Abstract
Objective:
Infant amygdala connectivity correlates with maternal reports of infant temperament characterized by novelty-evoked distress and avoidance. However, no studies examine how human infant amygdala connectivity relates to direct observations of novelty-evoked distress in human infants. The current study examines the link between amygdala connectivity and infant novelty-evoked distress using direct-observation of temperament.
Method:
At four-months of age infants’ (N=90) novelty-evoked distress was assessed using a standardized reactivity assessment and parent-report. Within three weeks, resting state fMRI was collected in a subset of infants (n=34). Using a whole-brain voxel wise approach, we examine amygdala connectivity associated with positive and negative affect during the reactivity assessment. We then examined regions where the association of amygdala connectivity with negative affect was higher than with positive affect. Additionally, we examined associations between amygdala connectivity and parent-report of temperament.
Results:
Greater amygdala-cingulate and amygdala-superior frontal gyrus connectivity was associated with lower positive affect during the reactivity assessment. Results further indicated that the association between amygdala-cingulate connectivity was greater for negative affect compared to positive affect. There were no significant associations between latency to approach novelty (as measured by parent-report) and amygdala connectivity. Validation analyses conducted using a large independent longitudinal sample (N=323) demonstrate that negative reactivity is associated with increased child-reported anxiety symptoms in adolescence.
Conclusion:
These results provide novel insight into the developmental pathophysiology of novelty-evoked distress. This is consistent with research linking an altered cognitive control mechanism to temperamental risk for anxiety.
Keywords: infant MRI, temperament, negative reactivity, amygdala, functional connectivity
Introduction
Infants who exhibit distress to novelty face elevated risk for later-life anxiety1,2. Several findings implicate perturbed amygdala connectivity in this association3–8. However, no studies examine how amygdala connectivity relates to novelty-evoked distress in human infants assessed with direct-observation methods. While parent report is valuable, these ratings of infant behavior can be biased by parent personality and they only modestly correlate with direct observation of temperament9–12. Thus, research is needed in infants combining behavioral assessments of temperament with measures of amygdala connectivity. The current report provides such data.
Infants’ responses to novelty vary from distressed (high negative affect, low positive affect) to happy/excited (high positive affect, low negative affect)13. Individual differences in distress to novelty at four-months of age (i.e., negative reactivity) predict later behavioral inhibition (BI)13–16. BI, in turn, predicts elevated risk for anxiety1,17–20. Thus, high distress/negative affect in response to novelty in infancy may represent an important risk phenotype20. Broadly, amygdala functional connectivity is linked to emotion dysregulation21. In behaviorally inhibited children, several lines of work link risk for anxiety to patterns of connectivity among brain regions associated with threat detection (e.g., amygdala) and cognitive control (prefrontal cortex)3–8. However, the emergence of such connectivity patterns and their association with behavior remains unknown. In part, this is because most imaging research on BI measures brain function in middle childhood and adulthood3,7—in many cases nearly 10 years after temperaments first emerge. Uncovering correlates of BI requires concurrent measurement of novelty-evoked distress and amygdala connectivity in infants before BI emerges.
Recent studies have measured brain function and temperament in infancy5,6,8,22. However, these studies possess two key limitations. First, they rely solely on maternal report of infant temperament6,22,5,8. While this is valuable, behavioral assessments of distress to novelty robustly predict anxiety in large longitudinal studies2,23. Direct observation of behavior also is not biased by influences of parental personality or psychopathology. Thus, the current study uses direct observation of temperament. Second, most infant imaging studies examining associations with infant temperament have acquired functional magnetic resonance imaging (fMRI) data within the first weeks of life. At present, no studies have demonstrated that connectivity patterns found in neonates persist beyond this period to ages when temperament is quantified using observational measures. The current study examines amygdala functional connectivity in 4-month-olds. Concurrent assessments of temperament and brain function at 4 months can provide novel insight into whether brain connectivity patterns evaluated in newborns are present when temperament can be reliably assessed.
To address these gaps, the current study examined amygdala connectivity and reactivity to novelty in four-month-old infants. Infant temperament was assessed via both behavioral assessment and parental report—allowing us to directly compare amygdala connectivity associated with these two measures. Within one month of these temperament assessments, fMRI was conducted during natural sleep. We test the hypothesis that amygdala-prefrontal cortex connectivity is associated with high negative affect and/or low positive affect. We additionally perform exploratory analyses to examine amygdala connectivity linked to parental report of temperament. Lastly, we make use of a large longitudinal validation sample to provide data on the clinical significance of negative reactive temperament.
Method
Participants
90 healthy full-term 4-month-old infants (Mage=4 months 5 days; range= 3 months 6 days – 4 months 28 days; 40 female infants) were recruited for a longitudinal study examining the neural correlates of early social behavior between July 2018 and March 2020. Infants were recruited via community outreach events, moms’ groups, mailings, and social media advertisements. Infants were excluded if they were premature (gestation length of less than 36 weeks), low birth weight (<2,500 grams), severe birth complications, known developmental disabilities, history of neurological problems, metallic implants, or had known uncorrected visual or auditory impairments. Parents were informed prior to the first visit that this study utilized neuroimaging and that they could elect to participate in either a follow-up MRI visit, a follow-up EEG visit, or both. Prior to participation in each visit parents provided informed consent. Families were compensated (Reactivity-$30 + toy, MRI-$60 + t-shirt +souvenir scan) at each visit.
All 90 infants completed the reactivity assessment and provided parent report measures of temperament. See Table 1 for sample demographics. Of these 90 infants, 59 (Mage=4 months 24 days, range= 3 months 23 days – 5 months 26 days) elected to participate in the follow-up MRI visit. Whether a parent elected to participate in the MRI follow-up was not significantly associated with age, maternal anxiety (assessed via Beck Anxiety Inventory24) infant reactivity (motor, positive or negative) and did not differ by infant sex, maternal education, or race/ethnicity (ps> .172; See Table S1, available online, for detailed breakdown). Eleven of these 59 (19%) infants failed to sleep during the visit, generating no imaging data, yielding data in 48 (81%) infants. 14 of these 48 (29%) infants were excluded from resting state analyses because they awoke during scanning, terminating the resting-state run. The successful acquisition of a resting state run was not related to age, sex, race/ethnicity, maternal anxiety, any aspect of infant reactivity, or visit duration (Mvisit duration=3 hours 49 minutes; ps>.178). The final sample of infants with temperament and resting state data was n=34.
Table 1.
