Abstract
Postlaparoscopic shoulder pain (PLSP) is a common clinical problem that needs to be addressed by medical professionals who are currently perform laparoscopic surgeries. The purpose of this study was to determine the perioperative clinical factors and demographic characteristics associated with PLSP. A prospective observational study was performed with 442 inpatients undergoing laparoscopic surgery for infertility. The pain visual analogue scale was used as the measuring instrument. To identify the predictors of PLSP, we performed multivariate conditional logistic regression. PLSP was correlated with body mass index (BMI, odds ratio = 0.815). The incidence of shoulder pain and more severe shoulder pain in patients with a lower BMI was significantly higher than it was in patients with a higher BMI, and BMI was significantly negatively correlated with PLSP. Most of the patients (95%) began to experience shoulder pain on the first postoperative day, and it rarely occurred on the day of surgery. Patients with lower BMI presented a higher risk of reporting shoulder pain on the first postoperative day. We should identify high-risk patients in advance and make specific treatment plans according to the characteristics of their symptoms.
Subject terms: Anatomy, Health care, Medical research, Risk factors
Introduction
Laparoscopic-assisted surgery has largely replaced traditional open surgery1–3. The main reason is that laparoscopic-assisted surgery has the advantages of decreased postoperative pain4–8, earlier return of bowel function, lower morbidity and mortality, quicker postoperative recovery and early hospital discharge9–11.
In China, an increasing number of patients have chosen laparoscopic-assisted surgery for infertility assessment and treatment for various reasons in recent years. For patients with infertility undergoing laparoscopic-assisted surgery, the procedure aims to diagnose and solve specific causes of infertility. Most patients will be able to undergo pregnancy and childbirth after surgery. Therefore, in addition to the advantages mentioned above, the choice of laparoscopic-assisted surgery is largely due to the small abdominal incision, minimal scarring and the low incidence of abdominal adhesions, which is conducive to the subsequent pregnancy and delivery process.
Although the abdominal incision is small, unfortunately, many patients complain of postlaparoscopic shoulder pain (PLSP)12–16. The reported incidence of PLSP varies (30%-90%) with different surgical methods and studies. Because there are so many patients undergoing laparoscopic surgery every year, even if we estimate the minimum rate of 30%, the number of patients with this clinical problem is quite alarming17. Similar to other types of laparoscopic surgery, PLSP is a problem that cannot be ignored in infertility patients after laparoscopic-assisted surgery18–20.
According to previous studies, PLSP can last up to 7 days, and sometimes more than 5 weeks12. In many cases, the degree of shoulder pain is far greater than that of the incision and internal organs. Most importantly, 72% of patients did not take any opioids to relieve their shoulder pain15. It has also been found that PLSP is less responsive to treatment than incision and visceral pain21. If we do not give effective treatment immediately, continuous pain will not only increase the discomfort of the patients but also may lead to an increase in the incidence of various postoperative complications and delayed rehabilitation, which will significantly increase the cost of care14,17. Furthermore, continuous pain will strongly reduce the satisfaction of the patients. All of these effects are contrary to the original intentions of performing laparoscopic assisted surgery. Therefore, PLSP is increasingly being recognized as a serious clinical problem.
The precise mechanism of PLSP has remained unclear until now. At present, most scholars believe that PLSP is associated with referred pain. The central diaphragmatic pleura and the mediastinal pleura are supplied by phrenic innervation (C3-5), while the supraclavicular nerve (C3-4) conducts sensory input from the acromium process. Pain occurs in the neck or scapula when the phrenic nerve is irritated16,17,22.
Many intervention clinical studies aimed at solving PLSP have been conducted24–32. Unfortunately, these intervention studies have often found quite varied and sometimes even conflicting results regarding the effectiveness of these interventions, including using drainage19,32–34, intraperitoneal instillation of local anesthetics13,35–37, pulmonary recruitment manoeuvre38–40, gasless laparoscopy41–43, warm and humidified dioxide44–46, low-pressure pneumoperitoneum30,31,47, intraperitoneal normal saline infusion48,49 and drugs preventatively or therapeutically20,50,51. However, few studies have attempted to identify the risk factors for PLSP52. The purpose of this study was to determine the risk factors for PLSP through observation.
Materials and methods
This study was conducted at the Affiliated Yantai Yuhuangding Hospital of Qingdao University from March 2016 to October 2017. It was approved by the clinical trial ethics committee of Yantai Yuhuangding Hospital of Qingdao University (2016–11) and was registered with the Chinese Clinical Trial Registry (ChiCTR-OOC-16008044). This clinical trial is consistent with the ethical principles of the Helsinki declaration. All patients volunteered to participate in the study and signed written informed consent before the trial.
A total of 442 hospitalized patients undergoing elective laparoscopic surgery for infertility were included in this study. The exclusion criteria were chronic pain syndromes such as fibromyalgia or neck and shoulder pain, a history of long-term use of daily opioids, allergies to medications used in this study such as fentanyl, propofol, and midazolam, impaired cognitive function or inability to understand the study protocol, communication barriers, unstable cardiovascular disease and hypertension, central nervous system disease, endocrine system diseases, and liver or kidney dysfunction.
