ASD symptom severity in individuals with ASD based on the overall comparison score derived from the ADOS. Associations were only examined in the HK-ASD group, and not re-examined in the US-ASD group.
Ethical approval
All procedures performed in this study were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Results
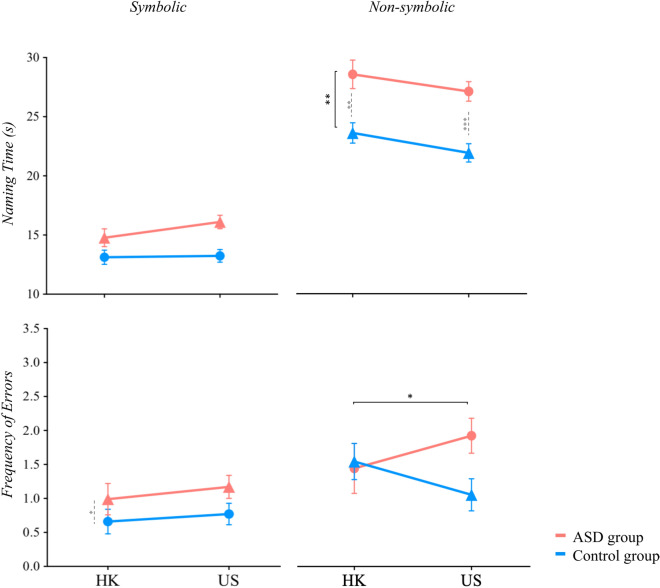
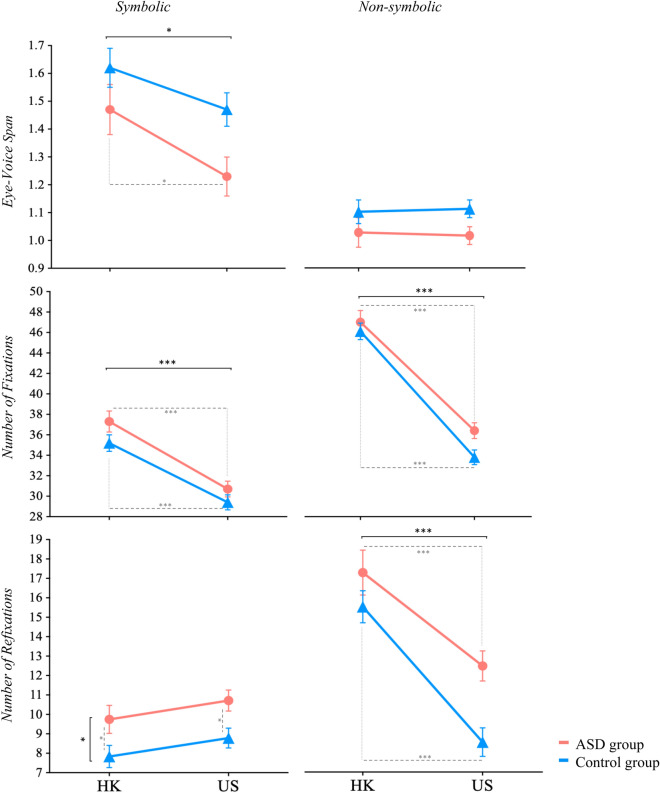
Descriptive statistics and group statistical comparisons are presented in Table 2 and Fig. 1 (Naming performance) and Fig. 2 (Eye movement during RAN), and summarized below.
Table 2.
Summary of results.
| Variable | Condition | Hong Kong | United States | Culture effect | Diagnosis effect | Diagnosis X culture interaction | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | ASD | Control | ASD | |||||||||||||||||
| M (SE) | M (SE) | M (SE) | M (SE) | Est | SE | t | p | padj | Est | SE | t | p | padj | Est | SE | t | p | padj | ||
| Performance | ||||||||||||||||||||
| Naming time (s) | Symbolic | 13.13 (0.60) | 14.77 (0.76) | 13.24 (0.54) | 16.11 (0.57) | 1.34 | 0.90 | 1.50 | 0.137 | 0.274 | −1.64 | 0.97 | −1.68 | 0.095 | 0.317 | −1.24 | 1.20 | −1.03 | 0.305 | 0.763 |
| Non-symbolic | 23.18 (0.84) | 27.98 (1.17) | 21.54 (0.75) | 26.57 (0.80) | −1.41 | 1.37 | −1.03 | 0.304 | 0.380 | −4.80 | 1.44 | −3.33 | 0.001 | 0.010 | −0.23 | 1.77 | −0.13 | 0.896 | 0.984 | |
| Errors | Symbolic | 0.66 (0.18) | 0.99 (0.23) | 0.77 (0.16) | 1.17 (0.17) | 0.18 | 0.27 | 0.66 | 0.509 | 0.566 | −0.33 | 0.30 | −1.10 | 0.273 | 0.341 | −0.07 | 0.37 | −0.20 | 0.846 | 0.984 |
| Non-symbolic | 1.51 (0.26) | 1.41 (0.36) | 1.03 (0.23) | 1.88 (0.25) | 0.47 | 0.43 | 1.11 | 0.270 | 0.380 | 0.11 | 0.45 | 0.24 | 0.812 | 0.812 | −0.96 | 0.55 | −1.74 | 0.084 | 0.763 | |
| Eye tracking | ||||||||||||||||||||
| Eye-voice span | Symbolic | 1.62 (0.07) | 1.47 (0.09) | 1.47 (0.06) | 1.23 (0.07) | −0.24 | 0.11 | −2.18 | 0.031 | 0.077 | 0.16 | 0.12 | 1.37 | 0.174 | 0.341 | 0.08 | 0.14 | 0.55 | 0.582 | 0.984 |
| Non-symbolic | 1.09 (0.04) | 1.02 (0.05) | 1.10 (0.03) | 1.01 (0.03) | −0.01 | 0.06 | −0.09 | 0.927 | 0.927 | 0.07 | 0.06 | 1.14 | 0.256 | 0.341 | 0.02 | 0.08 | 0.22 | 0.825 | 0.984 | |
| Total fixations | Symbolic | 35.20 (0.82) | 37.30 (1.04) | 29.40 (0.74) | 30.70 (0.78) | −6.52 | 1.24 | −5.25 | < .0001 | < .0001 | −2.03 | 1.34 | −1.51 | 0.133 | 0.332 | 0.65 | 1.66 | 0.39 | 0.698 | 0.984 |
| Non-symbolic | 46.1 (0.81) | 47.0 (1.13) | 33.8 (0.72) | 36.4 (0.77) | −10.53 | 1.32 | −7.95 | < .0001 | < .0001 | −0.80 | 1.40 | −0.57 | 0.567 | 0.630 | −1.86 | 1.72 | −1.08 | 0.280 | 0.763 | |
| Refixations | Symbolic | 7.84 (0.57) | 9.74 (0.72) | 8.79 (0.51) | 10.71 (0.54) | 0.97 | 0.86 | 1.13 | 0.260 | 0.380 | −1.90 | 0.92 | −2.06 | 0.042 | 0.208 | −0.02 | 1.14 | −0.02 | 0.984 | 0.984 |
| Non-symbolic | 15.46 (0.82) | 17.21 (1.15) | 8.54 (0.73) | 12.44 (0.78) | −4.77 | 1.34 | −3.56 | 0.001 | 0.002 | −1.75 | 1.42 | −1.24 | 0.218 | 0.341 | −2.15 | 1.74 | −1.24 | 0.218 | 0.763 | |
Bold indicates significance p < .05; padj included Benjamini–Hochberg correction at a false discovery rate of .10.
Figure 1.
RAN performance (naming time and error rates) across cultures, diagnostic groups, and conditions. A significant diagnosis effect emerged in the non-symbolic condition for naming time (Estimate = − 4.80 p < .01). Error bars represent standard error of the mean (SEM). For US-ASD versus US-control group comparisons, refer to Nayar et. al., 20188. *p < .05, **p < .01, ***p < .001. Black overall bars denote significance of the overall model (i.e., group effect, diagnosis effect), and dashed grey lines indicate pair-wise comparisons.