Sample Demographics and data collected
| Characteristic | N (% of sample) | Mean (SD) |
|---|---|---|
| N | 90 | -- |
| Sex (female) | 40 (44.4%) | -- |
| Child Race/Ethnicity | ||
| White/Non-Hispanic | 60 (66.7%) | -- |
| African American/Non-Hispanic | 2 (2.2%) | -- |
| Asian/Non-Hispanic | 4 (4.4%) | -- |
| Multi-racial/Non-Hispanic | 17 (19.0%) | -- |
| Other/ Non-Hispanic | -- | -- |
| White/Hispanic | 1 (1.1%) | -- |
| African American/Hispanic | 1 (1.1%) | -- |
| Asian/Hispanic | -- | -- |
| Multi-racial/Hispanic | 4 (4.4%) | -- |
| Other/Hispanic | 1 (1.1%) | -- |
| Maternal Education | ||
| High school graduate | 1 (1.1%) | -- |
| GED | 2 (2.2%) | -- |
| Some college | 7 (7.8%) | -- |
| 2 Year Professional Degree | 2 (2.2%) | -- |
| 4 Year College Degree | 22 (24.5%) | -- |
| Advanced Degree | 56 (62.2%) | -- |
| Maternal Anxiety (assessed via Beck Anxiety Inventory) | 29.0 (5.62) | |
| High | 11 (12.2%) | -- |
| Moderate | 68 (75.6%) | -- |
| Low | 4 (4.4%) | -- |
| No data | 7 (7.8%) | -- |
| Data Collected | ||
| Reactivity Assessment | 90 (100%) | |
| Parental report of Temperament | 90 (100%) | -- |
| MRI Assessment | 59 (65.6%) | -- |
| No MRI data obtained (failure to sleep) | 11 (18.6%) | -- |
| Woke up before resting state | 7 (11.9%) | -- |
| Resting state run incomplete | 7 (11.9%) | -- |
| Resting state run complete | 34 (57.6%) |
Reactivity Assessment
A standardized assessment of infant reactivity16,25,26 was conducted at age 4-months (Mage=4 months 5 days, SDage=17 days). Infants sat in an infant seat while experimenters (out of sight) presented novel toys (i.e., visual blocks) and sounds (i.e., auditory blocks) to the child. Stimuli were presented in 4 blocks (2 visual and 2 auditory) of fixed order with visual and auditory blocks alternating. Each visual block was presented as hanging mobiles. Mobiles were comprised of 1, 3 or 6 animals. All mobiles were presented for 20 seconds each, separated by a 10-second interval for a total of 9 trials per block. Each auditory block included sentences or syllables. The sentence block presented 8 sentences. Every two sentences the number of voices speaking and, consequently, the volume of the presentation increased by one (e.g., first two sentences were spoken by one voice, second two sentences by two voices, etc). The duration of each sentence was approximately 6 seconds followed by a 2-second interval between sentences. Syllables (ma, pa, ga) were presented in 3 consecutive 10-second trials, with a 5-second interval between syllable repetitions. Speakers were 6 feet from the infant seat and the computer volume was standardized across all visits. During the presentation of the stimuli, if the infant cried continuously for more than 10 seconds, the experimenter paused the session to calm the infant, resuming the session once the infant was calm.
The entire assessment was video recorded for later coding of infant motor and emotional behavior. Motor responses (leg kicks, arm waves, back arching, hyper-extensions), positive affect (smiling, laughing), and negative affect (fussing, crying) during the presentation of the stimuli were coded on a 7-point Likert scale (1 indicative of low motor or affect; 7 indicative of high motor or affect) for each block. A second coder double coded all blocks for all subjects. Reliability ranged between .65 and .89 (Mmotor=.75, Mnegative=.86, Mpositive=.76) indicating good agreement between coders. All analyses were conducted on the primary coders scores. Summary scores for motor, negative affect, and positive affect were computed by averaging the respective scores across the four blocks. Table 2 provides the mean and standard deviations for all temperament measures and illustrates the correlations between all temperament measures. See Supplement 1, available online, for data that make use of support vector machine (SVM) learning to integrate all three dimensions of reactivity into one continuous factor score. See Supplement 1 and Figure S1, available online, provide details on how the SVM factor score was computed.
Table 2.
Descriptive statistics (N=90) for all temperament measures including correlations among all temperament measures and maternal anxiety.
| Mean | SD | (1) | (2) | (3) | (4) | (5) | (6) | (7) | (8) | (9) | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Reactivity Assessment | |||||||||||
| (1) Motor reactivity | 3.97 | 1.19 | |||||||||
| (2) Negative affect | 2.37 | 1.56 | .325** | ||||||||
| (3) Positive affect | 1.78 | .95 | .254* | −.274* | |||||||
| IBQ | |||||||||||
| (4) Activity Level | 3.89 | .78 | .068 | .055 | .048 | ||||||
| (5) Distress to limitations | 3.19 | .68 | −.112 | .182 | −.142 | .211* | |||||
| (6) Latency to approach novelty | 2.14 | .46 | −.084 | .281** | −.239* | .148 | .395** | ||||
| (7) Duration Orienting | 3.76 | 1.07 | −.091 | .013 | .094 | .264* | −.112 | −.010 | |||
| (8) Smiling | 4.15 | .87 | .039 | .106 | −.041 | .319** | −.008 | −.134 | .356** | ||
| (9) Soothability | 4.54 | .80 | −.177 | .139 | −.126 | .193 | −.104 | .148 | .366** | .256* | |
| BAI | |||||||||||
| (10) Maternal Anxiety | 28.96 | 5.62 | .200 | .142 | .045 | .016 | −.030 | .147 | −.072 | −.127 | .193 |
IBQ = Infant behavior questionnaire
BAI = Beck anxiety inventory
p<.05,
p<.01.
Infant Behavior Questionnaire
During the reactivity assessment, parents (out of sight) filled out questionnaires including the Infant Behavior Questionnaire (IBQ27) which evaluates infant temperament. Of note, two parents failed to complete all items for the soothability subscale, thus n=88 for any analyses using the soothability subscale. See Table 2 for the association between all temperament measurements.
MRI Acquisition
Infants MRI assessments were conducted during natural sleep and within 8 weeks of the initial reactivity assessment (M=19.17 days; range=1 day - 50 days; Mage=4 months 24 days; SDage=18 days). Visits were conducted in the evening. Upon arrival, infants were measured, changed and swaddled using an MR-safe Velcro swaddle. Parents were then given privacy to feed their baby and begin their bedtime routine. Once the infant was asleep for 20 minutes hearing protection (i.e., silicone earplugs and MiniMuff noise attenuators) was applied for the infant. The infant was then transferred to the scanner bed where several foam pads were placed around the infant’s head to minimize motion and sandbags were placed on each side of the infant’s body. The infant slept on the scanner bed for an additional 15 minutes before acquisition was initiated. A trained research assistant remained in the scan room to monitor the infant and alert the MR operator as needed. If the infant woke up, scanning was paused until the baby was soundly asleep again. This process of soothing and scanning was repeated until either all data were collected or the parents decided to end the session. On average MRI visit duration was 3.5 hours.
