Abstract
BACKGROUND:
Regulatory T cell (Treg) therapy is a promising approach to amelioration of allograft rejection and promotion of organ transplant tolerance. However, the fate of infused Treg, and how this relates to their therapeutic efficacy using different immunosuppressive regimens is poorly understood. Our aim was to analyze the tissue distribution, persistence, replicative activity and phenotypic stability of autologous, donor antigen allo-reactive Treg (darTreg) in ATG-lymphodepleted, heart-allografted cynomolgus monkeys.
METHODS:
darTreg were expanded ex vivo from flow-sorted, circulating Treg using activated donor B cells and infused post-transplant into recipients of MHC-mismatched heart allografts. Fluorochrome-labeled darTreg were identified and characterized in peripheral blood, lymphoid and non-lymphoid tissues and the graft by flow cytometric analysis.
RESULTS:
darTreg selectively suppressed autologous T cell responses to donor antigens in vitro. However, following their adoptive transfer after transplantation, graft survival was not prolonged. Early (within 2 weeks post-transplant; under ATG, tacrolimus and anti-IL-6R) or delayed (6–8 weeks post-transplant; under rapamycin) darTreg infusion resulted in a rapid decline in transferred darTreg in peripheral blood. Following their early or delayed infusion, labeled cells were evident in lymphoid and non-lymphoid organs and the graft at low percentages (< 4% CD4+ T cells). Notably, infused darTreg showed reduced expression of immunoregulatory molecules (Foxp3 and CTLA4), Helios, the proliferative marker Ki67 and anti-apoptotic Bcl2, compared with pre-infusion darTreg and endogenous CD4+CD25hi Treg.
CONCLUSION:
Lack of therapeutic efficacy of infused darTreg in lymphodepleted heart graft recipients appears to reflect loss of a regulatory signature and proliferative and survival capacity shortly after infusion.
INTRODUCTION
Cell therapy using regulatory T cells (Treg)1, 2 has shown considerable promise for promotion of transplantation tolerance in animal models.3–6 As a result, several centers have embarked upon early phase clinical trials designed primarily to assess the feasibility and safety of Treg therapy in organ transplantation.7–11 Key questions yet to be resolved include the timing of cell infusion, optimal cell dosage, and selection of appropriate immunosuppressive and other drugs to preserve or enhance the cells’ regulatory function.5, 12–14 Moreover, the in vivo fate, tissue distribution, stability and longevity of adoptively-transferred Treg15, 16 are largely unknown.
Nonhuman primates (NHP) are important pre-clinical models in organ transplant research.17 We and others have observed18, 19 that, when infused systemically into non-transplanted monkeys, fluorochrome-labeled, ex vivo-expanded autologous polyclonal Treg decline rapidly in peripheral blood during the first week post-infusion, though they persist at low levels in blood for at least 3 weeks.19 In these studies, distribution of the infused Treg in native organs was not evaluated and could account for the diminished numbers of these cells in peripheral blood. Moreover, the dynamics of Treg migration, their preponderance relative to T effector cells, and their ability to suppress naïve versus memory T cell function20 may variably impact their therapeutic efficacy after infusion.
We reported previously21 that infusion of ex vivo-expanded autologous polyclonal Treg did not prolong survival of heart allografts in monkeys treated with anti-thymocyte globulin (ATG), an agent reported to expand Treg in vitro22, 23 and promote Treg in vivo,23–25 including in kidney-transplanted monkeys.26 In the current study, we aimed to assess graft survival and to monitor infused donor antigen alloreactive (dar)Treg in peripheral blood, lymphoid and non-lymphoid tissues and the allograft of similarly-treated, NHP heart transplant recipients. To our knowledge, this is the first report on tracking and characterization of adoptively-transferred Treg in a preclinical NHP organ transplant model.
MATERIALS AND METHODS
Animals
Male cynomolgus monkeys (Macaca fascicularis) of Indonesian origin (3–5 kg; 5–7 years old) were obtained from specific pathogen-free colonies at Alpha Genesis, Inc, or the National Institute of Allergy and Infectious Diseases colony (both Yamassee, SC). Experiments were conducted under an Institutional Animal Care and Use Committee-approved protocol. All animals received humane care in compliance with the “Principles of Laboratory Animal Care” formulated by the National Society for Medical Research and the “Guide for the Care and Use of Laboratory Animals” prepared by the Institute of Laboratory Animal Resources and published by the NIH (publication number 86–23. Revised 1996). Environmental enrichment was provided.
Heart transplantation
Ten monkeys (including historical controls) received heart grafts from ABO-compatible, MHC-mismatched donors. MHC genotyping was performed as described.27 Anesthesia, heterotopic intra-abdominal heart transplantation and graft monitoring were performed as detailed previously21 and in the Supplementary Methods. In darTreg recipients CM115 and CM121, grafts were monitored until complete cessation of cardiac contraction, at which time the animals were euthanized. Graft survival was compared to historical controls with either no Treg infusion (CM117, CM116 and CM123) or with ex vivo expanded polyclonal Treg infusion (CM120 and CM118), reported previously.21 darTreg recipients CM102, CM103 and CM220 were electively euthanized on days 18, 19 and 63 post-transplant, respectively.
Immunosuppression
Immunosuppressive regimens used are described in detail in the Supplementary Methods.
Expansion and function of donor Ag alloreactive (dar) Treg
CD4+T cells were negatively enriched from fresh PBMC using NHP CD4+T cell isolation kits (Miltenyi Biotech, Auburn, CA). CD4+CD25+CD127− Treg were then flow-sorted using a BD FacsAria (BD Biosciences, San Jose, CA). Isolated Treg were expanded using CD154-stimulated donor B cells for one week, followed by polyclonal stimulation using, artificial antigen-presenting cells (aAPCs; L-32 cells), kindly provided by Dr. Levings, University of British Columbia, Vancouver, Canada, as described19, 21, 28 and in the Supplementary Methods (Figure S1). The ability of the expanded darTreg to suppress autologous alloreactive VPD450-labeled CD3+CD25−T cell (obtained from the same Treg donor) proliferative responses to prospective donor or third-party PBMC was determined in 5-day MLR.29
Tracking of infused darTreg
Expanded darTreg were labeled with either 4 μM carboxyfluorescein succinimidyl ester (CFSE; Invitrogen) or BD Horizon™ Violet Proliferation Dye 450 (VPD450, BD Biosciences) before infusion, as described.19 At various times after infusion, mononuclear cells were isolated from blood or tissues and the incidence of infused darTreg determined. Phenotypic analysis of the cells was performed in comparison with darTreg before infusion and endogenous (native) Treg. Tissue samples obtained from various organs were minced and digested with collagenase. The resulting cell suspensions were overlaid on Ficoll-Paque (GE Healthcare), centrifuged for 20 min at 1500 rpm and the buffy coat collected. .
Phenotypic analyses
Phenotypic analyses were performed using monoclonal (m) Abs directed against CD3 (clone SP34–2), CD4 (L200), CD8 (RPA-T8), CTLA4 (BNI3) and bcl-2 (Bcl-2/100) from BD Pharmingen (Franklin Lakes, NJ). Anti-CD25 mAb (BC96) was from eBioscience (San Diego, CA). Ki-67 (20Raj1) and Foxp3 (PCH101) mAbs were from Invitrogen (Carlsbad, CA), and CXCR3 (G025H7) and Helios (22F6) mAbs from Biolegend (San Diego, CA). Following live/dead staining with Zombie Aqua Fixable Viability Kit (BioLegend) at 4°C for 15 min, surface staining was performed with anti-CD3, CD4, CD8, CD25, CXCR3, and CTLA4. Afterwards, the cells were fixed and permeabilized for 45 min at 4°C using Fixation/Permeabilization buffer (eBioscienceTM; Invitrogen). Thereafter, intracellular staining was performed for Foxp3, Helios, Bcl2, Ki67 and CTLA4. Data were acquired on a LSR II or LSR Fortessa (BD Bioscience) flow cytometer and analyzed with FlowJo software (Tree Star).
RESULTS
Heart allograft survival
Two heart allograft recipients CM115 and CM121 (Figure 1A) received 4 weekly infusions of autologous, unlabeled ex vivo-expanded darTreg (Figure 1B) that exhibited superior ability to suppress autologous CD3+T cell proliferation in response to donor than to 3rd party Ags at 1:8, 1:32 (p<0.01) and 1:128 ratios (Figure 1C). Moreover, their suppressive potency for MLR responses to donor was greater than that of ex vivo-expanded polyclonal Tregs30 (Figure S2). Furthermore, darTreg expressed higher levels of CD25, Foxp3, CTLA4 and Helios, as well CXCR3 compared to autologous effector T cells (Figure 1D). Each recipient received 4 darTreg infusions on days 3, 10, 17 and 24 (Figure 1E).
FIGURE 1.