Figure 2.
RAN eye-movement variables across cultures, diagnostic groups, and conditions. Culture effect for EVS symbolic condition (Estimate = − 0.23, p < .05) and total fixations across conditions (Estimates < -6.52, ps < .0001). Diagnostic effect for refixations during symbolic condition (Estimate = −0.190, p < .05) and culture effect during non-symbolic condition (Estimate =− 4.77, p < .001). Error bars represent standard error of the mean (SEM). For US-ASD versus US-control group comparisons, refer to Nayar et. al., 20188. *p < .05, **p < .01, ***p < .001. Black overall bars denote significance of the overall model (i.e., group effect, diagnosis effect), and dashed grey lines indicate pair-wise comparisons.
Naming performance
Naming time
There were no significant culture, diagnosis, or culture X diagnosis effects during the symbolic conditions. For non-symbolic conditions, a significant diagnosis effect emerged (Estimate = − 4.80, p = 0.001), such that individuals with ASD across both cultures were significantly slower than their respective control groups (HK: t(134) = 3.33, p = 0.001; US: t(134) = 4.51, p < 0.0001). There was no significant effect of culture or culture X diagnosis interaction for the non-symbolic conditions.
Frequency of errors
There were no significant culture, diagnosis, or culture X diagnosis effects in error rates across both symbolic and non-symbolic conditions. Mann–Whitney U tests revealed significant differences in error rates during symbolic trials for HK groups, showing that the HK-ASD group committed a greater number of errors than their respective HK control group, (U = 4.89, p = 0.027). Additionally, the HK control group committed a greater number of errors than the US control group during non-symbolic trials (U = 5.86, p = 0.015).
Eye tracking performance
Eye-voice span (EVS)
During the symbolic condition, there was a significant culture effect (Estimate = − 0.23, p = 0.031)—individuals with ASD from HK had a significantly longer EVS than individuals with ASD from the US (t(141) = 2.18, p = 0.031). No diagnosis or culture X diagnosis interaction emerged for the symbolic conditions. Similarly, there were no significant culture or diagnosis effects or interactions during the non-symbolic conditions.
Number of fixations
Across both conditions, a significant culture effect (symbolic: Estimate = − 6.52, p < 0.0001 and non-symbolic: Estimate = − 10.53, p < 0.0001) was detected, with both groups from HK making a significantly greater number of fixations compared to their US counterparts (symbolic: ASD t(141) = 5.25, p < 0.0001, TD t(141) = 5.35, p < 0.0001; non-symbolic: ASD t(135) = 7.95, p < 0.0001, TD t(135) = 11.45, p < 0.0001). No significant diagnosis or culture X diagnosis interactions emerged in either condition.
Refixations
During the symbolic condition, a significant diagnosis effect (Estimate = − 1.90, p = 0.042) revealed that across both cultures, individuals with ASD made a greater number of regressions and perseverations than their respective control groups (HK: t(141) = 2.06, p = 0.042, US: t(141) = 2.53, p = 0.012). No significant culture or culture X diagnosis interaction emerged for the symbolic condition. During the non-symbolic condition, a significant culture effect emerged (Estimate = − 4.77, p = 0.001), where both groups from HK made a greater number of regressions and perseverations than their US counterparts (ASD: t(135) = 3.56, p = 0.001, TD: t(135) = 6.32, p < 0.0001). No significant diagnosis or culture X diagnosis interaction emerged during the non-symbolic conditions.
