Abstract
Risk-stratification has contributed to a dramatic improvement in survival in pediatric acute lymphoblastic leukemia (ALL). This study evaluated the utility of prephase response and day 15 bone marrow when a minimal residual disease (MRD) assessment was available. A file review of children aged ≤ 15 years diagnosed with precursor-B ALL from 2014 to 2019 was performed. The protocol used for risk stratification and treatment was based on a UKALL-2003 backbone. All patients received one week of prephase therapy comprised of intravenous dexamethasone in the first 48 h followed by oral prednisolone. The median age of the 255 patients in the study was 5 years. Following the prephase, the peripheral blood absolute blast count was 0 and ≥ 1000/µL blasts in 141 (56%) and 29 (11%), respectively. Ten of 199 (5%) patients with an evaluable day 15 bone marrow had M3 status. At the end of induction, 30 (12%), 127 (50%) and 98 (38%) patients belonged to the standard-risk, intermediate-risk and high-risk (HR) groups, respectively. An M3 day15 bone marrow was the sole reason for escalation in three (3%) of the patients in the HR group. A lack of complete clearance of peripheral blood blasts post-prephase [HR: 2.45 (1.04–5.75), p = 0.040] and a positive MRD [HR: 3.00 (1.28–7.02), p = 0.011] independently predicted risk of relapse. Complete blast clearance is superior to the traditional cut-off of 1000/µL in predicting relapse. The role of a day 15 bone marrow morphology is diminished when an end of induction MRD is available.
Electronic supplementary material
The online version of this article (10.1007/s12288-020-01354-0) contains supplementary material, which is available to authorized users.
Keywords: Childhood leukemia, Early bone marrow, Poor prednisolone response, Prognostic marker, Rapid early response
Introduction
The overall survival in children with acute lymphoblastic leukemia (ALL) currently approaches 90% [1]. The key strategy behind the improved survival is a risk-based adaptation of treatment intensity [2]. Risk-stratified chemotherapy implies an aggressive treatment in patients with a high risk of relapse, and a de-escalated approach in favourable risk groups [3]. The criteria employed for identifying risk categories encompass patient-related (age), disease-related (presenting leukocyte count, extramedullary disease, genetics) and treatment response-related factors. The rapidity of treatment response to induction chemotherapy is a strong predictor of survival [1]. Peripheral blood blast clearance and morphological bone marrow response during, and post induction therapy are conventional cytomorphological measures of early treatment response. Minimal residual disease (MRD) evaluation has emerged as a sensitive, strong and independent predictor of treatment response and survival in the last two decades [1, 3]. This study evaluates the relevance of day 8 peripheral blood blast clearance and day 15 bone marrow status during induction therapy, when a sensitive end of induction MRD assessment is available.
Methods
The study was performed as a retrospective file review. The inclusion criteria were: age ≤ 15 years, newly diagnosed precursor-B lineage ALL (B-ALL) confirmed by immunophenotyping and initiation of therapy between January 2014 and September 2019. Exclusion criteria included: patients who received more than one week of treatment elsewhere prior to presentation and patients transferred after diagnosis to another center for treatment.. The diagnosis of B-ALL was confirmed by morphology and immunophenotyping in all patients. Reverse-transcriptase polymerase chain reaction was employed to detect the following mutations: ETV6-RUNX1, TCF3-PBX1, BCR-ABL1 and KMT2A-AFF1. Conventional karyotyping was performed to identify numerical and structural chromosomal aberrations. Patients were classified based on National Cancer Institute (NCI) criteria, central nervous system (CNS) status, extramedullary disease, early treatment response, genetics and MRD into standard risk (SR), intermediate risk (IR) and high risk (HR). Risk stratification and treatment was based on the Medical Research Council United Kingdom Acute Lymphoblastic Leukaemia (UKALL 2003) backbone [4, 5]. Important modifications included the use of a corticosteroid prephase in all patients, and high dose methotrexate administration in intermediate and high-risk patients. The prephase therapy employed intravenous dexamethasone in the first 48 h followed by oral prednisolone for 5 days. MRD was measured with a flow-cytometry based technique that utilized multiparametric immunophenotyping and evaluation for single marker leukemia associated immunophenotype [6]. All BCR-ABL positive patients were treated with the addition of imatinib at a dose of approximately 300 mg/m2/day throughout the duration of therapy. The details of risk stratification and treatment are outlined in supplemental Tables 1 and 2.