During the preoperative visit, basic patient information was collected and recorded. The information included the patient's medical history, age, years of education, height, weight, waist circumference and hip circumference. Body mass index (BMI), waist-to-hip ratio, and waist-to-height ratio were calculated.
All patients received similar general anaesthetic and surgical regimens. No premedication was used. Heart rate, arterial blood pressure, and oxygen saturation were monitored in all patients upon arrival at the anaesthesia room. General anaesthesia was induced with midazolam (0.1 mg/kg), fentanyl (4 μg/kg) and propofol (1–2 mg/kg). Cisatracurium infusion (0.15 mg/kg) was used to facilitate tracheal intubation and obtain intraoperative muscle relaxation. Anaesthesia was maintained with oxygen in air (1:2), sevoflurane, propofol and remifentanil. All patients were ventilated with a volume-controlled ventilator (VT10 ml/kg, RR 12/min). The airway plateau pressure, peak airway pressure and CO2 pressure in the exhaled air (PetCO2) were recorded before and 15 min after the establishment of artificial pneumoperitoneum. Then, tidal volume and respiratory rate were adjusted according to the airway pressure and PetCO2. Ondansetron (8 mg) was administered intravenously by the anaesthesiologists to minimize postoperative nausea and vomiting when the surgeons began to close the umbilical trocar sites. Neuromuscular relaxation was reversed pharmacologically at the end of surgery with atropine and neostigmine.
All patients were placed in the lithotomy and Trendelenburg positions during the operation. Laparoscopy was performed with abdominal insufflation of CO2 at 12 mmHg using a standard automated insufflator30,31. The insufflation gas was not heated or humidified with additional devices44–46. All operations were conducted by experienced laparoscopic surgeons (more than 10 years in laparoscopic surgery, involving more than 200 laparoscopic operations) using the standard technique with one 10-mm and two 5-mm trocars. CO2 was evacuated at the end of the procedure by manual compression of the abdomen with open trocars21. All patients were kept for observation in the PACU until their condition stabilized before moving them to their designated wards.
The following prophylactic analgesic standard treatment was used: intravenous propacetamol (1 g) was used approximately 20 min before the end of surgery, and either intravenous pentazocine (30 mg in the PACU) or oral ibuprofen sustained release capsules (300 mg in the ward) were administered on demand.
Assessment of pain
The degree of pain was assessed at 6 h, 12 h, 24 h, 48 h and 72 h postoperatively before the patients left the PACU by a 10-cm visual analogue pain scale (0 = no pain to 10 = unbearable pain). We selected the highest value of shoulder pain score at each time point for statistical analysis. The patients were classified into two groups: the absence of shoulder pain (scores equal to zero) and shoulder pain (scores higher than zero).
Sample size
The sample size was calculated on the basis of the expected incidence of PLSP derived from previous studies18–20,32. The formula was as follows:
where n: estimated sample size; π: expected prevalence of PLSP, 60% was used; δ: allowing a double-sided type I error rate within 5%, δ = 0.05; α = 0.05, uα = 1.96.
The estimated sample size for the survey was 368. An additional 20% of patients were recruited to overcome dropouts, refusals and exclusions. The study was completed with 442 patients.
Statistical analysis
The patients were divided into shoulder pain group and absence shoulder pain group. Independent sample t test and Mann Whitney U test were used to compare the differences between the two groups. In order to evaluate the impact of each variable on PLSP, we used univariate binary logistic regression analysis. Then, the influencing factors of PLSP were included in the positive stepwise multiple regression analysis. The patients were divided into four groups according to the Asian BMI standard: group A (BMI < 18.5), group B (18.5 ≤ BMI < 23), group C (23 ≤ BMI < 30) and group D (BMI ≥ 30). The highest VAS score of shoulder pain within 72 h after the operation was compared. Independent-samples t-tests and Pearson’s chi-squared tests were used to compare variables between the groups. P < 0.05 was considered statistically significant. SPSS for Windows (version 18.0, SPSS Inc., Chicago, IL, USA) was used for the statistical analyses.
Results
Fifty patients were excluded from this study. One case was converted to open surgery because of ovarian cancer detected during the operation, two cases underwent an emergency operation because of intra-abdominal haemorrhage after the laparoscopic operation, and 47 cases were treated by cauterization because of endometriosis found in the diaphragm and upper abdomen during the operation. Finally, 392 patients were included in the statistical analysis.