Clinical-behavioral correlates of RAN ability in individuals with ASD from HK
No significant associations between any RAN performance or gaze variables with ADOS scores emerged for individuals with ASD from HK.
Discussion
This study explored the coordination of gaze and language during rapid automatized naming (RAN) in individuals with ASD and controls from both Hong Kong and the US, to examine whether RAN impairments previously documented in ASD might extend beyond linguistic and cultural boundaries, and constitute a marker of language-related impairments associated with ASD. Specifically, prior work studying English-speaking individuals with ASD and their relatives reported key differences in rapid naming across both performance (i.e., longer naming time) and gaze (i.e., longer eye-voice span, elevated total fixations, and increased rates of refixations)8–10. The HK-ASD group showed some similarities with this profile, but an apparent cultural effect was observed in some areas. Specifically, the HK-ASD group did not differ from controls in eye voice span (EVS) or in their overall fixation rate, and both the ASD and control HK groups differed from the US groups on these component skills, suggesting a cultural effect likely stemming from visual processing and attentional cultural differences. In contrast, as was previously documented in US-ASD groups, the HK-ASD group demonstrated longer naming times and elevated rates of refixations compared to controls. It is thus possible that this specific pattern of naming and gaze during RAN may be tied to ASD-risk genes, particularly as they have been documented in ASD across cultures, among first-degree relatives of ASD8,9, as well as among carriers of the FMR1 premutation (a gene heavily implicated in ASD)8,47.
Diagnostic effects detected for naming time in the non-symbolic conditions across both HK and US groups are consistent with prior findings from US samples, demonstrating longer naming time among individuals with ASD8,9, as well as their siblings9 and parents8,10. That no cultural or culture by diagnosis effects emerged during these non-symbolic trials (or during symbolic conditions), where participants from HK spoke in their native language, is consistent with prior work demonstrating similar RAN naming in control groups between cultures e.g., 40,42,67. More importantly, group differences in the absence of such culture effects suggest that RAN naming time in non-symbolic trials may be more sensitive to ASD-related deficits across cultures than the symbolic (i.e., letters and numbers) condition, perhaps reflecting the increased cognitive demands inherent to non-symbolic than symbolic conditions (Norton & Wolf, 2012). Norton and Wolf (2012)3 also highlighted the role of cognitive processes involved in lower-level functions (e.g., visual processing, phonological representation) to be accurate and automatic. When the lower-level functions are not operating efficiently, this may have downstream effects on the allocation of resources to multiple high-order language skills such as reading, conversation, and narrative. Indeed, a recent meta-analysis documented relationships between RAN symbolic conditions and reading speed (a lower level process) in contrast to relationships between RAN non-symbolic conditions and reading comprehension (a more advance reading skill)68. Prior work has also indicated that the stream from perceptual encoding to articulatory processes in RAN is more susceptible to interference during non-symbolic conditions60, with greater challenges suggesting reduced automaticity due to inefficient linguistic and executive processes. Given the overlap in reading and language networks and brain regions implicated in RAN, it is then not surprising that individuals with a language-related disorder such as ASD, and those with a greater genetic liability to ASD (siblings/parents) will have greater difficulty during non-symbolic conditions than their control counterparts, regardless of culture or language.
Findings that the control and ASD groups from HK showed comparable naming time during the symbolic trials contrast with prior work from the US8,9, and may be attributable to the requirement that Cantonese-speaking participants name letters in English (for stimuli consistency). Both groups took approximately two seconds longer to name letters than numbers, and this lag may have dampened possible diagnostic effects. In terms of naming error rates, while non-parametric findings revealed a greater number of errors in the HK-ASD group compared to HK controls (and reported for US group in Nayar et al. (2018)8, and in HK control compared to US control groups, there were very few errors overall (~ 1–2 errors on average), reducing interpretability owing to this variable’s lack of sensitivity e.g., 8,9.
Findings from eye movement analyses, which highly complement the performance-based measures of time and errors by reflecting underlying strategies or mechanisms, revealed more complex differences across groups and cultures, and arguably provided a more sensitive index of RAN-related deficits than global measures of naming and error performance. Specifically, culture effects were found in EVS, wherein EVS was longer in both HK groups compared to those from the US, particularly during the symbolic condition. Moreover, though not statistically significant, EVS in both the ASD groups was shorter than their respective controls, indicating comparable diagnostic group patterns across English- and Cantonese-speaking individuals with ASD. It is possible that systematic education that starts from as early as 2 years and 8 months in HK, may have conditioned children in HK to pay closer attention to symbolic information. In addition to indexing automaticity and coordinating visual-vocal processing, EVS may also tap global visual processing, known to be different across Eastern and Western cultures. For instance, the lead in eye movement may signify a more seamless and efficient ability to process visual information more rapidly, and therefore, globally69–71—perhaps processing information further along in the stimulus more rapidly. According to Hofstede’s ‘individualism–collectivism framework’72,73, Western individualistic cultures (e.g., U.S.) value independence and self-expression, whereas Eastern collectivist cultures (e.g., Japan, China) emphasize social connection and harmony with the environment. Additionally, consistent with observations that Eastern and Western cultures differentially emphasize collectivism versus individualism, evidence from studies of visual perception suggest that members of East Asian cultures have demonstrated a greater propensity toward processing global information (background or the “gist”) versus Western cultures52,74. It is therefore possible that global processing in ASD is only somewhat dampened in East Asian cultures, and therefore protective against an ASD effect, as compared to individuals with ASD in Western cultures who have the tendency to process information more locally75. This may have resulted in an EVS that was comparable to that of the US control groups’, but longer (and therefore more efficient overall; as reflected by relationships with faster naming across groups and cultures; data not shown given prior work consistently evidencing this relationship)8,9,26,27,60,67 than that of the US-ASD group. Finally, differences in EVS during symbolic conditions between ASD groups across cultures may have risen due to methodological differences in naming languages, although there were no culture or diagnosis differences in the non-symbolic conditions, indicating their efficacy in tapping into skills that are not influenced by culture or ASD8. As such, differences between the HK and US groups are unlikely due to language differences, particularly as the words in each task are largely mono-syllabic.