Table 1.
Patient distribution according to the criteria used for risk-stratification
| Criterion | N (%) |
|---|---|
| Age (years) | |
| > 1 and < 10 | 228 (89) |
| ≥ 10 | 26 (11) |
| < 1 | 1 |
| Presenting white blood cell count (/µL) | |
| < 50,000 | 205 (80) |
| ≥ 50,000 | 50 (20) |
| Bulky disease | |
| Yes | 116 (46) |
| No | 139 (54) |
| Central nervous system disease | |
| Yes | 2 (0.8) |
| No | 253 (99.2) |
| Testicular disease | |
| Yes | 1 (0.4) |
| No | 254 (99.6) |
| Prephase response | |
| Good | 222 (87) |
| Poor | 29 (11) |
| Not assessed | 4 (1.6) |
| Genetics | |
| ETV6-RUNX1 | 39 (15) |
| High Hyperdiploidy | 80 (31) |
| TCF3-PBX1 | 13 (5) |
| BCR-ABL1 | 8 (3) |
| KMT2A-AFF1 | 0 |
| Hypodiploidy | 2 (0.8) |
| Not assessed | 4 (1.6) |
| No aberrations detected | 110 (43) |
| Day 15 bone marrow | |
| M1/M2 | 189 (95) |
| M3 | 10 (5) |
| Not evaluable | 56 (22) |
| Day 35 bone marrow | |
| M1 | 243 (95) |
| M2 | 12 (5) |
| Day 35 minimal residual disease | |
| < 0.01% | 190 (75) |
| ≥ 0.01% | 63 (25) |
| Not evaluable | 2 (0.8) |
Table 2.
Univariate analysis of prognostic factors associated with relapse
| Prognostic factor | HR (95% CI) | P |
|---|---|---|
| National Cancer Institute High risk | 1.90 (0.97–3.71) | 0.062 |
| Bulky disease | 1.06 (0.55–2.06) | 0.859 |
| Poor Prephase response (blast count cut-off: 1000/µL) | 1.19 (0.42–3.39) | 0.745 |
| Poor Prephase response (blast count cut-off: 0/µL) | 2.45 (1.23–4.89) | 0.011 |
| Favourable genetics | 0.6 (0.29–1.25) | 0.175 |
| High risk genetics | 2.85 (0.86–9.38) | 0.085 |
| Day 15 M3 bone marrow | 0.64 (0.09–4.74) | 0.664 |
| Positive Day 35 minimal residual disease | 2.92 (1.50–5.69) | 0.002 |
Definitions
Prephase response: Absolute blast count in the peripheral blood on day 8 of induction therapy of < 1000/µL and ≥ 1000/µL were defined as a good prephase response and a poor prephase response (PPR), respectively.
Bone marrow morphological status on day 15 and day 35: M1 < 5% blasts, M2 5–25% blasts and M3 ≥ 25% blasts.
Minimal residual disease (MRD): Flow-cytometry based assessment, positive MRD ≥ 0.01%
High risk genetics: Hypodiploidy (≤ 39 chromosomes), BCR-ABL1 (Philadelphia chromosome), KMT2A-AFF1.
Favourable genetics: High hyperdiploidy (51–65 chromosomes), ETV6-RUNX1.
Event: induction failure (M3 bone marrow at the end of induction), relapse and death.