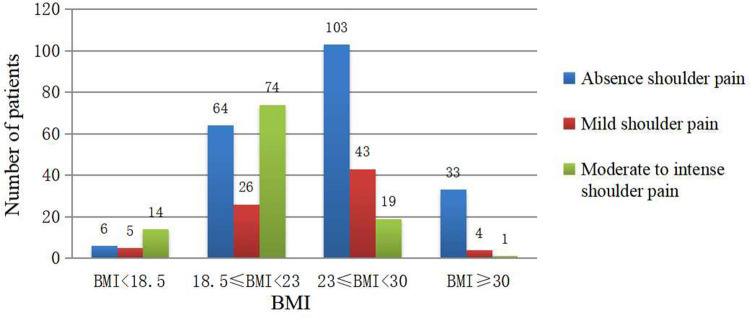
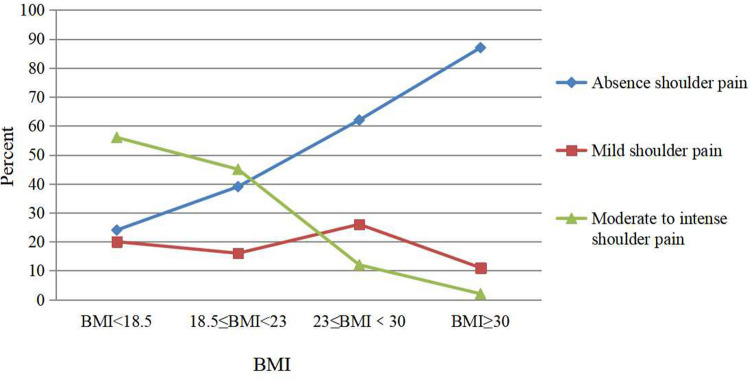
The basic characteristics of the patients’ shoulder pain are shown in Table 1. Nearly half of the patients (186/392, 47.7%) felt unilateral or bilateral shoulder pain during hospitalization. Half of the patients (92/186, 49.4%) had bilateral shoulder pain. The vast majority of patients (95%) began to have shoulder pain on the first day after surgery and rarely on the day of surgery. Table 2 shows that age, height, weight, BMI, waist circumference, hip circumference, waist hip ratio, waist height ratio, peak airway pressure before pneumoperitoneum (P peak1), peak airway pressure 15 min after pneumoperitoneum (P peak2) and the difference between them (P peak2-p peak1), airway plateau pressure before pneumoperitoneum (P platform1), airway plateau pressure 15 min after pneumoperitoneum (P platform 2) and the difference between them (P peak2-P peak1) of patients have influence on PLSP. However, the number of years in school, duration of surgery, duration of pneumoperitoneum, end-tidal carbon dioxide pressure before pneumoperitoneum (PetCO2)1 or 15 min after pneumoperitoneum (PetCO2)2, and the difference between (PetCO2)1 and (PetCO2)2 had no effect on PLSP. Stepwise positive regression analysis showed that BMI was an independent predictor of PLSP (OR = 0.815). The scatter plot of BMI and pain score showed that patients with low BMI were more likely to have shoulder pain after operation, and the degree of shoulder pain was also more severe. (Fig. 1). Comparing the highest VAS score of shoulder pain within 72 h after the operation, it was found that there was no significant difference between group A and group B, and there was a significant difference between the other two groups (Table 3). There were significant differences in the incidence of shoulder pain and more severe shoulder pain among the groups. The incidence of shoulder pain and more severe shoulder pain in patients with a lower BMI was significantly higher than it was in patients with a higher BMI, and BMI was significantly negatively correlated with PLSP (Fig. 2, Fig. 3).
Table 1.
Basic characteristics of the patients’ shoudler pain (n = 392). Values are presented as number or proportion.
| No | Percent (%) | ||
|---|---|---|---|
| /392 | /186 | ||
| Some pain | 186 | 47.4 | 100 |
| Occasional | 56 | 14.3 | 30.1 |
| Intermittent | 72 | 18.4 | 38.7 |
| Constant | 58 | 14.7 | 31.2 |
| Site of pain | |||
| L-shoulder Only | 39 | 9.9 | 21 |
| R-shoulder Only | 55 | 14 | 29.6 |
| Both shoulders | 92 | 23.5 | 49.4 |
| Quality of shoulder pain | |||
| Prick/Sharp pain | 26 | 6.6 | 14 |
| Dull/Distending/Aching pain | 160 | 40.8 | 86 |
| Presence of shoulder pain | |||
| Operative day | 6 | 1.5 | 3 |
| The first postoperative day | 176 | 44.9 | 95 |
| The second postoperative day | 4 | 1 | 2 |
Table 2.
Comparison between the two groups and univariate binary logistic regression analysis of the potential influencing factors of LPSP. Values are presented as mean ± standard deviation or median (interquartile range). *P < 0.05.