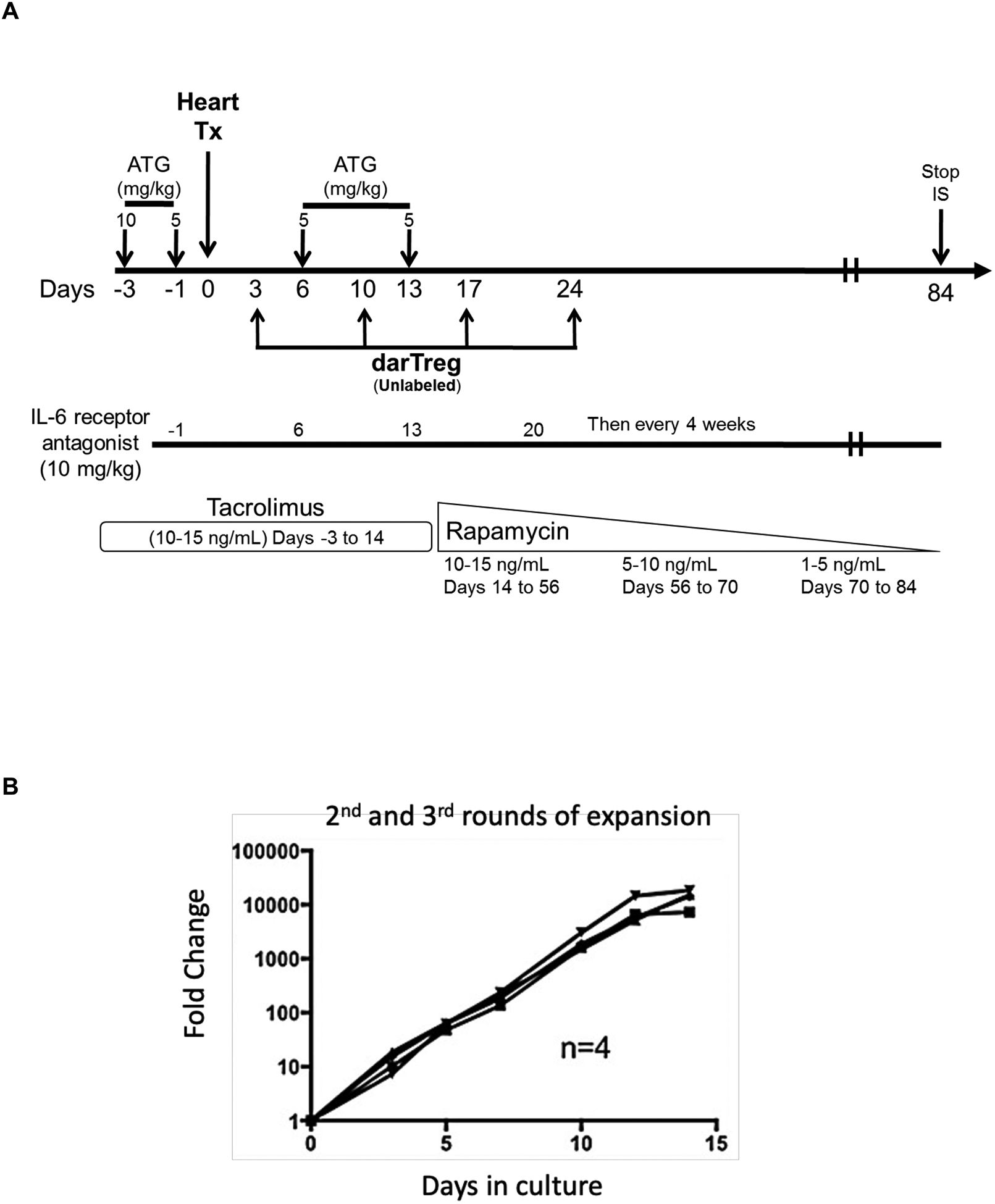
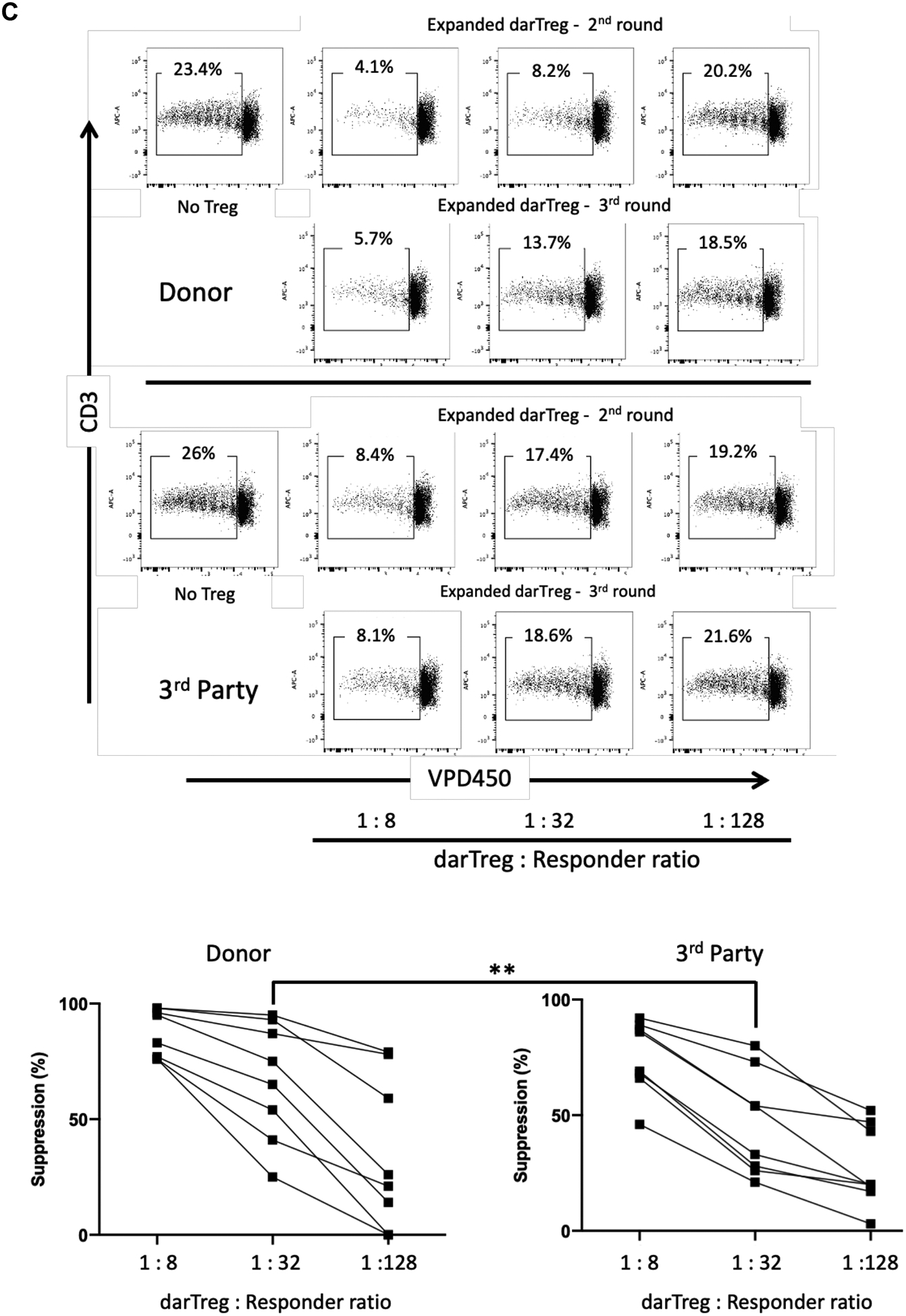
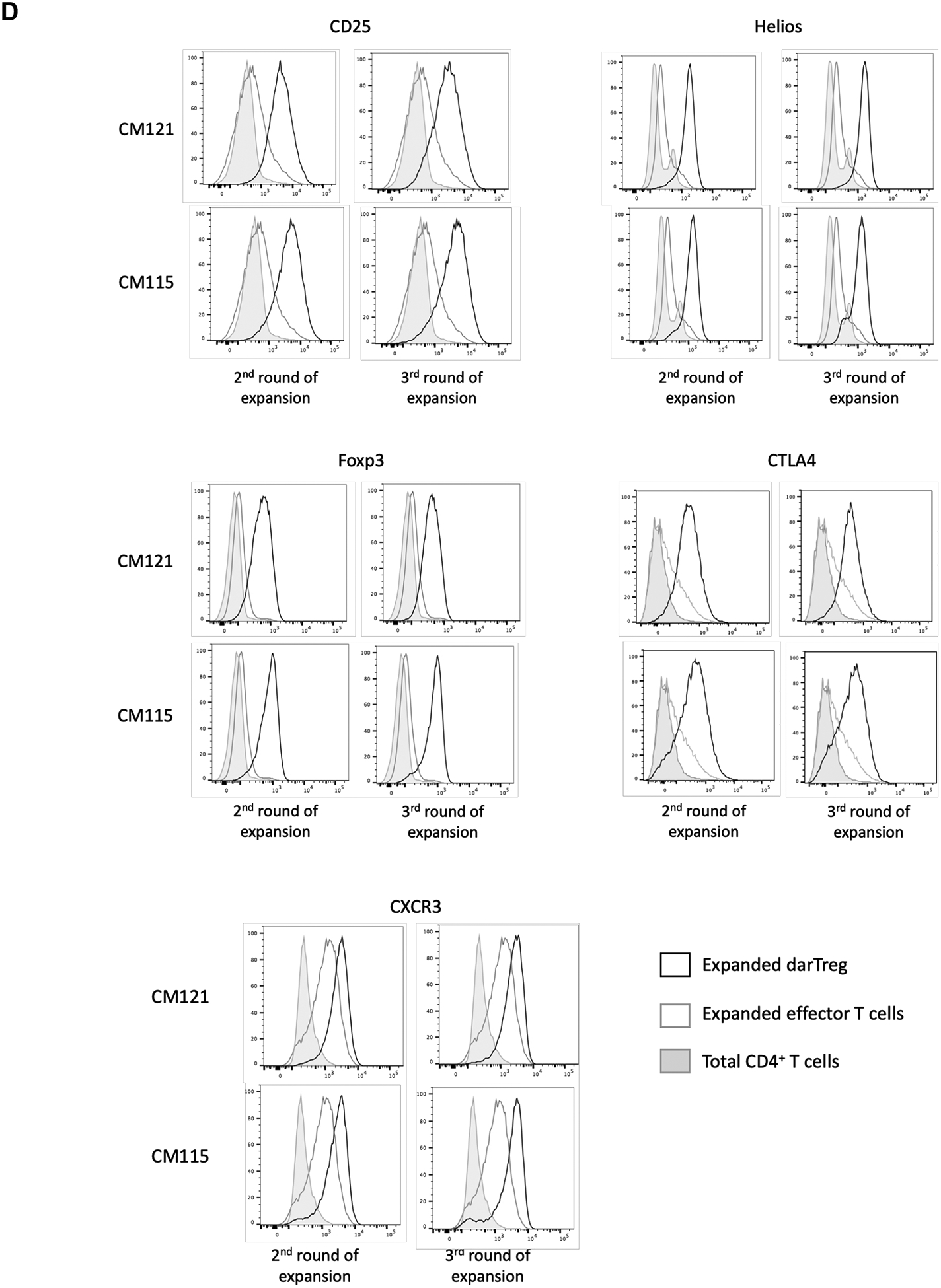
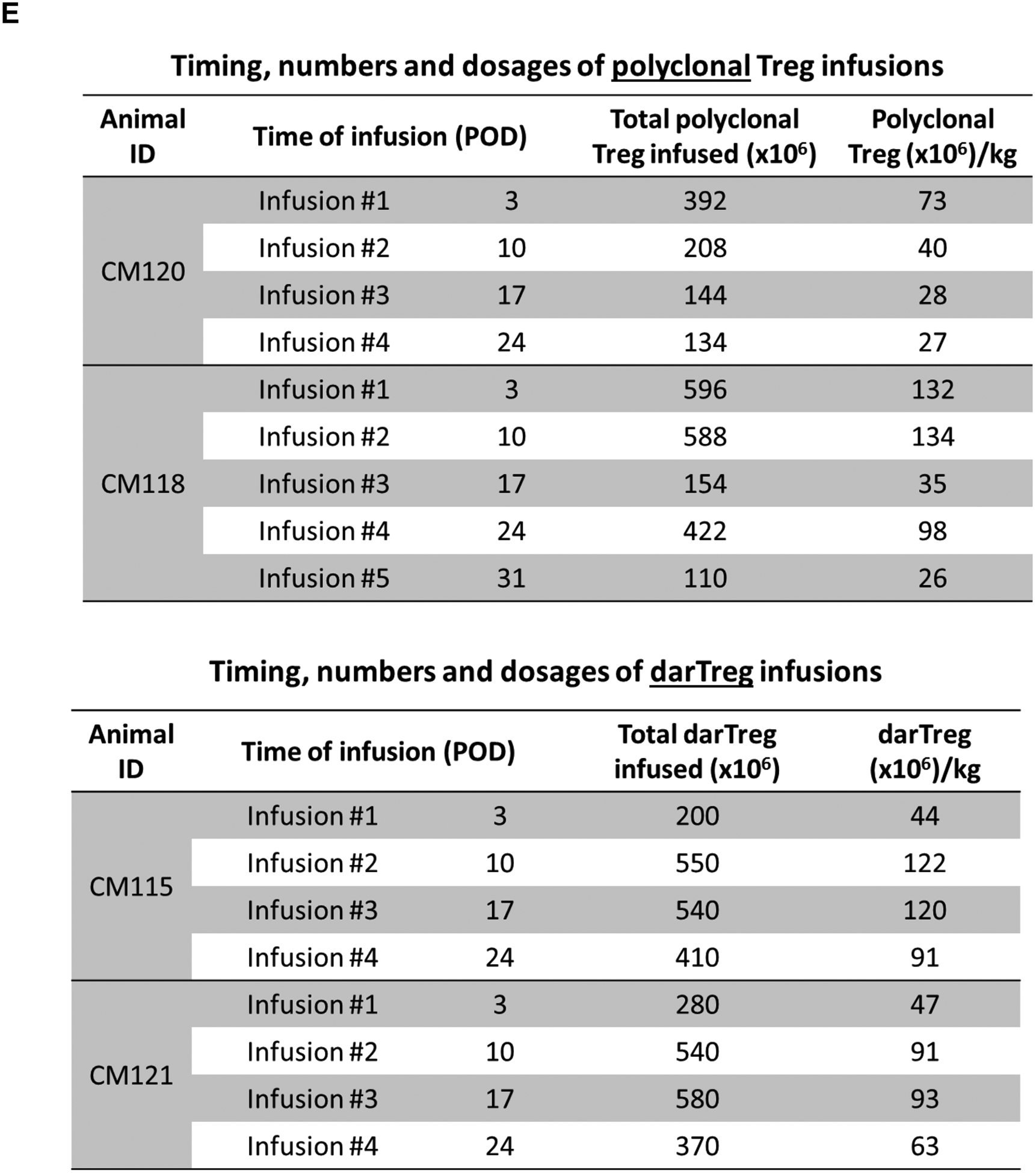
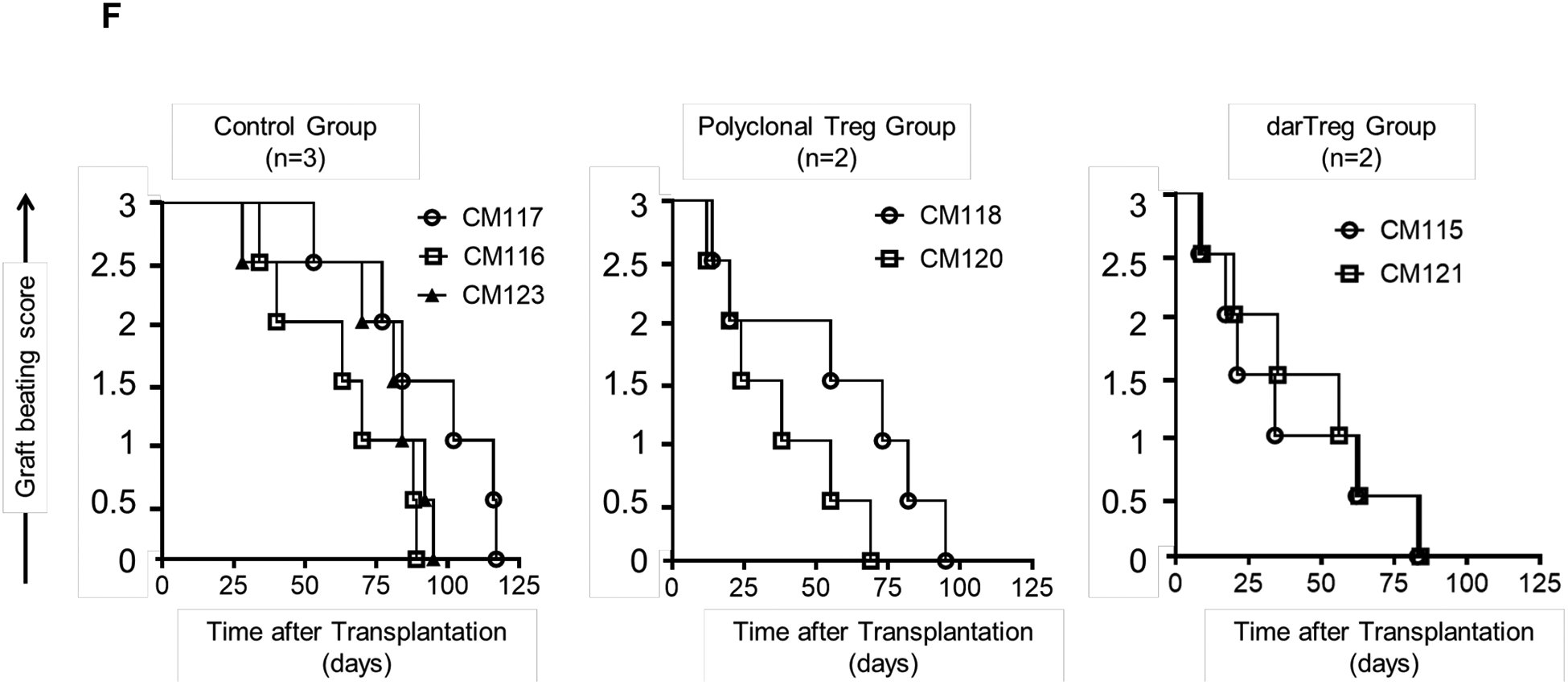
Heart allograft survival in ATG-treated monkeys given autologous donor Ag alloreactive (darTreg) infusions or for comparison, polyclonal Treg infusions. A, Immunosuppressive regimen. Cynomolgus monkeys received rabbit ATG intravenously (i.v.) over 4 hours on days −3 and −1 before, and on days 6 and 13 after transplant at doses of 10, 5, 5 and 5 mg/kg, respectively. Methylprednisolone was given before each ATG infusion at doses of 5, 2.5, 2.5 and 2.5 mg/kg, respectively. Anti-IL-6 receptor antagonist mAb was administered i.v. over 1 hour at 10 mg/kg on days −1, 6, 13 and 20, and then once every 4 weeks. Tacrolimus was given by intramuscular (i.m.) injection from day −3 to 14 (target whole blood trough levels: 10–15 ng/ml), followed by rapamycin (i.m.) from days 14 to 56 (target trough levels: 10–15 ng/ml), after which rapamycin was weaned slowly and discontinued completely on day 84. B, ex vivo-expansion of darTreg (n= 4 separate ex vivo-expanded darTreg preparations); C, suppression of the proliferation of autologous T cells stimulated by donor or third-party stimulators by darTreg in MLR (top). darTreg were obtained from 2nd or 3rd rounds of expansion and are representative of 8 separate suppressive assays (bottom). Percent suppression of T cell proliferation is presented on the y-axis. x-axis shows darTreg : effector cell ratios. D, Phenotype of darTreg obtained from 2nd or 3rd rounds of ex vivo expansion. E, Timing, numbers and dosages of polyclonal (top) and darTreg (bottom). POD = post-operative day. F, Graft beating scores at various times post-transplant in monkeys infused with darTreg (n=2) and in non-infused (n=3) or polyclonal Treg-infused historical controls (n=2)
Reductions in graft beating score were first observed on day 8 and 9, respectively. Graft contraction declined progressively thereafter until total cessation on days 84 and 85, respectively. Graft survival did not differ significantly from that of ATG-treated historical controls21 that did not receive Treg infusion or received post-transplant infusions of polyclonal Treg (Figure 1F). These data indicate that infusion of darTreg into lymphodepleted recipient monkeys did not prolong heart graft survival, similarly to our previous observations using polyclonal Treg.
