Abstract
MYCN activation is a hallmark of advanced neuroblastoma (NB) and a known master regulator of metabolic reprogramming, favoring NB adaptation to its microenvironment. We found that the expression of the main regulators of the molecular clock loops is profoundly disrupted in MYCN-amplified NB patients, and this disruption independently predicts poor clinical outcome. MYCN induces the expression of clock repressors and downregulates the one of clock activators by directly binding to their promoters. Ultimately, MYCN attenuates the molecular clock by suppressing BMAL1 expression and oscillation, thereby promoting cell survival. Reestablishment of the activity of the clock activator RORα via its genetic overexpression and its stimulation through the agonist SR1078, restores BMAL1 expression and oscillation, effectively blocks MYCN-mediated tumor growth and de novo lipogenesis, and sensitizes NB tumors to conventional chemotherapy. In conclusion, reactivation of RORα could serve as a therapeutic strategy for MYCN-amplified NBs by blocking the dysregulation of molecular clock and cell metabolism mediated by MYCN.
Subject terms: Cancer, Cell biology
MYCN is frequently amplified in neuroblastomas. Here, the authors show that MYCN disrupts the molecular clock by downregulating clock activator RORα and that the reactivation of RORα restores BMAL1 activity, and inhibits lipid metabolism and neuroblastoma growth
Introduction
Oncogenic expression of the MYC family members (e.g., MYC and MYCN) drives many human cancers;1 however, strategies aiming at chemically disrupting their functions have proven difficult to develop. The MYCN oncogene is amplified in almost half of all high-risk neuroblastomas (NBs) and is the primary oncogene driving this malignancy2. Overexpressed MYCN causes spontaneous, high-penetrance NB in mice, originating from neuroblastic precursor cells3. In tumors with activated MYC or MYCN, the high demand for growth and biomass accumulation required for tumor progression is achieved by metabolic reprogramming4. MYC drives several metabolic pathways associated with cell growth, including increased glucose5 and glutamine uptake6, stimulation of mitochondrial biogenesis7 and nucleotide synthesis8, and enhanced de novo lipid synthesis9. In particular, MYC directly activates the expression of lipogenic enzymes (e.g., ACACA, FASN, and SCD1), promoting de novo fatty acid (FA) synthesis from citrate10. In addition, MYC interacts with the related transcriptional regulator MondoA to control SREBP1-dependent lipid biosynthesis11. However, far less is known about MYCN regulation of cell metabolism. MYCN loss of function suppresses FA β-oxidation and leads to lipid droplet accumulation in NB cells12, suggesting that aggressive NBs require FA as an energy source.
Cellular metabolism and circadian rhythm intimately crosstalk13, and MYC regulates rhythmic cell metabolism14. Moreover, genetic ablation of clock genes enhances glucose and glutamine consumption in vivo15. The core molecular clock is composed of the heterodimer CLOCK/BMAL1, which activates transcription of the PER and CRY genes via binding at specific E-box sequences. In turn, PER1/2 and CRY1/2 suppress CLOCK/BMAL116. This transcriptional feedback loop ensures a 24-h rhythm. Retinoic acid receptor-related orphan receptor alpha (RORα) and REV-ERBα (also known as nuclear receptor subfamily 1, group D member 1, NR1D1) are the two major regulators of both expression and oscillation of the clock central component BMAL1 (ARNTL) and compete for the ROR response element (RORE) within the BMAL1 promoter17,18. RORα activates, whereas REV-ERBα suppresses BMAL1 transcription. MYC and MYCN have been shown to directly induce REV-ERBα expression, dampening BMAL1 expression and oscillation14. In addition, MYC/Myc-interacting zinc-finger protein-1 (MIZ1) complexes are recruited to non-E-box sites in the BMAL1 and CLOCK promoters independently of REV-ERB19. However, MYCN regulation of the molecular clock and its effects on tumor phenotype remain largely unknown. The clock activator RORα has been identified as a putative tumor suppressor and is repressed in many cancer types20–23. Mechanistically, RORα acts by inducing SEMA3F20 and p53 signaling24 and suppressing Wnt/βcatenin signaling25. Similarly, BMAL1 is epigenetically inactivated in hematologic malignancies26, and ectopic expression enhances sensitivity to chemotherapy27 and reduces clonogenicity of NB cells14, further supporting its potential tumor-suppressive role.
RORs and REV-ERBs are known as orphan nuclear receptors; however, the subsequent identification of their endogenous ligands28,29 led to the development of synthetic ligands targeting these receptors. Because the clock components can affect tumorigenesis, pharmacological modulation of the circadian clock is emerging as a promising approach for therapeutic intervention. Recent reports found two REV-ERBs agonists (SR9009 and SR9011) were lethal to cancer cells by targeting de novo lipogenesis and autophagy30. These clock-modulating compounds are also active against metabolic diseases, including obesity and diabetes18,31. The synthetic ligand SR1078 functions as a RORα/γ agonist29 and selectively binds to the ligand-binding domain of RORα/γ receptors, inducing conformational changes resulting in recruitment of co-activators and stimulation of transcription29. In this study, we asked whether dysregulation of the MYCN-operated clock had functional significance and whether restoring the positive arm of the clock would interfere with that dysregulation, opposing MYCN-driven oncogenesis. Mechanistically, we have shown that MYCN attenuates the clock in NB by downregulating clock activators and inducing clock repressors. Restoration of the molecular clock reestablishes cellular lipid metabolism and efficiently blocks NB tumor growth.
Results
Clock gene expression is altered in MYCN-amplified patients and predicts poor clinical outcomes
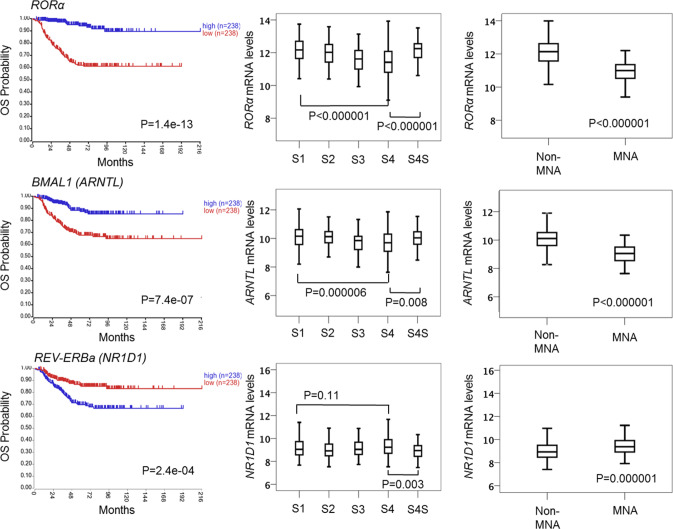
To establish the potential clinical relevance of MYCN regulation of the clock, we examined how the expression of the main clock component (BMAL1) and its activator (RORα) and repressor (REV-ERBα) related to clinical outcome and MYCN amplification in human NB primary tumors. We used transcriptomic data from three patient cohorts: Kocak (n = 649, GEO: GSE45547, patient cohort 1), Versteeg (n = 88, GEO: GSE16476 88/122, patient cohort 2), and NB Research Consortium (NRC; n = 283, GEO: GSE85047, patient cohort 3) (R2: genomics analysis and visualization platform, http://r2.amc.nl). Kaplan–Meier analysis in cohort 1 revealed that high RORα and BMAL1 (ARNTL) expression is strongly associated with a favorable clinical outcome and stage, while high REV-ERBα (NR1D1) expression correlates with a poor outcome and stage (Fig. 1). These findings were validated across multiple patient cohorts (Supplementary Fig. 1a, patient cohort 2) and mark the positive arm of the clock as a potential tumor suppressor. Notably, the expression of both RORα and BMAL1 is strongly repressed in MYCN-amplified (MNA) NB (cohort 1, Fig. 1; cohort 2, Supplementary Fig. 1a), and this correlates with poor overall (OS) and progression-free survival (PFS) in all patient cohorts (Fig. 1 and Supplementary Fig. 1). This association is retained when the analysis is restricted to stage 3 and 4 patients (Supplementary Fig. 1b, c). Conversely, REV-ERBα expression is significantly higher in MNA NB (cohort 1, Fig. 1; cohort 2, Supplementary Fig. 1a). Notably, we found that RORα expression predicts outcome independently of current prognostic factors, including age at diagnosis, International Neuroblastoma Staging System (INSS) stage, and MYCN status (Supplementary Table 1). Collectively, our data define the low expression of the positive arm of the clock (RORα-BMAL1) as an unfavorable prognostic marker in NB.
Fig. 1. Clock gene expression is altered in primary NB tumor samples and correlates with poor clinical outcomes.
Kaplan-Meier analysis of overall survival (OS) in patient cohort 1 (Kocak n = 476, GEO: GSE45547). Graphs depict p-values corrected for multiple testing (Bonferroni correction) of cutoff levels for RORα, BMAL1, and REV-ERBα. Correlations between clock gene expression, INSS stages, and MYCN amplification status are shown. MNA = MYCN-amplified; S1–4S = stage1-4S; 476 of the 649 samples were annotated with survival data. The box plot is defined by two lines at the 25th and 75th percentile. A line is drawn inside the box at the 50th percentile.
MYCN disrupts the molecular clock by direct binding to clock gene promoters
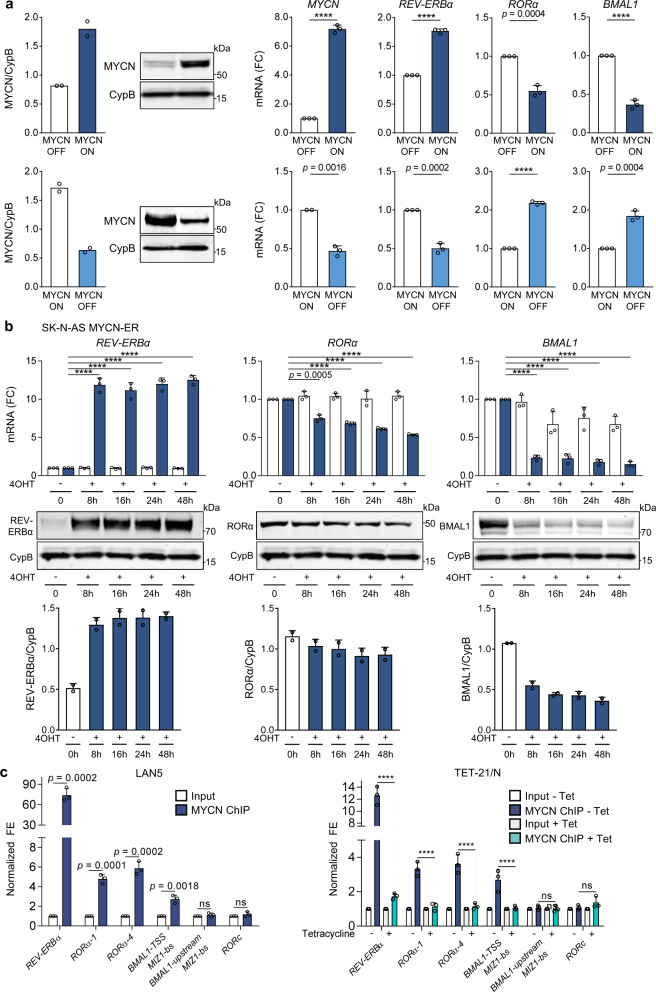
Due to the significant association between circadian genes and MYCN expression in patients, we next examined whether MYCN could alter clock gene expression. To test this hypothesis, we conditionally overexpressed MYCN in MYCN3 (Tet-ON) cells and conditionally silenced MYCN in LAN5 MNA cells (LAN5 ShMYCN). Ectopic MYCN expression resulted in significant upregulation of the circadian repressor REV-ERBα, and downregulation of the circadian activators RORα and BMAL1. However, depletion of MYCN completely restored their expression levels (Fig. 2a). We then used a human NB line SK-N-AS that lacks amplification of MYCN and stably expresses inducible wild-type MYCN-ERTM (estrogen receptor tamoxifen mutant). When stimulated with 4-hydroxytamoxifen (4OHT), MYCN-ER translocates into the nucleus and upregulates MYCN targets14. MYCN transcriptional activation led to significant upregulation of REV-ERBα and downregulation of RORα and BMAL1 both at mRNA and protein levels (Fig. 2b). As a control, we demonstrated that both doxycycline (DOX) and 4OHT do not alter clock gene expression (DBP, BMAL1, and PER2) in parental LAN5 and SK-N-AS cells (Supplementary Fig. 2). Collectively, these data support a role for MYCN in altering circadian gene expression, and specifically suppressing the positive arm of the clock.