| Variable | Absence shoulder pain (n = 206) | Have shoulder pain (n = 186) | Odds ration | 95% CI |
|---|---|---|---|---|
| Age (years) | 31.96 ± 3.56 | 29.96 ± 3.56 | 0.940 | (0.894–0.988)* |
| Height (m) | 1.62 ± 0.05 | 1.64 ± 0.04 | 5059.128 | (51.627–495,760.092)* |
| Weight (kg) | 66.63 ± 11.88 | 60.16 ± 9.53 | 0.944 | (0.925–0.964)* |
| BMI (kg/m2) | 25.3 ± 4.33 | 22.35 ± 3.31 | 0.815 | (0.767–0.866)* |
| Waist width (cm) | 84.89 ± 11.03 | 78.66 ± 8.32 | 0.934 | (0.913–0.957)* |
| Hip width (cm) | 96.29 ± 8.69 | 91.78 ± 6.68 | 0.927 | (0.901–0.954)* |
| Waist/Hip Ratio | 0.87 ± 0.05 | 0.86 ± 0.05 | 0.000 | (0.000–0.005)* |
| Waist/Height ratio | 0.52 ± 0.07 | 0.48 ± 0.05 | 0.000 | (0.000–0.000)* |
| Education (years) | 11.38 ± 2.9 | 11.38 ± 2.9 | 1.053 | (0.982–1.129) |
| Duration of surgery (min) | 85(60–115) | 85(57.75–120) | 0.999 | (0.995–1.004) |
| Duration of pneumoperitoneum (min) | 77(52–108) | 76.5(15–210) | 0.999 | (0.995–1.004) |
| Plateau airway pressure (P plat) | ||||
| before pneumoperitoneum (P plat1) | 11.66 ± 2.48 | 10.44 ± 1.91 | 0.763 | (0.685–0.850)* |
| 15 min after pneumoperitoneum (P plat2) | 19.7 ± 3.83 | 17.36 ± 3.1 | 0.820 | (0.768–0.876)* |
| P plat2-P plat1 | 8.04 ± 2.45 | 6.92 ± 2.15 | 0.807 | (0.736–0.885)* |
| Peak airway pressure (P peak) | ||||
| before pneumoperitoneum (P peak1) | 13.82 ± 3.11 | 12.21 ± 2.45 | 0.798 | (0.732–0.870)* |
| 15 min after pneumoperitoneum (P peak2) | 22.01 ± 4.38 | 19.51 ± 3.65 | 0.853 | (0.807–0.902)* |
| P peak2-P peak1 | 8.2 ± 2.51 | 7.3 ± 2.2 | 0.848 | (0.776–0.927)* |
| Partial pressure of end-tidal carbon dioxide(PetCO2) | ||||
| before pneumoperitoneum (PetCO2)1 | 31(29–34) | 32(30–34) | 1.011 | (0.945–1.080) |
|
15 min after pneumoperitoneum (PetCO2)2 |
37.5(36–40) | 38(36–40) | 1 | (0.939–1.066) |
| (PetCO2)2- (PetCO2)1 | 6(5–8) | 6(5–8) | 0.995 | (0.928–1.067) |
Figure 1.
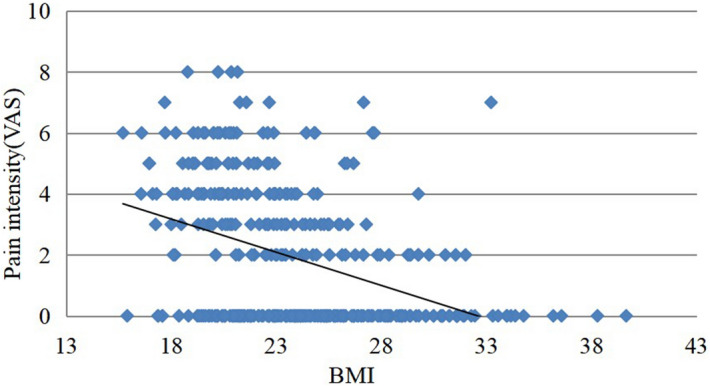
Shoulder pain scores in patients with different BMIs.
Table 3.
Incidence and intensity of shoudler pain between different BMI patients. Values are presented as mean ± standard deviation or number. There was no significant difference between BMI < 18.5 Group and 18.5 ≤ BMI < 23 group. There was significant difference between the other two groups (p < 0.05).
| BMI | Absence shoulder pain (n = 206) |
Mild shoulder pain (n = 78) | Moderate to intense shoulder pain (n = 108) | The highest VAS score (72 h after operation) |
|---|---|---|---|---|
| BMI < 18.5 (n = 25) | 6 | 5 | 14 | 4.76 ± 1.35 |
| 18.5 ≤ BMI < 23 (n = 164) | 64 | 26 | 74 | 4.94 ± 1.46 |
| 23 ≤ BMI < 30 (n = 165) | 103 | 43 | 19 | 3.71 ± 1.48 |
| BMI ≥ 30 (n = 38) | 33 | 4 | 1 | 3.15 ± 1.51 |
Figure 2.
The relationship between PLSP and BMI. The patients were grouped according to the Asian BMI standard. The distribution of the number of people with different degrees of shoulder pain in each group.
Figure 3.
The relationship between PLSP and BMI. The patients were grouped according to the Asian BMI standard. The proportion of patients with different degrees of shoulder pain in each group is shown.
Discussion
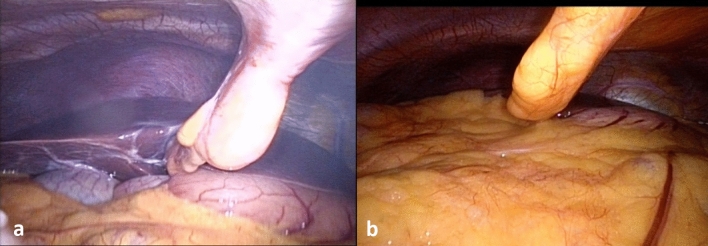
PLSP is generally considered to develop due to diaphragmatic irritation from direct injury or stretching of CO2 gas53. This study found that BMI was negatively correlated with PLSP. When placing the patient in the Trendelenburg position, we could clearly observe the anatomical structures of the left and right lobes of the liver, the lower margin of the liver, the gallbladder and the diaphragm in thin patients. There is a large space in the upper abdomen. There may be a lot of gas trapped here after the operation. In obese patients, we can only observe the omentum since the rest of the anatomical structure is covered by the omentum, and the upper abdominal space is very small. Gas is unlikely to be retained here after surgery (Fig. 4). Previous imaging studies have found that thin patients are more likely to experience free gas in the abdominal cavity whether they are undergoing laparotomy or laparoscopic surgery54,55. The volume of the residual pneumoperitoneum is positively correlated with the intensity of shoulder pain after laparoscopy53,56. This is one explanation for why PLSP is more common in thin patients.