MRI was acquired on a 3T Siemens MAGNETOM Trio/Tim scanner with a 32-channel head coil. Scanning began with the acquisition of structural images which were used for registration. The T1-weighted scans used MPRAGE with TR/TE= 1900ms/2.43ms, flip=9°, voxel size .8mm isotropic. The T2-weighted scans use MPRAGE with TR/TE=3200ms/488ms, flip=9°, voxel size= .7x.8x.8. Resting state data was acquired using multi-band radiofrequency pulses (multi-band acceleration factor=6) to excite several slices in a single TR to acquire high-resolution data faster. 10-minute resting state runs were obtained using a gradient echo, echo-planar-image (EPI) sequence sensitized to T2* blood oxygen level dependent (BOLD) contrast using the following parameters: TR/TE=1250ms/39.4ms, flip=90°, voxel size= 2 mm isotropic. Framewise Integrated Real-time MRI Monitoring (FIRMM28), motion tracking software, was used for real-time motion tracking and to determine if additional 10 minute runs were necessary to obtain at least 10 minutes of data in which all frames had <.2mm framewise displacement (FD). Data was acquired until at least 10 minutes of low movement data were obtained or the infant woke up. All 34 infants had at least 1 run of data acquired (7 infants slept long enough to acquire two 10-minute runs).
Resting state fMRI Processing
Resting state data were processed using AFNI and the CONN toolbox v.18b. CONN is Matlab/SPM-based software for the analysis of resting state functional connectivity data. CONN makes use of Matlab v.2017b and SPM8 (SPM8 allowed for infant-specific adaptations to CONN’s default pipeline). Pre-processing of functional data began with slice timing correction (conducted using AFNI’s 3dTshift function). Subsequent pre-processing steps were conducted using the CONN toolbox29,30 and included spatial realignment (compensating for head motion), and co-registration to T1-weighted image. Structural images were then normalized to age-specific template (4.5-month template from the MRI Study of Normal Brain Development31), and MNI space, and tissue segmentation was conducted (classifying gray matter, white matter and cerebral spinal fluid). Subsequently, confounding effects were identified using anatomical component-based noise correction procedure (aCompCor32) rather than global signal regression (GSR). Analyses incorporating GSR are presented in Supplement 1, available online. The Artifact Detection Tool (ART; https://www.nitrc.org/projects/artifact_detect/) was utilized for outlier detection. Frames with FD>.25mm and global BOLD signal z-scores>5 were censored via ART33. For connectivity processing, data were centered and detrended (ignoring censored frames) and nuisance variables (estimates of head motion, white matter, CSF, and frames marked as outliers) were regressed out and data were band-pass filtered (.009 – .08 Hz) and spatially smoothed using a Gaussian kernel (6mm). In line with prior infant imaging studies5, a minimum of 4 minutes of fMRI data was required, excluding censored frames. After the removal of censored frames, the median amount of data retained was 9 minutes 22 seconds (range=4 minutes 53 seconds - 18 minutes 45 seconds; See Figure S2, available online, for histogram). Across all subjects, mean FD and percent censored frames were .195mm and 15%, respectively. This criterion did not result in the exclusion of any subjects—all 34 infants had data that survived this stringent volume-censoring threshold. See Supplement 1 and Figures S3–S4, available online, for scatterplots illustrating no significant association between FD and left amygdala connectivity and distance dependent biases in the FD-functional connectivity correlations.
Amygdala Functional Connectivity
Amygdala functional connectivity was assessed using a seed-based, whole-brain voxel-wise approach, using the average time course of left and right amygdala as the seed (see Supplement 1, available online for analyses from the left and right amygdala separately). The Pearson correlation coefficient of each voxel with the seed time course was calculated and converted to a z-score using Fisher’s r-to-z transformation. Analyses examined regions where amygdala functional connectivity is associated with negative affect and positive affect. Additionally, we examine regions where amygdala functional connectivity has a stronger positive association with negative affect compared to positive affect (negative affect > positive affect). To test these effects, we used a general linear model with functional connectivity values as the dependent variable, both negative affect and positive affect as predictors of interest, and motor, age at MRI assessment and sex as nuisance variables. Age and sex were included as covariates because of the wide age range and associations between temperament and sex have been reported in the literature34,35. Results were corrected voxel-wise at p<.001 and corrected for multiple testing to provide family-wise error (FWE) control for clusters (p<.05; cluster size=155 voxels). Additional details on the methods used to define the cluster size criterion are provided in Supplement 1, available online.
Next, we examined connectivity associated with parent reported fearful temperament. To do so, we first identified a parent-report measure that mapped onto observed measures of infant reactivity to provide a parallel to studies that utilize parent-report measures. We began by conducting an exploratory analysis to determine which subscales of the IBQ were positively associated with negative affect and negatively associated with positive affect (See Table 2). The latency to approach novelty subscale emerged as the only parent report measure linked to high negative affect and low positive affect (See also validation analyses). Next, we examined amygdala connectivity associated with parent report of latency to approach novelty, controlling for age and sex of the child. In Supplement 1, available online, we provide additional exploratory analyses examining connectivity associated with the other subscales of the IBQ (i.e., activity level, distress to limitations, duration orienting, smiling and soothability).
Validation Analyses
To facilitate greater generalization of our results we conducted two validation analyses using a large independent sample. First, we validated the link between negative (vs. positive) reactive temperament and parent reported temperament. Second, we validated the claim that behavioral assessments of reactivity provide robust links to later psychopathology by examining differences in child and parent report of anxiety as a function of negative vs. positive reactive temperament (assessed via direct observation). We also examine the association between parent-report of infant fearful temperament (i.e., IBQ-latency to approach novelty) and later child and parent report of anxiety. Both validation analyses were conducted using data from negative and positive reactive infants (N=323) from two large longitudinal cohorts (Cohort 1: n=101; Cohort 2: n=222). Infants were screened for negative and positive reactive temperament at 4 months of age using a standardized temperamental reactivity assessment (See14 for details). Infants were classified as negative reactive, if their negative affect and motor scores exceeded the group median. Infants were classified as positive reactive, if their positive affect and motor scores exceeded the median. Infants who met criteria for both groups were placed into the group where corresponding to the affect dimension they scored highest on14,25,26. Children were then longitudinally followed into adolescence (cohort 1 and 2) and adulthood (at present, only the cohort 1 sample has aged into this assessment time point).
For the first validation analysis, examining the link between reactivity and parent-report of infant temperament, we combine reactivity and IBQ data from the two cohorts. Cohort 1’s initial IBQ assessment was conducted at 9 months, whereas Cohort 2 collected IBQ data beginning at 4 months. For the purposes of these analyses, we combine parent report across these ages (See Supplement 1, available online, for analyses controlling for cohort). In total, 288 parents of negative and positive reactive infants completed the IBQ (Cohort 1: n=78; 77%; Cohort 2: n=210; 95%). Whether a parent did or did provide IBQ data was not associated with reactivity (p>.521).
