Abstract
Background and Aims:
Successful liver transplantation offers the possibility of improved survival among patients with decompensated cirrhosis. However, there is wide variability in access to care and promptness of the transplant evaluation process in the United States.
Methods:
We performed a multi-center retrospective study of 1,118 patients who underwent evaluation for liver transplantation at the six Veterans Affairs’ transplant centers from 2013–2018. Of these, 832 patients were evaluated within 30 days and 286, more than 30 days after referral. We studied the differential effects of the time from referral to evaluation on pre and post-transplant mortality and transplant list drop out and explored predictors of early transplant evaluation.
Results:
Patients in the early evaluation group had a shorter adjusted time from referral to listing by 29.5 days (95% CI −50.4, −8.5, p<0.006), and referral to transplantation by 115.1 days (95% CI −179.5, −50.7, p<0.0001). On a multivariable Cox hazard model, evaluation within 30 days of referral was associated with a significantly lower pre-transplant mortality (adjusted Hazard Ratio aHR 0.70, 95% CI 0.54–0.91, p-value<0.01), but not associated with transplant list dropout (aHR 0.95, 95% CI 0.65–1.39, p=0.79) or post-transplant death (aHR 1.88, 95% CI 0.72–4.9, p=0.20). An early evaluation within 30 days was positively associated with a higher MELD at referral (aHR 1.03, 95% CI 1.01–1.06, p=0.006) and negatively associated with distance from the transplant center (aHR 0.99, 95% CI 0.99–0.99, p=0.045).
Conclusion:
Evaluation of patients referred for liver transplantation within 30 days is associated with a reduction in pre-transplant mortality.
INTRODUCTION:
Liver transplantation (LT) is curative therapy for patients with decompensated cirrhosis and liver cancer.1,2 While a MELD (Model End Stage Liver Disease) threshold of 15 is widely used to begin LT evaluation, patients are often not transplanted until the MELD scores are much higher, and this is based on organ availability.3 While LT has shown a mortality benefit in patients with cirrhosis, it is not uniformly available throughout the United States, with 13 out of 50 states without LT centers.4 There is also wide variability in the number of centers in each state with large metropolitan communities typically having multiple. There is limited published data on the outcomes of “all comers” referred for transplantation, and on the association of time from referral to initial evaluation at a transplant center or the time to listing on outcomes. The United Network for Organ Sharing (UNOS) data lacks this information because they track only listed patients from their time of listing. Because transplantation rates and waitlist survival are typically tracked only from the time of listing, this artificially inflates transplant rates and excludes outcomes of patients who are referred but end up not listed. While many transplant centers strive to improve access to expedite patient care and ease referrals from the community, the association of the time from referral to initial evaluation on outcomes in the liver transplant process has not been studied. The VA recommends that patients referred for liver transplantation be evaluated within 30 days of referral, but this recommendation is not based on published data. The aims of our study was to analyze a cohort of all patients referred to transplantation, and to compare the association of early evaluation (time from referral to initial transplant hepatologist evaluation) on pre-transplant mortality, transplant list dropout and post-transplant mortality.
MATERIALS AND METHODS:
Study Design and Data Sources:
We performed a retrospective multi-center cohort study using data assembled from the Veterans Affairs Health system of all patients referred for liver transplantation between July 2013 and March 2018. We captured detailed demographic, clinical, laboratory, and administrative data using validated methodology from the VA corporate data warehouse (CDW).5 Data on patients evaluated for transplant within the VA system was obtained using information from Transplant Referral and Cost Evaluation/Reimbursement Application (TRACER). Mortality data were obtained from the Vital Status File, and transplantation outcomes were cross-referenced from (UNOS) Standard Transplant Analysis and Research data file (as of 3/31/2019).6
Transplant referral process:
In the VA system, LT is offered at 1 of 6 Veterans Health Administration Transplant Centers (VATC) distributed geographically across the United States, in Houston, TX, Pittsburgh, PA, Nashville, TN, Portland, OR, Richmond, VA and Madison, WI.7 The process is initiated at the patient’s local VA and is commonly done by a gastroenterologist/ hepatologist, and in the absence of one, by a primary care physician. In patients with cirrhosis, though there are no national guidelines, a referral is often initiated when the MELD-Na score is ≥15 or with a condition eligible for MELD exemption.1 A standardized checklist including labs, imaging, non-invasive cardiac testing, and psychosocial evaluations are completed by the referring provider in preparation for LT evaluation. The results from these tests are assembled and forwarded electronically, through the TRACER system to a VATC, based on patient/physician choice. The workup is remotely reviewed by a transplant hepatologist and is either provisionally accepted or rejected. Those who are provisionally accepted then undergo an initial evaluation with the transplant hepatologist. This evaluation is done either by a traditional in-person evaluation at the transplant center or by telehealth.
During the initial evaluation, a detailed history of the patient’s liver disease, co-morbidities, social history (particularly substance abuse and social support), counseling about the transplant listing process, waiting list, MELD scores, transplant procedure, immunosuppression, complications, and post-transplant outcomes are discussed. After the initial evaluation, if no contraindications are identified, patients continue the evaluation process at the VATC with the aim of listing. During this time, patients may undergo specialized testing (such as cardiac catheterization), initial treatments (such as down-staging or bridging of a patient with HCC with local-regional therapy), and presentation at multidisciplinary tumor board and transplant selection committees. In some cases, the listing was deferred if MELD scores improved after referral until scores went up again. If approved at the multidisciplinary transplant selection committee, patients are listed and allowed to return home, where they are co-managed by the referring physician and the transplant center. Those veterans not deemed to be acceptable candidates for LT are sent back to the referring center.
The primary aim of the study was to identify the association of early evaluation on pre-transplant mortality. The secondary aims were to identify the association of time from referral to listing on waitlist drop out and post-transplant survival.
Subject Identification:
Patients referred for transplantation were first identified from the Transplant Referral and Cost Evaluation/Reimbursement Application (TRACER). This is an electronic database that tracks every patient who is referred for transplantation within the VA system from the time of referral until transplantation, death, or removal from the waitlist. This data was cross-linked with data from the VA Corporate Data Warehouse. Cirrhosis was identified using ICD9/10 codes for 1 inpatient or 2 outpatient ICD-9-CM or ICD-10 diagnosis codes for cirrhosis (ICD-9-CM codes 571.2 and 571.5; ICD-10 codes K74.6x and K70.3x)8. We excluded patients who were referred emergently (typically acute liver failure), referred from the local transplant center, referred but were deemed by the transplant center to be not candidates without further evaluation, and those who had no cirrhosis. All patients were followed until death, transplantation, or June 30th, 2019, whichever came first.
Covariates and Outcomes:
The following laboratory values were obtained from the time of referral to transplantation and throughout listing and pre-transplant: albumin, serum sodium, creatinine, total bilirubin, α-fetoprotein, HCV RNA, international normalized ratio, and platelet count. Baseline values that were closest to the date of referral and listing were obtained from 90 days before to 30 days after the event. For transplantation, the labs closest to the date of transplantation, obtained from the UNOS data file were used. Tobacco use at the time of transplant referral was characterized as current use, former use, or lifetime non-use using a previously validated approach.9 Alcohol use characterized by Alcohol Use Disorders Identification Test (AUDIT-C) scores obtained in the year before referral.10 AUDIT-C scores ≥4 in males ≥3 in females were considered alcohol misuse.11 Disease etiology was assigned using ICD codes and laboratory tests according to that described by Beste et al.12 Data on patients with hepatocellular carcinoma, including tumor size and number, BCLC stage, presence of disease outside of Milan criteria, details of tumor downstaging, tumor progression while on the waitlist and post-transplant HCC recurrence were abstracted from patient records. We identified the presence of medical comorbidities using the validated cirrhosis comorbidity (CirCom) score by Jepsen et al, which classified patients with cirrhosis into 7 groups based on the presence of one or more of the following: acute myocardial infarction, chronic kidney disease, chronic obstructive pulmonary disease, epilepsy, heart failure, metastatic cancer, non-metastatic or hematologic cancer, peripheral arterial disease, and substance abuse other than alcoholism.13
Distance was calculated between the patient’s referral VA and the location of the transplant center (in miles).14 MELD scores were calculated at the time of referral using laboratory values closest to the listing date and MELD scores at the time of listing and transplantation were obtained from listing labs with UNOS. Data on transplant list drop out and reasons were abstracted directly from the records. Death was directly abstracted from the chart. The institutional review board at each participating VA site approved the study.