Figure 4.
Upper abdominal imaging of two patients in the Trendelenburg position (30°). (A) 31Y, BMI = 19.3; (B) 32Y, BMI = 34.3.
Our study found that PLSP in the vast majority of patients occurred on the first day after surgery. Previous studies have reported that after laparoscopic surgery, visceral pain predominates in the first 24 h but subsides from a peak soon after the operation, whereas shoulder pain, minor on the operative day, worsens and becomes significant on the following day57. In addition, PLSP is less responsive to analgesics than wound pain21. Proponents of pre-emptive analgesia believe that the most effective pre-emptive analgesia programmes are those that can limit the sensitization of the nervous system throughout the perioperative period58,59. Because the time characteristics of PLSP are different from abdominal pain17,58, if we do not consider the time characteristics of PLSP but treat PLSP according to the same plan used for the prevention and treatment of pain of the incision and viscera, the peak plasma concentration may not coincide with the peak PLSP. This may be one of the reasons why traditional interventions cannot effectively alleviate PLSP59,60.
Predicting the postoperative pain and/or analgesic needs of individual patients as accurately as possible will help us tailor-made personalized postoperative analgesia methods for each patient, which will certainly reduce the occurrence of severe postoperative pain. Thus, patients undergoing so-called minor surgery should be monitored more closely15. For the management of PLSP, we can comply with existing methods but change their timing or duration of administration58. This requires us to identify high-risk patients in advance and develop specific programmes according to the time characteristics of their occurrence. These need further study.
The main limitation of our study is that we did not measure the amount of carbon dioxide left in the abdominal cavity. Therefore, the correlation between the amount of residual carbon dioxide in the abdominal cavity and the severity of pain, as well as its correlation with BMI, is not clear.
Conclusion
PLSP was negatively correlated with BMI. The occurrence of PLSP has obvious specific time characteristics. The results of the present study suggest that a series of studies according to the above clinical characteristics are required to explore measures to prevent or minimize shoulder pain resulting from this now well-standardized surgical procedure.
Acknowledgements
The authors would like to thank the nurses at the reproductive medicine centre of Yantai Yuhuangding Hospital of Qingdao University for assisting in data collection.
Author contributions
K.Z.L. and X.Y.L. initiated and designed the trial. M.T. and A.Z.L. contributed to the data collection. C.L.H. performed the primary data analysis. X.Y.L. drafted the manuscript. All authors contributed to the revision of the report for crucial intellectual content. K.Z.L. and X.Y.L. are the guarantors of this work, have full access to all of the data in the study, and assume full responsibility for the integrity of the data and the accuracy of the data analysis.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Eypasch E, Sauerland S, Lefering R, Neugebauer EA. Laparoscopic versus open appendectomy: Between evidence and common sense. Dig. Surg. 2002;19:518–522. doi: 10.1159/000067608. [DOI] [PubMed] [Google Scholar]
- 2.Wadlund DL. Laparoscopy: Risks, benefits and complications. Nurs. Clin. North. Am. 2006;41:219–229. doi: 10.1016/j.cnur.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 3.Maddern GJ, Rudkin G, Bessell JR, Devitt P, Ponte L. A comparison of laparoscopic and open hernia repair as a day surgical procedure. Surg. Endosc. 1994;48:1404–1408. doi: 10.1007/BF00187345. [DOI] [PubMed] [Google Scholar]
- 4.Ortega AE, Hunter JG, Peters JH, Swanstrom LL, Schirmer B. A prospective, randomized comparison of laparoscopic appendectomy with open appendectomy. Laparoscopic Appendectomy Study Group. Am. J. Surg. 1995;169:208–212. doi: 10.1016/S0002-9610(99)80138-X. [DOI] [PubMed] [Google Scholar]
- 5.Mais V, et al. Laparoscopic versus abdominal myomectomy: A prospective, randomized trial to evaluate benefits in early outcome. Am. J. Obstet. Gynecol. 1996;174:654–658. doi: 10.1016/S0002-9378(96)70445-3. [DOI] [PubMed] [Google Scholar]