Findings related to fixations may have arisen from methodological differences that placed an unequal task burden in Cantonese than in English. Specifically, both groups from HK tended to make a greater number of fixations compared to both groups from the US, and the number of refixations differed by group and culture, depending on the condition. There were cultural effects in the non-symbolic condition where both groups from HK refixated more often than both groups from the US. Increased refixation rates during this non-symbolic condition appeared to also prolong naming time for both HK groups, and error rates for the HK-control group, which may be related to methodological differences in language used for naming. While for English, initial sounds in items displayed in the object and color trials were phonetically unique (e.g., pen, star, fish, chair, boat, key), there were a greater number of overlapping initial-phonemes in Cantonese (e.g., bat1, seng1, jyu4, dang3, syun4, so2 si4). Given, prior work evidencing the co-activation between phonological codes of phonologically similar words and fixations during reading, which prolonged reading times76–78, it is possible that similarities in initial-phoneme sounds may have therefore impacted the ability for HK groups to efficiently and automatically convert the visual information into phonetic information, potentially influencing both naming time and fixation style.
During symbolic trials, during which both US and HK participants labeled letters in English, a diagnosis effect emerged, such that individuals with ASD across both cultures committed elevated rates of refixations than their control counterparts. Furthermore, in line with the local–global visual processing hypothesis, both groups from the US made a greater number of refixations (i.e., greater local processing) than both groups from HK, with both ASD groups demonstrating this visual rigidity or “stickiness” more prominently compared to their control counterparts. Importantly, this fixation pattern may reflect ASD genetic influence as it robustly differentiated groups in two independent studies of RAN and eye-voice patterns conducted with first-degree relatives of individuals with ASD, as well as among carriers of the FMR1 gene in its premutation (a gene heavily implicated in ASD)8,47.
Finally, the lack of associations with ASD symptomatology in the current study is not necessarily surprising, given the relatively limited battery of clinical-behavioral measures available, consisting of a single overall symptom severity score derived from a clinical measure (ADOS) that was normed on individuals from Western cultures. Prior work has demonstrated relationships with more detailed information about social-communication traits and RRBs from spontaneous narrations of story-books and conversational interaction in parents of individuals with ASD8, suggesting that it will be important for future work to examine relationships between RAN and a broader range of phenotypic measures, including those that are normed in East Asian cultures, that may more sensitively capture more nuanced ASD-related atypicalities.
Conclusions and future directions
Taken together, differences detected in ASD across cultures in naming time and refixations in particular have implications for understanding the language phenotype of ASD. Links with language-related phenotypes have been previously identified with evidence showing reduced synchronization of voice and eye movement during RAN were related to narrative skills in ASD8. Additionally, findings that similar differences in RAN have been documented among populations with subtle pragmatic (social) language atypicalities13–16, children with dyslexia, and cross-linguistically in ASD from this study, point to the possibility that RAN deficits may contribute to the language-related differences observed among these groups. Finally, evidence from studies of RAN in children also indicates clear stages of brain development in language-related regions related to RAN skills79, and shows that RAN naming time improves with developmental increases in myelin production and subsequent language development as well80. As such, together with findings from prior studies of RAN in ASD (and siblings and parents of individuals with ASD) in English-language samples, results from this study point towards such RAN differences as potential markers of ASD-related language impairments, with the possibility for revealing mechanistic underpinnings (specifically, refixations) of clinical phenotypes in ASD across different cultures and languages. It is also likely that findings in the present study were influenced by broader language, executive functioning, and processing speed differences not exclusive to ASD, particularly given evidence that RAN ability taps an extensive neural network reflecting a wide set of neuropsychological skills3. It is possible that the present findings documenting weaknesses in RAN in ASD cross-culturally are unlikely due to a deficit in one specific skill, but rather reflect atypicalities in a constellation of cognitive processes often functioning in concert as a result of the extensive neural network tapped by RAN3. Importantly, evidence that impairments in RAN performance (i.e., naming and error rates) are present across other language-related and neurodevelopmental conditions e.g., 81, highlights RAN’s utility for studying potentially shared neural and genetic mechanisms of impairment across conditions. This approach has been fruitfully explored in cross-population genetic studies, that have revealed many common genomic variations across disorders82. Future work would benefit from inclusion of an expanded battery of phenotypic correlates to further explore the clinical impact of RAN across cultures, and to disentangle language-related deficits associated with RAN1,3,47,60,83 from executive and visual-perceptual processes. Such fine-grained analyses will help to establish whether potential RAN deficits in ASD is language-related or not.
A consideration for future cross-linguistic studies of RAN is that naming in a non-native language for letter runs (1 of 4 conditions) in HK groups may have influenced the present study’s findings; however, this did not appear to impact ASD-related findings. In particular, naming time and refixations were the primary two indices revealing diagnostic effects only (i.e., without culture or interaction effects), suggesting that naming letters in English did not impact primary findings of diagnostic differences in refixations and naming time in both languages/cultures. Additionally, naming of letter runs occurred in a separate block or run, which ensured that only one language was being utilized within a specific block. Relatedly, non-symbolic naming may have been influenced by the stimuli included in these trials. Because Cantonese is a non-alphanumeric language and direct translations from English to Cantonese may cause initial phonemes to be similar, future work should consider the development and usage of items with unique initial-word sounds that may be important to determine if the diagnostic or cultural effects emerging from the current study in the non-symbolic conditions hold true. While prior work has indeed utilized Chinese characters for some RAN conditions46,84,85, these have not yet been normed, limiting application to cross-linguistic research studies and clinical settings. It may, however, be important to examine RAN performance in monolingual participants only, given known differences in bilingual studies of RAN86. However, as noted previously, findings reported here are not consistent with differences reported in studies of bilingual individuals, suggesting that bilingualism did not appear to impact current findings. Finally, it will be important to investigate whether differences in RAN extend to other Eastern Asian languages and cultures.