Fig. 2. MYCN directly binds to clock gene promoters and downregulates the positive arm of the clock.
a Left panel: MYCN protein expression in MYCN3 (MYCN Tet-ON) and LAN5 ShMYCN cells after DOX (1 µg/ml) induction for 48 and 72 h, respectively. Protein expression was analyzed by densitometry (Image J v1.42q). CypB served as a loading control and MYCN/CypB ratio was determined (n = 2 independent experiments). Right panel: mRNA levels of MYCN, REV-ERBα, RORα, and BMAL1 in MYCN3 (MYCN Tet-ON) and LAN5 ShMYCN cells following DOX induction for 48 and 72 h, respectively. Data are mean ± SD (n = 3; ****p < 0.0001; two-tailed unpaired t-test). b mRNA and protein expression of REV-ERBα, RORα, and BMAL1 in SK-N-AS MYCN-ER cells with and without 4OHT (1 µM) for 0, 8, 16, 24, and 48 h. mRNA data are mean ± SD (n = 3; ****p < 0.0001; two-tailed unpaired t-test). Protein expression data were analyzed by densitometry (Image J v1.42q). CypB served as loading control and REV-ERBα/RORα/BMAL1/CypB ratios were determined (n = 2). REV-ERBα and RORα were run on the same gel. c MYCN ChIP-qPCR assays in LAN5 and Tet-21/N (MYCN Tet-OFF) cells following tetracycline treatment (2 μg/ml for 48 h). Input (white bars) and MYCN IP (blue bars) samples were analyzed by q-PCR using specific primers for REV-ERBα, RORα, BMAL1, and RORc (Supplementary Table 3). Data are mean ± SD (n = 3; ****p < 0.0001; two-way ANOVA with Dunnett’s multiple comparisons test). FC fold change, FE fold enrichment.
We then asked whether MYCN could also disrupt circadian rhythmicity. We subjected DOX-induced MYCN3 and LAN5 ShMYCN cells to real-time luminescence analysis using a previously established Per2-luc adenovirus reporter32 and found that MYCN induction also impairs the circadian oscillation of PER2 by both lengthening the circadian period and reducing the circadian amplitude (Supplementary Fig. 3). These results are consistent with previously reported impaired BMAL1 oscillation in MYC overexpressing cell lines14.
To address the mechanism of MYCN regulation of the clock, we performed MYCN chromatin immunoprecipitation (ChIP)-qPCR analysis in both MNA cells and MYCN Tet-OFF (Tet-21/N) cells, in which MYCN is turned off upon DOX treatment (Fig. 2c and Supplementary Fig. 4). We found significant enrichment of MYCN binding to the promoter regions of REV-ERBα, RORα (transcript variants 1 and 4), and BMAL1 in both MNA cell lines. Moreover, turning off MYCN fully abrogates MYCN binding (Fig. 2c). These findings suggest that MYCN directly attenuates the clock by repressing the clock activators RORα and BMAL1, and inducing the clock repressor REV-ERBα. Because MYCN induces transcriptional repression by indirectly binding to DNA in part through interactions with MIZ133, we performed MYCN ChIP-qPCR analysis in LAN5 cells upon genetic depletion of MIZ1 (LAN5 ShMIZ1) to determine whether loss of MIZ1 altered MYCN occupancy at clock activators genes. Effective knockdown of MIZ1 (Supplementary Fig. 5a, b) significantly reduces MYCN binding at the promoter regions of BMAL1 and RORα (Supplementary Fig. 5c, d), suggesting that MYCN repression of the clock requires MIZ1.
RORα activity rescues MYCN-mediated repression of BMAL1 expression and oscillation
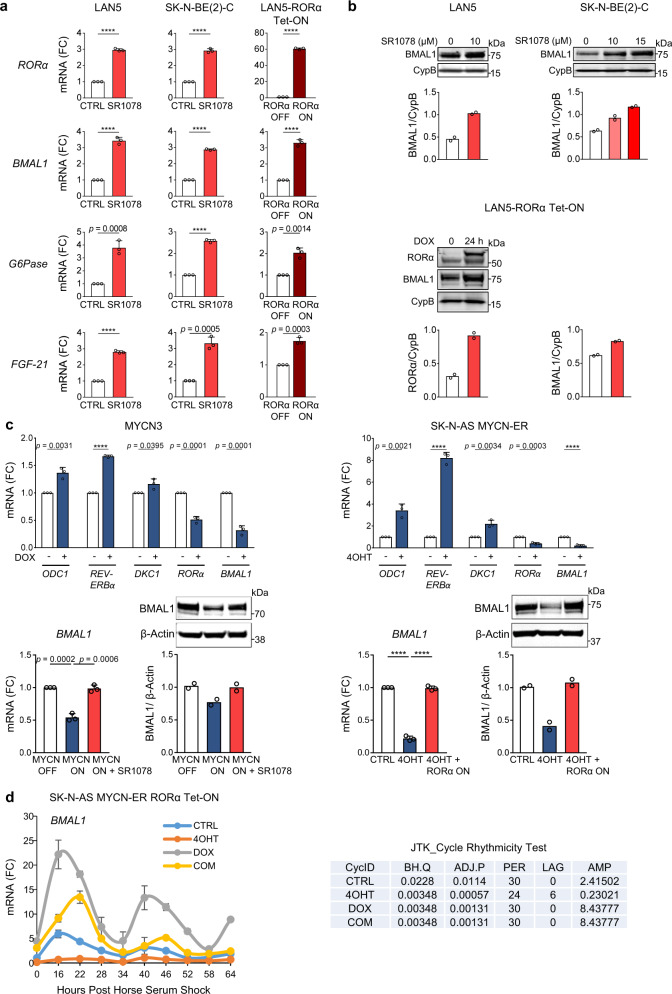
Synthetic ligands for RORs have been recently characterized, including the RORα/γ agonist SR107834. Unlike most family members, the RORs recognize and bind as monomers to specific sequences of DNA (ROR response elements, RORE) in the regulatory region of the target genes. When bound to these elements, they constitutively recruit co-activators resulting in continual transcriptional activation35. To test whether RORα signaling is active in NB cells, we used two approaches: RORα pharmacological activation (via the agonist SR1078) and RORα genetic overexpression. Two MNA cell lines (LAN5 and SK-N-BE(2)-C) were treated with SR1078 and the mRNA expression of RORα target genes containing a RORE in their promoters (G6Pase, FGF-21, and BMAL1)36 was determined. SR1078 effectively activates target gene transcription in both cell lines (Fig. 3a). We next examined whether genetic overexpression of RORα could activate downstream signaling as well. To ectopically express RORα, we inserted a lentiviral DOX-inducible vector containing the sequence of RORα (isoform 1) into MNA LAN5 cells because these cells showed relatively low RORα expression compared to other cell lines tested. Conditional RORα overexpression resulted in robust transcriptional activation of its target genes34 (Fig. 3a). Furthermore, SR1078 and the genetic overexpression of RORα efficiently induced BMAL1 protein expression in both systems (Fig. 3b).
Fig. 3. RORα activity rescues MYCN-mediated repression of BMAL1 expression and oscillation.
a mRNA expression of RORα target genes (BMAL1, G6Pase, and FGF-21) in MNA LAN5 and SK-N-BE(2)-C cells treated with SR1078 (10 µM for 24 h), and LAN5 RORα Tet-ON cells cultured with (RORα-ON) and without (RORα-OFF) DOX (2 µg/ml) for 48 h. Data are mean ± SD (n = 3; ****p < 0.0001; two-tailed unpaired t-test). b Upper panel: BMAL1 protein expression in LAN5 and SK-N-BE(2)-C cells treated with SR1078. Lower panel: RORα and BMAL1 protein expression in LAN5 RORα Tet-ON cells cultured with (RORα-ON) and without (RORα-OFF) DOX (2 µg/ml) for 24 h. Data were analyzed by densitometry (Image J v1.42). CypB served as a loading control and BMAL1/RORα/CypB ratios were determined (n = 2). c Upper panel: mRNA expression of MYCN target genes in MYCN3 cells treated with (MYCN-ON) and without (MYCN-OFF) DOX (1 µg/ml) for 48 h, and in SK-N-AS MYCN-ER cells treated with and without 4OHT (1 µM) for 48 h. Data are mean ± SD (n = 3; ****p < 0.0001; two-tailed unpaired t-test). Lower panel: BMAL1 mRNA and protein expression in MYCN3 cells under MYCN-OFF, MYCN-ON, and MYCN-ON + SR1078 (5 µM for 24 h) conditions. BMAL1 mRNA and protein expression in SK-N-AS MYCN-ER RORα Tet-ON cells upon MYCN activation (4OHT 1 µM for 24 h) with and without RORα overexpression (DOX 2 µg/ml for 24 h). Protein expression data were analyzed by densitometry (Image J v1.42q) and BMAL1/CypB ratios determined (n = 2). d SK-N-AS MYCN-ER RORα cells were cultured with and without 4OHT (MYCN-ON/OFF), DOX (RORα-ON/OFF), and 4OHT + DOX for 24 h and then synchronized with 50% horse serum for 2 h. Cells were collected every 6 h from 16 to 64 h after synchronization. 4-OHT and DOX treatments were applied during and after cell synchronization. BMAL1 mRNA expression was determined by q-PCR and normalized to 18 S expression. Data are mean ± SEM (n = 2, p < 0.0001 at all conditions and time points by two-way ANOVA with Tukey’s multiple comparisons test). BMAL1 oscillates at all conditions (p < 0.05) according to the JTK-rhythmicity test. FC fold change.
We then asked whether SR1078 and RORα overexpression could rescue MYCN-mediated repression of BMAL1 expression and oscillation. For this purpose, we employed two inducible MYCN cell systems: MYCN3 (Tet-ON) and SK-N-AS MYCN-ER cells. As expected, all known and identified MYCN-target genes (ODC1, REV-ERBα, DKC1, RORα, and BMAL1) were significantly induced or repressed upon turning on MYCN. Notably, BMAL1 mRNA and protein levels were repressed in both systems upon MYCN activation. However, SR1078 treatment and RORα genetic overexpression were both able to completely rescue BMAL1 mRNA and protein levels (Fig. 3c). Moreover, active MYCN-ER (MYCN-ON) greatly impaired circadian oscillation of BMAL1 in SK-N-AS MYCN-ER cells. However, overexpression of RORα was capable of both restoring BMAL1 expression, and also fully rescuing its circadian oscillation (Fig. 3d). Similarly, turning ON RORα highly enhanced BMAL1 oscillation in MNA cells (Supplementary Fig. 6). Collectively, these data indicate that RORα activity effectively counteracts MYCN-mediated repression of BMAL1 expression and oscillation.
MYC and other cell cycle regulators have been shown to be clock-regulated37, suggesting that multiple feedback loops exist between MYC and the components of the molecular clock. Thus, we asked whether restoration of the clock could alter c-MYC and MYCN expression. MYCN protein stabilization was notably decreased by ROR agonist SR1078 in MNA cells (LAN5 and SK-N-BE(2)C); however, no changes in c-MYC protein levels were detected in non-MNA c-MYC overexpressing cells (SH-SY5Y) (Supplementary Fig. 7), suggesting that this clock-mediated regulation is MYCN-dependent.