The second validation analysis examines the longitudinal association between infant temperament (assessed both behaviorally and via parent report) and later anxiety. First, we examined whether negative and positive reactive infants (classified via direct observation) differed in terms of child or parent reported anxiety symptoms. Second, we examine the association between parent-report of infant temperament (IBQ) and anxiety symptoms later in life. Anxiety symptoms were assessed in adolescence via the Screen for Child Anxiety Related Disorders (SCARED)36; a clinically validated questionnaire whereby participants report anxiety experienced over the past three months. In total, 185 participants (Cohort 1: n=59; 58%; Cohort 2: n=126; 57%) completed the child report SCARED and 193 participants (Cohort 1: n=54; 53%; Cohort 2: n=139; 63%) completed the parent report SCARED. Whether or not a child or parent participated in this follow-up visit did not differ as a function of reactivity (ps>.089). Supplement 1, available online, provides results controlling for cohort.
Results
In line with prior research, the amygdala exhibited widespread functional connectivity6,37. Functional activity in the amygdala was positively correlated with activity in the cingulate cortex, insula, operculum, superior frontal cortex, temporal lobe, and subcortical regions. Additionally, there were widespread negative correlations with activity in prefrontal, parietal, and occipital lobe areas (See Figure S5, available online). Focal analyses first examined amygdala connectivity associated with negative and positive affect. Results demonstrated a negative association between amygdala-cingulate and superior frontal gyrus and positive affect—such that greater connectivity was associated with lower positive affect (Figure 1). There was no main effect of negative affect. Next, we examined amygdala functional connectivity that was more positively associated with negative affect compared to positive affect. Results indicated that greater amygdala-cingulate connectivity (i.e., anterior cingulate and paracingulate) had a stronger positive association with negative affect compared to positive affect (Figure 2). There was no correlation between FD (mean or max) and connectivity in this region (ps>.38). See Figures S6, available online, for results with lowered voxel-wise threshold. See Figure S7 and S8, available online, for results of right and left amygdala seeds, respectively. Results incorporating GSR were similar See Figures S9–11 and Supplement 1, available online.
Figure 1.
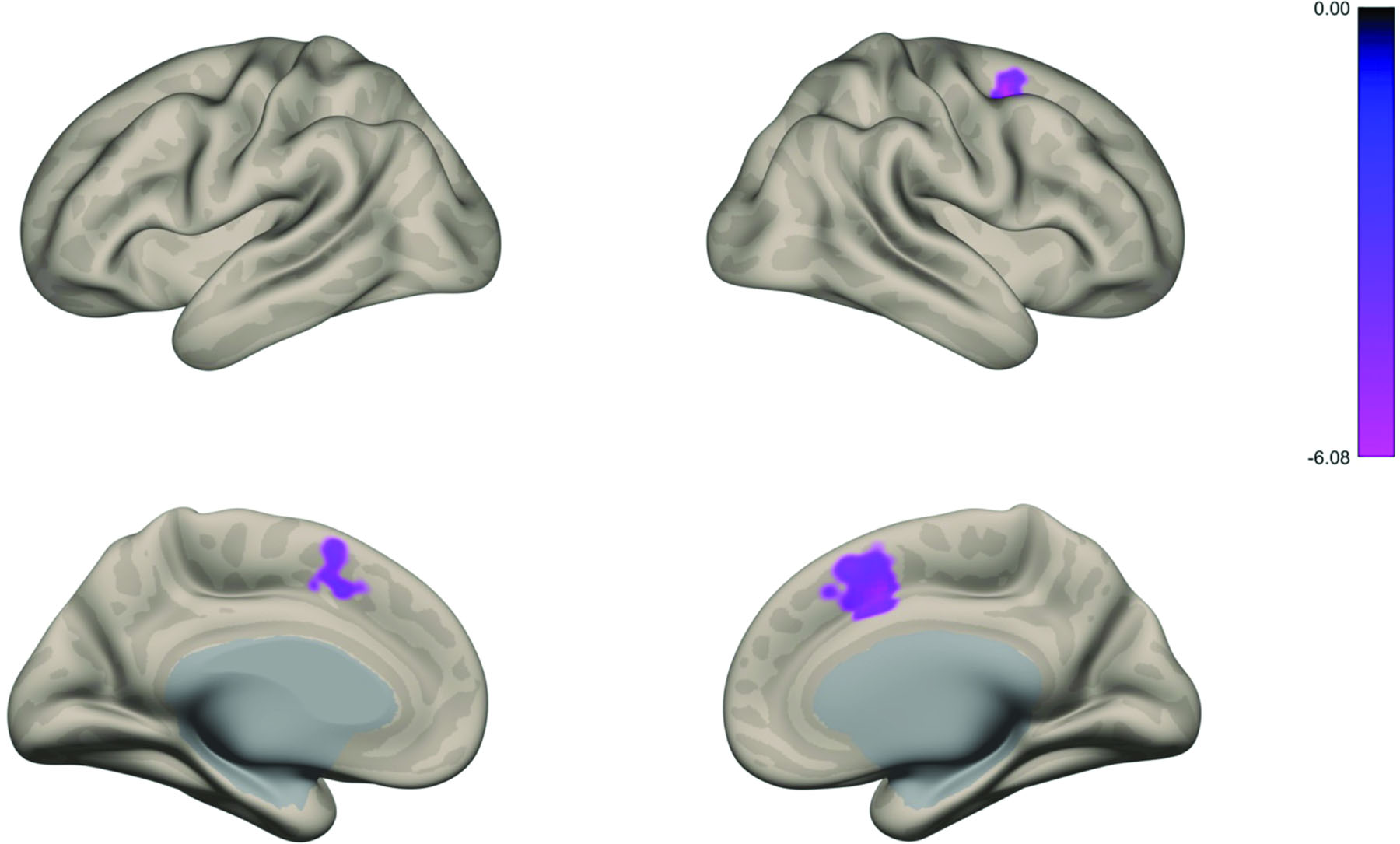
Amygdala connectivity associated with lower levels of positive affect
Note: (A) Whole brain voxel-wise map illustrates that greater amygdala-cingulate and amygdala-superior frontal gyrus connectivity was associated with less positive affect. Regions highlighted include: paracingulate gyrus, anterior cingulate, superior frontal gyrus, supplementary motor cortex, and middle frontal gyrus.
Figure 2.