Acknowledgements
We are grateful to the individuals who participated in this study and to all the staff and students who assisted with data collection and processing, including Judy Kwan and Carol Kit-sum To.
Abbreviations
- ADOS
Autism diagnostic observation schedule
- ANOVA
Analysis of variance
- AOI
Area of interest
- ASD
Autism spectrum disorder
- CTOPP
Comprehensive test of phonological processing
- EVS
Eye-voice span
- RAN
Rapid automatized naming
- RRB
Restricted and repetitive behaviors
Author contributions
M.L. and P.W. conceived and designed the study and oversaw all data collection, analyses, and writing of the manuscript. K.N. and X.K. assisted in acquisition of data. K.N. led development of eye-tracking variables, data processing, analysis, and manuscript preparation. X.K. contributed to data processing and manuscript preparation. J.X. contributed to manuscript preparation. P.C.G. contributed to conceptualization of the project, and development and processing of eye-tracking variables. All authors read and approved the final manuscript.
Funding
This research was supported by grants from the National Institutes of Health (R01DC010191, PI: Losh), the National Science Foundation (BCS-0820394, PI: Losh), P30 HD03110 (PI: Larson), the Health and Medical Research Fund (HKSAR) (02130846, PI: Wong), the University Grants Committee (HKSAR) (RGC/GRF) (14117514 & 34000118, PI: Wong), and the Global Parent Child Resource Centre Limited and Dr. Stanley Ho Medical Development Foundation awarded to PW.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Wolf M, Bowers PG, Biddle K. Naming-speed processes, timing, and reading: A conceptual review. J. Learn. Disabil. 2000;33:387–407. doi: 10.1177/002221940003300409. [DOI] [PubMed] [Google Scholar]
- 2.Denckla MB, Cutting LE. History and significance of rapid automatized naming. Ann. Dyslexia. 1999;49:29–42. doi: 10.1007/s11881-999-0018-9. [DOI] [Google Scholar]
- 3.Norton ES, Wolf M. Rapid automatized naming (RAN) and reading fluency: Implications for understanding and treatment of reading disabilities. Annu. Rev. Psychol. 2012;63:427–452. doi: 10.1146/annurev-psych-120710-100431. [DOI] [PubMed] [Google Scholar]
- 4.Cummine J, Chouinard B, Szepesvari E, Georgiou GK. An examination of the rapid automatized naming-reading relationship using functional magnetic resonance imaging. Neuroscience. 2015;305:49–66. doi: 10.1016/j.neuroscience.2015.07.071. [DOI] [PubMed] [Google Scholar]
- 5.He, Q. H. et al. Decoding the neuroanatomical basis of reading ability: A multivoxel morphometric study. J. Neurosci.33, 12835. 10.1523/Jneurosci.0449-13.2013 (2013). [DOI] [PMC free article] [PubMed]
- 6.Saur D, et al. Ventral and dorsal pathways for language. Proc. Natl. Acad. Sci. USA. 2008;105:18035–18040. doi: 10.1073/pnas.0805234105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schwartz MF, Faseyitan O, Kim J, Coslett HB. The dorsal stream contribution to phonological retrieval in object naming. Brain. 2012;135:3799–3814. doi: 10.1093/brain/aws300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nayar K, et al. Links between looking and speaking in autism and first-degree relatives: Insights into the expression of genetic liability to autism. Mol. Autism. 2018;9:51. doi: 10.1186/s13229-018-0233-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hogan-Brown, A. L., Hoedemaker, R. S., Gordon, P. C. & Losh, M. Eye-voice span during rapid automatized naming: evidence of reduced automaticity in individuals with autism spectrum disorder and their siblings. J. Neurodev. Disord.6. 10.1186/1866-1955-6-33 (2014). [DOI] [PMC free article] [PubMed]
- 10.Losh M, Esserman D, Piven J. Rapid automatized naming as an index of genetic liability to autism. J. Neurodev. Disord. 2010;2:109–116. doi: 10.1007/s11689-010-9045-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nation K, Clarke P, Wright B, Williams C. Patterns of reading ability in children with autism spectrum disorder. J. Autism Dev. Disord. 2006;36:911–919. doi: 10.1007/s10803-006-0130-1. [DOI] [PubMed] [Google Scholar]
- 12.Saldana D, Carreiras M, Frith U. Orthographic and phonological pathways in hyperlexic readers with autism spectrum disorders. Dev. Neuropsychol. 2009;34:240–253. doi: 10.1080/87565640902805701. [DOI] [PubMed] [Google Scholar]
- 13.Landa R, et al. Social language use in parents of autistic individuals. Psychol. Med. 1992;22:245–254. doi: 10.1017/s0033291700032918. [DOI] [PubMed] [Google Scholar]
- 14.Losh M, Childress D, Lam K, Piven J. Defining key features of the broad autism phenotype: A comparison across parents of multiple- and single-incidence autism families. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2008;147B:424–433. doi: 10.1002/ajmg.b.30612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nayar K, et al. Elevated polygenic burden for autism spectrum disorder is associated with the broad autism phenotype in mothers of individuals with autism spectrum disorder. Biol. Psychiatry. 2021;89:476–485. doi: 10.1016/j.biopsych.2020.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Piven J, Palmer P, Jacobi D, Childress D, Arndt S. Broader autism phenotype: Evidence from a family history study of multiple-incidence autism families. Am. J. Psychiatry. 1997;154:185–190. doi: 10.1176/ajp.154.2.185. [DOI] [PubMed] [Google Scholar]