Statistical analysis
The data was entered in IBM-SPSS software (version 20.0, 2011, IBM-SPSS, Inc., Chicago, IL, USA). Baseline variables were analysed by descriptive statistics. Variables with normal and skewed distribution were represented as mean with standard deviation and median with range, respectively. Chi-square and Fisher exact tests were used to compare proportions. A p value < 0.05 was considered as significant. Kaplan–Meier method was utilized for survival analysis. Log-rank test was used to compare survival among different patient categories. A cox proportional hazard analysis was performed to evaluate the effect of different factors on relapse.
Results
Figure 1 illustrates the recruitment of the study patients. Of the 255 patients who were treated for B-ALL, 140 (55%) were boys. The median age was 5 years (range: 0.4–15). Based on the age and presenting white blood cell count, 54 (21%) and 200 (78%) patients were assigned to the SR and IR categories, respectively. One patient who presented with CNS disease was treated as HR upfront. Table 1 depicts the frequencies of different risk-defining criteria. Of the 251 patients who underwent a peripheral blood blast count evaluation after the prephase, the absolute blast count was 0 /µL, 1–999 /µL and ≥ 1000 /µL in 141 (56%), 81 (32%) and 29 (12%) patients, respectively. In the remaining four patients, parenteral chemotherapy was administered prior to day 8 of induction for hyperleukocytosis. Hence, the day 8 blast count was not reflective of the effect of prephase therapy.
Fig. 1.
Flow diagram illustrating inclusion of eligible patients for the study
Twenty patients were excluded due to day 15 bone marrow not being performed, the reasons including, unwell clinical status: 11, low blood counts: 3, already high risk: 1, peripheral blood blasts present on day 15: 1, indeterminate reason: four. The day 15 bone marrow aspirate was evaluable in 199 (78%) patients. Of the 199 patients, 173 (87%), 16 (8%) and 10 (5%) patients, had an M1, M2 and M3 status, respectively. Among the ten patients with M3 status, six had additional HR features. One patient was treated as IR as an individualised decision. M3 status in the day 15 bone marrow was the sole reason for escalation to HR in 3 (3%) of the 98 patients who were assigned to the HR group. The commonest causes for escalation to HR were PPR and positive MRD. Eighteen (18%) HR patients had more than one reason for risk escalation. At the end of induction, 30 (12%), 127 (50%) and 98 (38%) patients were SR. IR and HR, respectively. The end of induction MRD was positive in 63 (25%) and negative in 190 (75%) patients. It was not evaluable in 2 patients due to a dilute marrow aspirate sample. Post-prephase complete blast clearance did not differ between patients with a positive and negative MRD (45% vs. 59%, P = 0.053), respectively. The proportion of patients with M3 status in the day 15 bone marrow did not differ among the positive and negative MRD groups (6% vs 5%, P = 0.207), respectively.
The median duration of follow up was 24 months (range: 3–57). Thirty-five (13.7%) patients relapsed. The 3-year event-free survival (EFS) and the 3-year overall-survival of the cohort was 76.7 ± 3.5% and 84.9 ± 2.9%. On univariate analysis, HR criteria such as NCI risk, bulky disease, genetics, PPR and day 15 M3 bone marrow status did not predict risk of relapse (Table 2). Lack of complete clearance of peripheral blood blasts after prephase and positive day 35 bone marrow MRD significantly predicted risk of relapse on univariate analysis, and they further emerged as independent predictors of relapse on multiple cox regression analysis [HR: 2.45 (1.04–5.75), P = 0.040 and HR: 3.00 (1.28–7.02), P = 0.011, Table 3]. Figure 2 contrasts the relapse-free survival using different peripheral blast count cut-offs for defining post steroid prephase response. Patients with complete peripheral blast clearance had significantly better 3-year relapse-free survival compared to the other groups (0 /µL: 89.2 ± 3.4%, 1–999 /µL: 68.9 ± 7.0% and ≥ 1000 /µL: 76.2 ± 10.9%, P = 0.023).
Table 3.