Statistical analysis:
Data were summarized using mean (standard deviation) when normally distributed, otherwise median (interquartile range) for continuous variables, and number (percentage) for categorical variables. Differences between the groups were examined using Student’s t-test for normally distributed continuous variables, Kruskal-Wallis equality-of-populations rank test for skewed continuous variables, and chi-square test for binary and categorical variables. To examine the relationship between time to evaluation and time to different milestones in the transplant process, we used generalized linear regression models. Cox proportional hazard models were used to test the effect from time to evaluation on pre and post-transplant mortality based on an intention to treat analysis. Analyses were performed using SAS 9.4 (SAS Inc., Cary, NC).
RESULTS:
Baseline characteristics
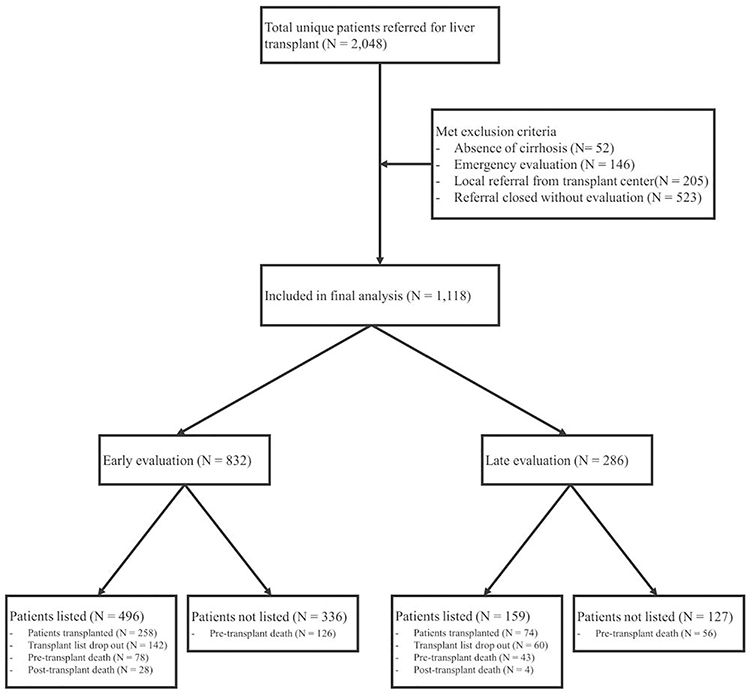
During the study period, 2,048 patients were referred for liver transplantation to the six VATCs from 07/2013 to 03/2018, of whom, 1,118 patients met the inclusion criteria (Figure 1).
Figure 1:
Flow of patients referred for liver transplantation (n=2048)
We excluded patients who were referred emergently (typically acute liver failure, n=146), referred from the local transplant center (n=205), referred but were deemed by the transplant center to be not candidates without further evaluation (n=523), and those who had no cirrhosis (n=52). Of these 523 excluded subjects who did not undergo evaluation after referral, 99 (18.9%) were too well for transplant, 184 (35.2%) had medical contraindications (58 were deconditioned or too sick for transplant, 55 had progression of HCC, 29 had cardiovascular disease, 21 died before evaluation, 12 had an extra-hepatic malignancy, and 9 due to other reasons), 113 had psychosocial contraindications, 85 were evaluated at a later time, 39 were evaluated at non-VA sites and 3 were still pending evaluation.
Of the patients who were accepted for further evaluation, 655 were approved and listed for transplantation, of which 334 patients were transplanted. A total of 303 died after referral but before receiving a transplant (Table 1). Briefly, the population was predominately male (97.3%), non-Hispanic Caucasian (65.0%), with a median age of 62 years (Table 1). The median Body Mass Index (BMI) was 29 kg/m2 (IQR 6.8) and 21.8% had coronary artery disease (CAD). HCC was present in 45.6% of patients. Of the 1,118 patients, 832 were in the early evaluation group and 286 were in the delayed evaluation group.
Table 1:
Baseline characteristics of patients referred for liver transplantation by time to evaluation
| Total | Early evaluation | Late evaluation | P Value | |
|---|---|---|---|---|
|
| ||||
| Total | 1118 | 832 | 286 | |
|
| ||||
| Referral Year, N (%) | <0.0001 | |||
| 07/2013–12/2013 | 124 | 37(29.8%) | 87(70.2%) | |
| 2014 | 233 | 154(66.1%) | 79(33.9%) | |
| 2015 | 226 | 173(76.5%) | 53(23.5%) | |
| 2016 | 280 | 237(84.6%) | 43(15.4%) | |
| 2017 | 212 | 191(90.1%) | 21(9.9%) | |
| 01/2018–03/2018 | 43 | 40(93.0%) | 3(7.0%) | |
|
| ||||
| Center, N (%) | <0.0001 | |||
| Station 1 | 170 | 131(77.1%) | 39(22.9%) | |
| Station 2 | 113 | 89(78.8%) | 24(21.2%) | |
| Station 3 | 226 | 187(82.7%) | 39(17.3%) | |
| Station 4 | 292 | 197(67.5%) | 95(32.5%) | |
| Station 5 | 152 | 66(43.4%) | 86(56.6%) | |
| Station 6 | 165 | 162(98.2%) | 3(1.8%) | |
|
| ||||
| Etiology, N (%) | 0.3127 | |||
| Autoimmune hepatitis | 8 | 5(0.6%) | 3(1.1%) | |
| Cryptogenic Cirrhosis | 6 | 5(0.6%) | 1(0.4%) | |
| Alcohol | 282 | 225(27%) | 57(19.9%) | |
| HBV | 24 | 20(2.4%) | 4(1.4%) | |
| HCV | 200 | 140(16.8%) | 60(21.0%) | |
| HCV + Alcohol | 430 | 309(37.1%) | 121(42.3%) | |
| HFE | 17 | 14(1.7%) | 3(1.1%) | |
| NAFLD | 111 | 84(10.1%) | 27(9.4%) | |
| PBC | 30 | 22(2.6%) | 8(2.8%) | |
| PSC | 10 | 8(1.0%) | 2(0.7%) | |
|
| ||||
| Age, Median (IQR) | 62.0(8.0) | 62.0(9.0) | 62.0(7.0) | 0.2863 |
|
| ||||
| Gender, N (%) | 0.3239 | |||
| Female | 30 | 20(2.4%) | 10(3.5%) | |
| Male | 1088 | 812(97.6%) | 276(96.5%) | |
|
| ||||
| Race/Ethnicity, N (%) | 0.3420 | |||
| White | 727 | 540(64.9%) | 187(65.4%) | |
| Black | 171 | 125(15.0%) | 46(16.1%) | |
| Other | 58 | 48(5.8%) | 10(3.5%) | |
| Hispanic / Latino | 102 | 71(8.5%) | 31(10.8%) | |
| Unknown | 60 | 48(5.8%) | 12(4.2%) | |
|
| ||||
| BMI at referral, Median (IQR) | 29.0(6.8) | 28.9(6.6) | 29.1(7.3) | 0.4503 |
|
| ||||
| BMI at referral, N (%) | 0.7653 | |||
| Underweight (less than 18.5) | 23 | 18(2.2%) | 5(1.8%) | |
| Normal Weight (18.5 to 25) | 193 | 142(17.1%) | 51(17.8%) | |
| Overweight (25 to 30) | 436 | 333(40%) | 103(36%) | |
| Obese (more than 30) | 466 | 339(40.8%) | 127(44.4%) | |
|
| ||||
| Diabetes at referral, N (%) | 0.6576 | |||
| No | 410 | 302(36.3%) | 108(37.8%) | |