Restoration of BMAL1 via RORα activation inhibits cell growth and lipogenic gene expression
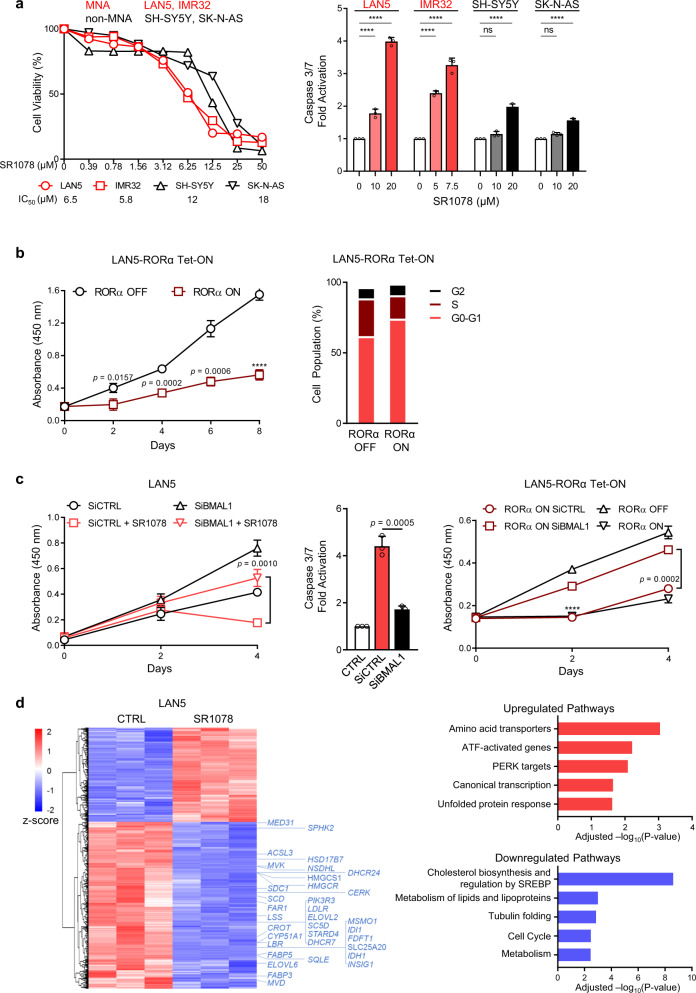
Because MYCN repression of the clock correlates with poor clinical outcomes, we then asked whether restoration of the clock via activation of RORα could have a functional significance and alter NB cell phenotype. SR1078 inhibits cell growth in both MNA and non-MNA cell lines. However, this effect is greater in MNA compared to non-MNA cells. Similarly, MNA cells show higher caspase-mediated apoptosis following SR1078 treatment (Fig. 4a). Genetic overexpression of RORα (RORα Tet-ON) also profoundly restricts cell growth and induces cell cycle arrest (Fig. 4b and Supplementary Fig. 8). However, depletion of BMAL1 (Supplementary Fig. 9) promotes cell growth and almost completely rescues the block in cell viability and the apoptosis induced both by SR1078 and RORα overexpression (Fig. 4c), suggesting that the anti-tumor activity of RORα is dependent on BMAL1. Because cell viability assays such as MTT and CCK-8 sense metabolic processes, we used cell counting as an alternative method and confirmed the same changes in cell proliferation (Supplementary Fig. 10). In addition, because SR1078 can stabilize p5324, we tested whether this also occurs in NB. We detected no changes in p53 protein levels upon SR1078 treatment in multiple MNA lines. Moreover, genetic depletion of p53 did not affect the response to SR1078 (Supplementary Fig. 11), suggesting that SR1078 alters cell viability in a p53-independent manner.
Fig. 4. RORα activation inhibits cell survival via BMAL1 and constrains lipogenic gene expression.
a Cell viability (MTT assay) and apoptosis (caspase 3/7 assay) in MNA (LAN5, IMR32) and non-MNA (SH-SY5Y, SK-N-AS) cells following treatment with SR1078 for 24 h (apoptosis) and 72 h (cell viability). IC50 values are shown in the lower panel. Cell viability data are mean ± SD and are representative from n = 2 biological replicates (n = 4 technical replicates). MNA cell lines in red; non-MNA cell lines in black. Apoptosis data are mean ± SD (n = 3; two-way ANOVA with Tukey’s multiple comparisons test). b Cell proliferation of LAN5 RORα Tet-ON cells cultured with (RORα-ON) and without (RORα-OFF) DOX over 8 days. Data are mean ± SD (n = 3; ****p < 0.0001; two-tailed unpaired t-test). Cell cycle analysis of LAN5 RORα-OFF and LAN5 RORα-ON cells following 10 days of DOX (2 µg/ml) induction. Data are mean ± SD (n = 2). c Left panel: cell growth of LAN5 SiCTRL and LAN5 SiBMAL1 cells in the presence and absence of SR1078 (5 µM) for 4 days. Data are mean ± SD (n = 3; two-tailed unpaired t-test). Middle panel: Caspase 3/7 activation in LAN5 SiCTRL and LAN5 SiBMAL1 cells following SR1078 treatment (10 µM for 24 h). Data are mean ± SD (n = 3; two-tailed unpaired t-test). Right panel: cell growth of LAN5 RORα SiCTRL and LAN5 RORα SiBMAL1 cells treated with (+Tet, RORα-ON) and without (−Tet, RORα-OFF) DOX for 4 days. Data are mean ± SD (n = 3, ****p < 0.0001; two-tailed unpaired t-test). d Left panel: Heat map of differentially expressed genes in LAN5 cells following SR1078 treatment (10 μM for 8 h). Right panel: Most enriched up- and downregulated pathways by SR1078 in LAN5 cells (p < 0.05). Blue = downregulated genes, red = upregulated genes.
To further elucidate the molecular mechanisms by which SR1078 impairs cell survival, we performed RNA-sequencing analysis in LAN5 cells with and without SR1078 treatment for 8 h. A total of 712 genes were differentially regulated (p < 0.05): 459 downregulated and 253 upregulated (Fig. 4d). Reactome pathway enrichment analysis showed that the most downregulated biological processes were cholesterol biosynthesis and regulation by SREBP (adjusted p = 2.44E−09), metabolism of lipids and lipoproteins (adjusted p = 1.01E−03), and tubulin folding (adjusted p = 1.39E−03), while amino acid transporters (adjusted p = 9.13E−04) were the most upregulated (Fig. 4d and Supplementary Data 1), suggesting that restoration of the clock alters cell metabolism. Overall, 34 out of the 459 SR1078-downregulated genes belonged to the top enriched lipid pathways (cholesterol biosynthesis and regulation by SREBP, and metabolism of lipids and lipoproteins, in blue, Supplementary Fig. 12a). We further selected ten genes specifically involved in cholesterol synthesis (IDI-1, HMGR, HMGCS1, and MVK2) and FA metabolism (FABP3, FABP5, ELOVL2, ELOVL6, ACSL3, and SCD1) and validated their expression after SR1078 treatment by q-PCR in MNA cells. Both these gene sets were significantly downregulated by SR1078 in LAN5 cells (Supplementary Fig. 12b). Cell metabolism is tightly controlled by the molecular clock38 as both metabolites and metabolic gene expression are subject to circadian oscillations39. Altogether, these data indicate that activation of RORα inhibits NB cell survival by restoring BMAL1 expression and constraining lipogenic gene expression.
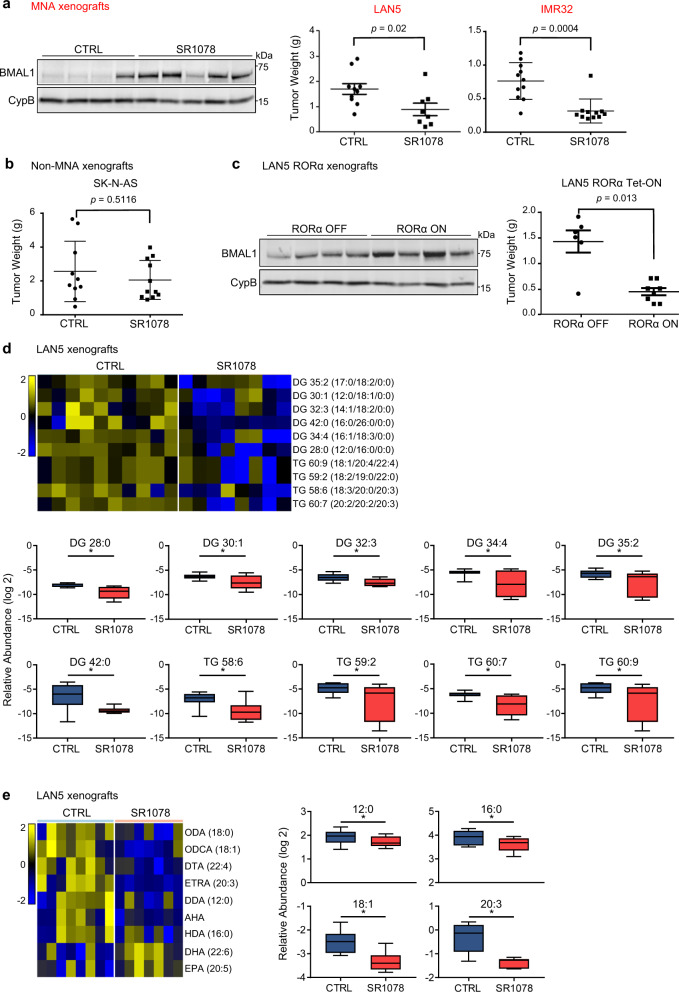
Activation of RORα restores BMAL1 and blocks NB tumor growth by inhibiting lipid metabolism
Our patient and in vitro data both suggest that clock activators RORα and BMAL1 are downregulated in MNA NB. These data also suggest that activation of RORα restricts cell survival. We, therefore, examined the in vivo anti-tumor activity of restoring RORα function via SR1078 and genetic overexpression. For this purpose, we used an orthotopic NB mouse model and generated MNA xenografts by implanting MNA cells under the renal capsule of nude mice. This model faithfully recapitulates the aggressive and highly vascular phenotype of primary NB40. LAN5 cells were engrafted and SR1078 treatment was initiated 2 weeks after implantation. Vehicle control and SR1078 (15 mg/kg) were given i.p. daily for 14 days, and the effect on tumor growth was compared between groups at the end of the treatment. SR1078 treatment resulted in significant inhibition of tumor growth (p = 0.02). The ability of SR1078 to block tumor growth was confirmed in a second MNA xenograft model, derived from MNA IMR32 cells (p = 0.0004). Notably, SR1078 anti-tumor activity was associated with the restoration of BMAL1 protein levels in the xenograft tumors (Fig. 5a). However, SR1078 was not able to block tumor growth in a non-MNA xenograft model of SK-N-AS cells (p = 0.5116) (Fig. 5b), suggesting that the tumor-suppressive function of RORα activation is MYCN-dependent. The general health conditions of the mice and their body weights were recorded weekly throughout the studies and no apparent signs of toxicity related to SR1078 treatment were noted (Supplementary Fig. 13). We then engrafted LAN5 RORα Tet-ON cells and evaluated their ability to grow in nude mice with and without DOX treatment to induce RORα overexpression. Overexpression of RORα significantly blocked tumor growth (p = 0.013), and DOX-induced tumors also showed higher BMAL1 protein levels (Fig. 5c). In addition, compared to control tumors, tumor cell proliferation was significantly lower in tumors that were both treated with SR1078 and overexpressing RORα (p < 0.0001 and p = 0.0012, respectively). Although SR1078 induced a robust apoptotic response in the treated tumors compared to controls (p = 0.0358), only a mild effect on cell apoptosis was observed in RORα-overexpressing tumors (Supplementary Fig. 14). Collectively, our data suggest that restoration of BMAL1 is tumor suppressive in NB.