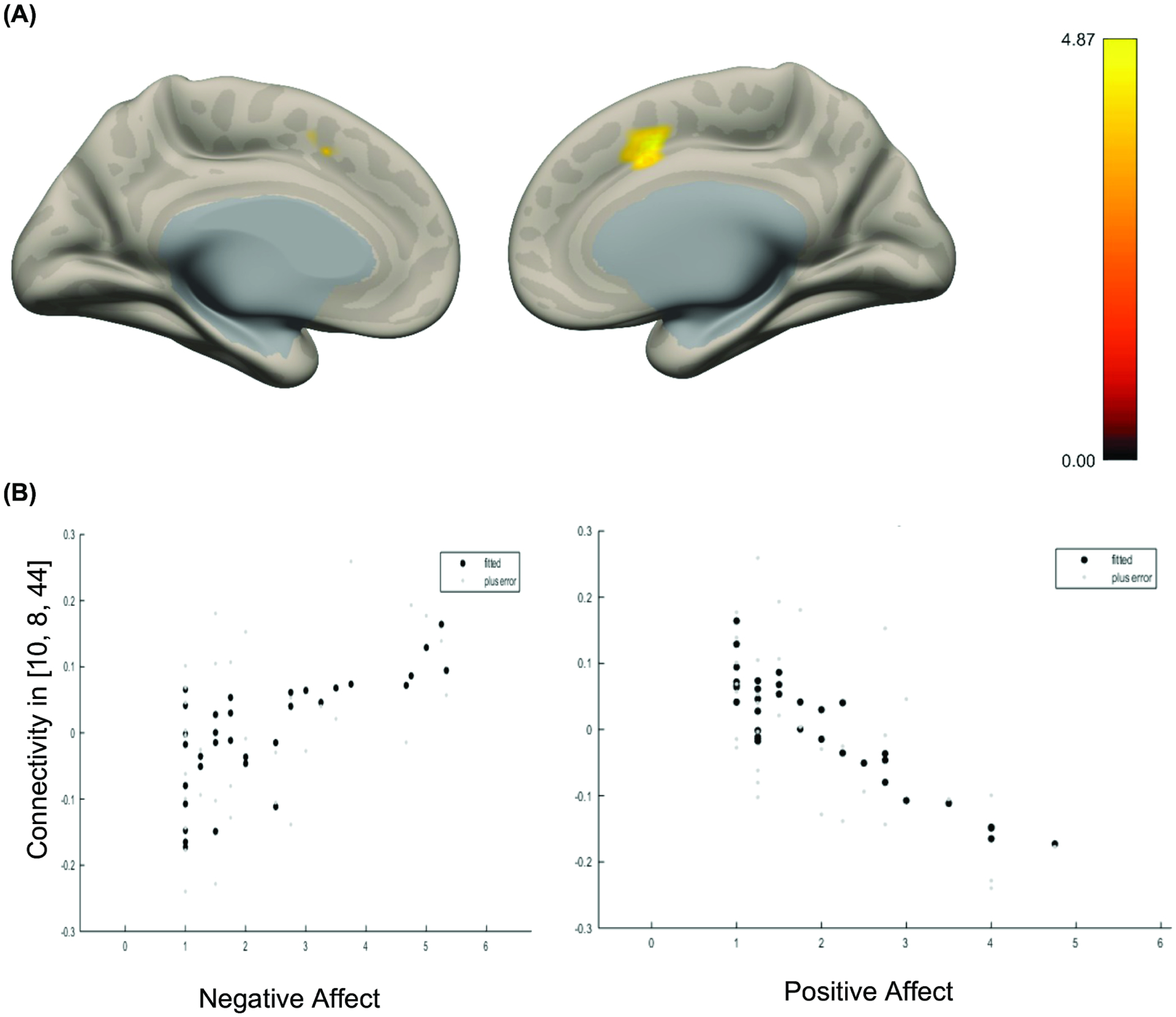
Amygdala connectivity associated with greater negative affect relative to positive affect
Note: (A) Whole brain voxel-wise map illustrates that amygdala-cingulate connectivity was associated greater negative affect compared to positive affect. Regions highlighted include: paracingulate gyrus, anterior cingulate gyrus, and supplemental motor area. Color bar indicates t-statistic. (B) Plots fitted response between negative affect (left) and positive affect (right) and connectivity represented in A. Error is indicated in light gray dots.
Next, to provide comparison to the existing parent report literature, we aimed to identify a parent report measure associated with direct observation of high negative affect and low positive affect. Results indicated that the IBQ-latency to approach novelty subscale was significantly positively associated with negative affect and negatively associated with positive affect (See Table 2). Also, as expected, the latency to approach novelty subscale was also positively associated with the SVM continuous factor score of reactivity. Thus, longer latency to approach novelty was associated with greater negative reactivity. No other subscales of the IBQ showed this association. This is consistent with prior literature that utilizes the IBQ-latency to approach (renamed the “fear” subscale) as an index of infant risk for the development of internalizing disorders later in life.
Using the results of these analyses, we then examined connectivity associated with parent report of latency to approach novelty, controlling for age and sex of the child. Results indicated that amygdala connectivity was not significantly associated with latency to approach novelty. Exploratory follow-ups lowering the voxel-wise threshold to p<.01 did not yield significant results. See Figure S12, available online, for results of analyses examining connectivity associated with SVM factor scores.
Validation Analyses
To validate the results reported above we conducted analyses using data from a large independent cohort. First, to validate the association between observational measures of negative reactive temperament and parent report measures of latency to approach novelty we examined whether infants classified as negative and positive reactive using observational assessment differed in average latency to approach novelty (as assessed via IBQ). Results indicated that relative to positive reactive (M=2.27) infants, negative reactive (M=2.56) infants exhibited a longer latency to approach novelty (F(1, 271)=7.20, p<.008). No other subscale of the IBQ was significantly greater for negative reactive infants compared to positive reactive infants (p>.261; See Supplement 1 and Table S2, available online, for descriptives on all other IBQ subscales as a function of reactivity). These results demonstrate convergence between our current study sample and a large sample of infants selected for negative and positive reactivity.
Second, to validate the claim that negative reactivity is associated with greater anxiety later in life, we conducted univariate ANOVAs examining whether parent or child-reported anxiety in adolescence differed as a function of infant reactivity. Results demonstrated that negative reactive (M=20.29) infants exhibited higher child-reported anxiety in adolescence than positive reactive (M=16.65) infants (F(1,183)=4.486, p<.036). However, there were no group differences in parent-reported anxiety (Mnegative=11.43, Mpositive=10.57; p>.561). Of the IBQ parent reported measures of infant temperament neither the IBQ-latency to approach novelty subscale nor any other subscale of the IBQ were associated with parent (ps>.174) or child reports (ps<.097) of anxiety in adolescence. These results provide supporting evidence of the clinical significance of negative reactivity and demonstrate that observed temperament is a robust predictor of later anxiety.