| Yes | 708 | 530(63.7%) | 178(62.2%) | |
|
| ||||
| Tobacco Use at Diagnosis of Cirrhosis, N (%) | 0.2028 | |||
| Current smoker | 480 | 352(42.3%) | 128(44.8%) | |
| Former smoker | 280 | 200(24.0%) | 80(28.0%) | |
| Never smoker | 345 | 269(32.3%) | 76(26.6%) | |
| Unknown | 13 | 11(1.3%) | 2(0.7%) | |
|
| ||||
| AUDIT-C Score at referral, N (%) | 0.0449 | |||
| Low | 983 | 722(86.8%) | 261(91.3%) | |
| High | 135 | 110(13.2%) | 25(8.7%) | |
|
| ||||
| HCV viremia at referral, N (%) | 0.3954 | |||
| No | 838 | 629(75.6%) | 209(73.1%) | |
| Yes | 280 | 203(24.4%) | 77(26.9%) | |
|
| ||||
| Cirrhosis comorbidity index at referral, N (%) | 0.6242 | |||
| 0 | 752 | 562(67.6%) | 190(66.4%) | |
| 1+0 | 241 | 179(21.5%) | 62(21.7%) | |
| 1+1 | 56 | 37(4.5%) | 19(6.6%) | |
| 3+0 | 39 | 29(3.5%) | 10(3.5%) | |
| 3+1 | 29 | 24(2.9%) | 5(1.8%) | |
| 5+1 | 1 | 1(0.1%) | - | |
|
| ||||
| CAD at referral, N (%) | 0.2183 | |||
| No | 874 | 643(77.3%) | 231(80.8%) | |
| Yes | 244 | 189(22.7%) | 55(19.2%) | |
|
| ||||
| Listed outside the VA, N (%) | 0.0250 | |||
| No | 1063 | 784(94.2%) | 279(95.5%) | |
| Yes | 55 | 48(5.8%) | 7(2.5%) | |
|
| ||||
| Transplanted out of VA, N (%) | 0.1936 | |||
| No | 1072 | 794(95.4%) | 278(97.2%) | |
| Yes | 46 | 38(4.6%) | 8(2.8%) | |
|
| ||||
| Liver cancer at referral, N (%) | 0.2809 | |||
| No | 578 | 438(52.6%) | 140(48.9%) | |
| Yes | 540 | 394(47.4%) | 146(51.1%) | |
|
| ||||
| Disease within Milan criteria at referral, N (%) (N=540) | 0.4916 | |||
| No | 46 | 35(4.2%) | 11(3.8%) | |
| Yes | 494 | 359(43.2%) | 135(47.2%) | |
|
| ||||
| Down-staging of HCC, N (%) (N=46) | 0.2760 | |||
| No | 13 | 12(1.4%) | 1(0.4%) | |
| Yes | 33 | 23(2.8%) | 1(3.5%) | |
|
| ||||
| Tumor progression while waiting, N (%) (N=524) | 0.3681 | |||
| No | 392 | 282(33.9%) | 110(38.5%) | |
| Yes | 132 | 101(12.1%) | 31(10.8%) | |
|
| ||||
| Number of tumors, N (%) (N=524) | 0.7927 | |||
| 0 | 42 | 32(3.9%) | 10(3.5%) | |
| 1 | 306 | 222(26.7%) | 84(29.4%) | |
| 2 | 119 | 86(10.3%) | 33(11.6%) | |
| 3 | 46 | 35(4.2%) | 11(3.9%) | |
| 4 | 7 | 4(0.5%) | 3(1.1%) | |
| 5 | 3 | 3(0.4%) | - | |
| 6 | 1 | 1(0.1%) | - | |
|
| ||||
| Tumor Size, (median, IQR) | 2.4(1.3) | 2.3(1.3) | 2.5(1.3) | 0.2209 |
|
| ||||
| BCLC staging at listing, N (%) (N=524) | 0.1532 | |||
| 0 | 78 | 65(7.8%) | 13(4.6%) | |
| A | 384 | 271(32.6%) | 113(39.5%) | |
| B | 60 | 46(5.5%) | 14(4.9%) | |
| C | 1 | - | 1(0.4%) | |
| D | 1 | 1(0.1%) | - | |
|
| ||||
| Distance from referring VA to transplant center in miles (median, IQR) | 317.1(389.8) | 291.9(332.7) | 426.5(582.1) | <0.0001 |
|
| ||||
| MELD at referral (median, IQR) | 12.5(9.4) | 12.6(9.7) | 12.2(8.3) | 0.1706 |
| MELD at referral, of patients without MELD exception (median, IQR) | 15.2(6.9) | 15.5(7.0) | 14.6(5.6) | 0.0393 |
|
| ||||
| MELD at evaluation (median, IQR) | 12.9(9.5) | 13.1(10.0) | 12.2(8.7) | 0.1317 |
| MELD at evaluation, of patients without MELD exception (median, IQR) | 16.0(7.5) | 16.2(7.6) | 15.4(7.4) | 0.3770 |
|
| ||||
| MELD at listing (median, IQR) | 15.0 (11.0) | 15.0 (12.0) | 15.0 (11.0) | 0.6925 |
| MELD at listing, of patients without MELD exception (median, IQR) | 21.0(10.0) | 21.0 (10.0) | 20.0 (10.5) | 0.6888 |
|
| ||||
| MELD at transplant (median, IQR) | 20.0(17.0) | 20.0(17.0) | 18.0(16.0) | 0.2161 |
| MELD at transplant, of patients without MELD exception (median, IQR) | 26.0(11.0) | 26.0 (1.00) | 25.5(13.0) | 0.8778 |
|
| ||||
| Event | ||||
| Listing, N (%) | 655 | 496(59.6%) | 159(55.6%) | 0.2337 |
| Transplant, N (%) | 334 | 259(31.1%) | 75(26.2%) | 0.1179 |
| Transplant list drop out, N (%) | 226 | 161(19.4%) | 65(22.7%) | 0.2200 |
| Pre-transplant Death, N (%) | 303 | 204(24.4%) | 99(34.6%) | 0.0009 |
| Post-transplant Death, N (%) | 32 | 28(3.4%) | 4(1.4%) | 0.0853 |
|
| ||||
| Time to Event | ||||
| Days from referral to evaluation (median, IQR) | 26.0 (11.0) | 23.0 (10.0) | 39.0 (16.0) | <0.0001 |
| Days from referral to listing (median, IQR) | 71.0 (77.0) | 69.0 (77.0) | 77.0 (82.0) | 0.1268 |
| Days from referral to transplant (median, IQR) | 244.5(271.0) | 223.0 (246.0) | 367.0 (366.0) | 0.0009 |
| Days from Referral to pre-transplant death (median, IQR) | 291.0 (361.0) | 242.0 (281.0) | 402.0 (396.0) | <0.0001 |
| Days from Referral to post-transplant death (median, IQR) | 568.5(701.5) | 521.0 (566.5) | 947.0 (213.5) | 0.0353 |
The early and delayed evaluation groups were similar with regards to the distribution of gender, age etiology of liver disease, race/ethnicity, BMI, diabetes, tobacco use, cirrhosis comorbidity index, and CAD. However, patients in the early evaluation group were more likely to be located closer to the transplant center (291.9 vs. 426.5 days, p<0.0001), have a higher AUDIT-C score at one year before referral (13.2% vs. 8.7%, p=0.04), and a higher MELD at the time of referral (15.5 vs. 14.6, p=0.04).