Fig. 5. SR1078 and RORα genetic overexpression block tumor growth and inhibit lipid metabolism.
a Tumor weights (mean ± SD) from MNA LAN5- and IMR32-derived xenografts treated with vehicle control or SR1078 (15 mg/kg i.p. for 14 days) (LAN5: p = 0.02, Mann–Whitney test, control group n = 10 and SR1078 group n = 8; IMR32: p = 0.0004, Mann-Whitney test, control group n = 11 and SR1078 group n = 11). BMAL1 protein expression in representative LAN5-xenografted tumor lysates from SR1078 treated mice and controls. b Tumor weights (mean ± SD) from non-MNA SK-N-AS derived xenografts treated with vehicle control or SR1078 (15 mg/kg i.p. for 14 days) (p = 0.5116, Mann–Whitney test, control group n = 10 and SR1078 group n = 10). c. Tumor weights (mean ± SD) from DOX-induced (RORα-ON) and controls (RORα-OFF) mice (p = 0.013, Mann–Whitney test, RORα-OFF group n = 6 and RORα-ON group n = 8). BMAL1 protein expression in tumor lysates from representative RORα-ON and RORα-OFF mice. d Heat map and relative levels of altered DG and TG in LAN5-derived xenografts treated with vehicle control (n = 10) or SR1078 (n = 8; 15 mg/kg i.p. for 14 days). Groups were compared by a two-tailed unpaired t-test. P-values were adjusted by the Benjamini–Hochberg method to obtain FDR. Changes with FDR < 0.25 were selected for heat map presentation; *=FDR < 0.25. Yellow = upregulated lipids; blue=downregulated lipids. e Heat map and relative levels of altered intratumoral FAs (*=FDR < 0.25) in LAN5-derived xenografts treated with vehicle control (n = 8) or SR1078 (n = 7; 15 mg/kg i.p. for 14 days). Groups were compared by a two-tailed unpaired t-test. P-values were adjusted by the Benjamini-Hochberg procedure to obtain FDR. Changes with FDR < 0.25 were selected for heat map presentation; *=FDR < 0.25. AHA aminoheptanoic acid, DDA dodecanoic acid, DG diglycerides, DHA docosahexaenoic acid, DTA docosatetraenoic acid, EPA eicosapentaenoic acid, ETRA eicosatrienoic acid, HDA hexadecanoic acid, ODA octadecanoic acid (stearic acid), ODCA octadecenoic acid (oleic acid); TG triglycerides. For panels (d) and (e): the box plot is defined by two lines at the 25th and 75th percentile. A line is drawn inside the box at the 50th percentile.
The RNA-seq data indicated that SR1078 inhibits NB cell survival in part by suppressing genes involved in lipid metabolism (Fig. 4d). To determine whether SR1078 suppresses tumor growth by perturbing lipid metabolism, we performed lipidomics analysis in control (n = 10) and SR1078-treated (n = 8) LAN5 tumors. Supporting our RNA-seq data, we found that intratumoral levels of glycerolipids, such as triglycerides (TGs) and their precursors diglycerides (DGs) were significantly reduced by SR1078 (FDR < 0.25; Fig. 5d and Supplementary Data 2), suggesting that activation of RORα effectively inhibits in vivo lipogenesis. In contrast, the intratumoral cholesterol esters were increased by SR1078 (Supplementary Fig. 15). This finding challenges our RNA-seq data in which cholesterol biosynthesis genes were suppressed by SR1078, suggesting a potential compensatory upregulation of cholesterol uptake in these tumors. Because FAs are required for DG and TG synthesis, we further determined what changes in FA composition were induced by SR1078 using liquid chromatography-mass spectrometry (LC-MS)-based FA profiling. Notably, SR1078 significantly reduces the levels of intratumoral FAs including C12:0, C16:0, C18:1, and C20:3, all of which serve as side chains of the reduced DG and TG groups (Fig. 5e and Supplementary Table 2). Altogether, these data suggest that SR1078 blocks tumor growth by inhibiting FA metabolism and glycerolipid synthesis.
Activation of RORα opposes MYCN-driven lipogenesis
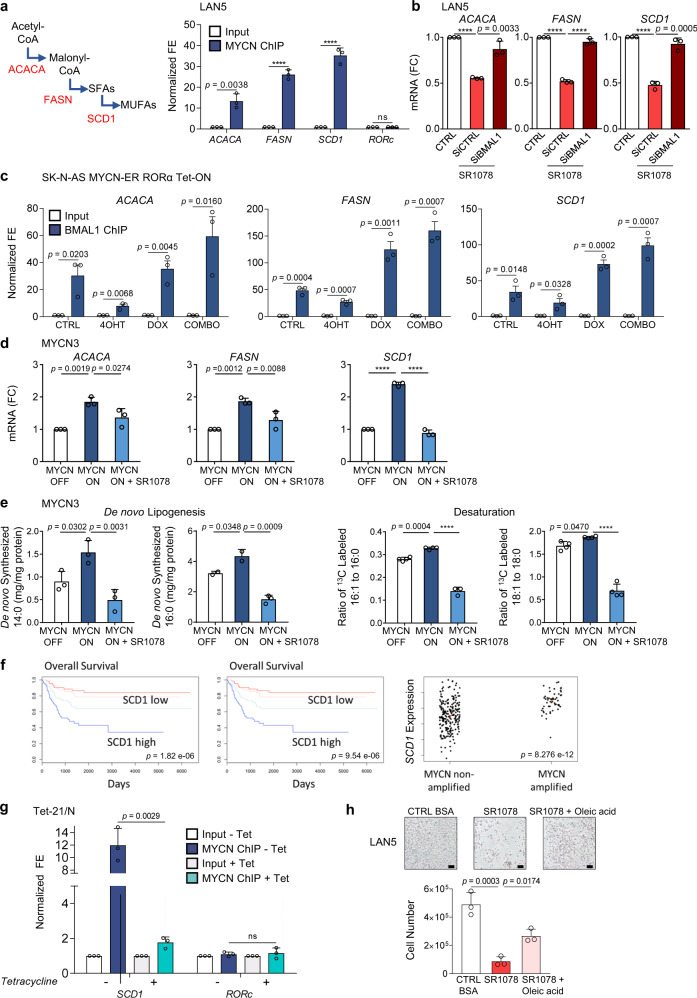
Recent reports have emphasized the role of MYC in reprogramming lipid metabolism to enhance tumorigenesis41. MYC induces SREBP1c-dependent de novo FA synthesis and directly activates the transcription of key lipogenic enzymes to support cancer growth42. On the other hand, RORs are also key regulators of lipid metabolism with tissue-dependent effects35. Nobiletin (a natural flavone)-mediated activation of RORα/γ protects against metabolic syndrome, suggesting a role in metabolic regulation43. Moreover, our RNA-seq and in vivo lipidomics data suggest that SR1078 acts by inhibiting cell metabolic processes, in particular lipid biosynthesis. Unlike normal cells, cancer cells highly depend on de novo lipogenesis44. We first asked whether the main lipogenic genes (Acetyl-CoA Carboxylase Alpha (ACACA), FA synthase (FASN), and stearoyl-CoA desaturase 1 (SCD1)) behave as direct MYCN targets. ChIP-qPCR analyses confirmed a significant enrichment of MYCN binding at the promoter regions of all lipogenic genes (Fig. 6a and Supplementary Fig. 16). We then tested whether activation of RORα could repress MYCN-driven lipogenic gene expression. The mRNA levels of the lipogenic genes were all significantly repressed following SR1078 treatment. However, depletion of BMAL1 completely reestablished their levels, suggesting that SR1078 inhibition of lipogenic gene expression is mediated by BMAL1 (Fig. 6b). This observation was further supported by BMAL1 ChIP-qPCR analysis in SK-N-AS MYCN-ER RORα cells, which confirmed reduced BMAL1 binding at the promoters of all three lipogenic enzymes upon activation of MYCN and increased binding upon overexpression of RORα (Fig. 6c), suggesting that MYCN and BMAL1 may compete for binding at these loci. Moreover, SR1078 treatment rescued the induction of lipogenic gene expression mediated by MYCN in MYCN3 (Tet-ON) cells (Fig. 6d).
Fig. 6. SR1078 opposes MYCN-induced lipogenesis.
a Left panel: schematic representation of the main enzymatic steps of de novo lipogenesis. Right panel: MYCN ChIP assays in LAN5 cells (input = white bars; MYCN IP = blue bars). Samples were analyzed by q-PCR using specific primers for lipogenic enzymes (ACACA, FASN, and SCD1). RORc served as negative control (Supplementary Table 3). Data are mean ± SD (n = 3 biological replicates; ****p < 0.0001; two-tailed unpaired t-test). b mRNA expression of lipogenic enzymes in LAN5 SiCTRL and SiBMAL1 cells upon SR1078 treatment (10 µM for 24 h). Data are mean ± SD (n = 3; ****p < 0.0001; two-tailed unpaired t-test). c BMAL1 ChIP-qPCR assays in SK-N-AS MYCN-ER RORα cells following 4OHT (1 µM for 24 h), DOX (2 µM for 24 h), and 4OHT + DOX treatments. Data are mean ± SD (n = 3; two-tailed unpaired t-test). d mRNA expression of lipogenic enzymes in MYCN3 (MYCN-ON) cells upon SR1078 treatment (10 µM for 24 h). Data are mean ± SD (n = 3; ****p < 0.0001; one-way ANOVA with Dunnett’s multiple comparisons test). e Determination of de novo lipogenesis and SCD1 activity in MYCN3 (MYCN-ON) cells upon SR1078 treatment (5 µM for 72 h). Data are mean ± SD (n = 4 biological replicates; ****p < 0.0001; one-way ANOVA with Dunnett’s multiple comparisons test). f Kaplan–Meier analysis of overall survival (OS) and event-free survival (EFS) in the NB NRC patient cohort (n = 101) based on SCD1 mRNA expression. Significant (p = 8.276 E−12 by Fisher’s exact test) association with MYCN amplification is shown. g MYCN ChIP assays in Tet-21/N cells in conditions of MYCN-ON and MYCN-OFF (DOX for 48 h) (input = white bars; MYCN IP = blue bars). Samples were analyzed by q-PCR using specific primers for SCD1 and RORc (Supplementary Table 3). Data are mean ± SD (n = 3 biological replicates; two-tailed unpaired t-test). h Cell viability of LAN5 cells treated with SR1078 (10 μM for 48 h) in control (CTRL) media (0.3 mM BSA) or CTRL media supplemented with oleic acid (500 μM for 48 h). Data are mean ± SD (n = 3 biological replicates; one-way ANOVA with Dunnett’s multiple comparisons test). Representative images (100×) of LAN5 cells; scale bar =50 µM. FC fold change, FE fold enrichment.
To further determine the effects of SR1078 on MYCN-induced de novo lipogenesis and desaturation activity, stable isotope labeling and gas chromatography-mass spectrometry (GC-MS) were employed for FA determination. Turning on MYCN expression significantly increases the concentration of de novo synthesized C14:0 (myristic acid) and C16:0 (palmitic acid), and enhances SCD1 activity as indicated by the 13C16-labeled C16:1 (palmitoleic acid) to 13C16-labeled C16:0 ratio. However, SR1078 restores these FA levels, suggesting that activation of RORα efficiently blocks MYCN-mediated de novo FA synthesis and desaturation (Fig. 6e). Because the most profound effect of SR1078 was on SCD1 expression and activity, we next examined how SCD1 expression relates to NB clinical outcome. SCD1 expression is strongly associated with poor clinical outcomes and MYCN amplification (Fig. 6f). Moreover, ChIP-qPCR analyses in MYCN Tet-OFF cells confirmed a significant enrichment of MYCN binding at the SCD1 promoter, which is fully abrogated upon turning off MYCN (Fig. 6g), suggesting that MYCN directly transcriptionally induces SCD1 to promote lipogenesis. Selected FAs (e.g., oleic acid), the main product of SCD1, have been shown to rescue cell viability in cancer cells treated with FA synthesis inhibitors (e.g., TOFA)36. We found that culture media supplemented with oleic acid partially rescues the viability of MNA cells treated with SR1078 (Fig. 6h), suggesting that SR1078 targets MYCN-mediated SCD1 activity.