Infusion of labeled darTreg early post-transplant
To track and characterize labeled darTreg infused early post-transplant, 2 monkeys (CM102 and CM103) were infused on days 3 and 10 (Figure 2A). The first darTreg infusion was labeled with CFSE and the second with VPD450 to distinguish the 2 populations in vivo. Of note, the first darTreg infusion was given 3 days before, while the second infusion 4 days after the third (final) ATG dose (on day 6). The recipients were electively euthanized on days 18 and 19 post-transplant, respectively, at which times graft-beating scores as shown in Figure 2B.
FIGURE 2.
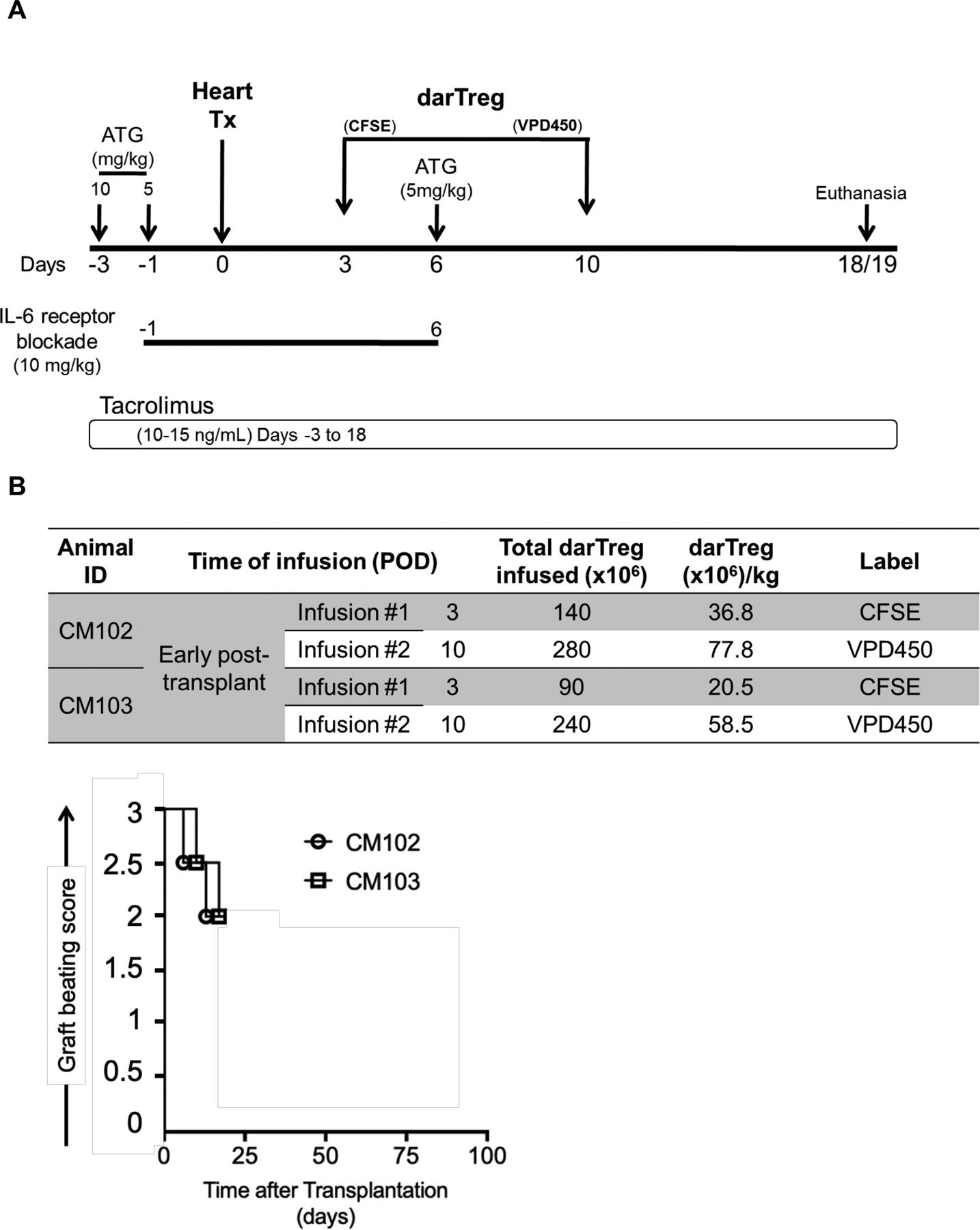
Infusion of CFSE- and VPD450-labeled darTreg early post-transplant. A, The immunosuppressive drug regimen and darTreg infusion time points (days post-transplant) for 2 heart allograft recipient monkeys (CM102 and CM103) are shown. In each recipient, CFSE-labeled darTreg were infused on post-transplant day 3 (i.e. 3 days before ATG) and VPD450-labeled darTreg were infused on day 10 (4 days after ATG). Graft recipients were electively euthanized on day 18 (CM102) or 19 (CM103) for blood, allograft and host tissue sampling. B, Number of labeled darTeg infused at each time point into each graft recipient (top). Graft beating scores at various times post-transplant in CM102 and CM103 (bottom).
Tracking of darTreg infused early post-transplant in peripheral blood
CFSE- and VPD450-labeled darTreg were monitored in peripheral blood following their infusion. The incidences of CFSE-labeled darTreg (% CD4+ T cells) on days 5 and 6 after transplantation (i.e. 2 and 3 days after their infusion) were 1.4% and 0.4% in CM102, and 0.5% and 0.1% in CM103, respectively (Figure 3A). The incidences of VPD450-labeled darTreg on days 10, 12, 13 and 15 after transplantation (i.e. 30 min, 2, 3 and 5 days after infusion) were 21.8%, 26.7% 6.7% and 1.4%, respectively in CM102 and 12.1%, 21.9%, 8.4% and 1.4%, in CM103 respectively. Meanwhile, the percentages of CFSE-labeled darTreg in peripheral blood at the same time points were <0.1% in both recipients (Figure 3B). Although Treg transfer was not performed in the absence of ATG, these observations suggest that lymphodepletion may negatively impacts the incidences of infused darTreg, as evidenced by their more pronounced reduction after ATG infusion.
FIGURE 3.