As noted in Table 1, the percentage of patients who were evaluated in less than 30 days increased yearly from 29.8% in 2013 to 93% in 2018 (p<0.0001). There was also a significant center-to-center variation in the relative proportion of patients evaluated in less than 30 days from as low as 43.4% in station 5 to as high as 98.2% in station 6 (p<0.0001). There were no differences in characteristics of HCC between the two groups, including BCLC stage, disease within Milan, need for downstaging, median tumor size or number of lesions. The MELD scores at the time of evaluation, listing, and transplantation were similar between the two groups. There was no difference in the proportion of patients who were listed and transplanted between the two groups; however, pre-transplant death was lower in the early evaluation group (24.4% vs. 34.6%, p<0.0009) (Table 1).
Association of Early Transplant Evaluation with Pre and post-transplant Mortality
On an adjusted analysis, patients in the early evaluation group had a shorter time to listing by 29.5 days (95% CI −50.4, −8.5, p<0.006), to transplantation by 115.1 days (95% CI −179.5, −50.7, p<0.0001), as well as, to pre-transplant death by 123.5 days (95% CI −191.5,−55.4, p=0.0004) (Table 2).
Table 2:
Time from referral to listing, transplant and pre-transplantation death, by early/late evaluation (Generalized linear regression)
| VAR | Value | Time to listing | Time to transplant | Time to pre-transplantation death |
|---|---|---|---|---|
|
| ||||
| Estimate (CI) | Estimate (CI) | Estimate (CI) | ||
|
| ||||
| Unadjusted | ||||
| Time from referral to evaluation | More than 30 days | REF | REF | REF |
| Less than 30 days | −15.27(−34.88, 4.34) | −104.67(−165.86, −43.48) | −171.48(−237.15, −105.81) | |
| P=0.1268 | P=0.0009 | P<0.0001 | ||
|
| ||||
| Adjusted | ||||
| Time from referral to evaluation | More than 30 days | REF | REF | REF |
| Less than 30 days | −29.49(−50.44, −8.54) | −115.07(−179.47, −50.67) | −123.45(−191.49, −55.41) | |
| P=0.0059 | P<0.0001 | P=0.0004 | ||
On a multivariable Cox hazard model, age at the time of referral (adjusted Hazard Ratio aHR 1.03, 95%CI, 1.01–1.05, p=0.003), alcohol associated liver disease (aHR 0.58, 95% CI 0.37–0.90, p=0.01), race/ethnicity other than white, black, or Hispanic (aHR 2.46, 95% CI, 1.60–3.77, p<0.0001), diabetes (aHR 0.67, 95% CI 0.53–0.86, p=0.001), liver cancer at referral (aHR 0.68, 95% CI 0.22–0.94, p<0.001), disease within Milan criteria at referral (aHR 0.13, 95% CI 0.05–0.31, p<0.0001), successful downstaging of HCC (aHR 0.27, 95% CI 0.10–0.77, p=0.01), BCLC stage A at listing (vs. BCLC 0, aHR 2.70, 95% CI 1.37–5.33, p=0.004), and MELD score at referral (HR 1.04, 95% CI 1.02–1.06, p<0.0001) were associated with higher pre-transplant mortality (Table 3). After adjusting for potential confounders, an early evaluation within 30 days of referral was associated with a significantly lower hazard of pre-transplant mortality (aHR 0.70, 95% CI 0.54–0.91, p-value<0.01) (Table 3 and Figure 2). There was no association between early transplant evaluation and post-transplant mortality (aHR 1.88, 95% CI 0.72–4.90, p=0.20). (Table S1 http://links.lww.com/TP/C102 ).
Table 3:
Unadjusted and adjusted cox hazard model for hazard of pre-transplant death, by early/late evaluation (use 30 days as cutoff)
| Univariable | Multivariable | ||||
|---|---|---|---|---|---|
|
| |||||
| Parameter | Value | HR (95%CI) | P | aHR (95%CI) | P |
|
| |||||
| Time from referral to evaluation | Late Evaluation | REF | REF | ||
| Early Evaluation | 0.74 (0.58,0.94) | 0.0125 | 0.70 (0.54,0.91) | 0.0070 | |
|
| |||||
| Age | Age at Referral | 1.02 (1.00,1.04) | 0.0403 | 1.03 (1.01,1.05) | 0.0025 |
|
| |||||
| Center | Station 1 | REF | REF | ||
| Station 2 | 0.59 (0.36,0.98) | 0.0420 | 0.56 (0.33,0.97) | 0.0371 | |
| Station 3 | 0.68 (0.46,1.01) | 0.0565 | 0.64 (0.42,0.99) | 0.0456 | |
| Station 4 | 1.22 (0.87,1.70) | 0.2493 | 1.03 (0.72,1.47) | 0.8849 | |
| Station 5 | 0.70 (0.46,1.08) | 0.1103 | 0.64 (0.40,1.02) | 0.0595 | |
| Station 6 | 0.79 (0.53,1.20) | 0.2757 | 0.84 (0.53,1.32) | 0.4441 | |
|
| |||||
| Etiology | NAFLD | REF | REF | ||
| Autoimmune hepatitis | 0.67 (0.16,2.76) | 0.5751 | 0.63 (0.12,3.27) | 0.5842 | |
| Cryptogenic Cirrhosis | 1.33 (0.32,5.50) | 0.6985 | 1.75 (0.32,9.66) | 0.5193 | |
| Alcohol | 0.70 (0.47,1.05) | 0.0859 | 0.58 (0.37,0.90) | 0.0146 | |
| HBV | 0.59 (0.23,1.51) | 0.2714 | 0.63 (0.24,1.65) | 0.3461 | |
| HCV | 0.69 (0.45,1.06) | 0.0865 | 0.63 (0.38,1.02) | 0.0614 | |
| HCV + Alcohol | 0.81 (0.56,1.17) | 0.2534 | 0.80 (0.51,1.26) | 0.3342 | |
| HFE | 0.50 (0.16,1.63) | 0.2524 | 0.67 (0.20,2.31) | 0.5289 | |
| PBC | 0.83 (0.40,1.72) | 0.6123 | 0.84 (0.41,1.70) | 0.6200 | |
| PSC | 2.00 (0.84,4.75) | 0.1154 | 1.55 (0.74,3.23) | 0.2414 | |
|
| |||||
| Gender | Male | REF | REF | ||
| Female | 1.52 (0.83,2.78) | 0.1731 | 1.78 (0.95,3.31) | 0.0712 | |
|
| |||||
| Race/Ethnicity | White | REF | REF | ||
| Black | 0.83 (0.59,1.17) | 0.2808 | 0.75 (0.52,1.08) | 0.1173 | |
| Other | 2.08 (1.39,3.11) | 0.0004 | 2.46 (1.60,3.77) | <.0001 | |