- 17.Folstein SE, et al. Predictors of cognitive test patterns in autism families. J. Child Psychol. Psychiatry. 1999;40:1117–1128. doi: 10.1111/1469-7610.00528. [DOI] [PubMed] [Google Scholar]
- 18.Losh M, et al. Developmental markers of genetic liability to autism in parents: A longitudinal, multigenerational study. J. Autism Dev. Disord. 2017;47:834–845. doi: 10.1007/s10803-016-2996-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Byrne B, et al. Longitudinal twin study of early literacy development: Preschool through Grade 1. Read Writ. 2007;20:77–102. doi: 10.1007/s11145-006-9019-9. [DOI] [Google Scholar]
- 20.Altani A, Protopapas A, Georgiou GK. The contribution of executive functions to naming digits, objects, and words. Read Writ. 2017;30:121–141. doi: 10.1007/s11145-016-9666-4. [DOI] [Google Scholar]
- 21.Brooks AD, Berninger VW, Abbott RD. Letter naming and letter writing reversals in children with dyslexia: Momentary inefficiency in the phonological and orthographic loops of working memory. Dev. Neuropsychol. 2011;36:847–868. doi: 10.1080/87565641.2011.606401. [DOI] [PubMed] [Google Scholar]
- 22.Jacobson LA, et al. Working memory influences processing speed and reading fluency in ADHD. Child Neuropsychol. 2011;17:209–224. doi: 10.1080/09297049.2010.532204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leonard CM, et al. Brain anatomy, processing speed, and reading in school-age children. Dev. Neuropsychol. 2011;36:828–846. doi: 10.1080/87565641.2011.606398. [DOI] [PubMed] [Google Scholar]
- 24.Messer D, Henry LA, Nash G. The relation between executive functioning, reaction time, naming speed, and single word reading in children with typical development and language impairments. Br. J. Educ. Psychol. 2016;86:412–428. doi: 10.1111/bjep.12115. [DOI] [PubMed] [Google Scholar]
- 25.Baddeley, A. D. & Hitch, G. in Psychology of Learning and Motivation Vol. 8 47–89 (Academic Press, 1974).
- 26.Laubrock, J. & Kliegl, R. The eye-voice span during reading aloud. Front. Psychol.6. 10.3389/fpsyg.2015.01432 (2015). [DOI] [PMC free article] [PubMed]
- 27.Levin, H. & Addis, A. B. The Eye-Voice Span. (MIT Press, 1979).
- 28.Inhoff AW, Connine C, Eiter B, Radach R, Heller D. Phonological representation of words in working memory during sentence reading. Psychon. B Rev. 2004;11:320–325. doi: 10.3758/Bf03196577. [DOI] [PubMed] [Google Scholar]
- 29.Booth RW, Weger UW. The function of regressions in reading: Backward eye movements allow rereading. Mem. Cognit. 2013;41:82–97. doi: 10.3758/s13421-012-0244-y. [DOI] [PubMed] [Google Scholar]
- 30.Perea, M. & Carreiras, M. Regressions and eye movements: Where and when. Behav. Brain Sci.26, 497. 10.1017/S0140525x03420104 (2003).
- 31.Rayner K. Eye movements in reading and information processing: 20 years of research. Psychol. Bull. 1998;124:372–422. doi: 10.1037/0033-2909.124.3.372. [DOI] [PubMed] [Google Scholar]
- 32.Rayner K, Slattery TJ, Belanger NN. Eye movements, the perceptual span, and reading speed. Psychon. B Rev. 2010;17:834–839. doi: 10.3758/Pbr.17.6.834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leonard LB, Sabbadini L, Leonard JS, Volterra V. Specific language impairment in children: A cross-linguistic study. Brain Lang. 1987;32:233–252. doi: 10.1016/0093-934x(87)90126-x. [DOI] [PubMed] [Google Scholar]
- 34.Goswami U. Phonological representations, reading development and dyslexia: Towards a cross-linguistic theoretical framework. Dyslexia. 2000;6:133–151. doi: 10.1002/(SICI)1099-0909(200004/06)6:2<133::AID-DYS160>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 35.Wong AMY, Ciocca V, Yung S. The perception of lexical tone contrasts in cantonese children with and without specific language impairment (SLI) J. Speech Lang. Hear R. 2009;52:1493–1509. doi: 10.1044/1092-4388(2009/08-0170). [DOI] [PubMed] [Google Scholar]
- 36.Wang X, Wang S, Fan Y, Huang D, Zhang Y. Speech-specific categorical perception deficit in autism: An event-related potential study of lexical tone processing in Mandarin-speaking children. Sci. Rep. 2017;7:43254. doi: 10.1038/srep43254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McBride-Chang C, Shu H, Zhou AB, Wat CP, Wagner RK. Morphological awareness uniquely predicts young children's Chinese character recognition. J. Educ. Psychol. 2003;95:743–751. doi: 10.1037/0022-0663.95.4.743. [DOI] [Google Scholar]
- 38.McBride-Chang C, Liu PD, Wong T, Wong A, Shu H. Specific reading difficulties in Chinese, English, or both: Longitudinal markers of phonological awareness, morphological awareness, and RAN in Hong Kong Chinese children. J. Learn Disabil.-Us. 2012;45:503–514. doi: 10.1177/0022219411400748. [DOI] [PubMed] [Google Scholar]
- 39.Shu H, McBride-Chang C, Wu S, Liu HY. Understanding Chinese developmental dyslexia: Morphological awareness as a core cognitive construct. J. Educ. Psychol. 2006;98:122–133. doi: 10.1037/0022-0663.98.1.122. [DOI] [Google Scholar]