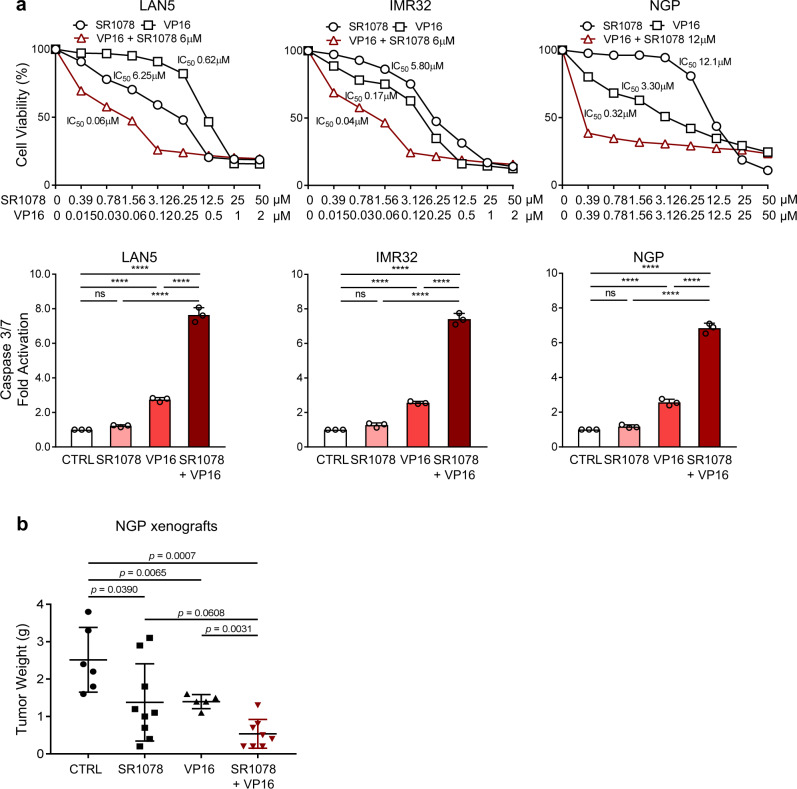
Activation of RORα sensitizes NB tumors to conventional chemotherapy
We hypothesized that activation of RORα, by restoring the molecular clock and cell metabolism, could sensitize NB to conventional therapy. To investigate whether SR1078 alters the sensitivity of NB cells to conventional therapies such as etoposide (VP16), MNA cells were exposed to different concentrations of SR1078 and VP16 as single agents and in combination (IC50 doses). VP16 IC50 values were significantly reduced (10.3, 4.2, and 10.3 fold for LAN5, IMR32, and NGP cells, respectively) when cells were exposed to the combination therapy (Fig. 7a). SR1078 IC50 values were also robustly reduced (13.6, 29, and 40 fold, respectively) when cells were treated with the combination therapy (Supplementary Fig. 17). Moreover, the addition of low-dose SR1078 strongly enhanced the cell apoptosis induced by VP16 in all three cell lines (Fig. 7a). We then tested the anti-tumor activity of our combination therapy in vivo, using orthotopic xenografts generated from MNA NGP cells. As expected, single agents SR1078 and VP16 significantly blocked tumor growth (p < 0.05), and SR1078 strongly sensitized NB tumors to VP16 (p = 0.0031) (Fig. 7b), suggesting that modulation of the clock could be a viable therapeutic approach.
Fig. 7. SR1078 sensitizes NB to conventional chemotherapy.
a Cell viability (72 h) and caspase 3/7 activation (24 h) of MNA cells (LAN5, IMR32, and NGP) upon treatment with SR1078, etoposide (VP16), and their combination. Cell viability data are means and are representative from n = 2 biological replicates (n = 4 technical replicates). Apoptosis data (SR1078 2.5 μM; VP16 100–200 nM) are means ± SD (n = 3; ****p < 0.0001; one-way ANOVA with Tukey’s multiple comparisons test). b Tumor weights (mean ± SD) from NGP-derived xenografts treated with vehicle control, SR1078 (20 mg/kg i.p. daily), VP16 (10 mg/kg i.p. 3 times/week), and their combination (in red). All treatments continued for 14 days (Mann–Whitney test; control group n = 6; SR1078 group n = 9; VP16 group n = 5; combination group n = 8).
Discussion
We have shown here that MYCN suppresses the molecular clock in NB. In turn, restoration of the clock effectively counteracts MYCN-mediated cell growth and metabolism. MYCN-amplification, which occurs in 50% of high-risk NB, distinguishes tumors with a high relapse rate and poor survival2,45. We found that MYCN directly activates the clock repressor REV-ERBα while suppressing the activators RORα and BMAL1. This dysregulation is a powerful NB prognostic marker: low RORα and BMAL1 expression and high REV-ERBα expression strongly associated with poor clinical outcomes, independently of other prognostic factors.
The downregulation of the clock by MYCN is consistent with previous studies showing that ectopic MYC expression activates REV-ERBα to dampen BMAL1 expression and oscillation46 and downregulates core clock gene expression via MIZ119. Indeed, in the context of transcriptional repression MYC does not directly contact DNA but associates with DNA-bound transcription factors, such as MIZ1 and/or Sp147. Our data show that depletion of MIZ1 reduces the MYCN binding at the promoter regions of BMAL1 and RORα, suggesting that MIZ1 mediates MYCN repression of the clock in NB cells. This is further supported by the observation that MYCN physically binds the BMAL1 promoter despite the absence of a functional E-box and in the region where MIZ1 binds. Importantly, we show that MYCN suppresses the clock activators RORα and BMAL1 to sustain cell proliferation and rewire cell metabolism, suggesting that this dysregulation has a functional significance and may contribute to NB oncogenesis. Depending on the context, BMAL1 can function as a tumor suppressor14 or oncogene48. Interestingly, MYC and BMAL1 are basic helix-loop-helix DNA-binding transcription factors, which recognize the same E-box sequences in the regulatory regions of target genes49,50. Our ChIP-qPCR data revealed reduced BMAL1 occupancy of target E-box sites in lipogenic genes, which is rescued by ectopic RORα. Thus, we can speculate that oncogenic MYCN plays a role in disrupting BMAL1-controlled transcription and metabolism. RORα and BMAL1 gene silencing is found in several other cancers, including hematological malignancies26. RORs also play important roles in tumor metabolism and inflammation. Genetic studies have shown that attenuated RORα leads to various metabolic abnormalities in mice, including hyperglycemia and glucose intolerance51. Moreover, RORα inhibits inflammation by interfering with NF-kB signaling52.
These observations prompted us to investigate whether restoring the clock could counteract the effects of MYCN on cell growth and metabolism. To restore the core clock component BMAL1 and clock function, we pharmacologically activated the circadian activator RORα via SR1078, a synthetic RORα/γ ligand agonist that selectively binds to the ligand-binding domain of the receptors promoting the transcription of target genes, such as BMAL134. Our data indicate that restoration of the molecular clock is tumor suppressive in NB. Both SR1078 and genetic overexpression of RORα effectively restore BMAL1 expression and oscillation, and block tumor growth in MNA, but not in non-MNA orthotopic xenografts, suggesting that the anti-tumor effect of SR1078 is MYCN-dependent. Importantly, the anti-tumor activity of SR1078 and RORα is abolished following depletion of BMAL1, further supporting that the core clock component BMAL1 functions as a tumor suppressor in NB. These emerging connections between clock disruption and oncogenesis suggest that circadian interventions could be employed for anti-cancer treatment. Supporting this concept, recent work suggests that pharmacological agonists of REV-ERBs are selectively lethal to cancer cells and block glioblastoma (GBM) and GBM stem cell growth30,48. We further hypothesized that SR1078, by restoring the molecular clock and cell metabolism, could be used in combination with conventional therapies to reduce toxicity and enhance chemotherapeutic viability. We find that SR1078 significantly improves the anti-tumor activity of the standard-of-care drug etoposide in MNA xenografts, suggesting that pharmacological modulation of the clock could be explored in future combination studies.
Metabolic rewiring is a key function of MYC oncogenes. Although several c-MYC metabolic functions are well characterized, the role of MYCN in metabolic reprogramming is less defined. MYC, in coordination with SREBP1, controls lipogenesis, which is required for both initiation and maintenance of tumorigenic growth53. Moreover, inhibition of FA oxidation dampens tumor growth of triple-negative breast cancers overexpressing MYC54. Interestingly, the activity of some of these lipogenic enzymes has been previously reported to be clock-controlled55 and linked to oncogenesis56, suggesting that changes in their circadian activity may affect tumor growth. We demonstrate here that MYCN directly induces lipogenic gene expression and lipid intermediates, which are constrained by SR1078 in a BMAL1-dependent fashion. Moreover, SR1078 profoundly inhibits lipid metabolism in vivo by reducing glycerolipids and their FA components. Thus, we postulate that MYCN-mediated repression of the clock could induce the unconstrained expression of lipogenic genes and activate lipid metabolism to support tumor growth. However, restoration of the clock effectively constrains MYCN-mediated lipogenesis. We show that MYCN directly binds and upregulates the stearoyl-CoA desaturase-1 (SCD1), which catalyzes the synthesis of monounsaturated from saturated FA. In turn, activation of RORα blocks MYCN-induced SCD1 expression and activity. Moreover, supplementation with oleic acid, the major product of SCD1, rescues the viability of NB cells, confirming that SR1078 functions by blocking MYCN-mediated lipogenesis. SCD1 expression is frequently elevated in cancer57 and its inhibition has shown some anti-tumor activity58. We find that high SCD1 expression predicts poor clinical outcomes in NB and its expression directly correlates with MYCN amplification. This could represent a potentially targetable metabolic vulnerability.
Overall, our results demonstrated that restoration of the molecular clock via activation of RORα blocks MYCN-driven tumor growth and lipid metabolism (Supplementary Fig. 18). These novel findings implicate RORα activation as a potential therapeutic strategy for blocking MYCN-mediated dysregulation of the molecular clock and metabolism in high-risk NB.
Methods
Cell lines and culture conditions
SH-SY5Y, IMR32, SK-N-BE(2)-C, SK-N-AS, NGP (ATCC), LAN5, and CHLA255 (Metelitsa, BCM, Houston, TX), and MYCN3 (Shohet, BCM, Houston, TX) NB cell lines were maintained in RPMI 1640 medium. Tet-21/N (Perini, Bologna, Italy) cells were grown in DMEM high glucose medium and kept in selection with neomycin (G418, Santa Cruz Biotechnology# sc-29065a, 0.2 mg/ml) and hygromycin (Sigma Aldrich# H3274, 0.150 mg/ml). All cell lines were validated by genotyping and regularly tested for mycoplasma. To turn off MYCN, doxycycline (DOX; Santa Cruz Biotechnology# sc-204734A) was added at a final concentration of 2 µg/mL. SK-N-AS MYCN-ER (Altman, Rochester, USA) cells were maintained in DMEM high glucose and sodium pyruvate. To activate MYCN transcription, cells were treated with 1 µM 4-hydroxytamoxifen (Sigma Aldrich# T176) for 48 h. Cell lines were validated by genotyping and confirmed by expression of CD56, Nestin, MYCN, and tyrosine hydroxylase within the past 12 months. SR1078 (Calbiochem# 557352) was used at a concentration of 5–15 μM for in vitro studies. BSA-conjugated oleic acid (Sigma Aldrich# O3008) was supplemented in the culture media (10–500 µM).
Primary NBs survival data
mRNA gene expression analyses were performed within R2: a genomics analysis and visualization platform (http://r2.amc.nl), using datasets: NB Kocak (n = 649, GEO: GSE45547, patient cohort 1), NB Versteeg (n = 88, GEO: GSE16476 88/122, patient cohort 2), and NB Research Consortium (NRC; n = 283, GEO: GSE85047, patient cohort 3). In the Kocak dataset, 476 of the 649 samples were annotated with survival data. To test the association of gene expression with survival, individual gene expression profiles were dichotomized by a median split into “high” or “low” groups, and Kaplan–Meier survival curves were plotted for each group. A Cox regression model was used to test for the independent predictive ability of RORα expression after adjustment for other significant factors: MYCN amplification, age, and INSS stages. The Mann–Whitney U test was used to evaluate the significant differences among quantitative variables. The significance of correlation analyses between MYCN and RORα was evaluated by the Spearman test.