| Hispanic / Latino | 0.97 (0.65,1.44) | 0.8674 | 1.07 (0.67,1.70) | 0.7905 | |
| Unknown | 0.66 (0.36,1.21) | 0.1753 | 0.64 (0.33,1.25) | 0.1903 | |
|
| |||||
| BMI at referral | Normal Weight | REF | REF | ||
| Underweight | 1.27 (0.61,2.67) | 0.5257 | 1.49 (0.72,3.11) | 0.2862 | |
| Overweight | 0.86 (0.62,1.19) | 0.3573 | 1.01 (0.72,1.42) | 0.9522 | |
| Obese | 1.00 (0.73,1.37) | 0.9870 | 1.18 (0.84,1.66) | 0.3463 | |
|
| |||||
| Diabetes at referral | No | REF | REF | ||
| Yes | 0.71 (0.56,0.89) | 0.0029 | 0.67 (0.53,0.86) | 0.0013 | |
|
| |||||
| Tobacco Use at Diagnosis of Cirrhosis | Never smoker | REF | REF | ||
| Current smoker | 1.20 (0.91,1.57) | 0.2059 | 1.11 (0.80,1.53) | 0.5432 | |
| Former smoker | 1.29 (0.95,1.75) | 0.1007 | 1.22 (0.89,1.66) | 0.2193 | |
| Unknown | 1.49 (0.54,4.05) | 0.4398 | 1.83 (0.72,4.67) | 0.2035 | |
|
| |||||
| AUDIT-C Score at referral | Low | REF | REF | ||
| High | 1.10 (0.78,1.54) | 0.5928 | 1.17 (0.8,1.71) | 0.4153 | |
|
| |||||
| HCV viremia at referral | No | REF | REF | ||
| Yes | 1.03 (0.8,1.33) | 0.8107 | 1.04 (0.76,1.41) | 0.8172 | |
|
| |||||
| Cirrhosis comorbidity index at referral | 0 | REF | REF | ||
| 1+0 | 1.14 (0.86,1.50) | 0.3564 | 1.26 (0.92,1.73) | 0.1486 | |
| 1+1 | 1.28 (0.79,2.08) | 0.3116 | 1.39 (0.80,2.42) | 0.2363 | |
| 3+0 | 1.49 (0.85,2.61) | 0.1638 | 1.18 (0.62,2.23) | 0.6129 | |
| 3+1 | 1.55 (0.84,2.85) | 0.1580 | 1.53 (0.85,2.75) | 0.1552 | |
|
| |||||
| CAD at referral | No | REF | REF | ||
| Yes | 1.11 (0.86,1.45) | 0.4221 | 1.14 (0.87,1.50) | 0.3340 | |
|
| |||||
| Liver cancer at referral | No | REF | REF | ||
| Yes | 0.82 (0.65,1.03) | 0.0842 | 0.68 (0.22,0.94) | <.0001 | |
|
| |||||
| Disease within Milan criteria at referral | No | REF | REF | ||
| Yes | 0.57 (0.34,0.94) | 0.0279 | 0.13 (0.05,0.31) | <.0001 | |
|
| |||||
| Down-staging of HCC | No | REF | REF | ||
| Yes | 0.30 (0.12,0.78) | 0.0130 | 0.27 (0.10,0.77) | 0.0142 | |
|
| |||||
| Tumor progression while waiting | No | REF | REF | ||
| Yes | 2.59 (1.83,3.67) | <.0001 | 2.69 (1.88,3.84) | <.0001 | |
|
| |||||
| BCLC staging at listing | 0 | REF | REF | ||
| A | 2.49 (1.26,4.92) | 0.0085 | 2.70 (1.37,5.33) | 0.0041 | |
| B | 1.27 (0.51,3.21) | 0.6083 | 0.68 (0.23,1.99) | 0.4814 | |
|
| |||||
| Distance (in miles) | Distance from referring VA to transplant center in miles | 0.99 (0.99,1.01) | 0.3970 | 0.99 (0.99,1.01) | 0.8634 |
|
| |||||
| MELD at referral | MELD Score at Referral | 1.03 (1.01,1.04) | <.0001 | 1.04 (1.02,1.06) | <.0001 |
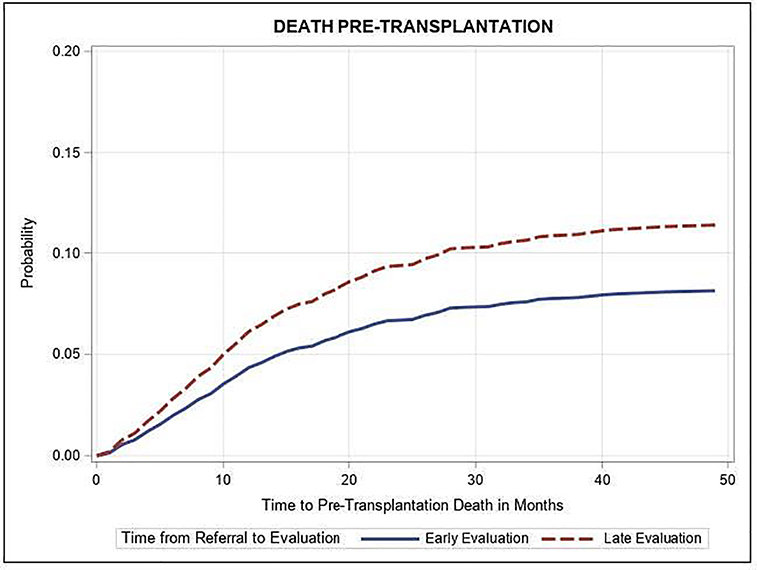
Figure 2:
Figure 2: Adjusted time from referral to pre-transplant death, by early (≤30 days) versus late (>30 days) time from referral to evaluation
Predictors of Transplant List Dropout
We performed a multivariable logistic regression to identify the predictors of transplant list dropout. We found that transplant list dropout was positively associated with tumor progression while awaiting transplantation (aOR 4.89, 95% CI 2.96–8.08, p<0.001), and negatively associated with being evaluated at station 2 (aOR 0.29, 95% CI 0.15–0.57, p<0.001), station 3 (aOR 0.28, 95% CI 0.16–0.48, p<0.001), station 5 (aOR 0.34, 95% CI 0.15–0.63, p<0.001), station 6 (aOR 0.27, 95% CI 0.14–0.51, p<0.001, all stations compared with station 1), or a history of diabetes mellitus (aOR 0.60, 95% CI 0.43–0.84, p=0.003). Early evaluation within 30 days of referral was not associated with transplant list dropout (aHR 0.95, 95% CI 0.65–1.39, p=0.80). (Table 4)
Table 4:
Univariate and multivariate logistic regression model for predictors of transplant list drop-out, by early (≤30 days) versus late (>30 days) time from referral to evaluation
| Univariable | Multivariable | ||||
|---|---|---|---|---|---|
|
| |||||
| Parameter | Value | OR (95%CI) | P | aOR (95%CI) | P |
|
| |||||
| Time from referral to evaluation | Late Evaluation | REF | REF | ||
| Early Evaluation | 0.82 (0.59,1.13) | 0.2205 | 0.95 (0.65,1.39) | 0.8030 | |
|
| |||||
| Age | Age at Referral | 1.02 (0.99,1.04) | 0.1443 | 1.02 (0.99,1.04) | 0.2379 |
|
| |||||
| Center | Station 1 | REF | REF | ||
| Station 2 | 0.33 (0.18,0.62) | 0.0109 | 0.29 (0.15,0.57) | <.0001 | |
| Station 3 | 0.28 (0.17,0.47) | <.0001 | 0.28 (0.16,0.48) | <.0001 | |
| Station 4 | 0.96 (0.64,1.44) | 0.1814 | 0.90 (0.57,1.41) | 0.1268 | |
| Station 5 | 0.38 (0.22,0.66) | 0.0136 | 0.34 (0.18,0.63) | 0.0214 | |
| Station 6 | 0.26 (0.15,0.47) | <.0001 | 0.27 (0.14,0.51) | 0.0123 | |
|
| |||||
| Etiology | NAFLD | REF | REF | ||
| Autoimmune hepatitis | 1.84 (0.34,9.91) | 0.6630 | 2.92 (0.45,19.06) | 0.3746 | |
| Cryptogenic Cirrhosis | 1.11 (0.12,10.06) | 0.8553 | 1.46 (0.14,15.07) | 0.9610 | |
| Alcohol | 1.59 (0.88,2.86) | 0.4269 | 1.39 (0.74,2.62) | 0.9888 | |
| HBV | 0.79 (0.21,2.94) | 0.3743 | 0.83 (0.20,3.40) | 0.4124 | |
| HCV | 1.47 (0.79,2.73) | 0.6755 | 1.45 (0.70,3.00) | 0.8653 | |
| HCV + Alcohol | 1.36 (0.77,2.41) | 0.9032 | 1.17 (0.59,2.32) | 0.5269 | |
| HFE | 1.19 (0.31,4.57) | 0.8505 | 1.50 (0.35,6.36) | 0.9011 | |
| PBC | 2.01 (0.77,5.25) | 0.3163 | 1.93 (0.69,5.40) | 0.4604 | |