Discussion
Individual differences in distress to novelty emerge in infancy and predict behavioral inhibition14. At present, few infant imaging studies have examined the neural correlates of distress to novelty and all rely on parent-report of temperament5,6,8,22. The current study is the first to link observed temperament in 4-month-old infants and amygdala connectivity. We found that greater amygdala-cingulate and -superior frontal gyrus connectivity is associated with lower positive affect. We further demonstrated that amygdala-cingulate connectivity is more positively associated with negative affect than positive affect. At present, it remains unclear whether the combination of reduced positive and increased negative affect or reduced positive affect alone is central to amygdala-cingulate connectivity. This report additionally provides some of the first evidence that observed distress to novelty but not parent-report of temperament is associated with differences in children’s self-reported anxiety 15 years later. Thus, these findings highlight the value of standardized observation-based assessments of infant temperament.
Studies in older subjects link connectivity between the amygdala and cingulate gyrus to emotion regulation38,39. Thus, the current findings linking amygdala-cingulate connectivity to observed temperament are consistent with this broader literature. Greater amygdala-cingulate connectivity could implicate coupled cortical-subcortical engagement as a process that regulates behavior. Beyond a primary perturbation in connectivity, altered function in either the amygdala or cingulate gyrus also could underlie the observed associations. Longitudinal work assessing brain connectivity and activation could clarify how brain function relates to temperament at particular ages. For example, both the amygdala and cingulate gyrus are part of the brain’s salience network, which is involved in error monitoring, a process heightened in children with a history of BI40,41. Heightened error monitoring may moderate the link between BI and anxiety. Longitudinal research tracking relations among connectivity, temperament, and error-related activation could clarify how early brain function relates to the emergence of anxiety.
Using gold-standard behavioral assessments, the current study is among the first to identify neural correlates of infant temperament. To date, five other studies have used parent ratings to evaluate the link between neonatal brain connectivity and infant temperament or closely-related internalizing problems in the first few years of life5,6,8,22,37. Three of these five studies related stronger amygdala-cortical connectivity in infancy to later-emerging temperament or internalizing problems. Findings in these three studies manifested between the amygdala and salience-network nodes, including the insula5,8, medial prefrontal cortex8, and cingulate gyrus5,6. Our results extend these findings using an observational measure of temperament. Of note, a fourth study connected infant fear to decreased connectivity between the salience/ventral attention networks and the default mode network22. The fifth study linked anxiety at age 4 to increased amygdala-default-mode network and amygdala-visual areas connectivity (over the first two years of life) and decreased amygdala-sensorimotor connectivity37. Together, this infant imaging work fits well with the larger neurocognitive literature on markers of novelty-evoked distress.26,35,40,42,43.
In addition to extending the temperament literature, this work sharpens questions about age-related changes in brain function and the emergence of anxious behavior. Indeed, not all reactive infants will go on to exhibit BI or anxiety13. Nevertheless, at a group level we tend to see similar neurobiological profiles between temperaments characterized by novelty-evoked distress and clinical disorders associated with anxiety44–47. It has been argued by some that this overlap in biological correlates of temperament and clinical disorders suggests continuity among phenotypes48 although others find evidence to the contrary49,50. Stronger evidence for such continuity requires repeated imaging from the neonatal period to adolescence. This study is among the first to examine amygdala connectivity in four-month-old infants—an age at which temperamental distress to novelty first emerges behaviorally. However, replication with large, diverse, longitudinal samples is necessary particularly for identifying early targets for intervention.
The strengths of this study include the use of in-lab behavioral assessment of temperament, concurrent brain imaging at 4 months of age, and use of an independent large longitudinal sample to provide key validating evidence. The use of this independent sample provides evidence of the generalizability (specifically for the association between observed reactivity and parent-report) and the clinical significance of negative reactivity. As well, there are several limitations of this work. First, the sample size reported here is relatively small. It could be that our failure to replication prior studies using maternal report is due to limited statistical power. Second, the sample reported here was largely homogeneous in ethnicity and SES, a problem in other studies of this nature. Parents of children in this sample were primarily White and the majority of mothers holding advanced degrees. Future studies should aim to replicate in a diverse sample to address to the generalizability of these results. Third, this study evaluated amygdala connectivity at only one time point--four months; thus, we cannot know whether these effects have been present since birth or emerge at four months. While prior studies suggest similar patterns are present in neonates, longitudinal assessments of amygdala connectivity and temperament are necessary to determine how stable these patterns are over time. Finally, the validation analyses do provide novel evidence of a link between negative reactivity at four-months and self-reported anxiety in adolescence. Nevertheless, these results come from an independent dataset. Thus, they cannot clarify whether or not amygdala-cingulate connectivity mediates the link between negative reactivity and later anxiety. This is a possibility that should be explored in future studies. Moreover, while research collectively suggests that an altered cognitive control mechanism may underlie the development of anxiety, the present data cannot distinguish between normative variation and disrupted patterns of brain connectivity.
Overall these results provide novel insight into the developmental pathophysiology of novelty-evoked distress. This study demonstrates that greater amygdala-cingulate and amygdala-superior frontal gyrus connectivity is associated with low positive affect in response to novelty. Further, we show that amygdala-cingulate connectivity exhibits a more positive association with negative affect than positive affect. Critically, this is the first infant imaging study to link behavioral assessments of temperament in infancy with amygdala connectivity. Future work should aim to identify the extent to which amygdala-cingulate connectivity is stable within an individual and across time. These stable neural correlates of novelty-evoked distress may act as potential risk markers of anxiety. In summary, these results provide additional evidence of a link between brain circuitry associated with cognitive control and temperamental risk for anxiety.
Supplementary Material
Disclosure:
Dr. Sylvester has received grant or research support from NIMH, the McDonnell Foundation, the Taylor Family Institute, the Parker Fund, and the Brain and Behavior Research Foundation. He has a patent in preparation for ‘Amygdala Precision Functional Mapping.’ Drs. Filippi, Winkler, Pine, Fox and Mss. Ravi and Bracy have reported no biomedical financial interests or potential conflicts of interest.
This research was supported by a NARSAD Young Investigator Grant from the Brain and Behavior Research Foundation (#28024) awarded to Dr. Filippi, a Maryland Neuroimaging Center Seed Grant (awarded to Dr. Fox), and NIMH grant U01MH093349 (awarded to Dr. Fox). Additional support for this project is through the Intramural Research Program of the NIMH through project ZIA-MH-002782 (Dr. Pine) and NIMH grants R01MH122389 (Dr. Sylvester) and K23MH109983 (CMS).