- 6.Ellström M, et al. Pain and pulmonary function following laparoscopic and abdominal hysterectomy: A randomized study. Acta. Obstet. Gynecol. Scand. 1998;77:923–928. [PubMed] [Google Scholar]
- 7.Murphy AA, et al. Operative laparoscopy versus laparotomy for the management of ectopic pregnancy: A prospective trial. Fertil. Steril. 1992;57:1180–1185. doi: 10.1016/S0015-0282(16)55070-5. [DOI] [PubMed] [Google Scholar]
- 8.Holzer A, Jirecek ST, Illievich UM, Huber J, Wenzl RJ. Laparoscopic versus open myomectomy: A double-blind study to evaluate postoperative pain. Anesth. Analg. 2006;102:1480–1484. doi: 10.1213/01.ane.0000204321.85599.0d. [DOI] [PubMed] [Google Scholar]
- 9.Song T, et al. A prospective comparative study of cosmetic satisfaction for three different surgical approaches. Eur. J. Obstet. Gynecol. Reprod. Biol. 2015;190:48–51. doi: 10.1016/j.ejogrb.2015.04.014. [DOI] [PubMed] [Google Scholar]
- 10.Wei HB, et al. Laparoscopic versus open appendectomy: a prospective randomized comparison. Surg. Endosc. 2010;24:266–269. doi: 10.1007/s00464-009-0563-7. [DOI] [PubMed] [Google Scholar]
- 11.Valla JS, et al. Laparoscopic appendectomy in children: Report of 465 cases. Surg. Laparosc. Endosc. 1991;1:166–172. [PubMed] [Google Scholar]
- 12.Dixon JB, Reuben Y, Halket C, O’Brien PE. Shoulder pain is a common problem following laparoscopic adjustable gastric band surgery. Obes. Surg. 2005;15:1111–1117. doi: 10.1381/0960892055002149. [DOI] [PubMed] [Google Scholar]
- 13.Cunniffe MG, McAnena OJ, Dar MA, Calleary J, Flynn N. A prospective randomized trial of intraoperative bupivacaine irrigation for management of shoulder-tip pain following laparoscopy. Am. J. Surg. 1998;176:258–261. doi: 10.1016/S0002-9610(98)00150-0. [DOI] [PubMed] [Google Scholar]
- 14.Tobias JD. Pain management following laparoscopy: Can we do better? Saudi. J. Anaesth. 2013;7:3–4. doi: 10.4103/1658-354X.109553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gerbershagen HJ, et al. Pain intensity on the frst day afer surgery: A prospective cohort study comparing 179 surgical procedures. Anesthesiology. 2013;118:934–944. doi: 10.1097/ALN.0b013e31828866b3. [DOI] [PubMed] [Google Scholar]
- 16.Mouton WG, Bessell JR, Otten KT, Maddern GJ. Pain after laparoscopy. Surg. Endosc. 1999;13:445–448. doi: 10.1007/s004649901011. [DOI] [PubMed] [Google Scholar]
- 17.Alexander JI. Pain after laparoscopy. Br. J. Anaesth. 1997;79:369–378. doi: 10.1093/bja/79.3.369. [DOI] [PubMed] [Google Scholar]
- 18.Shokeir TA, Shalan HM, El-Shafei MM. Combined diagnostic approach of laparoscopy and hysteroscopy in the evaluation of female infertility: Results of 612 patients. J. Obstet. Gynaecol. Res. 2004;30:9–14. doi: 10.1111/j.1341-8076.2004.00147.x. [DOI] [PubMed] [Google Scholar]
- 19.Leelasuwattanakul N, Bunyavehchevin S, Sriprachittichai P. Active gas aspiration versus simple gas evacuation to reduce shoulder pain after diagnostic laparoscopy: A randomized controlled trial. J. Obstet. Gynaecol. Res. 2016;42:190–194. doi: 10.1111/jog.12868. [DOI] [PubMed] [Google Scholar]
- 20.Bunyavejchevin S, Prayoonwech C, Sriprajittichai P. Preemptive analgesic efficacy of parecoxib vs placebo in infertile women undergoing diagnostic laparoscopy: randomized controlled trial. J. Minim. Invasive Gynecol. 2012;19:585–588. doi: 10.1016/j.jmig.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 21.Lee DH, Song T, Kim KH, Lee KW. Incidence, natural course, and characteristics of postlaparoscopic shoulder pain. Surg. Endosc. 2018;32:160–165. doi: 10.1007/s00464-017-5651-5. [DOI] [PubMed] [Google Scholar]
- 22.Kim HY, et al. A randomized clinical trial on the effect of a lidocaine patch on shoulder pain relief in laparoscopic cholecystectomy. Sci. Rep. 2021;11:1052. doi: 10.1038/s41598-020-80289-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berberoglu M, Dilek ON, Ercan F, Kati I, Ozmen M. The effect of CO2 insufflation rate on the postlaparoscopic shoulder pain. J. Laparoendosc. Adv. Surg. Tech. A. 1998;8:273–277. doi: 10.1089/lap.1998.8.273. [DOI] [PubMed] [Google Scholar]
- 24.Asgari, Z. et al. Spinal anesthesia and spinal anesthesia with subdiaphragmatic lidocaine in shoulder pain reduction for gynecological laparoscopic surgery: a randomized clinical trial. Pain Res. Manag.2017, 1721460 (2017). [DOI] [PMC free article] [PubMed]