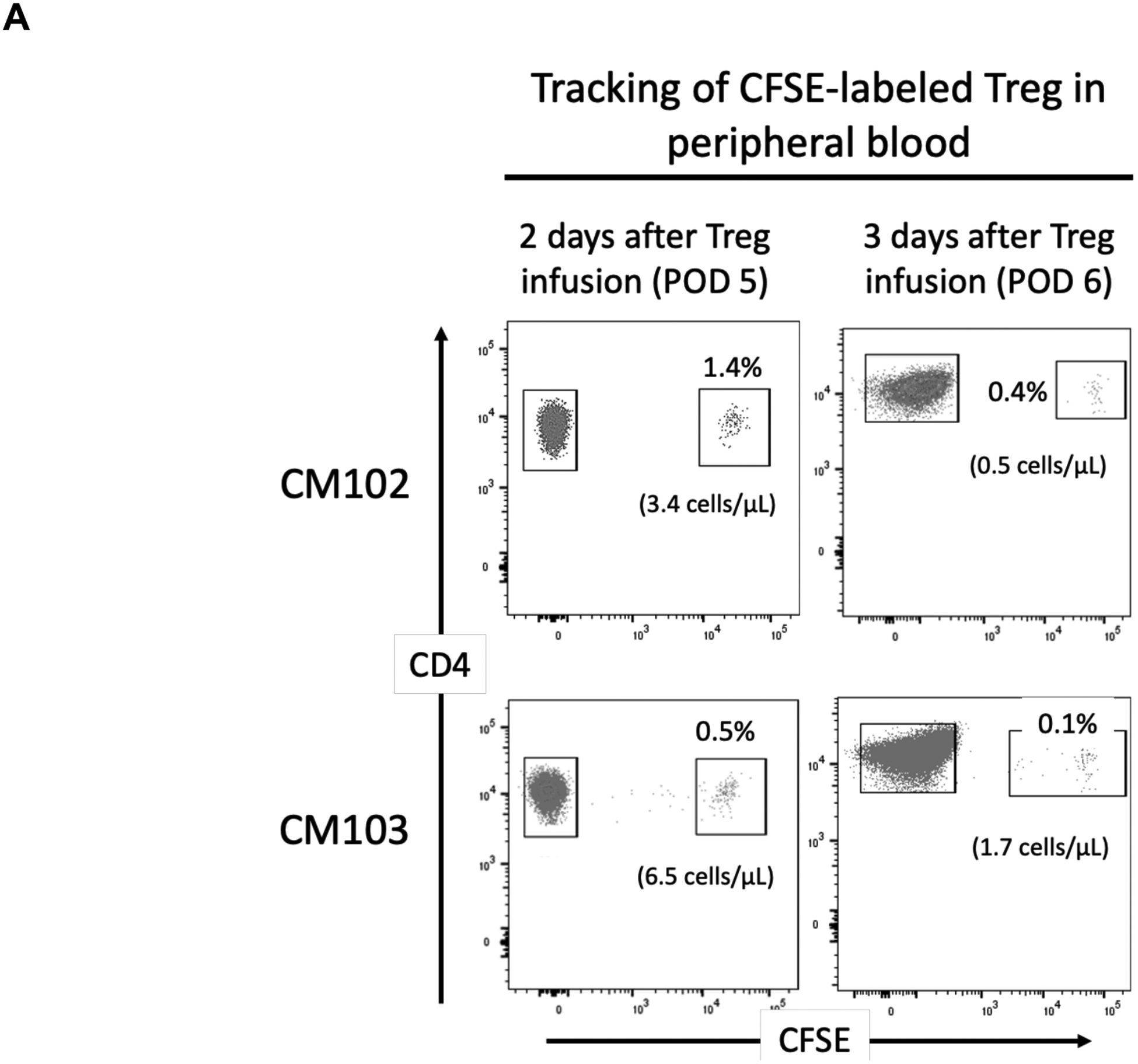
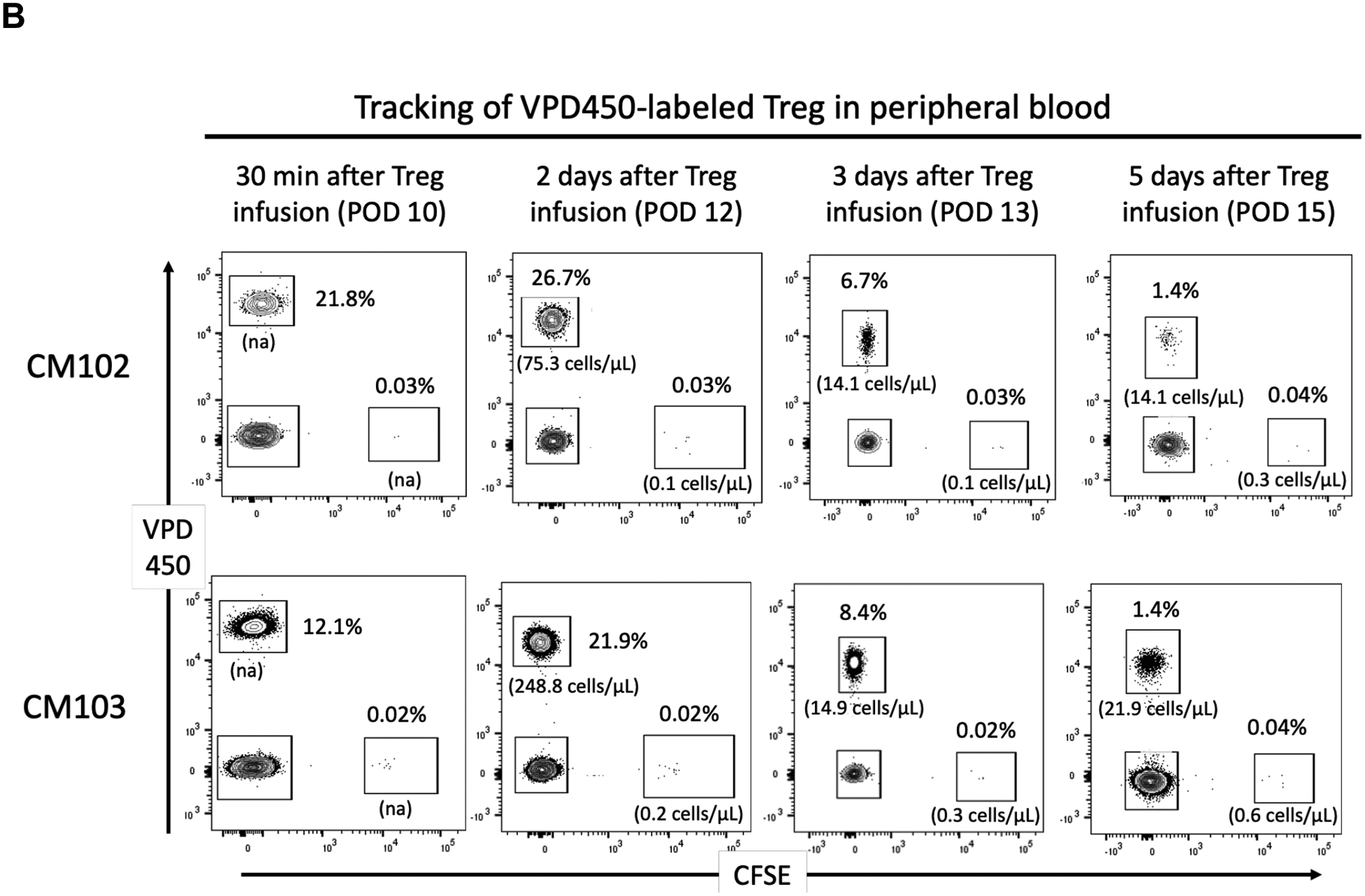
In vivo tracking of CFSE- and VPD450-labeled darTreg infused early post-transplant in peripheral blood. A, Peripheral blood analysis of CFSE-labeled darTreg in heart allograft recipients (CM102 and CM103) on post-operative day (POD) 5 and 6 (i.e. 2 and 3 days respectively after their infusion). B, Peripheral blood analysis of VPD450- and CFSE-labeled darTreg in the same heart allograft recipients on POD 10, 12, 13, and 15 (i.e. 30 min, and 2, 3 and 5 days respectively after infusion of the VPD450-labeled darTreg,). Dot plots represent flow data after gating on total CD4+ T cells. Absolute numbers of labeled darTreg (cells/μL) are also shown.
Detection of darTreg infused early post-transplant in host tissues and the allograft
Graft recipients CM102 and CM103 were electively euthanized on days 18 and 19 post-transplantation respectively, to examine CFSE- and VPD450-labeled darTreg in blood, lymphoid and non-lymphoid tissues and the allograft (Figure 4). In CM102, CFSE-labeled darTreg that were infused 3 days before ATG were not detected in peripheral blood or lymphoid or non-lymphoid tissues at the time of euthanasia. In CM103, CFSE-labeled darTreg were either minimally detected (0.01% of CD4+ T cells), or could not be detected at the time of euthanasia. In both recipients however, VPD450-labeled darTreg, that were infused 4 days after ATG, were evident at higher levels in blood (0.8% and 1.8%), the heart graft (0.4% and 1.8%), kidney (0.06% and 2.4%) and liver (0.3% and 2%), but not in the thymus (Figure 4A). In both recipients, CFSE-labeled darTreg were not detected in secondary lymphoid tissues or bone marrow. On the other hand, VPD450-labeled darTreg were detected at very low levels in mesenteric (0.04% and 0.2%), axillary (0.05% and 0.4%), inguinal (0.02% and 1.8%) lymph nodes (LNs), spleen (0.2% and 0.8%) and in bone marrow (0.06% and 0.05%) (Figure 4B).
FIGURE 4.
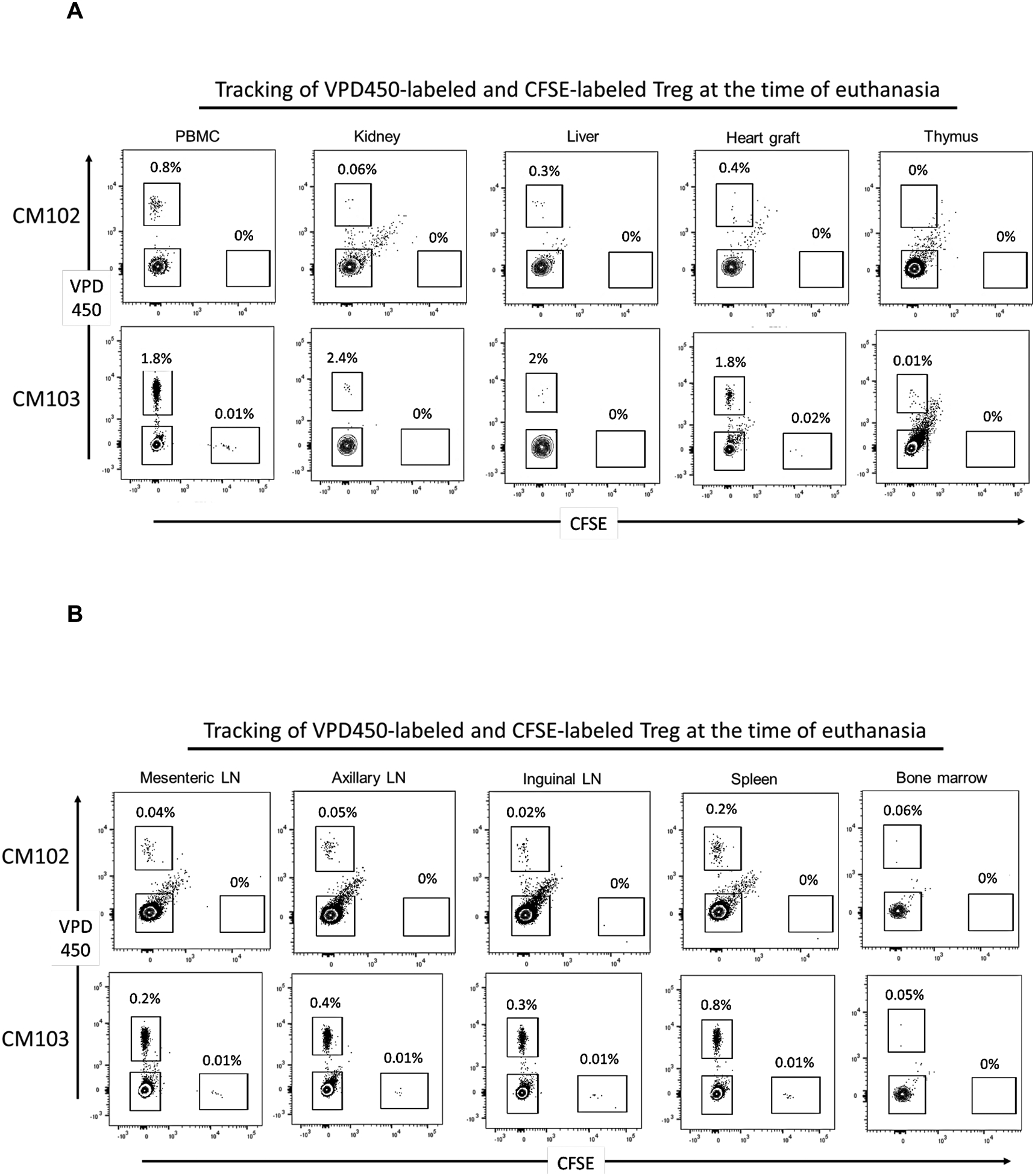
Incidences of CFSE- and VPD450-labeled darTreg infused early post-transplant in peripheral blood, lymphoid tissues, non-lymphoid organs and the allograft at the time of euthanasia. A, Incidences of VPD450- and CFSE-labeled darTreg infused into monkeys CM102 and CM103 were evaluated 18/19 days post-transplant in (blood, kidney, liver and the heart allograft, thymus, and B, mesenteric, axillary and inguinal lymph nodes (LN), spleen and bone marrow. Dot plots represent flow data obtained after gating on total CD4+ T cells. Absolute numbers of labeled darTreg (cells/μL) are also shown.