| PSC | 1.38 (0.27,7.08) | 0.9552 | 1.07 (0.19,6.09) | 0.7399 | |
|
| |||||
| Gender | Male | REF | REF | ||
| Female | 0.60 (0.21,1.74) | 0.3465 | 0.61 (0.20,1.87) | 0.3850 | |
|
| |||||
| Race/Ethnicity | White | REF | REF | ||
| Black | 0.84 (0.49,1.42) | 0.7917 | 0.92 (0.57,1.49) | 0.4866 | |
| Other | 0.88 (0.58,1.33) | 0.5892 | 1.09 (0.51,2.33) | 0.3211 | |
| Hispanic / Latino | 0.86 (0.43,1.69) | 0.7712 | 0.79 (0.43,1.42) | 0.9785 | |
| Unknown | 0.48 (0.22,1.08) | 0.1451 | 0.39 (0.16,0.95) | 0.0557 | |
|
| |||||
| BMI at referral | Normal Weight | REF | REF | ||
| Underweight | 0.38 (0.09,1.67) | 0.1837 | 0.33 (0.07,1.63) | 0.1101 | |
| Overweight | 1.00 (0.66,1.52) | 0.2703 | 1.25 (0.78,2.01) | 0.0936 | |
| Obese | 1.04 (0.68,1.57) | 0.1957 | 1.29 (0.80,2.08) | 0.0725 | |
|
| |||||
| Diabetes at referral | No | REF | REF | ||
| Yes | 0.61 (0.46,0.82) | 0.0012 | 0.60 (0.43,0.84) | 0.0031 | |
|
| |||||
| Tobacco Use at Diagnosis of Cirrhosis | Never smoker | REF | REF | ||
| Current smoker | 1.08 (0.76,1.53) | 0.9629 | 1.10 (0.73,1.67) | 0.8619 | |
| Former smoker | 1.13 (0.77,1.67) | 0.9623 | 1.03 (0.66,1.59) | 0.8661 | |
|
| |||||
| AUDIT-C Score at referral | Low | REF | REF | ||
| High | 0.93 (0.59,1.47) | 0.7682 | 0.86 (0.51,1.43) | 0.5592 | |
|
| |||||
| HCV viremia at referral | No | REF | REF | ||
| Yes | 1.04 (0.75,1.46) | 0.8089 | 0.87 (0.56,1.36) | 0.5482 | |
|
| |||||
| Cirrhosis comorbidity index at referral | 0 | REF | REF | ||
| 1+0 | 0.98 (0.68,1.41) | 0.9683 | 0.89 (0.59,1.35) | 0.9700 | |
| 1+1 | 1.48 (0.80,2.75) | 0.9591 | 1.37 (0.68,2.77) | 0.9604 | |
| 3+0 | 0.89 (0.38,2.05) | 0.9705 | 0.78 (0.31,1.97) | 0.9731 | |
| 3+1 | 1.54 (0.67,3.55) | 0.9582 | 1.54 (0.62,3.80) | 0.9578 | |
|
| |||||
| CAD at referral | No | REF | REF | ||
| Yes | 0.93 (0.65,1.33) | 0.6753 | 0.95 (0.64,1.41) | 0.8063 | |
|
| |||||
| Liver cancer at referral | No | REF | REF | ||
| Yes | 0.93 (0.69,1.24) | 0.6133 | 0.57 (0.18,1.89) | 0.3598 | |
|
| |||||
| Disease within Milan criteria at referral | No | REF | REF | ||
| Yes | 1.52 (0.70,3.33) | 0.3923 | 1.61 (0.39,6.60) | 0.8992 | |
|
| |||||
| Down-staging of HCC | No | REF | REF | ||
| Yes | 3.5 (0.39,31.12) | 0.3864 | 3.93 (0.33,46.72) | 0.2759 | |
|
| |||||
| Tumor progression while waiting | No | REF | REF | ||
| Yes | 5.04 (3.20,7.95) | <.0001 | 4.89 (2.96,8.08) | <.0001 | |
|
| |||||
| BCLC staging at listing | 0 | REF | REF | ||
| A | 0.87 (0.47,1.59) | 0.9663 | 0.98 (0.50,1.92) | 0.9684 | |
| B | 0.50 (0.20,1.27) | 0.9715 | 0.60 (0.19,1.82) | 0.9749 | |
|
| |||||
| Distance (in miles) | Distance from referring VA to transplant center in miles | 0.99 (0.99,1.01) | 0.0931 | 0.99 (0.99,1.01) | 0.1123 |
|
| |||||
| MELD at referral | MELD Score at Referral | 1.00 (0.98,1.02) | 0.7692 | 1.01 (0.98,1.03) | 0.6676 |
Predictors of Early Transplant Evaluation
We performed a multivariable logistic regression to identify the predictors of early transplant evaluation. We found that evaluation within 30 days was positively associated with race other than black, white or hispanic (aOR 2.29, 95% CI 1.01–5.21, p=0.02), a higher MELD score at referral (aOR 1.03, 95% CI 1.00–1.05, p=0.02), or being evaluated at station 6 (aOR 19.25, 95% CI 5.72–64.85, p<0.001). Early evaluation was negatively associated with evaluation in station 4 (aOR 0.62, 95% CI 0.39–0.99, p<0.001), station 5 (aOR 0.24, 95% CI 0.14–0.41, p<0.0001), BCLC Stage A (vs. stage 0, aOR 0.46, 95% CI 0.23–0.93, p<0.001), and greater distance between the referring and transplant centers (aOR 0.99, 95% CI 0.99–0.99, p=0.045). (Table 5)
Table 5:
Univariate and multivariate logistic regression model for predictors of early (≤30 days) time from referral to evaluation
| Univariable | Multivariable | ||||
|---|---|---|---|---|---|
|
| |||||
| Parameter | Value | OR (95%CI) | P | aOR (95%CI) | P |
|
| |||||
| Age | Age at Referral | 1.00 (0.98,1.02) | 0.7401 | 1.00 (0.98,1.03) | 0.7769 |
|
| |||||
| Center | Station 1 | REF | REF | ||
| Station 2 | 1.10 (0.62,1.96) | 0.6093 | 1.17 (0.63,2.16) | 0.6233 | |
| Station 3 | 1.43 (0.87,2.35) | 0.4393 | 1.52 (0.90,2.58) | 0.4446 | |
| Station 4 | 0.62 (0.40,0.95) | <.0001 | 0.62 (0.39,0.99) | <.0001 | |
| Station 5 | 0.23 (0.14,0.37) | <.0001 | 0.24 (0.14,0.41) | <.0001 | |
| Station 6 | 16.07 (4.86,53.17) | <.0001 | 19.25 (5.72,64.85) | <.0001 | |
|
| |||||
| Etiology | NAFLD | REF | REF | ||
| Autoimmune hepatitis | 0.54 (0.12,2.39) | 0.3124 | 0.45 (0.09,2.25) | 0.3110 | |
| Cryptogenic Cirrhosis | 1.61 (0.18,14.37) | 0.6783 | 1.46 (0.12,17.44) | 0.6988 | |
| Alcohol | 1.27 (0.75,2.14) | 0.4332 | 1.26 (0.70,2.27) | 0.2590 | |
| HBV | 1.61 (0.51,5.12) | 0.4292 | 1.05 (0.29,3.83) | 0.8582 | |
| HCV | 0.75 (0.44,1.27) | 0.1277 | 0.73 (0.38,1.41) | 0.3557 | |
| HCV + Alcohol | 0.82 (0.51,1.33) | 0.2103 | 0.84 (0.46,1.56) | 0.6593 | |
| HFE | 1.50 (0.40,5.62) | 0.5645 | 1.36 (0.31,5.86) | 0.5842 | |
| PBC | 0.88 (0.35,2.21) | 0.6543 | 1.09 (0.40,2.97) | 0.7522 | |
| PSC | 1.29 (0.26,6.43) | 0.7945 | 0.71 (0.12,4.20) | 0.7210 | |
|
| |||||
| Gender | Male | REF | REF | ||