- 40.Bowey JA, McGuigan M, Ruschena A. On the association between serial naming speed for letters and digits and word-reading skill: Towards a developmental account. J. Res. Read. 2005;28:400–422. doi: 10.1111/j.1467-9817.2005.00278.x. [DOI] [Google Scholar]
- 41.Cardoso-Martins C, Pennington BF. The relationship between phoneme awareness and rapid serial naming skills and literacy acquisition: The role of developmental period and reading ability. Sci. Stud. Read. 2004;8:27–52. doi: 10.1207/s1532799xssr0801_3. [DOI] [Google Scholar]
- 42.Yan M, Pan JG, Laubrock J, Kliegl R, Shu H. Parafoveal processing efficiency in rapid automatized naming: A comparison between Chinese normal and dyslexic children. J. Exp. Child Psychol. 2013;115:579–589. doi: 10.1016/j.jecp.2013.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ho CSH, Lai DNC. Naming-speed deficits and phonological memory deficits in Chinese developmental dyslexia. Learn Individ. Differ. 1999;11:173–186. doi: 10.1016/S1041-6080(00)80004-7. [DOI] [Google Scholar]
- 44.Wolf M, Bally H, Morris R. Automaticity, retrieval-processes, and reading—A longitudinal-study in average and impaired readers. Child Dev. 1986;57:988–1000. doi: 10.1111/j.1467-8624.1986.tb00260.x. [DOI] [PubMed] [Google Scholar]
- 45.Georgiou GK, Parrila R, Liao CH. Rapid naming speed and reading across languages that vary in orthographic consistency. Read Writ. 2008;21:885–903. doi: 10.1007/s11145-007-9096-4. [DOI] [Google Scholar]
- 46.Liao CH, Georgiou GK, Parrila R. Rapid naming speed and Chinese character recognition. Read Writ. 2008;21:231–253. doi: 10.1007/s11145-007-9071-0. [DOI] [Google Scholar]
- 47.Nayar K, et al. Language processing skills linked to FMR1 variation: A study of gaze-language coordination during rapid automatized naming among women with the FMR1 premutation. PLoS ONE. 2019;14:e0219924. doi: 10.1371/journal.pone.0219924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang QD, et al. Children with autism spectrum disorder prefer looking at repetitive movements in a preferential looking paradigm. J. Autism Dev. Disord. 2018;48:2821–2831. doi: 10.1007/s10803-018-3546-5. [DOI] [PubMed] [Google Scholar]
- 49.Fischer J, Koldewyn K, Jiang YV, Kanwisher N. Unimpaired attentional disengagement and social orienting in children with autism. Clin. Psychol. Sci. 2014;2:214–223. doi: 10.1177/2167702613496242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fischer J, et al. Unimpaired attentional disengagement in toddlers with autism spectrum disorder. Dev. Sci. 2016;19:1095–1103. doi: 10.1111/desc.12386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yi L, et al. Visual scanning patterns during the dimensional change card sorting task in children with autism spectrum disorder. Autism Res. Treat. 2012;2012:123053. doi: 10.1155/2012/123053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lee YJ, Greene HH, Tsai CW, Chou YJ. Differences in sequential eye movement behavior between Taiwanese and American viewers. Front. Psychol. 2016;7:697. doi: 10.3389/fpsyg.2016.00697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wagner RK, et al. Changing relations between phonological processing abilities and word level reading as children develop from beginning to skilled readers: A 5-year longitudinal study. Dev. Psychol. 1997;33:468–479. doi: 10.1037/0012-1649.33.3.468. [DOI] [PubMed] [Google Scholar]
- 54.Lord C, et al. Autism Diagnostic Observation Schedule—2nd Edition (ADOS-2) Western Psychological Corporation; 2012. [Google Scholar]
- 55.Lord C, Rutter M, Le Couteur A. Autism diagnostic interview-revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J. Autism Dev. Disord. 1994;24:659–685. doi: 10.1007/bf02172145. [DOI] [PubMed] [Google Scholar]
- 56.Mungkhetklang C, Crewther S, Bavin E, Goharpey N, Parsons C. Comparing measures of ability in adolescents with intellectual disabilities. J. Intell. Disabil. Res. 2016;60:801–801. [Google Scholar]
- 57.Wagner, R. K., Torgesen, J. K., Rashotte, C. A. & Pearson, N. A. Comprehensive Test of Phonological Processing: CTOPP. (Pro-ed Austin, 1999).
- 58.Meyer MS, Wood FB, Hart LA, Felton RH. Longitudinal course of rapid naming in disabled and nondisabled readers. Ann. Dyslexia. 1998;48:91–114. doi: 10.1007/s11881-998-0005-6. [DOI] [Google Scholar]
- 59.Yuan J, Liberman M. Speaker identification on the SCOTUS corpus. J. Acoust. Soc. Am. 2008;123:3878. doi: 10.1121/1.2935783. [DOI] [Google Scholar]
- 60.Gordon PC, Hoedemaker RS. Effective scheduling of looking and talking during rapid automatized naming. J. Exp. Psychol. Hum. Percept. Perform. 2016;42:742–760. doi: 10.1037/xhp0000171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wass SV, Smith TJ, Johnson MH. Parsing eye-tracking data of variable quality to provide accurate fixation duration estimates in infants and adults. Behav. Res. Methods. 2013;45:229–250. doi: 10.3758/s13428-012-0245-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bates D, Machler M, Bolker BM, Walker SC. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2015;67:1–48. doi: 10.18637/jss.v067.i01. [DOI] [Google Scholar]
- 63.R Core Team. (2018).