These observations show that ex vivo-expanded darTreg can be detected in secondary lymphoid tissues and native organs following their infusion into allograft recipient monkeys.
Characterization of darTreg infused early post-transplant in blood and lymphoid tissues
As CFSE-labeled darTreg were either minimally or not detectable, we could not evaluate their phenotype. Rather, we next examined the phenotype of VPD450-labeled darTreg at the time of euthanasia in blood, spleen and mesenteric, axillary and inguinal LNs. In both graft recipients (CM102 and CM103), VPD450-labeled darTreg were assessed for expression of Treg markers,- Foxp3, CTLA4, and Helios, the proliferation marker ki67, and the anti-apoptotic marker Bcl2, in comparison to endogenous (native) CD4+CD25hi Treg (Figure 5). Expression of Foxp3 and CTLA4 by VPD450-labeled darTreg in all tissues examined was lower than by endogenous darTreg, while Helios expression was comparable. Ki67 expression by VPD450-labeled darTreg was markedly reduced in comparison to that of endogenous darTreg. In CM102, Bcl2 expression by the infused darTreg was comparable to that of endogenous Treg, while in CM103, its expression by VPD450-labeled darTreg was lower than that by endogenous Treg. These observations suggest that ex vivo-expanded darTreg lose their regulatory signature, proliferative capacity and survival signals shortly after infusion into ATG-lymphodepleted allograft recipients.
FIGURE 5.
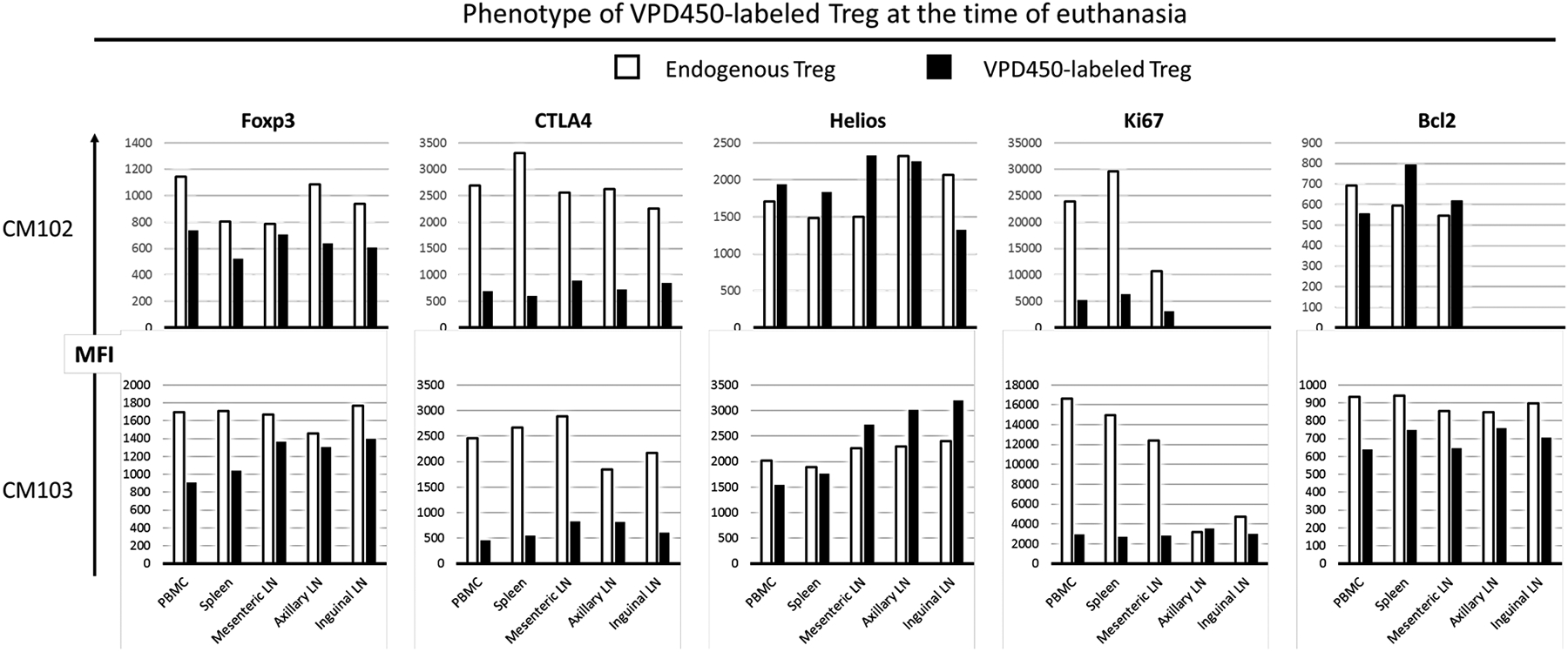
Phenotype of VPD450-labeled darTreg infused early post-transplant in peripheral blood and lymphoid tissues at the time of euthanasia. In the 2 heart graft recipients (CM102 and CM103), the phenotype of VPD450-labeled darTreg was evaluated in blood, mesenteric, axillary and inguinal lymph nodes (LN) and spleen at the time of euthanasia 18/19 days post-transplant. MFI (mean fluorescence intensity) values of VPD450-labeled darTreg were determined simultaneously with MFI of native (endogenous) CD4+CD25hi Treg.
Delayed infusion of autologous darTreg
Next, we evaluated whether “delayed” post-transplant infusion of ex vivo-expanded darTreg following ATG-mediated lymphodepletion would impact their survival and phenotype differently compared with early infusion. In one heart allograft recipient (CM220; Figure 6A), VPD450-labeled darTreg were infused intravenously on days 43, 50 and 57 post-transplant (Figure 6B). In this recipient, tacrolimus was discontinued and replaced by rapamycin on day 18, while no IL-6R blockade was administered.This recipient was electively euthanized on day 63. Graft beating score is shown in Figure 6B.
FIGURE 6.
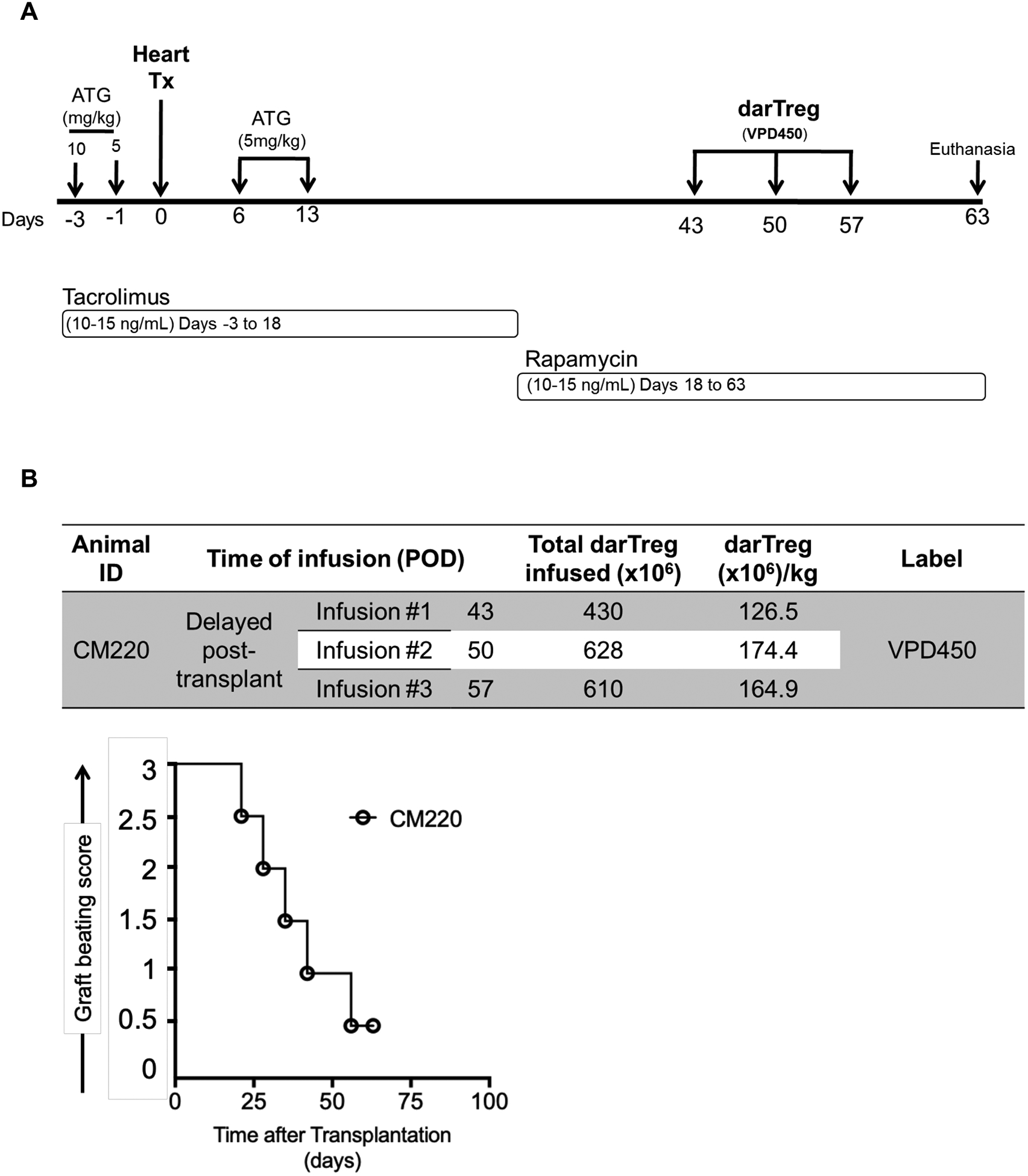
Delayed infusion of darTreg post-transplant. A, Immunosuppressive drug regimen and darTreg infusion time points (days post-transplant) in one heart allograft recipient cynomolgus monkey (CM220). Infusions of VPD450-labeled darTreg were given on post-operative day (POD) 43, 50 and 57 (i.e. 30, 37 and 42 days after the final ATG infusion). B, Numbers of labeled darTeg infused at each timepoint (top). Graft beating scores at various times post-transplant in CM220 (bottom). The graft recipient was electively euthanized on POD 63 for blood, allograft and host tissue sampling.