| Female | 0.68 (0.31,1.47) | 0.3265 | 1.04 (0.41,2.64) | 0.9404 | |
|
| |||||
| Race/Ethnicity | White | REF | REF | ||
| Black | 0.79 (0.50,1.25) | 0.0896 | 0.67 (0.43,1.05) | 0.0728 | |
| Other | 0.94 (0.65,1.37) | 0.3304 | 2.29 (1.01,5.21) | 0.0197 | |
| Hispanic / Latino | 1.66 (0.82,3.35) | 0.1698 | 0.93 (0.54,1.60) | 0.1876 | |
| Unknown | 1.39 (0.72,2.66) | 0.4265 | 2.24 (1.07,4.67) | 0.0112 | |
|
| |||||
| BMI at referral | Normal Weight | REF | REF | ||
| Underweight | 1.29 (0.46,3.66) | 0.6652 | 0.95 (0.30,3.02) | 0.9339 | |
| Overweight | 1.16 (0.79,1.71) | 0.7145 | 1.17 (0.75,1.81) | 0.3418 | |
| Obese | 0.96 (0.66,1.40) | 0.3949 | 0.85 (0.55,1.33) | 0.4068 | |
|
| |||||
| Diabetes at referral | No | REF | REF | ||
| Yes | 1.07 (0.81,1.41) | 0.6576 | 1.19 (0.86,1.64) | 0.2943 | |
|
| |||||
| Tobacco Use at Diagnosis of Cirrhosis | Never smoker | REF | REF | ||
| Current smoker | 0.78 (0.56,1.08) | 0.3167 | 0.75 (0.50,1.12) | 0.1614 | |
| Former smoker | 0.71 (0.49,1.02) | 0.1621 | 0.77 (0.51,1.17) | 0.1978 | |
| Unknown | 1.55 (0.34,7.16) | 0.4065 | 2.14 (0.40,11.42) | 0.2662 | |
|
| |||||
| AUDIT-C Score at referral | Low | REF | REF | ||
| High | 1.59 (1.01,2.51) | 0.0464 | 1.50 (0.90,2.52) | 0.1235 | |
|
| |||||
| HCV viremia at referral | No | REF | REF | ||
| Yes | 0.88 (0.65,1.19) | 0.3956 | 1.29 (0.87,1.92) | 0.2093 | |
|
| |||||
| Cirrhosis comorbidity index at referral | 0 | REF | REF | ||
| 1+0 | 0.98 (0.70,1.36) | 0.9607 | 1.06 (0.72,1.57) | 0.9615 | |
| 1+1 | 0.66 (0.37,1.17) | 0.9510 | 0.80 (0.41,1.55) | 0.9546 | |
| 3+0 | 0.98 (0.47,2.05) | 0.9608 | 1.10 (0.48,2.54) | 0.9624 | |
| 3+1 | 1.62 (0.61,4.31) | 0.9731 | 1.66 (0.59,4.69) | 0.9724 | |
|
| |||||
| CAD at referral | No | REF | REF | ||
| Yes | 1.24 (0.88,1.73) | 0.2189 | 1.09 (0.75,1.59) | 0.6470 | |
|
| |||||
| Liver cancer at referral | No | REF | REF | ||
| Yes | 0.88 (0.67,1.15) | 0.3537 | 1.15 (0.40,3.29) | 0.8015 | |
|
| |||||
| Disease within Milan criteria at referral | No | REF | REF | ||
| Yes | 1.21 (0.66,2.21) | 0.8385 | 4.31 (1.30,14.35) | 0.6822 | |
|
| |||||
| Down-staging of HCC | No | REF | REF | ||
| Yes | 0.44 (0.08,2.34) | 0.4979 | 0.64 (0.09,4.82) | 0.5828 | |
|
| |||||
| Tumor progression while waiting | No | REF | REF | ||
| Yes | 1.27 (0.80,2.01) | 0.5055 | 1.50 (0.88,2.55) | 0.9973 | |
|
| |||||
| BCLC staging at listing | 0 | REF | REF | ||
| A | 0.45 (0.23,0.88) | <.0001 | 0.46 (0.23,0.93) | <.0001 | |
| B | 0.64 (0.27,1.54) | 0.9760 | 0.71 (0.26,1.99) | 0.9869 | |
|
| |||||
| Distance (in miles) | Distance from referring VA to transplant center in miles | 0.99 (0.99,0.99) | <.0001 | 0.99 (0.99,0.99) | <.0001 |
|
| |||||
| MELD at referral | MELD Score at Referral | 1.02 (1.01,1.04) | 0.0205 | 1.03 (1.00,1.05) | 0.0188 |
DISCUSSION:
This study evaluates the association of the time from referral to initial transplant hepatologist evaluation, on the liver transplant process. The current data shows that early evaluation within 30 days of referral was associated with lower pre-transplant mortality. The data also shows that patients evaluated early had a shorter time to both listing and transplantation.
The data we present is different from most published studies on transplant outcomes because we tracked every patient who was referred for liver transplantation on an intention to treat basis. On initial appearance, the rates of transplantation appear lower than published literature because only 335 subjects out of 2048 patients referred for transplantation received a transplant during the study period. However, in our cohort, only 1118 subjects underwent transplant hepatologist evaluation and 665 were listed. Most centers and published data track subjects only from the time of listing. When tracking our number of transplants as a denominator of listed patients, our transplant rates of >50% is similar to published data. However, we believe that it is important to track all patients referred to transplantation because centers can select out subjects who are listed, and tracking all referred patients is a way to better study transplant center performance by reducing this bias.
The findings are novel because we are unaware of any prior data looking at time from referral to transplant evaluation as a potential quality metric. The transplant evaluation process, as well as, the ease of access to a transplant hepatologist varies widely from center-to-center. Many centers are actively trying to improve access to ease the burden on the referring physicians and promote transplant referrals. Despite the great interest of many centers on the metric of transplant evaluation wait times, we are unaware of any published data investigating the association of an early evaluation on liver transplant outcomes. This data is not widely available across centers because UNOS only tracks patients from their time of listing. The VA tracks the transplant referral process using a centralized online tracking system and is, therefore, a good platform to study the transplant process from referral through evaluation, listing, and transplantation.