- 64.Benjamini Y, Hochberg Y. Controlling the false discovery rate—A practical and powerful approach to multiple testing. J. R. Stat. Soc. B. 1995;57:289–300. doi: 10.1111/j.2517-6161.1995.tb02031.x. [DOI] [Google Scholar]
- 65.APA. Publication Manual of the American Psychological Association. 4th edn. (American Psychiatric Association, 1994).
- 66.Lenth RV. Least-squares means: The R package lsmeans. J. Stat. Softw. 2016;69:1–33. doi: 10.18637/jss.v069.i01. [DOI] [Google Scholar]
- 67.Pan J, Yan M, Laubrock J, Shu H, Kliegl R. Eye-voice span during rapid automatized naming of digits and dice in Chinese normal and dyslexic children. Dev. Sci. 2013;16:967–979. doi: 10.1111/desc.12075. [DOI] [PubMed] [Google Scholar]
- 68.Borokhovski E, Bernard RM, Segalowitz N, Sokolovskaya A. Systematically mapping connection between rapid automatized naming task and reading performance: A meta-analysis of correlational data. Russ. Psychol. J. 2018;15:46–76. doi: 10.21702/rpj.2018.1.3. [DOI] [Google Scholar]
- 69.Colombo J, Mitchell DW, Coldren JT, Freeseman LJ. Individual differences in infant visual attention: Are short lookers faster processors or feature processors? Child Dev. 1991;62:1247–1257. doi: 10.2307/1130804. [DOI] [PubMed] [Google Scholar]
- 70.Freeseman LJ, Colombo J, Coldren JT. Individual differences in infant visual attention: Four-month-olds' discrimination and generalization of global and local stimulus properties. Child Dev. 1993;64:1191–1203. doi: 10.2307/1131334. [DOI] [PubMed] [Google Scholar]
- 71.Poirel N, Pineau A, Mellet E. What does the nature of the stimuli tell us about the global precedence effect? Acta Psychol. (Amst) 2008;127:1–11. doi: 10.1016/j.actpsy.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 72.Hofstede G. Cultural-differences in teaching and learning. Int. J. Intercult. Rel. 1986;10:301–320. doi: 10.1016/0147-1767(86)90015-5. [DOI] [Google Scholar]
- 73.Hofstede, G. Culture's Consequences: Comparing Values, Behaviors, Institutions and Organizations Across Nations. (SAGE Publications, 2001).
- 74.McKone E, et al. Asia has the global advantage: Race and visual attention. Vis. Res. 2010;50:1540–1549. doi: 10.1016/j.visres.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 75.Van der Hallen R, Evers K, Brewaeys K, Van den Noortgate W, Wagemans J. Global processing takes time: A meta-analysis on local-global visual processing in ASD. Psychol. Bull. 2015;141:549–573. doi: 10.1037/bul0000004. [DOI] [PubMed] [Google Scholar]
- 76.Lee HW, Rayner K, Pollatsek A. The time course of phonological, semantic, and orthographic coding in reading: Evidence from the fast-priming technique. Psychon. Bull. Rev. 1999;6:624–634. doi: 10.3758/bf03212971. [DOI] [PubMed] [Google Scholar]
- 77.Lee YA, Binder KS, Kim JO, Pollatsek A, Rayner K. Activation of phonological codes during eye fixations in reading. J. Exp. Psychol. Hum. Percept. Perform. 1999;25:948–964. doi: 10.1037//0096-1523.25.4.948. [DOI] [PubMed] [Google Scholar]
- 78.Rayner K, Sereno SC, Lesch MF, Pollatsek A. Phonological codes are automatically activated during reading—Evidence from an eye-movement priming paradigm. Psychol. Sci. 1995;6:26–32. doi: 10.1111/j.1467-9280.1995.tb00300.x. [DOI] [Google Scholar]
- 79.Denckla MB. Color-naming defects in dyslexic boys. Cortex. 1972;8:164–176. doi: 10.1016/s0010-9452(72)80016-9. [DOI] [PubMed] [Google Scholar]
- 80.Dougherty RF, et al. Temporal-callosal pathway diffusivity predicts phonological skills in children. Proc. Natl. Acad. Sci. U S A. 2007;104:8556–8561. doi: 10.1073/pnas.0608961104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Semrud-Clikeman M, Guy K, Griffin JD, Hynd GW. Rapid naming deficits in children and adolescents with reading disabilities and attention deficit hyperactivity disorder. Brain Lang. 2000;74:70–83. doi: 10.1006/brln.2000.2337. [DOI] [PubMed] [Google Scholar]
- 82.Bulik-Sullivan, B. et al. An atlas of genetic correlations across human diseases and traits. Nat. Genet.47, 1236. 10.1038/ng.3406 (2015). [DOI] [PMC free article] [PubMed]
- 83.Wolf M, Bowers PG. Naming-speed processes and developmental reading disabilities: An introduction to the special issue on the double-deficit hypothesis. J. Learn. Disabil. 2000;33:322–324. doi: 10.1177/002221940003300404. [DOI] [PubMed] [Google Scholar]
- 84.Chang YJ, et al. The contribution of rapid automatized naming to Chinese character recognition. Learn. Individ. Differ. 2014;34:43–50. doi: 10.1016/j.lindif.2014.05.010. [DOI] [Google Scholar]
- 85.Liao CH, et al. The role of rapid naming in reading development and dyslexia in Chinese. J. Exp. Child Psychol. 2015;130:106–122. doi: 10.1016/j.jecp.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 86.Kana RK, Libero LE, Moore MS. Disrupted cortical connectivity theory as an explanatory model for autism spectrum disorders. Phys. Life Rev. 2011;8:410–437. doi: 10.1016/j.plrev.2011.10.001. [DOI] [PubMed] [Google Scholar]