Multiple Cox regression for prognostic factors associated with relapse
| Parameter | HR (95% CI) | P |
|---|---|---|
| National Cancer Institute risk category | 1.67 (0.69–4.08) | 0.258 |
| High risk genetics | 2.94 (0.65–13.24) | 0.160 |
| Poor prephase response (blast count cut-off: 0/µL) | 2.45 (1.04 – 5.75) | 0.040 |
| Day 15 M3 bone marrow | 0.45 (0.06–3.47) | 0.441 |
| Minimal residual disease ≥ 0.01% on day 35 | 3.00 (1.28–7.02) | 0.011 |
Fig. 2.
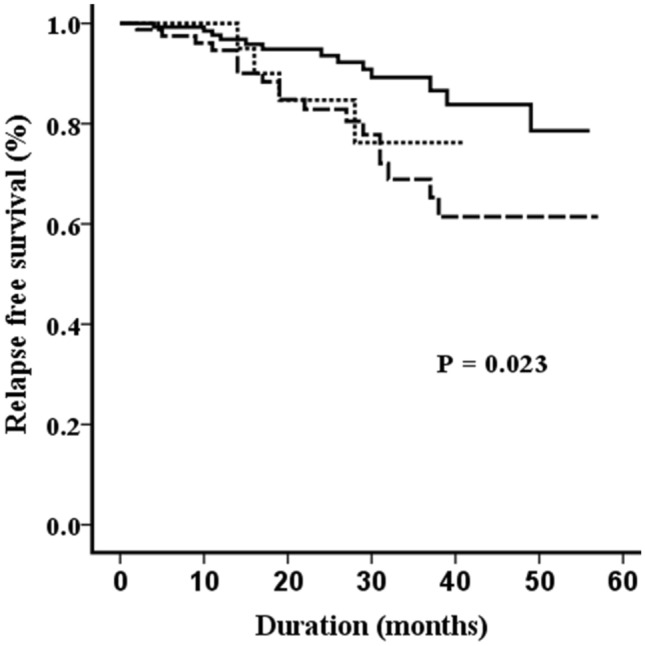
Comparison of relapse-free survival using different absolute peripheral blood blast count cut-offs to define steroid prephase response (solid line: 0 /µL, dashed line: 1–999 /µL and dotted line: ≥ 1000 /µL). Patients with complete peripheral blast clearance had significantly better survival compared to the other groups
Discussion
The survival in low- and middle-Income Countries (LMIC) lags behind the figures reported by high-income countries.[1, 7]. High treatment-related mortality in LMIC, which approximates 9–16% is an important reason for this survival gap [7, 8]. High-risk protocols which involve more intense chemotherapy independently predict mortality in studies from LMIC [8, 9]. A precise risk stratification system identifies high-risk patients who require intense chemotherapy and low-risk patients in whom the cytotoxic therapy can be de-escalated. Risk-stratification is therefore, fundamental to improving survival in ALL in LMIC.
ALL is a biologically heterogenous disease [10]. Rapidity of treatment response, which is an indicator of favourable biology, is a vital criterion in the risk classification of ALL [11]. The Berlin-Frankfurt-Münster (BFM) study group established the importance of response to a prednisolone prephase in children with ALL [12]. A peripheral blood blast cell count of ≥ 1,000/µL after a 7-day exposure to prednisone was seen in 10% of patients and was associated with a significantly inferior prognosis [12]. However, the cut-off of 1,000/µL did not significantly predict relapse in our study. Notably, absence of complete peripheral blood clearance after prephase, which was seen in more than half of our patients, emerged as an independent predictor of relapse. A study from Japan demonstrated that one third of children with ALL had no peripheral blasts on day 8, after a week of prednisolone monotherapy [13]. Complete blast clearance emerged as an independent predictor of outcome on multivariate analysis, akin to our study [13]. A Turkish study compared children with ALL treated with prednisolone versus high dose methylprednisolone [14]. Patients achieved a more rapid blast clearance with methylprednisolone, and had better survival, demonstrating that the type of glucocorticoid used may influence the response [14]. The prephase utilized in our study used dexamethasone for the first 48 h, followed by prednisolone. At the time of diagnosis, children with ALL are often febrile and unwell with a diminished oral intake. Further, a significant proportion of patients experience complications such as hyperleukocytosis and tumor lysis syndrome at presentation. Hence, steroids were administered parenterally in the form of dexamethasone for the first 48 h.