The authors would also like acknowledge the following individuals from the research team at the University of Maryland for their contributions to data collection: Emma Margolis, BS, Apongnwu Fopenawoh, BS, Gabby Suarez, BA, Stephanie Leach, BS, Keara Neuman, BS, and Abby Brustad, BA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sandstrom A, Uher R, Pavlova B. Prospective Association between Childhood Behavioral Inhibition and Anxiety: a Meta-Analysis. J Abnorm Child Psychol. 2020;48(1):57–66. doi: 10.1007/s10802-019-00588-5 [DOI] [PubMed] [Google Scholar]
- 2.Tang A, Crawford H, Morales S, Degnan KA, Pine DS, Fox NA. Infant behavioral inhibition predicts personality and social outcomes three decades later. Proc Natl Acad Sci. 2020;117(18):9800–9807. doi: 10.1073/pnas.1917376117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abend R, Swetlitz C, White LK, et al. Levels of early-childhood behavioral inhibition predict distinct neurodevelopmental pathways to pediatric anxiety. Psychol Med. Published onlineJanuary8, 2019:1–11. doi: 10.1017/S0033291718003999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Birn RM, Shackman AJ, Oler JA, et al. Extreme early-life anxiety is associated with an evolutionarily conserved reduction in the strength of intrinsic functional connectivity between the dorsolateral prefrontal cortex and the central nucleus of the amygdala. Mol Psychiatry. 2014;19(8):853–853. doi: 10.1038/mp.2014.85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Graham AM, Buss C, Rasmussen JM, et al. Implications of newborn amygdala connectivity for fear and cognitive development at 6-months-of-age. Dev Cogn Neurosci. 2016;18:12–25. doi: 10.1016/j.dcn.2015.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rogers CE, Sylvester CM, Mintz C, et al. Neonatal Amygdala Functional Connectivity at Rest in Healthy and Preterm Infants and Early Internalizing Symptoms. J Am Acad Child Adolesc Psychiatry. 2017;56(2):157–166. doi: 10.1016/j.jaac.2016.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roy AK, Benson BE, Degnan KA, et al. Alterations in amygdala functional connectivity reflect early temperament. Biol Psychol. 2014;103:248–254. doi: 10.1016/j.biopsycho.2014.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thomas E, Buss C, Rasmussen JM, et al. Newborn amygdala connectivity and early emerging fear. Dev Cogn Neurosci. Published onlineDecember2018:100604. doi: 10.1016/j.dcn.2018.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hayden EP, Durbin CE, Klein DN, Olino TM. Maternal Personality Influences the Relationship Between Maternal Reports and Laboratory Measures of Child Temperament. J Pers Assess. 2010;92(6):586–593. doi: 10.1080/00223891.2010.513308 [DOI] [PubMed] [Google Scholar]
- 10.Bornstein MH, Gaughran JM, Segui I. Multimethod Assessment of Infant Temperament: Mother Questionnaire and Mother and Observer reports evaluated and compared at five months using the infant temperament measure. Int J Behav Dev. 1991;14(2):131–151. [Google Scholar]
- 11.Goldsmith HH, Campos JJ. The Structure of Temperamental Fear and Pleasure in Infants: A Psychometric Perspective. Child Dev. 1990;61(6):1944–1964. [PubMed] [Google Scholar]
- 12.Bishop G, Spence SH, McDonald C. Can Parents and Teachers Provide a Reliable and Valid Report of Behavioral Inhibition? Child Dev. 2003;74(6):1899–1917. [DOI] [PubMed] [Google Scholar]
- 13.Fox NA, Henderson HA, Rubin KH, Calkins SD, Schmidt LA. Continuity and Discontinuity of Behavioral Inhibition and Exuberance: Psychophysiological and Behavioral Influences across the First Four Years of Life. Child Dev. 2001;72(1):1–21. doi: 10.1111/1467-8624.00262 [DOI] [PubMed] [Google Scholar]
- 14.Fox NA, Snidman N, Haas SA, Degnan KA, Kagan J. The Relations between Reactivity at 4 Months and Behavioral Inhibition in the Second Year: Replication across Three Independent Samples. Infancy. 2015;20(1):98–114. doi: 10.1111/infa.12063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kagan J, Snidman N, Arcus D. Childhood Derivatives of High and Low Reactivity in Infancy. Child Dev. 1998;69(6):1483–1493. doi: 10.1111/j.1467-8624.1998.tb06171.x [DOI] [PubMed] [Google Scholar]
- 16.Kagan J, Snidman N. Infant Predictors of Inhibited and Uninhibited Profiles. Psychol Sci. 1991;2(1):40–44. doi: 10.1111/j.1467-9280.1991.tb00094.x [DOI] [Google Scholar]
- 17.Biederman J, Hirshfeld-Becker DR, Rosenbaum JF, et al. Further Evidence of Association Between Behavioral Inhibition and Social Anxiety in Children. Am J Psychiatry. 2001;158(10):1673–1679. doi: 10.1176/appi.ajp.158.10.1673 [DOI] [PubMed] [Google Scholar]
- 18.Essex MJ, Klein MH, Slattery MJ, Goldsmith HH, Kalin NH. Early Risk Factors and Developmental Pathways to Chronic High Inhibition and Social Anxiety Disorder in Adolescence. Am J Psychiatry. 2010;167(1):40–46. doi: 10.1176/appi.ajp.2009.07010051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hirshfeld DR, Rosenbaum JF, Biederman J, et al. Stable Behavioral Inhibition and Its Association with Anxiety Disorder. J Am Acad Child Adolesc Psychiatry. 1992;31(1):103–111. doi: 10.1097/00004583-199201000-00016 [DOI] [PubMed] [Google Scholar]
- 20.Kagan J, Snidman N, Zentner M, Peterson E. Infant temperament and anxious symptoms in school age children. Dev Psychopathol. 1999;11(2):209–224. doi: 10.1017/S0954579499002023 [DOI] [PubMed] [Google Scholar]
- 21.Beauchaine TP, Cicchetti D. Emotion dysregulation and emerging psychopathology: A transdiagnostic, transdisciplinary perspective. Dev Psychopathol. 2019;31(3):799–804. doi: 10.1017/S0954579419000671 [DOI] [PubMed] [Google Scholar]
- 22.Sylvester CM, Smyser CD, Smyser T, et al. Cortical Functional Connectivity Evident After Birth and Behavioral Inhibition at Age 2. Am J Psychiatry. 2018;175(2):180–187. doi: 10.1176/appi.ajp.2017.17010018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kagan J, Fox N. Biology, Culture, and Temperamental Biases. In: Handbook of Child Psychology. Vol VI. 6th ed.John Wiley & Sons; 2006:167–224. [Google Scholar]
- 24.Beck AT, Brown G, Epstein N, Steer RA. An Inventory for Measuring Clinical Anxiety: Psychometric Properties. J Consult Clin Psychol. 1988;56(6):893–897. [DOI] [PubMed] [Google Scholar]