Plasmid constructs
To generate MYCN-inducible MYCN3 cells, MYCN cDNA was cloned into a pTR2-Hygro vector (BD Biosciences) containing a tetracycline-responsive promoter. The construct was then transfected into a SHEP sub-clone stably expressing the tetracycline response element and selected with hygromycin59. For LAN5 ShMYCN cells, GIPZ human MYCN lentiviral clones (V2LHS_36755 and V3LHS_322662) were obtained from the Cell-Based Screening Service core at Baylor College of Medicine (BCM). MYCN–shRNA sequences were inserted in the lentiviral gene silencing vector pInducer11, which has a dual fluorescent system consisting of a constitutive cassette (rtTA3 and eGFP) to detect infection efficiency and a turboRFP (tRFP)-shRNA cassette activated upon DOX treatment. To generate LAN5 and SK-N-AS RORα conditional overexpressing cells, RORα isoform 1 cDNA (Origene# RC202926) was cloned into the pENTR/D-TOPO vector (Invitrogen) and then gateway cloned into a pInducer21 lentiviral vector with RORα-HA under the control of the tetracycline-inducible promoter. LAN5 cells were transduced with lentiviral vectors at a multiplicity of infection sufficient to achieve greater than 90% infection as determined by the presence of GFP fluorescence. BMAL1 siRNAs (scrambled and BMAL1 targeted) were obtained from Thermo Scientific (Dharmacon SMARTpool siRNAs, which consists of a pool of four siRNA sequences, #SO-2564383G). To generate LAN5 Shp53 cells, second-generation lentiviruses expressing Shp53 and ShLuc control were used as previously described60. Briefly, 293 T cells were transfected with pLSLPw construct along with pVSVG and pLV-CMV-delta 8.2 by using lipofectamine. Virus-containing supernatants were collected at 48 h and 72 h and NB cells transduced in the presence of 8 mg/ml polybrene (Sigma).
In vitro functional assays
To test the effect of SR1078 on cell viability, 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay was carried out as previously described61. Cell proliferation was assessed using the CCK-8 Kit (Dojindo, Kumamoto, Japan) according to the manufacturer’s instructions. Briefly, 3–5 × 103 cells/well were seeded in 96-well plates. At determined times, CCK-8 reagent (10 µl) was added to the wells and incubated at 37 °C for 4 h, and absorbance (450 nM) was measured. Caspase 3/7-mediated apoptosis was determined using the Caspase-Glo assay kit (Promega). Briefly, 5 × 103 cells/well were seeded in 96-well plates and subjected to different treatments for 24–48 h. Equal volumes of caspase reagent were added to the wells, and luminescence was measured in a plate-reading luminometer after 3 h incubation. Cell cycle distribution was measured by PI Flow Cytometry Kit (abcam# 139418). LAN5 RORα cells were harvested and fixed (70% ethanol for 2 h). Cells were stained with 50 µg/ml PI for 30 min before FACS analysis (BD LSRII with BD FACSDIVA v6.1.3). Cell population (%) in G0-G1, S, and G2 phases was analyzed by FlowJo v7.6.1.
Real-time q-PCR and western blotting
Total RNA was extracted and purified with RNeasy kit (QIAGEN). Real-time PCR was performed with SYBR green reagent using a 50 ng template in a 25 μl reaction mixture according to the manufacturer’s protocol. Levels of GAPDH and CUL1 were used for normalization. All primers were designed with one primer overlapping an exon boundary. Primer sequences are listed in Supplementary Table 3. For western blotting analyses, cells and tumor tissue were lysed with RIPA buffer (Sigma) containing protease inhibitors (Roche). Protein concentration was measured by Bradford assay (Bio-Rad reagent) and 50–75 μg protein was electrophoresed and transferred. Cyclophilin B (CypB) was used as the protein loading control. Primary antibodies: MYCN (Cell Signaling# 9405 S; 1:500 dilution); c-MYC (Abcam# ab32072; 1:500 dilution); RORα (Santa Cruz Biotechnology# sc-6062; 1:500 dilution); p53 (Santa Cruz Biotechnology# sc-126; 1:500 dilution); BMAL1 (Cell Signaling# 14020 S; 1:1000 dilution); REV-ERBα (Cell Signaling# 13418 S; 1:500 dilution) and CypB (Santa Cruz Biotechnology# sc-130626; 1:1000 dilution). Secondary antibodies: anti-mouse IgG IRDye680RD (Licor# 926-68070; 1:10000 dilution) and anti-rabbit IgG IRDye800CW (Licor# 926-3211; 1:10000 dilution). Blots were scanned on an Odyssey infrared imaging system (LI-COR) and analyzed by Odyssey® Application Software v3.0. Protein expression was quantified by densitometry (ImageJ v1.42q). All uncropped blot scans are provided in the Source Data file.
RNA-seq analysis
We generated libraries with the Illumina TruSeq Stranded mRNA (p/n 20020594) kit starting with 150 ng of RNA, and we sequenced with the NextSeq 500, PE75, v2.5 reagent kit. Differential expression programs from RNA-seq profiles of LAN5 cells treated with SR1078 (10 μM) for 8 h were identified. Fastq files were aligned to the reference genome (hg38.p12) using STAR (v2.4) with default settings to produce transcripts per million (tpm) estimates for each ensembl gene62. When identifying up- and downregulated genes, we required genes to be significantly dysregulated by t-test and with a consistent fold change above 1.5 or below 0.67 compared to controls. Expression values were offset by adding a pseudo count of 1 tpm for each gene to exclude genes with low expression estimates. Gene set enrichment for Reactome pathways based on dysregulated genes was performed in Enrichr63. Pathways in the same category were grouped. Significant genes were sorted by p-value and associated with pathway terms.
Chromatin immunoprecipitation (ChIP)
The MYCN ChIP assays were performed as follows. Briefly, 1 × 107 cells were cross-linked using 1% formaldehyde, and the reaction was stopped using 0.125 M Glycine. The cell pellet was resuspended in cell lysis buffer and following 3500×g centrifugation, RIPA sonication buffer was added to complete nuclei lysis. DNA shearing was performed by sonication using the Bioruptor PLUS (Diagenode). A small aliquot of sonicated material was put aside, and the remaining sample was immunoprecipitated using 5 μg of ChIP-grade antibodies (B8.4.B, Santa Cruz Biotechnology# sc-53993). Rec-sepharose Protein A or G beads (Invitrogen) were used to immobilize immuno-complexes and RNAse-A treatment (37 °C 1 h) and reverse crosslinking was performed using Proteinase K (Roche) for 6 h at 65 °C. Immunoprecipitated DNA was purified using phenol/chloroform and ethanol precipitation techniques. DNA was analyzed by q-PCR and normalized by the fold enrichment method (2−(ΔΔCT)) using the primers listed in Supplementary Table 3. The ABCA10 transcription start site was used as a negative control DNA region for MYCN. The BMAL1-ChIP assays were performed using the ChIP-IT® Express Enzymatic (Active Motif# 53009) following the manufacturer’s protocol. Approximately, 1.5 × 107 cells were fixed using 1% formaldehyde. The fixation reactions were stopped by adding Glycine Fix-Stop solution. After washing with ice-cold PBS, cells were collected in a solution containing PMSF and centrifuged at 4 °C. The cell pellet was disrupted with a Dounce homogenizer in 1 ml ice-cold lysis buffer containing protease inhibitor and PMSF. After centrifugation, nuclei were resuspended in digestion buffer containing protease inhibitor and PMSF, then incubated at 37 °C for 5 min. Chromatin was then sheared to 200–300 bp by adding an enzymatic shearing cocktail for 10 min at 37 °C. Samples were centrifuged at 21,000xg for 10 min at 4 °C. One percent aliquot of this material was retained as “input” DNA. The remaining chromatin sample was divided, one-half was immunoprecipitated with the BMAL1 antibody (Abcam# 93806), and the second half was used for a mock immunoprecipitation with a control IgG. Real-time PCR amplification was carried out using 2.5 μl of DNA sample, using primers listed in Supplementary Table 3.
Circadian oscillation studies
Real-time bioluminescence monitoring: MYCN3 and LAN5 cells were treated with or without 1 μg/ml DOX for 48 h before infection with Per2-dluc adenovirus as previously described64 for another 24 h. Cells were then serum shocked with 50% horse serum for 30 min before being subjected to real-time bioluminescence as previously described32. BMAL1 circadian oscillation: SK-N-AS MYCN-ER RORα cells were cultured with and without 4OHT (MYCN-ON/OFF), DOX (RORα-ON/OFF), and 4OHT + DOX for 24 h and then synchronized with 50% horse serum for 2 h. Cells were collected every 6 h from 16 to 64 h after synchronization. 4-OHT and DOX treatments were applied during and after cell synchronization. BMAL1 mRNA expression was determined by q-PCR and normalized to 18 S expression. To test for rhythmicity, JTK_Cycle65 was applied. For serum shock experiments, rhythmicity was determined from all values excluding the ZT0 (unsynchronized) time point.
FA measurement
FA tracing was performed using pentafluorobenzyl bromide (PFBBr) derivatization and GC-MS negative chemical ionization as previously described66. SP-2380 columns (Supelco, Inc.) were used to allow better peak separation of palmitoleic from sapienic acid. Data were acquired in selective ion monitoring mode. The fragment masses m/z of 227–229 were used to monitor M0–M2 in myristic acid; 255–257 and 271 for M0–M2 and M16 in palmitic acid; and 253–255 and 269 for M0–M2 and M16 in palmitoleic acid. Analyte and standards peak areas were determined. The ratio of the area from the sum (M0–M2) analyte-derived ions compared to that from the tridecanoic acid (C13:0) (m/z, 213–215) internal standard was calculated. The ratios were then compared with the calibration curves to determine the concentration of each FA. To determine de novo lipogenesis, deuterated water (D2O) (atom 99%, Cambridge Isotope Laboratory) was added to the media to reach an enrichment of 2.5%. The percentage contribution of newly made FA was calculated as total 2H-labeled FA/(2H-labeled body water × n) × 100 (n = number of exchangeable hydrogens). Total 2H-labeled FA was calculated as EM1 × 1 + EM2 × 2, where E is the enrichment of the isotopomer. The absolute amount of newly synthesized FA was determined as the percentage of newly made FA × the concentration of the total FA. The FA amount was then normalized by the protein content of each sample. For desaturase activity measurement, [U-13C] palmitic acid (99 atom%13C, Cambridge Isotope Laboratories) was added (1.0 mg/100 mL) to the incubation media and D2O mix. The concentration of [13C16] in palmitic or palmitoleic acid was calculated as the area of M16/sum area (M0–M2) × the concentration of FA. Desaturase activity was measured as the ratio of [13C16] palmitoleic acid to [13C16] palmitic.
In vivo, FA profiling was performed using high-performance liquid chromatography (HPLC) coupled to Agilent 6495 QQQ mass spectrometry (Agilent Technologies) using ESI negative ionization via single reaction monitoring. FAs were extracted as previously described67. Separation was performed on a Luna 3u phenyl-hexyl column (150 × 2 mm) using 10 mM ammonium acetate pH 8 (mobile phase A) and methanol (mobile phase B). The binary pump flow rate was 0.2 ml/min with a gradient starting at 40% B at 0 min, 50% at 8 min, 67% at 13 min, 100% at 23 min, and 40% at 30 min to the initial condition and re-equilibration till the end of the gradient at 37 min. Data were analyzed with Agilent mass hunter quantitative software (v10.0) and were normalized with an internal standard and log2 transformed per sample. For every FA in the normalized dataset, two-sample t-tests were conducted to compare expression levels between different groups. Differential metabolites were identified by adjusting the p-values for multiple testing at FDR < 0.25.