- 25.Rettenmaier MA, Micha JP, Lopez KL, Wilcox AM, Goldstein BH. A prospective, observational trial assessing the efficacy of abdominal compression in reducing laparoscopic-induced shoulder pain. Surg. Innov. 2017;24:552–556. doi: 10.1177/1553350617718080. [DOI] [PubMed] [Google Scholar]
- 26.Ghezzi F, et al. Minilaparoscopic versus conventional laparoscopic hysterectomy: results of a randomized trial. J. Minim. Invasive Gynecol. 2011;18:455–461. doi: 10.1016/j.jmig.2011.03.019. [DOI] [PubMed] [Google Scholar]
- 27.Mohamed KS, Abd-Elshafy SK, El Saman AM. The impact of magnesium sulfate as adjuvant to intrathecal bupivacaine on intra-operative surgeon satisfaction and postoperative analgesia during laparoscopic gynecological surgery: randomized clinical study. Korean J. Pain. 2017;30:207–213. doi: 10.3344/kjp.2017.30.3.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Asgari Z, Mozafar-Jalali S, Faridi-Tazehkand N, Sabet S. Intraperitoneal dexamethasone as a new method for relieving postoperative shoulder pain after gynecologic laparoscopy. Int. J. Fertil. Steril. 2012;6:59–64. [PMC free article] [PubMed] [Google Scholar]
- 29.Shin HY, Kim SH, Lee YJ, Kim DK. The effect of mechanical ventilation tidal volume during pneumoperitoneum on shoulder pain after a laparoscopic appendectomy. Surg. Endosc. 2010;24:2002–2007. doi: 10.1007/s00464-010-0895-3. [DOI] [PubMed] [Google Scholar]
- 30.Bogani G, et al. Low vs standard pneumoperitoneum pressure during laparoscopic hysterectomy: prospective randomized trial. J. Minim. Invasive Gynecol. 2014;21:466–471. doi: 10.1016/j.jmig.2013.12.091. [DOI] [PubMed] [Google Scholar]
- 31.Madsen MV, et al. Postoperative shoulder pain after laparoscopic hysterectomy with deep neuromuscular blockade and low-pressure pneumoperitoneum: A randomised controlled trial. Eur. J. Anaesthesiol. 2015;32:1–7. doi: 10.1097/EJA.0000000000000174. [DOI] [PubMed] [Google Scholar]
- 32.Kerimoglu OS, et al. Effect of drainage on postoperative pain after laparoscopic ovarian cystectomy. J. Obstet. Gynaecol. 2015;35:287–289. doi: 10.3109/01443615.2014.948824. [DOI] [PubMed] [Google Scholar]
- 33.Raymond AP, et al. A comparative, single blind, randomized trial of pain associated with suction or non-suction drains after gynecological laparoscopy. J. Minim. Invasive Gynecol. 2010;17:16–20. doi: 10.1016/j.jmig.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 34.Craciunas L, Stirbu L, Tsampras N. The use of a peritoneal gas drain following gynecological laparoscopy: a systematic review. Eur. J. Obstet. Gynecol. Reprod. Biol. 2014;179:224–228. doi: 10.1016/j.ejogrb.2014.04.012. [DOI] [PubMed] [Google Scholar]
- 35.Marks JL, Ata B, Tulandi T. Systematic review and metaanalysis of intraperitoneal instillation of local anesthetics for reduction of pain after gynecologic laparoscopy. J. Minim. Invasive Gynecol. 2012;19:545–553. doi: 10.1016/j.jmig.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 36.Collins GG, et al. Surgical pain control with ropivacaine by atomized delivery (spray): A randomized controlled trial. J. Minim. Invasive Gynecol. 2016;23:40–45. doi: 10.1016/j.jmig.2015.07.018. [DOI] [PubMed] [Google Scholar]
- 37.Andrews V, Wright JT, Zakaria F, Banerjee S, Ballard K. Continuous infusion of local anaesthetic following laparoscopic hysterectomy—A randomised controlled trial. BJOG. 2014;121:754–760. doi: 10.1111/1471-0528.12610. [DOI] [PubMed] [Google Scholar]
- 38.Ryu K, Choi W, Shim J, Song T. The impact of a pulmonary recruitment maneuver to reduce post-laparoscopic shoulder pain: A randomized controlled trial. Eur. J. Obstet. Gynecol. Reprod. Biol. 2017;208:55–60. doi: 10.1016/j.ejogrb.2016.11.014. [DOI] [PubMed] [Google Scholar]
- 39.Tsai HW, et al. Prevention of postlaparoscopic shoulder and upper abdominal pain: a randomized controlled trial. Obstet. Gynecol. 2012;121:526–531. doi: 10.1097/AOG.0b013e318283fcca. [DOI] [PubMed] [Google Scholar]
- 40.Frishman GN. Shoulder pain following laparoscopic surgery: can we block the referral? J. Minim. Invasive. Gynecol. 2014;21:504. doi: 10.1016/j.jmig.2014.05.008. [DOI] [PubMed] [Google Scholar]
- 41.Johnson PL, Sibert KS. Laparoscopy. Gasless vs. CO2 pneumoperitoneum. J. Reprod. Med. 1997;42:255–259. [PubMed] [Google Scholar]