Detection of darTreg after delayed infusion in blood, host tissues and the allograft
The incidences of VPD450-labeled darTreg in peripheral blood on days 46, 50 and 57 post-transplant (i.e. 3, 7 and 14 days after their first infusion) were 9.2%, 3.4% and 3.5%, respectively (Figure 7A). At the time of euthanasia (Figure 7B), the darTreg were detected in blood (6.5%), the allograft (2.9%), native heart (1.6%) and graft-draining LN (1.5%). The VPD450-labeled darTreg were also detected in mesenteric (1%), right inguinal (2.5%), left inguinal (2.9%), right axillary (3.6%), left axillary (3.2%) LNs and spleen (2.7%). DarTreg were also found in low levels in kidney (0.7%), lung (0.7%), liver (2.4%), bone marrow (0.9%) and minimally in the thymus (0.09%).
FIGURE 7.
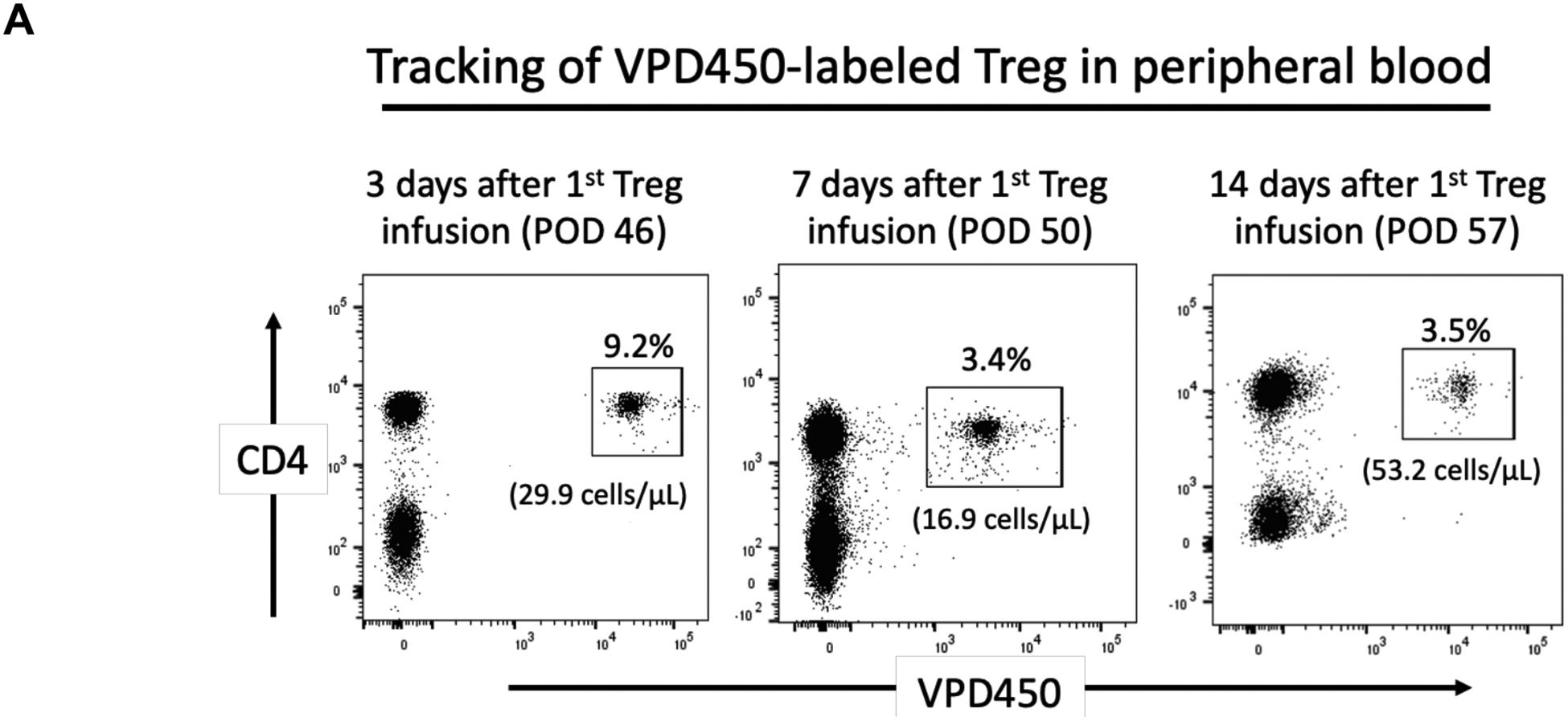
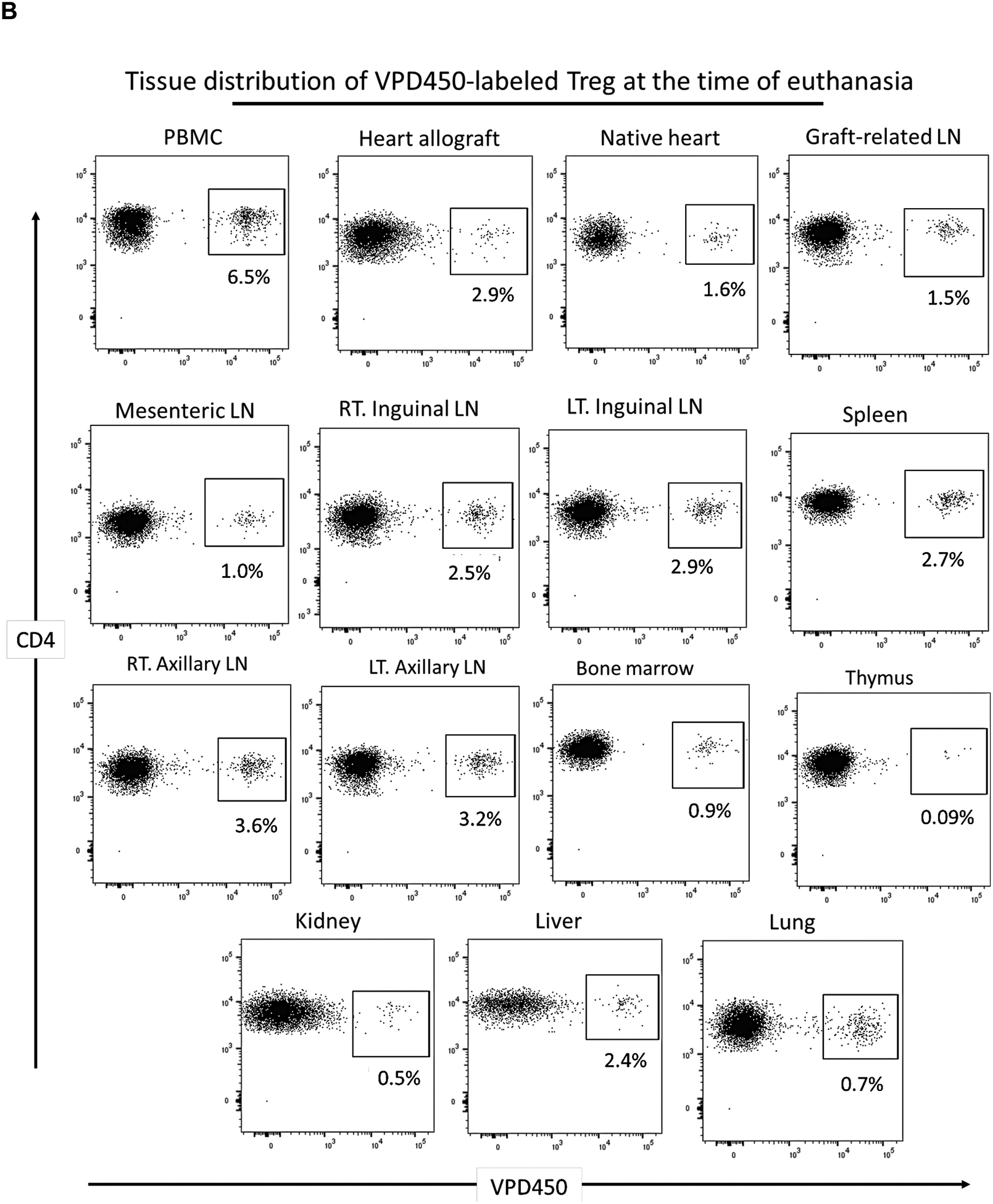
Incidences of VPD450-labeled darTreg following their delayed infusion post-transplant in peripheral blood, lymphoid tissues and non-lymphoid organs at the time of euthanasia. A, Peripheral blood analysis of VPD450-labeled darTreg in CM220 on post-operative day (POD) 46, 50 and 57 (i.e. 3, 7 and 14 days respectively after their infusion). Absolute numbers of labeled darTreg (cells/μL) are also shown. B, Incidences of VPD450-labeled darTreg in blood, the heart allograft, native heart, lung, kidney, liver, graft-draining lymph node (LN), mesenteric LN, right (RT) and left (LT) inguinal LN, spleen, RT and LT axillary LN, bone marrow and thymus at the time of euthanasia, 63 days post-transplant. Dot plots represent flow data after gating on total CD4+ T cells.
Characterization of darTreg in blood after delayed infusion
On days 43, 46 and 50 post-transplant, the phenotype of infused darTreg in peripheral blood was compared to that of native (endogenous) CD4+CD25hi Treg and VPD450-labeled darTreg immediately before infusion (Figure 8A). Thirty minutes after the first infusion (on post-transplant day 43), VPD450-labled darTreg in blood exhibited similar levels of Foxp3 and Helios to those of endogenous Treg. On post-transplant day 46 (3 days after the first infusion), Foxp3 and Helios expression was slightly reduced compared to native Treg. On post-transplant day 53, Foxp3 and Helios expression was similar. Of note, Foxp3 and Helios expression by VPD450-labeled darTreg on days 43, 46 and 53 after infusion was markedly lower compared to before infusion. CTLA4 expression by these cells was lower than that by native Treg and compared to before infusion. Ki67 and Bcl2 expression by VPD450-labeled darTreg was lower than that by native Treg. Notably, Ki67 expression by darTreg after infusion was markedly reduced compared to before infusion. These observations suggest that, in this setting, ex vivo-expanded darTreg in peripheral blood lose their regulatory phenotypic signature, proliferative and survival capacity over time after infusion.
FIGURE 8.