- 25.Hane AA, Fox NA, Henderson HA, Marshall PJ. Behavioral reactivity and approach-withdrawal bias in infancy. Dev Psychol. 2008;44(5):1491–1496. doi: 10.1037/a0012855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marshall PJ, Reeb BC, Fox NA. Electrophysiological responses to auditory novelty in temperamentally different 9-month-old infants. Dev Sci. 2009;12(4):568–582. doi: 10.1111/j.1467-7687.2008.00808.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gartstein MA, Rothbart MK. Studying infant temperament via the Revised Infant Behavior Questionnaire. Infant Behav Dev. 2003;26(1):64–86. doi: 10.1016/S0163-6383(02)00169-8 [DOI] [Google Scholar]
- 28.Dosenbach NUF, Koller JM, Earl EA, et al. Real-time motion analytics during brain MRI improve data quality and reduce costs. NeuroImage. 2017;161:80–93. doi: 10.1016/j.neuroimage.2017.08.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cox RW. AFNI: Software for Analysis and Visualization of Functional Magnetic Resonance Neuroimages. Comput Biomed Res. 1996;29(3):162–173. doi: 10.1006/cbmr.1996.0014 [DOI] [PubMed] [Google Scholar]
- 30.Whitfield-Gabrieli S, Nieto-Castanon A. Conn : A Functional Connectivity Toolbox for Correlated and Anticorrelated Brain Networks. Brain Connect. 2012;2(3):125–141. doi: 10.1089/brain.2012.0073 [DOI] [PubMed] [Google Scholar]
- 31.Sanchez CE, Richards JE, Almli CR. Neurodevelopmental MRI brain templates for children from 2 weeks to 4 years of age. Dev Psychobiol. 2012;54(1):77–91. doi: 10.1002/dev.20579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Behzadi Y, Restom K, Liau J, Liu TT. A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. NeuroImage. 2007;37(1):90–101. doi: 10.1016/j.neuroimage.2007.04.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nieto-Castanon A Handbook of Functional Connectivity Magnetic Resonance Imaging Methods in CONN. Hilbert Press; 2020. [Google Scholar]
- 34.Degnan KA, Fox NA. Behavioral inhibition and anxiety disorders: Multiple levels of a resilience process. Dev Psychopathol. 2007;19(03):729. doi: 10.1017/S0954579407000363 [DOI] [PubMed] [Google Scholar]
- 35.Schwartz CE, Kunwar PS, Greve DN, Kagan J, Snidman NC, Bloch RB. A phenotype of early infancy predicts reactivity of the amygdala in male adults. Mol Psychiatry. 2012;17(10):1042–1050. doi: 10.1038/mp.2011.96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Birmaher B, Khetarpal S, Brent D, et al. The Screen for Child Anxiety Related Emotional Disorders (SCARED): Scale Construction and Psychometric Characteristics. J Am Acad Child Adolesc Psychiatry. 1997;36(4):545–553. doi: 10.1097/00004583-199704000-00018 [DOI] [PubMed] [Google Scholar]
- 37.Salzwedel AP, Stephens RL, Goldman BD, Lin W, Gilmore JH, Gao W. Development of Amygdala Functional Connectivity During Infancy and Its Relationship With 4-Year Behavioral Outcomes. Biol Psychiatry Cogn Neurosci Neuroimaging. 2019;4(1):62–71. doi: 10.1016/j.bpsc.2018.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Blackford JU, Clauss JA, Avery SN, Cowan RL, Benningfield MM, VanDerKlok RM. Amygdala–cingulate intrinsic connectivity is associated with degree of social inhibition. Biol Psychol. 2014;99:15–25. doi: 10.1016/j.biopsycho.2014.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Etkin A, Egner T, Kalisch R. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends Cogn Sci. 2011;15(2):85–93. doi: 10.1016/j.tics.2010.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Buzzell GA, Troller-Renfree SV, Morales S, Fox NA. Relations between Behavioral Inhibition, Cognitive Control, and Anxiety: Novel Insights Provided by Parsing Subdomains of Cognitive Control. In: Pérez-Edgar K, Fox NA, eds. Behavioral Inhibition. Springer International Publishing; 2018:213–235. doi: 10.1007/978-3-319-98077-5_10 [DOI] [Google Scholar]
- 41.Filippi CA, Subar AR, Sachs JF, et al. Developmental pathways to social anxiety and irritability: The role of the ERN. Dev Psychopathol. Published onlineOctober28, 2019:1–11. doi: 10.1017/S0954579419001329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McDermott JM, Perez-Edgar K, Henderson HA, Chronis-Tuscano A, Pine DS, Fox NA. A History of Childhood Behavioral Inhibition and Enhanced Response Monitoring in Adolescence Are Linked to Clinical Anxiety. Biol Psychiatry. 2009;65(5):445–448. doi: 10.1016/j.biopsych.2008.10.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weinberg A, Meyer A, Hale-Rude E, et al. Error-related negativity (ERN) and sustained threat: Conceptual framework and empirical evaluation in an adolescent sample: ERN and sustained threat. Psychophysiology. 2016;53(3):372–385. doi: 10.1111/psyp.12538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hardee JE, Benson BE, Bar-Haim Y, et al. Patterns of Neural Connectivity During an Attention Bias Task Moderate Associations Between Early Childhood Temperament and Internalizing Symptoms in Young Adulthood. Biol Psychiatry. 2013;74(4):273–279. doi: 10.1016/j.biopsych.2013.01.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McClure EB, Monk CS, Nelson EE, et al. Abnormal Attention Modulation of Fear Circuit Function in Pediatric Generalized Anxiety Disorder. Arch Gen Psychiatry. 2007;64(1):97. doi: 10.1001/archpsyc.64.1.97 [DOI] [PubMed] [Google Scholar]
- 46.Monk CS, Nelson EE, McClure EB, et al. Ventrolateral Prefrontal Cortex Activation and Attentional Bias in Response to Angry Faces in Adolescents With Generalized Anxiety Disorder. Am J Psychiatry. Published online2006:7. [DOI] [PubMed] [Google Scholar]
- 47.Pérez-Edgar K, Roberson-Nay R, Hardin MG, et al. Attention alters neural responses to evocative faces in behaviorally inhibited adolescents. NeuroImage. 2007;35(4):1538–1546. doi: 10.1016/j.neuroimage.2007.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pérez-Edgar KE, Guyer AE. Behavioral Inhibition: Temperament or Prodrome? Curr Behav Neurosci Rep. 2014;1(3):182–190. doi: 10.1007/s40473-014-0019-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fox NA, Barker TV, White LK, J G. Suway, Pine DS. Commentary: To intervene or not? Appreciating or treating individual differences in childhood temperament - remarks on Rapee (2013). J Child Psychol Psychiatry. 2013;54(7):789–790. doi: 10.1111/jcpp.12101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Smith AR, White LK, Leibenluft E, et al. The Heterogeneity of Anxious Phenotypes: Neural Responses to Errors in Treatment-Seeking Anxious and Behaviorally Inhibited Youths. J Am Acad Child Adolesc Psychiatry. Published onlineMay2019. doi: 10.1016/j.jaac.2019.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