- 42.Goldberg JM, Maurer WG. A randomized comparison of gasless laparoscopy and CO2 pneumoperitoneum. Obstet. Gynecol. 1997;90:416–420. doi: 10.1016/S0029-7844(97)00279-2. [DOI] [PubMed] [Google Scholar]
- 43.Guido RS, Brooks K, McKenzie R, Gruss J, Krohn MA. A randomized, prospective comparison of pain after gasless laparoscopy and traditional laparoscopy. Am. Assoc. Gynecol. Laparosc. 1998;5:149–153. doi: 10.1016/S1074-3804(98)80081-9. [DOI] [PubMed] [Google Scholar]
- 44.Herrmann, A. & De Wilde, R. L. Insufflation with humidified and heated carbon dioxide in short-term laparoscopy: A double-blinded randomized controlled trial. Biomed. Res. Int. 2015, 412618 (2015). [DOI] [PMC free article] [PubMed]
- 45.Balayssac D, et al. Warmed and humidified carbon dioxide for abdominal laparoscopic surgery: meta-analysis of the current literature. Surg. Endosc. 2017;31:1–12. doi: 10.1007/s00464-016-4866-1. [DOI] [PubMed] [Google Scholar]
- 46.Demco L. Effect of heating and humidifying gas onpatients undergoing awake laparoscopy. J. Am. Assoc. Gynecol. Laparosc. 2001;8:247–251. doi: 10.1016/S1074-3804(05)60585-3. [DOI] [PubMed] [Google Scholar]
- 47.Sroussi J, et al. Low pressure gynecological laparoscopy (7mmHg) with AirSeal® System versus a standard insufflation (15mmHg): A pilot study in 60 patients. J. Gynecol. Obstet. Hum. Reprod. 2017;46:155–158. doi: 10.1016/j.jogoh.2016.09.003. [DOI] [PubMed] [Google Scholar]
- 48.Suginami R, Taniguchi F, Suginami H. Prevention of post laparoscopic shoulder pain by forced evacuation of residual CO2. JSLS. 2009;13:56–59. [PMC free article] [PubMed] [Google Scholar]
- 49.Thomson AJ, et al. Assessment of a method to expel intraperitoneal gas after gynecologic laparoscopy. J. Minim. Invasive Gynecol. 2005;12:125–129. doi: 10.1016/j.jmig.2005.01.020. [DOI] [PubMed] [Google Scholar]
- 50.Nutthachote P, Sirayapiwat P, Wisawasukmongchol W, Charuluxananan S. A randomized, double-blind, placebo-controlled trial of oral pregabalin for relief of shoulder pain after laparoscopic gynecologic surgery. J. Minim. Invasive Gynecol. 2014;21:669–673. doi: 10.1016/j.jmig.2014.01.018. [DOI] [PubMed] [Google Scholar]
- 51.van Ee R, Hemrika DJ, van der Linden CT. Pain relief following day-case diagnostic hysteroscopy-laparoscopy for infertility: A double-blind randomized trial with preoperative naproxen versus placebo. Obstet. Gynecol. 2015;82:951–954. [PubMed] [Google Scholar]
- 52.Rudin A, Wolner-HansseHellbom M, Werner MU. Prediction of postoperative pain after a laparoscopic tubal ligation procedure. Acta Anaesthesioln. Scand. 2008;52:938–945. doi: 10.1111/j.1399-6576.2008.01641.x. [DOI] [PubMed] [Google Scholar]
- 53.Jackson SA, Laurence AS, Hill JC. Does postlaparoscopy pain relate to residual carbon dioxide? Anaesthesia. 1996;51:485–487. doi: 10.1111/j.1365-2044.1996.tb07798.x. [DOI] [PubMed] [Google Scholar]
- 54.Gayer G, Hertz M, Zissin R. Postoperative pneumoperitoneum: prevalence, duration, and possible significance. Semin. Ultrasound CT. MR. 2004;25:286–289. doi: 10.1053/j.sult.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 55.Gayer G, et al. Postoperative pneumoperitoneum as detected by CT: Prevalence, duration, and relevant factors affecting its possible significance. Abdom. Imaging. 2000;25:301–305. doi: 10.1007/s002610000036. [DOI] [PubMed] [Google Scholar]
- 56.Song T, Kim KH, Lee KW. The intensity of postlaparoscopic shoulder pain is positively correlated with the amount of residual pneumoperitoneum. J. Minim. Invasive Gynecol. 2017;24:984–989.e1. doi: 10.1016/j.jmig.2017.06.002. [DOI] [PubMed] [Google Scholar]
- 57.Choi JB, et al. Pain characteristics after total laparoscopic hysterectomy. Int. J. Med. Sci. 2016;13:562–587. doi: 10.7150/ijms.15875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gottschalk A, Smith DS. New concepts in acute pain therapy: preemptive analgesia. Am. Fam. Physician. 2001;63:1979–1984. [PubMed] [Google Scholar]
- 59.Woolf CJ, Chong MS. Preemptive analgesia–treating postoperative pain by preventing the establishment of central sensitization. Anesth. Analg. 1993;77:362–379. doi: 10.1213/00000539-199377020-00026. [DOI] [PubMed] [Google Scholar]
- 60.Kissin I. Preemptive analgesia: Why its effect is not always obvious. Anesthesiology. 1996;84:1015–1019. doi: 10.1097/00000542-199605000-00001. [DOI] [PubMed] [Google Scholar]