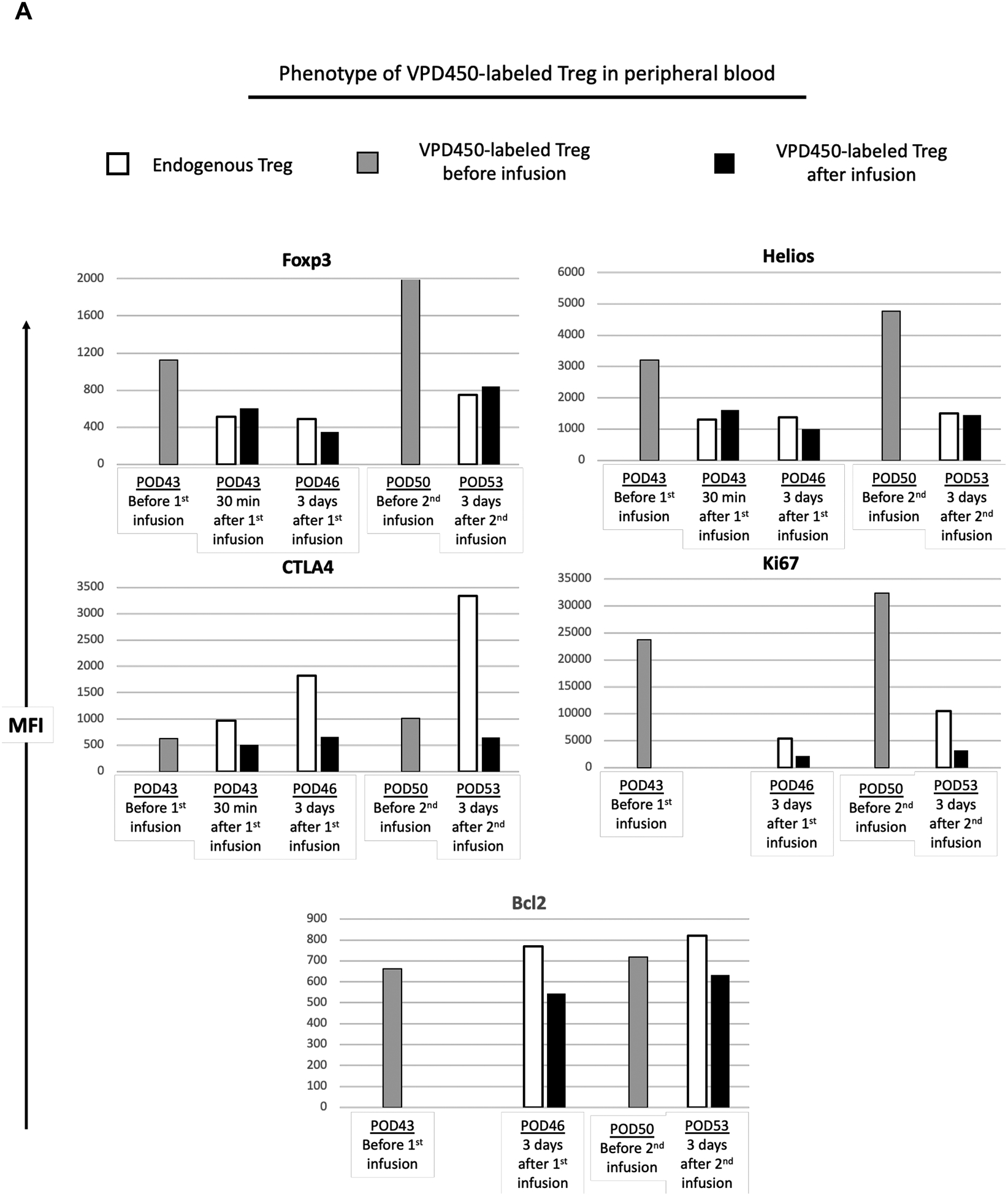
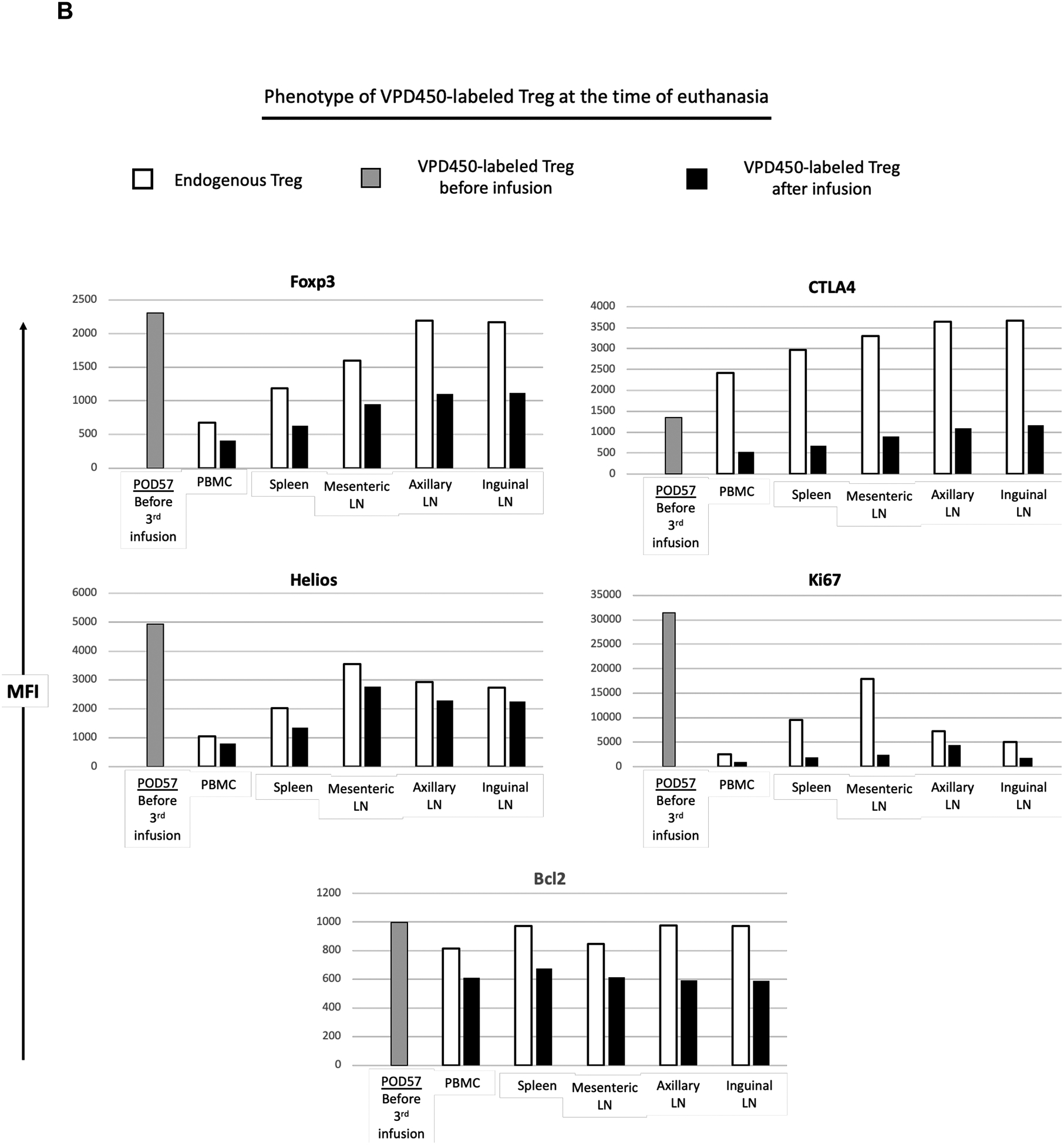
Phenotype of VPD450-labeled darTreg following their delayed infusion post-transplant in peripheral blood and lymphoid tissues at the time of euthanasia. A, In monkey CM220, PBMC samples collected on POD 43, 46 and 53 were evaluated for VPD450-labeled darTreg phenotype in comparison to endogenous (native) CD4+CD25hi Treg. For comparison, VPD450-labeled darTreg were analyzed on days 43 and 50 immediately before infusion. B, At the time of euthanasia, 63 days post-transplant, the phenotype of VPD450-labeled darTreg was evaluated in mesenteric, axillary and inguinal lymph nodes (LN), and spleen. MFI (mean fluorescence intensity) values of VPD450-labeled darTreg were determined simultaneously with MFI of native (endogenous) CD4+CD25hi Treg. In A and B, white bars indicate native (endogenous) Treg; black bars indicate VPD450-labeled darTreg in blood or lymphoid tissues after infusion. Gray bars indicate VPD450-labeled darTreg before infusion.
Characterization of darTreg in lymphoid tissues
Similarly, VPD450-labeled darTreg and native (endogenous) CD4+CD25hi Treg were evaluated in peripheral blood, spleen, mesenteric, axillary and inguinal LNs, and compared with the phenotype of VPD450-labeled darTreg immediately before infusion (Figure 8A,B). In peripheral blood and all lymphoid tissues, expression of Foxp3, Helios, CTLA4, Ki67 and Bcl2 by VPD450-labeled darTreg was markedly lower than that by endogenous Treg. Of note, at euthanasia (day 63), expression of each of these markers was markedly lower than that by VPD450-labeled darTreg before infusion. Collectively, these observations suggest that in this setting, infused darTreg lose their phenotypic signature, proliferative and survival capacity in native tissues, similarly to peripheral blood.
DISCUSSION
The kinetics of in vivo migration, tissue distribution, stability, replicative capacity and persistence/survival of ex vivo-expanded Tregs following their adoptive transfer in graft recipients are poorly understood. This is especially so in humans, in which early phase trials of polyclonal or darTreg in combination with various immunosuppressive regimens are currently underway in kidney and liver transplantation.5 To date, ex vivo-expanded human autologous polyclonal Treg labeled with deuterium have been monitored in peripheral blood following their infusion into patients with autoimmune disease31 or conventionally-immunosuppressed (tacrolimus, MMF, corticosteroid) kidney transplant recipients.8 To our knowledge, no studies have been reported of monitoring adoptively-transferred darTreg in humans.
Our earlier work21 showed that multiple infusions of polyclonal Treg into ATG-treated, profoundly lymphodepleted cynomolgus heart allograft recipients could exacerbate anti-donor immune responses. Others however,32 using a different model, have reported prevention of MHC class I- and II- mismatched renal transplant rejection in rhesus monkeys given host T cells rendered anergic to donor Ag following post-transplant cyclophosphamide administration. In the present study, multiple infusions of autologous ex vivo-expanded darTreg were not associated with improved heart graft function or delayed graft rejection, similar to our previous observations with polyclonal Treg.21
The doses of darTregs that we infused ranged from 20.5 to 120 × 106/kg for each of 2–4 infusions per heart graft recipient. These doses are similar to or greater than the doses of autologous T cells rendered anergic to donor Ag and infused (total 102 +/− 67 × 106) 13 days post-transplant that prolonged renal allograft survival in 6 rhesus monkeys, inducing donor-specific tolerance in 50%.32 They also resemble the single doses of similarly-generated Tregs (14–36 × 106/kg) infused 12 days post-transplant into living donor kidney transplant patients (n=16) that, by contrast, exhibited high rates of rejection upon subsequent immunosuppressive drug withdrawal.33 In other clinical studies of adoptive transfer of darTeg in living donor kidney transplantation, lower doses of darTreg cells have been targeted, i.e. 2 × 103-2×106/kg or 0.5–10×106/kg respectively in small numbers of patients at separate centers in the ONE Study10 in which overall safety of cell therapy and similar 1-year graft survival compared to a reference standard of care group were reported recently. In human living donor liver transplantation, doses of 23.3–14.4 ×106 T cells rendered anergic to donor and administered 13 days post-transplant induced operational tolerance in 7/10 recipients.7 A total of 300–500 × 106 darTreg have been targeted in a liver transplantation drug withdrawal study at UCSF (NCT02474199).
While in previous tracking studies19, 30 we monitored infused polyclonal Tregs in peripheral blood and secondary lymphoid tissue of immunosuppressed, non-transplanted cynomolgus monkeys, the present investigation, in which we have evaluated the therapeutic efficacy of infused darTreg and their fate in heart-allografted monkeys following lymphodepletion, provides new insights from this valuable, pre-clinical NHP model. Our dye labeling protocol does not impair NHP Treg viability or retention of Foxp3 expression following their in vivo transfer.19 Our findings show, that while infused labeled darTreg can readily be detected by flow analysis in peripheral blood shortly after their systemic infusion, the ability to detect these cells diminishes progressively over the ensuing several days. The rapidity and extent of this decline appears to depend on the proximity of darTreg infusion to that of ATG, in that (although the delayed regimen was tested in only one animal and the results are therefore preliminary) delaying initiation of cell infusion from several days to one month after ATG results in a higher incidence of infused darTregs in the peripheral circulation, lymphoid tissues and the allograft. While this suggests that ATG may negatively impact Tregs, taking into account differences in the immunosuppressive regimens with which early and delayed darTreg infusions were combined, the data could also indicate that, while tacrolimus negatively impacts Treg, rapamycin is superior to tacrolimus in preserving NHP Tregs following their adoptive transfer, as suggested previously.18 Under both experimental protocols however, we observed a rapid decline in transferred darTregs in the blood. This is consistent with our previous findings19 and those of others18 of an initial, short half-life of infused, dye-labeled polyclonal Tregs in the circulation of non-transplanted rhesus or cynomolgus monkeys, with very few cells persisting beyond 2 weeks. Importantly, this diminution in number could not be ascribed to proliferation of the darTreg (dye dilution), their selective accumulation in lymph nodes or bone marrow, or their phenotypic transformation. Cell death in the absence of appropriate expansion signals following their adoptive transfer, or immune-mediated elimination are possible underlying mechanisms. Notably, our findings are also in accord with similar pharmacokinetic profiles of adoptively-transferred human polyclonal Treg monitored using more human-applicable methods in hematopoietic stem cell transplant recipients34 and kidney transplant patients,8 and of chimeric Ag receptor (CAR) T cells in cancer patients.35, 36
Studies in mice37 have shown that transferred darTregs, identified by congenic marker expression in cyclophosphamide-lymphodepleted islet allograft recipients, constitute nearly 50% of all Tregs early (4 and 6 days) post-transplant. While their migration pattern was in keeping with the trafficking of endogenous Treg from inflamed (graft) tissue to draining lymph nodes reported earlier,38 the adoptively-transferred darTreg could barely be detected 14 days post-transplant, either in the graft or systemically,- in lymph nodes or spleen. This is in keeping with the low level of detection of transferred cynomolgus darTregs, either in the graft or lymphoid tissues, 1 or 4 weeks after their infusion early or late, respectively, after ATG administration in the present study. More recent monitoring of infused alloreactive (CAR) Tregs in mice using bioluminescence imaging has confirmed that they accumulate rapidly within skin grafts, with eventual migration to draining lymphoid tissue.39 Notably, expansion of adoptively-transferred darTregs has been reported in all tissues of mouse skin graft recipients when combined with IL‐2 administration,40 a possible means to mitigate their rapid loss/diminution.