In vivo studies
In vivo studies were approved by the Institutional Animal Care and Use Committee of BCM (AN7089). Orthotopic xenografts of human NB were generated as previously described40. Briefly, an inoculum of 106 tumor cells in 0.1 mL of PBS was injected under the renal capsule of 4–6-week-old female athymic Ncr nude mice (Taconic). Two weeks after cell implantation and confirmation of successful tumor engraftment by bioluminescent imaging (Xenogen IVIS 100 System, Caliper Life Sciences), mice were randomized and divided into treatment groups. Four to five weeks post-implantation mice were sacrificed, tumors resected, weighed, and preserved in 4% paraformaldehyde for immunohistochemical analysis. All animal data were compared using the Mann–Whitney test. To induce RORα expression DOX Hyclate (Sigma Aldrich# D9891) was given as an intraperitoneal injection (5 mg/kg) at day −1, 0, and +1 post-implantation and weekly for three weeks. DOX (2 mg/ml) was then supplemented in 5% sucrose drinking water. SR1078 (Calbiochem# 557352) was administered i.p. daily (15 mg–25 mg/kg in DMSO). Etoposide (VP16, Sigma Aldrich# E1383) was administered i.p. three times a week (15 mg/kg in DMSO) for two weeks. Vehicle control (PBS, PEG (5.4%) Tween 20 (5.4%), DMSO (4%)) was administered i.p. daily. Combination therapy of VP16 (10 mg/kg) and SR1078 (25mg/kg) was administered i.p. daily for two weeks. Immunohistochemistry staining: Assessment of tumor cell proliferation and apoptosis was performed as previously described68. Briefly, paraffin-embedded tumor sections were blocked with horse serum (10%) and incubated with Ki67 antibody (BD Bioscences# 550609; 1:400 dilution) or cleaved caspase 3 antibody (Cell Signaling# 9661 L; 1:400 dilution) at 4 °C overnight. Sections were washed with PBS and incubated with biotinylated anti-mouse (BA# 9200; 1:200 dilution) and anti-rabbit (BA# 1000; 1:200 dilution) antibodies at room temperature for 30 min, following incubation with the 3,3′-diaminobenzidine solution and counterstaining with hematoxylin. For tumor section scoring, four fields were evaluated with 500 tumor cells in each field using light microscopy, and percent positive tumor cells were calculated.
Lipidomics
Sample Preparation was as follows: Tumor samples were homogenized with an equal ratio of 0.15 M KCl and methanol followed by 400 µL of dichloromethane and 2 µL of acetic acid. Internal standards and quality control samples were prepared as previously described69. Briefly, an equimolar concentration of 10 µL of internal standards was added to each sample before adding water and dichloromethane (1:1). After centrifugation (5 min 6500×g), the lower organic layer was collected and dried under nitrogen. Before mass spectrometric (MS) analysis, the dried extract was suspended in 100 μL of solution (10:5:85 acetonitrile/water/isopropyl alcohol containing 10 mM ammonium acetate) and subjected to liquid chromatography/triple time-of-flight (TOF) for analysis (Shimadzu CTO-20A Nexera X2 41 UHPLC system). MS analysis was carried out on a Triple TOF 5600 equipped with Turbo VTM ion source (AB Sciex). Data were acquired with Analyst TF software v1.8 (AB Sciex). Lipids were separated on the acquity HSS UPLC T3 column (Waters) at 55 °C. The initial mobile phase consists of acetonitrile/water (40:60 v/v) with 10 mM ammonium acetate, and acetonitrile/water/isopropanol (10:5:85 v/v) with 10 mM ammonium acetate with gradient elution at a flow rate of 0.4 mL/min and an injection volume of 5 μL. Operating source conditions for Triple TOF scan for positive ionization: source voltage 5500 V, declustering potential (DP) 60 V, source T 450 °C, ion source gas 1 (GS1) 40 psi, ion source gas 2 (GS2) 45 psi, curtain gas (CUR) 30 psi, and collision energy 10 V. Conditions for negative ionization: source voltage −4500V, DP 60 V, GS1 40 psi, GS2 45 psi, CUR 30 psi, and collision −10 V. The MS/MS spectra were controlled by data-dependent acquisition with dynamic background subtraction, charge monitoring, and dynamic exclusion of former target ions for 9 s. Rolling collision energy spread was set to generate a collision energy ramp around a collision energy center point with the goal of providing a richer MS/MS spectrum of lipids. Mass accuracy was maintained by the use of an automated calibrant delivery system interfaced to the second inlet of the DuoSpray source. Data processing and statistical analysis were performed as previously described67. Briefly, data were normalized by median inter-quantile range normalization and were log2 transformed. The two groups were compared by Student’s t-test. P-values were adjusted by the Benjamini–Hochberg procedure to obtain FDR. Changes with FDR < 0.25 were selected for heat map presentation.
Statistical analysis
Data were collected in Microsoft Excel 2013 and analyzed in GraphPad Prism v7. All in vitro assays are expressed as mean ± standard deviation (SD) and performed in triplicate. Data are compared using a two-sided unpaired t-test, one or two-way ANOVA with Tukey’s, Sidak’s, and Dunnett’s multiple comparisons test. Tumor weights are expressed as mean ± SD and compared using Mann-Whitney tests; p-values < 0.05 were considered statistically significant. Rhythmicity was tested using the non-parametric test, JTK_Cycle, R version 4.0.3, package MetaCycle.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Supplementary information
Description of Additional Supplementary Files
Acknowledgements
We thank Brian J. Altman and Ronald Bernardi for proving SK-N-AS MYCN-ER and LAN5 ShMYCN cells, respectively. This project was supported by the Cell-Based Screening Service (C-BASS) core at BCM (core co-director: Dan Liu, Ph.D.), the Genomic and RNA Profiling Core at BCM (core directors: Dr. Lisa D. White, Ph.D., and Dr. Daniel Kraushaar, Ph.D.), and the Metabolomics Core at BCM (core director: Putluri Nagireddy, Ph.D.). E.B. is supported by the Alex’s Lemonade Stand Foundation for Childhood Cancer, the St. Baldrick’s Foundation, and the Cancer Prevention and Research Institute of Texas (CPRIT). G.P. is supported by Associazione Italiana per la Ricerca sul Cancro (Code: 15182). M.C. is supported by Fondazione Italiana per la Lotta al Neuroblastoma, Associazione Oncologia Pediatrica e Neuroblastoma, and Associazione Italiana per la Ricerca sul Cancro (Code: 19255). P.S. is partially funded by the European Union’s Horizon 2020 research and innovation program under grant agreements 668858 and 826121. The Metabolomics Core at BCM is supported by CPRIT Core Facility Support Award RP170005 “Proteomic and Metabolomic Core Facility,” NCI Cancer Center Support Grant P30CA125123, NIH/NCI R01CA220297, NIH/NCI R01CA216426, and intramural funds from the Dan L. Duncan Cancer Center (DLDCC).
Source data
Author contributions
Conception and design: M.M.-S. and E.B. Development of methodology: L.T., B.Z, M.A.M., S.V., N.P., K.E.-M., G.P., and E.B. Acquisition of data: M.M.-S., G.M., L.T., M.A.M., S.D.G., R.B., K.R.K.R., B.F., J.H., and E.B. Analysis and interpretation of data: M.M.-S., L.T., M.A.M., M.C., P.S., N.P., T.P.B., and E.B. Writing, review, and/or revision of the manuscript: M.M.-S., L.T., K.E.-M., G.P., T.P.B., and E.B. Study supervision: E.B.
Data availability
Publicly available clinical datasets GSE45547, GSE16476, and GSE85047. were analyzed with R2: Genomics Analysis and Visualization Platform (http://r2.amc.nl). RNA-sequencing data generated in this study have been deposited in the European Nucleotide Archive (ENA, https://www.ebi.ac.uk/ena/), accession number: PRJEB36158. All Figures and Supplementary Figures have associated raw data. Raw data of Figs. 1–7 and Supplementary Fig. 1–18, and uncropped blots are provided as one Source Data file. All the other data are available within the article and its Supplementary Information. Source data are provided with this paper.
Competing interests
The authors declare no competing interests.
Footnotes
Peer review information Nature Communications thanks the anonymous reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Giorgio Milazzo, Ling Tao.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-021-24196-4.
References
- 1.Eilers M, Eisenman RN. Myc’s broad reach. Genes Dev. 2008;22:2755–2766. doi: 10.1101/gad.1712408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maris JM. Recent advances in neuroblastoma. N. Engl. J. Med. 2010;362:2202–2211. doi: 10.1056/NEJMra0804577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weiss WA, Aldape K, Mohapatra G, Feuerstein BG, Bishop JM. Targeted expression of MYCN causes neuroblastoma in transgenic mice. EMBO J. 1997;16:2985–2995. doi: 10.1093/emboj/16.11.2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hsieh AL, Walton ZE, Altman BJ, Stine ZE, Dang CV. MYC and metabolism on the path to cancer. Semin. Cell Dev. Biol. 2015;43:11–21. doi: 10.1016/j.semcdb.2015.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cantor JR, Sabatini DM. Cancer cell metabolism: one hallmark, many faces. Cancer Discov. 2012;2:881–898. doi: 10.1158/2159-8290.CD-12-0345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DeBerardinis RJ, Cheng T. Q’s next: the diverse functions of glutamine in metabolism, cell biology and cancer. Oncogene. 2010;29:313–324. doi: 10.1038/onc.2009.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li F, et al. Myc stimulates nuclearly encoded mitochondrial genes and mitochondrial biogenesis. Mol. Cell. Biol. 2005;25:6225–6234. doi: 10.1128/MCB.25.14.6225-6234.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu YC, et al. Global regulation of nucleotide biosynthetic genes by c-Myc. PloS ONE. 2008;3:e2722. doi: 10.1371/journal.pone.0002722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morrish F, et al. Myc-dependent mitochondrial generation of acetyl-CoA contributes to fatty acid biosynthesis and histone acetylation during cell cycle entry. J. Biol. Chem. 2010;285:36267–36274. doi: 10.1074/jbc.M110.141606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Loven J, et al. Revisiting global gene expression analysis. Cell. 2012;151:476–482. doi: 10.1016/j.cell.2012.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carroll PA, et al. Deregulated Myc requires MondoA/Mlx for metabolic reprogramming and tumorigenesis. Cancer Cell. 2015;27:271–285. doi: 10.1016/j.ccell.2014.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zirath H, et al. MYC inhibition induces metabolic changes leading to accumulation of lipid droplets in tumor cells. Proc. Natl Acad. Sci. USA. 2013;110:10258–10263. doi: 10.1073/pnas.1222404110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mohawk JA, Green CB, Takahashi JS. Central and peripheral circadian clocks in mammals. Annu. Rev. Neurosci. 2012;35:445–462. doi: 10.1146/annurev-neuro-060909-153128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Altman BJ, et al. MYC disrupts the circadian clock and metabolism in cancer cells. Cell Metab. 2015;22:1009–1019. doi: 10.1016/j.cmet.2015.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Papagiannakopoulos T, et al. Circadian rhythm disruption promotes lung tumorigenesis. Cell Metab. 2016;24:324–331. doi: 10.1016/j.cmet.2016.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sato TK, et al. Feedback repression is required for mammalian circadian clock function. Nat. Genet. 2006;38:312–319. doi: 10.1038/ng1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cho H, et al. Regulation of circadian behaviour and metabolism by REV-ERB-alpha and REV-ERB-beta. Nature. 2012;485:123–127. doi: 10.1038/nature11048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kojetin DJ, Burris TP. REV-ERB and ROR nuclear receptors as drug targets. Nat. Rev. Drug Discov. 2014;13:197–216. doi: 10.1038/nrd4100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shostak A, et al. MYC/MIZ1-dependent gene repression inversely coordinates the circadian clock with cell cycle and proliferation. Nat. Commun. 2016;7:11807. doi: 10.1038/ncomms11807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xiong G, Wang C, Evers BM, Zhou BP, Xu R. RORalpha suppresses breast tumor invasion by inducing SEMA3F expression. Cancer Res. 2012;72:1728–1739. doi: 10.1158/0008-5472.CAN-11-2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kottorou AE, et al. Altered expression of NFY-C and RORA in colorectal adenocarcinomas. Acta Histochem. 2012;114:553–561. doi: 10.1016/j.acthis.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 22.Moretti RM, Montagnani Marelli M, Sala A, Motta M, Limonta P. Activation of the orphan nuclear receptor RORalpha counteracts the proliferative effect of fatty acids on prostate cancer cells: crucial role of 5-lipoxygenase. Int. J. Cancer. 2004;112:87–93. doi: 10.1002/ijc.20387. [DOI] [PubMed] [Google Scholar]