What would explain the correlation between prompt evaluation and pre-transplant mortality? The factors that lead to an early evaluation from the time of referral may be center or patient-related. Center related factors include the promptness with which the transplant center worked to schedule the initial evaluation. We recently published data showing that the ability to triage patients for transplantation using SCAN-ECHO or performing the initial evaluation via telehealth are both able to improve the efficiency of the transplant evaluation process. 15,16 An important factor that determines the promptness of evaluation is compliance of the patient and family. Delayed evaluation due to lack of patient and caregiver availability may be an indirect marker of social support. Identifying whether the reason for delayed evaluation was patient or center related, was challenging in this retrospective study. Initial liver transplant evaluation was performed either by telehealth or in person. Our group has recently published data on the benefits of telehealth evaluation in reducing time to listing in a single VATC; Though we did not have that data across multiple centers for this study.15 However, it was noted that centers that had a high adoption of telehealth also had shorter times to evaluation. Therefore, increased adoption of telehealth may also explain faster transplant evaluation and improved outcomes.15
Though patients in the early evaluation group had lower mortality, they died earlier; however, this is likely because of the variable total length of follow-up between the early and delayed evaluation groups. As noted in Table 1, the percentage of patients who were evaluated in less than 30 days increased yearly from 29.8% in 2013 to 93% in 2018. This would explain why the median total duration of follow-up was relatively lower in the early evaluation group. Because of the high collinearity between the year of referral and the time to evaluation, we did not include the year of referral in the Cox proportional hazard model. However, we adjusted for the duration of follow-up in the analysis of pre-transplant mortality.
Our study has several limitations. This was a retrospective cohort study where patients were not randomized to either intervention based on time to evaluation. Though we adjusted for known confounders, we acknowledge the possibility of residual confounding. Second, the study was done within the VA system where transplantation is centralized, and patients are referred across greater distances compared to the non-VA setting.17 The liver transplant evaluation and referral process in the VA is unique and is different compared to a non-VA setting. Therefore, our findings need to be validated in a non-VA setting. Thirdly, decisions on the timing of the listing, as well as, the MELD cutoff to list patients vary widely across centers based on their location and transplant wait times. We included all transplant centers in the VA system to mitigate some of these center effects. We believe that these limitations are outweighed by notable strengths. These include the multi-center study design with the distribution of transplant centers with variable wait times throughout the United States. The data were obtained from patients receiving care from a single national healthcare system with fairly uniform practices and guidelines across its facilities. Finally, the dates of referral, evaluation, listing, and transplantation were accurately monitored using a computerized national tracking system. We were successfully able to link patients and their data between the VA Corporate Data Warehouse, TRACER, and the UNOS/OPTN database.
In conclusion, our results support that a transplant hepatologist evaluation within 30 days of referral was associated with a significant reduction in pre-transplant mortality. This implies that although the shortage of donor organs remains the most important factor limiting LT other potentially limiting factors are also involved in determining the likelihood of undergoing a successful transplant for an individual patient. Although there has been an ongoing debate about organ allocation and awarding of additional MELD points for specific indications (most notably HCC), the promptness of transplant referral and listing also have a profound effect on individual patient outcomes. More studies are needed, particularly outside of the Veterans Administration Health System, to study center performance metrics, including the time from referral to evaluation and listing, as quality indicators of the liver transplant evaluation process.
Supplementary Material
Acknowledgments
Funding Sources: Services in support of the research project were generated by the VCU Massey Cancer Center Biostatistics Shared Resource, supported, in part, with funding from NIH-NCI Cancer Center Support Grant P30 CA016059.
List of Abbreviations:
- LT
Liver Transplantation
- MELD
Model End Stage Liver Disease
- UNOS
United Network of Organ Sharing
- VA
Veterans Affairs’
- TRACER
Transplant Referral and Cost Evaluation/Reimbursement Application
- eCTP
Electronic Child-Turcotte-Pugh
- AUDIT-C
Alcohol Use Disorders Identification Test
- HCC
Hepatocellular Carcinoma
- VOCAL
Veterans Outcomes and Costs Associated with Liver Disease
- VATC
Veterans Health Administration Transplant Centers
- BMI
Body Mass Index
- CAD
coronary artery disease
Footnotes
Conflict of Interest: None of the authors have a conflict of interest to declare.
References:
- 1.Martin P, DiMartini A, Feng S, et al. Evaluation for liver transplantation in adults: 2013 practice guideline by the American Association for the Study of Liver Diseases and the American Society of Transplantation. Hepatology. 2014; 59(3):1144. [DOI] [PubMed] [Google Scholar]
- 2.Merion R, Schaubel DE, Dykstra DM, et al. The survival benefit of liver transplantation. Am J Transplant 2005; 5(2):307–13 [DOI] [PubMed] [Google Scholar]
- 3.Hart A, Schladt DP, Zeglin J, et al. Predicting outcomes on the liver transplant waiting list in the United States: accounting for large regional variation in organ availability and priority allocation points. Transplantation. 2016;100(10):2153–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scientific Registry of Transplant Recipients. Find and compare programs. Available at https://www.srtr.org/transplant-centers/?&organ=liver&recipientType=adult&sort=volume&page=1. Accessed January 31, 2020.
- 5.Kaplan DE, Serper MA, Mehta R, et al. VOCAL Study Group. Effects of hypercholesterolemia and statin exposure on survival in a large national cohort of patients with cirrhosis. Gastroenterology. 2019; 156(6):1693–1706 [DOI] [PubMed] [Google Scholar]
- 6.Based on OPTN data accessed January 31st, 2020. In: United Network for Organ Sharing R, VA, ed, 2017. [Google Scholar]
- 7.Gunnar WP. Liver transplants among US veterans. JAMA. 2014;312(4):436–437. Doi: 10.1001/jama.2014.7152 [DOI] [PubMed] [Google Scholar]
- 8.Kaplan DE, Serper M, John BV, et al. VOCAL Study Group. Effects of metformin exposure on survival in a large national cohort of patients with diabetes and cirrhosis. Clin Gastroenterol Hepatol 2020. S1542–3565(20)31135–6. Doi: 10.1016/j.cgh.2020.08.026 [DOI] [PubMed] [Google Scholar]
- 9.Calhoun PS, Wilson SM, Hertzberg JS, et al. Validation of veterans affairs electronic medical record smoking data among Iraq- and Afghanistan-era veterans. J Gen Intern Med 2017; 32:1228–1234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bradley KA, DeBenedetti AF, Volk RJ, et al. AUDIT-C as a brief screen for alcohol misuse in primary care. Alcohol Clin Exp Res 2007; 31: 1208–1217 [DOI] [PubMed] [Google Scholar]
- 11.Williams EC, Rubinsky AD, Lapham GT, et al. Prevalence of clinically recognized alcohol and other substance use disorders among VA outpatients with unhealthy alcohol use identified by routine alcohol screening. Drug Alcohol Depend 2014; 135:95–103 [DOI] [PubMed] [Google Scholar]
- 12.Beste LA, Leipertz SL, Green PK, et al. Trends in burden of cirrhosis and hepatocellular carcinoma by underlying liver disease in US veterans, 2001–2013. Gastroenterology. 2015;149:1471–1482 e5 [DOI] [PubMed] [Google Scholar]
- 13.Jepsen P, Vilstrup H, Lash TL. Development and validation of a comorbidity scoring system for patients with cirrhosis. Gastroenterology. 2014;146(1):147–e16 [DOI] [PubMed] [Google Scholar]
- 14.Goldberg DS, French B, Forde KA, et al. Association of distance from a transplant center with access to waitlist placement, receipt of liver transplantation, and survival among US veterans. JAMA. 2014; 311(12):1234–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.John B, Love E, Dahman B, et al. Use of telehealth expedites evaluation and listing in patients referred for liver transplantation. Clin Gastroenterol Hepatol 2019. Doi: 10.1016/j.cgh.2019.12.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Konjeti VR, Heuman D, Bajaj JS, et al. Telehealth-based evaluation identifies patients who are not candidates for liver transplantation. Clin Gastroenterol Hepatol 2019; 17(1):207–209.e1. Doi: 10.1016/j.cgh.2018.04.048 [DOI] [PubMed] [Google Scholar]
- 17.Ross K, Patzer RE, Goldberg DS, et al. Sociodemographic determinants of waitlist and posttransplant survival among end-stage liver disease patients. Am J Transplant 2017; 17: 2879–2889 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.