While dye-labeling of infused darTreg may not be translatable to the clinical setting, it provided the important advantage (compared with deuterium labeling) of allowing us to directly examine the cells’ phenotype in blood and tissues. In all host tissues examined, the transferred Treg exhibited lower levels of Foxp3 and the cell proliferation marker Ki67 than endogenous Treg, or the expanded darTreg before their infusion. Loss of Foxp3 by infused polyclonal cynomolgus or rhesus Tregs has been reported previously18, 19 and taken to imply that the transferred cells lose their suppressive function. Unfortunately, as in these previous studies of polyclonal Treg, due to the low numbers of darTreg that persisted in the heart graft recipients, we were unable to isolate, re-purify and test their function directly. The current observations suggest that, as with polyclonal Tregs, the survival of transferred darTreg in lymphodepleted NHP recipients is short-lived and that, as reported in mice using different monitoring approaches, levels are maintained only at very low levels in lymphoid tissue and the allograft within the first few weeks post-infusion. Diminution of Foxp3 expression suggests loss of suppressive function over this period and that approaches are needed both to promote the longevity and to sustain the in vivo function of adoptively-transferred darTreg. Future application(s) of ex vivo-expanded Treg therapy may require approaches tailored to concomitant immunosuppressive regimens and the type of organ transplant.
Supplementary Material
ACKNOWLEDGMENTS
The work was supported by NIH Institutes of Health (NIH) grants U01 AI91197 and U19 131453 as part of the NIH Nonhuman Primate Transplantation Tolerance Cooperative Study Group sponsored by the National Institute of Allergy and Infectious Disease (NIAID). MHC genotyping assays were provided by Dr. David O’Connor’s laboratory at the University of Wisconsin-Madison as part of contract HHSN272201600007C from the NIAID.
Funding
The study was supported by NIH Institutes of Health (NIH) Nonhuman Primate Cooperative Study Group grant U01 AI91197. HZ was supported by an American Society of Transplantation Basic Science Fellowship and by NIH institutional T32 training grant AI74490. DJvdW was supported by NIH T32 AI 74490 and AP-G by a Starzl Transplantation Institute Clinical and Translational Research Fellowship.
ABBREVIATIONS
- Ag
antigen
- ATG
anti-thymocyte globulin
- CFSE
carboxyfluorescein succinimidyl ester
- CTLA4
cytotoxic T lymphocyte antigen 4
- Foxp3
forkhead box P3
- IS
immunosuppression
- MFI
mean fluorescence intensity
- NHP
nonhuman primate
- Teff
effector T cells
- Tmem
memory T cells
- Treg
regulatory T cells
- VPD450
violet proliferation dye 450
Footnotes
Disclosure statement
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Sicard A, Boardman DA, Levings MK. Taking regulatory T-cell therapy one step further. Curr Opin Organ Transplant 2018;23:509–515. [DOI] [PubMed] [Google Scholar]
- 2.Romano M, Tung SL, Smyth LA, et al. Treg therapy in transplantation: a general overview. Transpl Int 2017;30:745–753. [DOI] [PubMed] [Google Scholar]
- 3.Wood KJ, Bushell A, Hester J. Regulatory immune cells in transplantation. Nat Rev Immunol 2012;12:417–430. [DOI] [PubMed] [Google Scholar]
- 4.Kang SM, Tang Q, Bluestone JA. CD4+CD25+ regulatory T cells in transplantation: progress, challenges and prospects. Am J Transplant 2007;7:1457–1463. [DOI] [PubMed] [Google Scholar]
- 5.Tang Q, Vincenti F. Transplant trials with Tregs: perils and promises. J Clin Invest 2017;127:2505–2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bezie S, Anegon I, Guillonneau C. Advances on CD8+ Treg cells and their potential in transplantation. Transplantation 2018;102:1467–1478. [DOI] [PubMed] [Google Scholar]
- 7.Todo S, Yamashita K, Goto R, et al. A pilot study of operational tolerance with a regulatory T-cell-based cell therapy in living donor liver transplantation. Hepatology 2016;64:632–643. [DOI] [PubMed] [Google Scholar]
- 8.Chandran S, Tang Q, Sarwal M, et al. Polyclonal Regulatory T cell therapy for control of inflammation in kidney transplants. Am J Transplant 2017;17:2945–2954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mathew JM, HV J, LeFever A, et al. A Phase I clinical trial with ex vivo expanded recipient regulatory T cells in living donor kidney transplants. Sci Rep 2018;8:7428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sawitzki B, Harden PN, Reinke P, et al. Regulatory cell therapy in kidney transplantation (The ONE Study): a harmonised design and analysis of seven non-randomised, single-arm, phase 1/2A trials. Lancet 2020;395:1627–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sanchez-Fueyo A, Whitehouse G, Grageda N, et al. Applicability, safety, and biological activity of regulatory T cell therapy in liver transplantation. Am J Transplant 2020;20:1125–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Braza F, Durand M, Degauque N, et al. Regulatory T Cells in kidney transplantation: new directions? Am J Transplant 2015;15:2288–2300. [DOI] [PubMed] [Google Scholar]
- 13.Lam AJ, Hoeppli RE, Levings MK. Harnessing advances in T regulatory cell biology for cellular therapy in transplantation. Transplantation 2017;101:2277–2287. [DOI] [PubMed] [Google Scholar]
- 14.Gedaly R, De Stefano F, Turcios L, et al. mTOR inhibitor everolimus in regulatory T cell expansion for clinical application in transplantation. Transplantation 2019;103:705–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pesenacker AM, Broady R, Levings MK. Control of tissue-localized immune responses by human regulatory T cells. Eur J Immunol 2015;45:333–343. [DOI] [PubMed] [Google Scholar]
- 16.Lamarche C, Levings MK. Guiding regulatory T cells to the allograft. Curr Opin Organ Transplant 2018;23:106–113. [DOI] [PubMed] [Google Scholar]
- 17.Knechtle SJ, Shaw JM, Hering BJ, et al. Translational impact of NIH-funded nonhuman primate research in transplantation. Sci Transl Med 2019;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Singh K, Stempora L, Harvey RD, et al. Superiority of rapamycin over tacrolimus in preserving nonhuman primate Treg half-life and phenotype after adoptive transfer. Am J Transplant 2014;14:2691–2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang H, Guo H, Lu L, et al. Sequential monitoring and stability of ex vivo-expanded autologous and nonautologous regulatory T cells following infusion in nonhuman primates. Am J Transplant 2015;15:1253–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang J, Brook MO, Carvalho-Gaspar M, et al. Allograft rejection mediated by memory T cells is resistant to regulation. Proc Natl Acad Sci U S A 2007;104:19954–19959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ezzelarab MB, Zhang H, Guo H, et al. Regulatory T cell infusion can enhance memory T cell and alloantibody responses in lymphodepleted nonhuman primate heart allograft recipients. Am J Transplant 2016;16:1999–2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Feng X, Kajigaya S, Solomou EE, et al. Rabbit ATG but not horse ATG promotes expansion of functional CD4+CD25highFOXP3+ regulatory T cells in vitro. Blood 2008;111:3675–3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boenisch O, Lopez M, Elyaman W, et al. Ex vivo expansion of human Tregs by rabbit ATG is dependent on intact STAT3-signaling in CD4(+) T cells and requires the presence of monocytes. Am J Transplant 2012;12:856–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shimony O, Nagler A, Gellman YN, et al. Anti-T lymphocyte globulin (ATG) induces generation of regulatory T cells, at least part of them express activated CD44. J Clin Immunol 2012;32:173–188. [DOI] [PubMed] [Google Scholar]
- 25.Xia CQ, Chernatynskaya AV, Wasserfall CH, et al. Anti-thymocyte globulin (ATG) differentially depletes naive and memory T cells and permits memory-type regulatory T cells in nonobese diabetic mice. BMC Immunol 2012;13:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ma A, Qi S, Song L, et al. Adoptive transfer of CD4+CD25+ regulatory cells combined with low-dose sirolimus and anti-thymocyte globulin delays acute rejection of renal allografts in Cynomolgus monkeys. Int Immunopharmacol 2011;11:618–629. [DOI] [PubMed] [Google Scholar]
- 27.Shortreed CG, Wiseman RW, Karl JA, et al. Characterization of 100 extended major histocompatibility complex haplotypes in Indonesian cynomolgus macaques. Immunogenetics 2020;72:225–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Levings MK, Sangregorio R, Roncarolo MG. Human cd25(+)cd4(+) t regulatory cells suppress naive and memory T cell proliferation and can be expanded in vitro without loss of function. J Exp Med 2001;193:1295–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dons EM, Raimondi G, Cooper DK, et al. Non-human primate regulatory T cells: current biology and implications for transplantation. Transplantation 2010;90:811–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guo H, Zhang H, Lu L, et al. Thomson AW. Generation, cryopreservation, function and in vivo persistence of ex vivo expanded cynomolgus monkey regulatory T cells. Cell Immunol 2015;295:19–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bluestone JA, Buckner JH, Fitch M, et al. Type 1 diabetes immunotherapy using polyclonal regulatory T cells. Sci Transl Med 2015;7:315ra189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bashuda H, Kimikawa M, Seino K, et al. Renal allograft rejection is prevented by adoptive transfer of anergic T cells in nonhuman primates. J Clin Invest 2005;115:1896–1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Koyama I, Bashuda H, Uchida K, et al. A clinical trial with adoptive transfer of ex vivo-induced, donor-specific immune-regulatory cells in kidney transplantation—a second report. Transplantation 2020;Online First. [DOI] [PubMed] [Google Scholar]
- 34.Brunstein CG, Miller JS, Cao Q, et al. Infusion of ex vivo expanded T regulatory cells in adults transplanted with umbilical cord blood: safety profile and detection kinetics. Blood 2011;117:1061–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kowolik CM, Topp MS, Gonzalez S, et al. CD28 costimulation provided through a CD19-specific chimeric antigen receptor enhances in vivo persistence and antitumor efficacy of adoptively transferred T cells. Cancer Res 2006;66:10995–11004. [DOI] [PubMed] [Google Scholar]
- 36.Jensen MC, Popplewell L, Cooper LJ, et al. Antitransgene rejection responses contribute to attenuated persistence of adoptively transferred CD20/CD19-specific chimeric antigen receptor redirected T cells in humans. Biol Blood Marrow Transplant 2010;16:1245–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee K, Nguyen V, Lee KM, et al. Attenuation of donor-reactive T cells allows effective control of allograft rejection using regulatory T cell therapy. Am J Transplant 2014;14:27–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang N, Schroppel B, Lal G, et al. Regulatory T cells sequentially migrate from inflamed tissues to draining lymph nodes to suppress the alloimmune response. Immunity 2009;30:458–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dawson NA, Lamarche C, Hoeppli RE, et al. Systematic testing and specificity mapping of alloantigen-specific chimeric antigen receptors in regulatory T cells. JCI Insight 2019;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ratnasothy K, Jacob J, Tung S, et al. IL-2 therapy preferentially expands adoptively transferred donor-specific Tregs improving skin allograft survival. Am J Transplant 2019;19:2092–2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