- 23.Fu RD, Qiu CH, Chen HA, Zhang ZG, Lu MQ. Retinoic acid receptor-related receptor alpha (RORalpha) is a prognostic marker for hepatocellular carcinoma. Tumour Biol. 2014;35:7603–7610. doi: 10.1007/s13277-014-2007-9. [DOI] [PubMed] [Google Scholar]
- 24.Wang Y, Solt LA, Kojetin DJ, Burris TP. Regulation of p53 stability and apoptosis by a ROR agonist. PloS ONE. 2012;7:e34921. doi: 10.1371/journal.pone.0034921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee JM, et al. RORalpha attenuates Wnt/beta-catenin signaling by PKCalpha-dependent phosphorylation in colon cancer. Mol. Cell. 2010;37:183–195. doi: 10.1016/j.molcel.2009.12.022. [DOI] [PubMed] [Google Scholar]
- 26.Taniguchi H, et al. Epigenetic inactivation of the circadian clock gene BMAL1 in hematologic malignancies. Cancer Res. 2009;69:8447–8454. doi: 10.1158/0008-5472.CAN-09-0551. [DOI] [PubMed] [Google Scholar]
- 27.Zeng ZL, et al. Overexpression of the circadian clock gene Bmal1 increases sensitivity to oxaliplatin in colorectal cancer. Clin. Cancer Res. 2014;20:1042–1052. doi: 10.1158/1078-0432.CCR-13-0171. [DOI] [PubMed] [Google Scholar]
- 28.Kallen J, Schlaeppi JM, Bitsch F, Delhon I, Fournier B. Crystal structure of the human RORalpha Ligand binding domain in complex with cholesterol sulfate at 2.2 A. J. Biol. Chem. 2004;279:14033–14038. doi: 10.1074/jbc.M400302200. [DOI] [PubMed] [Google Scholar]
- 29.Wang Y, Kumar N, Crumbley C, Griffin PR, Burris TP. A second class of nuclear receptors for oxysterols: regulation of RORalpha and RORgamma activity by 24S-hydroxycholesterol (cerebrosterol) Biochim. Biophys. Acta. 2010;1801:917–923. doi: 10.1016/j.bbalip.2010.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sulli G, et al. Pharmacological activation of REV-ERBs is lethal in cancer and oncogene-induced senescence. Nature. 2018;553:351–355. doi: 10.1038/nature25170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cook, D. N., Kang, H. S. & Jetten, A. M. Retinoic acid-related orphan receptors (RORs): regulatory functions in immunity, development, circadian rhythm, and metabolism. Nucl. Receptor Res.2, 10.11131/2015/101185 (2015). [DOI] [PMC free article] [PubMed]
- 32.Zhu B, et al. Coactivator-dependent oscillation of chromatin accessibility dictates circadian gene amplitude via REV-ERB Loading. Mol. Cell. 2015;60:769–783. doi: 10.1016/j.molcel.2015.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peukert K, et al. An alternative pathway for gene regulation by Myc. EMBO J. 1997;16:5672–5686. doi: 10.1093/emboj/16.18.5672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang Y, et al. Identification of SR1078, a synthetic agonist for the orphan nuclear receptors RORalpha and RORgamma. ACS Chem. Biol. 2010;5:1029–1034. doi: 10.1021/cb100223d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jetten AM. Retinoid-related orphan receptors (RORs): critical roles in development, immunity, circadian rhythm, and cellular metabolism. Nucl. Receptor Signal. 2009;7:e003. doi: 10.1621/nrs.07003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chopra AR, et al. Absence of the SRC-2 coactivator results in a glycogenopathy resembling Von Gierke’s disease. Science. 2008;322:1395–1399. doi: 10.1126/science.1164847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kelleher FC, Rao A, Maguire A. Circadian molecular clocks and cancer. Cancer Lett. 2014;342:9–18. doi: 10.1016/j.canlet.2013.09.040. [DOI] [PubMed] [Google Scholar]
- 38.Masri S, Sassone-Corsi P. The emerging link between cancer, metabolism, and circadian rhythms. Nat. Med. 2018;24:1795–1803. doi: 10.1038/s41591-018-0271-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Altman BJ. Cancer clocks out for lunch: disruption of circadian rhythm and metabolic oscillation in cancer. Front Cell Dev. Biol. 2016;4:62. doi: 10.3389/fcell.2016.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Patterson, D. M., Shohet, J. M. & Kim, E. S. Preclinical models of pediatric solid tumors (neuroblastoma) and their use in drug discovery. Current protocols in pharmacology/editorial board, S.J. Enna Chapter 14, Unit 14 17, 10.1002/0471141755.ph1417s52 (2011). [DOI] [PubMed]
- 41.Dang, C. V. MYC, metabolism, cell growth, and tumorigenesis. Cold Spring Harbor Perspect. Med.3, 10.1101/cshperspect.a014217 (2013). [DOI] [PMC free article] [PubMed]
- 42.Dong Y, Tu R, Liu H, Qing G. Regulation of cancer cell metabolism: oncogenic MYC in the driver’s seat. Signal Transduct. Target Ther. 2020;5:124. doi: 10.1038/s41392-020-00235-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.He B, et al. The small molecule nobiletin targets the molecular oscillator to enhance circadian rhythms and protect against metabolic syndrome. Cell Metab. 2016;23:610–621. doi: 10.1016/j.cmet.2016.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rohrig F, Schulze A. The multifaceted roles of fatty acid synthesis in cancer. Nat. Rev. Cancer. 2016;16:732–749. doi: 10.1038/nrc.2016.89. [DOI] [PubMed] [Google Scholar]
- 45.Brodeur GM. Neuroblastoma: biological insights into a clinical enigma. Nat. Rev. Cancer. 2003;3:203–216. doi: 10.1038/nrc1014. [DOI] [PubMed] [Google Scholar]
- 46.Altman BJ, Hsieh AL, Gouw AM, Dang CV. Correspondence: oncogenic MYC persistently upregulates the molecular clock component REV-ERBalpha. Nat. Commun. 2017;8:14862. doi: 10.1038/ncomms14862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dang CV. Gene regulation: fine-tuned amplification in cells. Nature. 2014;511:417–418. doi: 10.1038/nature13518. [DOI] [PubMed] [Google Scholar]
- 48.Dong, Z. et al. Targeting glioblastoma stem cells through disruption of the circadian clock. Cancer Discov.10.1158/2159-8290.CD-19-0215 (2019). [DOI] [PMC free article] [PubMed]
- 49.Ripperger JA, Schibler U. Rhythmic CLOCK-BMAL1 binding to multiple E-box motifs drives circadian Dbp transcription and chromatin transitions. Nat. Genet. 2006;38:369–374. doi: 10.1038/ng1738. [DOI] [PubMed] [Google Scholar]
- 50.Walhout AJ, Gubbels JM, Bernards R, van der Vliet PC, Timmers HT. c-Myc/Max heterodimers bind cooperatively to the E-box sequences located in the first intron of the rat ornithine decarboxylase (ODC) gene. Nucleic Acids Res. 1997;25:1493–1501. doi: 10.1093/nar/25.8.1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Raichur S, et al. Identification and validation of the pathways and functions regulated by the orphan nuclear receptor, ROR alpha1, in skeletal muscle. Nucleic Acids Res. 2010;38:4296–4312. doi: 10.1093/nar/gkq180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Delerive P, et al. The orphan nuclear receptor ROR alpha is a negative regulator of the inflammatory response. EMBO Rep. 2001;2:42–48. doi: 10.1093/embo-reports/kve007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gouw AM, et al. The MYC oncogene cooperates with sterol-regulated element-binding protein to regulate lipogenesis essential for neoplastic growth. Cell Metab. 2019;30:556–572. doi: 10.1016/j.cmet.2019.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Camarda R, et al. Inhibition of fatty acid oxidation as a therapy for MYC-overexpressing triple-negative breast cancer. Nat. Med. 2016;22:427–432. doi: 10.1038/nm.4055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sahar S, et al. Circadian control of fatty acid elongation by SIRT1 protein-mediated deacetylation of acetyl-coenzyme A synthetase 1. J. Biol. Chem. 2014;289:6091–6097. doi: 10.1074/jbc.M113.537191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Comerford SA, et al. Acetate dependence of tumors. Cell. 2014;159:1591–1602. doi: 10.1016/j.cell.2014.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li J, et al. Partial characterization of a cDNA for human stearoyl-CoA desaturase and changes in its mRNA expression in some normal and malignant tissues. Int. J. Cancer. 1994;57:348–352. doi: 10.1002/ijc.2910570310. [DOI] [PubMed] [Google Scholar]
- 58.Roongta UV, et al. Cancer cell dependence on unsaturated fatty acids implicates stearoyl-CoA desaturase as a target for cancer therapy. Mol. Cancer Res. 2011;9:1551–1561. doi: 10.1158/1541-7786.MCR-11-0126. [DOI] [PubMed] [Google Scholar]
- 59.Slack A, et al. The p53 regulatory gene MDM2 is a direct transcriptional target of MYCN in neuroblastoma. Proc. Natl Acad. Sci. USA. 2005;102:731–736. doi: 10.1073/pnas.0405495102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Barbieri E, et al. A p53 drug response signature identifies prognostic genes in high-risk neuroblastoma. PloS ONE. 2013;8:e79843. doi: 10.1371/journal.pone.0079843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Barbieri E, et al. MDM2 inhibition sensitizes neuroblastoma to chemotherapy-induced apoptotic cell death. Mol. Cancer Ther. 2006;5:2358–2365. doi: 10.1158/1535-7163.MCT-06-0305. [DOI] [PubMed] [Google Scholar]
- 62.Dobin A, et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 2013;29:15–21. doi: 10.1093/bioinformatics/bts635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kuleshov MV, et al. Enrichr: a comprehensive gene set enrichment analysis web server 2016 update. Nucleic Acids Res. 2016;44:W90–W97. doi: 10.1093/nar/gkw377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stashi E, et al. SRC-2 is an essential coactivator for orchestrating metabolism and circadian rhythm. Cell Rep. 2014;6:633–645. doi: 10.1016/j.celrep.2014.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hughes ME, Hogenesch JB, Kornacker K. JTK_CYCLE: an efficient nonparametric algorithm for detecting rhythmic components in genome-scale data sets. J. Biol. Rhythm. 2010;25:372–380. doi: 10.1177/0748730410379711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mohammad MA, Haymond MW. Regulation of lipid synthesis genes and milk fat production in human mammary epithelial cells during secretory activation. Am. J. Physiol. Endocrinol. Metab. 2013;305:E700–E716. doi: 10.1152/ajpendo.00052.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vantaku V, et al. Multi-omics integration analysis robustly predicts high-grade patient survival and identifies CPT1B effect on fatty acid metabolism in bladder cancer. Clin. Cancer Res. 2019;25:3689–3701. doi: 10.1158/1078-0432.CCR-18-1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.White JJ, Sillitoe RV. Genetic silencing of olivocerebellar synapses causes dystonia-like behaviour in mice. Nat. Commun. 2017;8:14912. doi: 10.1038/ncomms14912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Piyarathna DWB, et al. Distinct lipidomic landscapes associated with clinical stages of urothelial cancer of the bladder. Eur. Urol. Focus. 2018;4:907–915. doi: 10.1016/j.euf.2017.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary Files
Data Availability Statement
Publicly available clinical datasets GSE45547, GSE16476, and GSE85047. were analyzed with R2: Genomics Analysis and Visualization Platform (http://r2.amc.nl). RNA-sequencing data generated in this study have been deposited in the European Nucleotide Archive (ENA, https://www.ebi.ac.uk/ena/), accession number: PRJEB36158. All Figures and Supplementary Figures have associated raw data. Raw data of Figs. 1–7 and Supplementary Fig. 1–18, and uncropped blots are provided as one Source Data file. All the other data are available within the article and its Supplementary Information. Source data are provided with this paper.