An early bone marrow performed at 2 weeks of induction, is the next landmark for assessment of treatment response. The ALL-BFM 95 trial identified patients with an M2 or M3 day 15 bone marrow to have a distinctly inferior survival when compared to those who had an M1 status [15]. The BFM and Children’s Cancer group (CCG) trials demonstrated that 11–12% of patients had an M2/M3 bone marrow on day 14 or 15 [15, 16]. Patients with a slow early response (SER) defined by M2/M3 marrow on day 14, had a survival that was 15–20% lesser than those with M1 marrow [16]. Augmentation of therapy in patients with SER was seen to improve outcomes in the CCG-1952 trial, but failed to do so in the Intercontinental IC-BFM 2002 trial [16, 17]. The proportion of patients with an M3 day 15 marrow in our study was 5%, which is lower than the figures reported in the above trials. Smaller numbers may have contributed to the lack of impact on relapse in our study. In comparison to the prephase response which is measured in a peripheral blood sample, bone marrow assessment involves an invasive procedure. During induction, low blood counts and unwell clinical status may preclude the investigation, as seen in our study. Further, the marrow sample may not be evaluable in a proportion of cases. In the ALL-BFM 95 trial, day 15 marrow could not be done in 20% of patients and of the marrows performed, 84% were evaluable [15].
End of induction MRD assessment has emerged as a strong and independent predictor of relapse in ALL. The Associazione Italiana di Ematologia Oncologia Pediatrica and the Berlin-Frankfurt-Münster Acute Lymphoblastic Leukemia (AIEOP-BFM) ALL 2000 trial established that MRD positivity was a strong and independent predictor of relapse [18]. The UKALL 2003 trials demonstrated benefit of treatment intensification in MRD positive patients and feasibility of treatment de-escalation in MRD negative patients [4, 5]. The impact of MRD on relapse was reiterated by our study. There is a paucity of literature pertaining to the relevance of early marrow morphology assessment with the availability of MRD assessment. A Children’s Oncology Group (COG) study concluded that a day 15 marrow is not essential if an end of induction MRD is measured, provided that a positive MRD is defined using a sensitive cut-off of 0.01% [19]. The same study observed that when a higher cut-off of 0.1% was used to intensify therapy, patients receiving augmented therapy for day 15 M2/M3 marrow appeared to have a better survival [19]. Our study which used an MRD cut-off of 0.01% failed to show an impact of day 15 marrow response on relapse.
The limitations of our study include the retrospective study design and the short follow up duration. Our study appraised the utility of cytomorphological response criteria such as day 8 blast count and day 15 bone marrow with the availability of MRD. We used a flow-cytometry based MRD technique, unlike the original UKALL trial which employed a PCR based method. Cytomorphological parameters are limited by their observer-dependent interpretation. Prephase response continues to play an important role in risk stratification, particularly in LMICs. Prospective studies are required to evaluate if patients with a day 8 blast count of 0 can be treated with less intensive therapy. Compared to prephase response, day 15 marrow is an invasive test. Day 15 marrow morphology status is not essential when using a sensitive end of induction MRD cut-off. However, it is useful for considering treatment escalation in patients in whom MRD is not evaluable due to sub-optimal sampling of the bone marrow or hypocellular state of the marrow.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgement
We are grateful to the Department of Hematology, Christian Medical College and Hospital, Vellore for performing and reporting the minimal residual disease in our patients.
Authors’ Contribution
TC: Collected and analysed the data, and wrote the manuscript. RJ, LLJ, HNS, DB: contributed to follow up and reviewed the final manuscript. TG: Contributed to laboratory aspect of study and reviewed the final manuscript. LGM: Study design, reviewed the final manuscript. ST: Study design, collected and analysed the data, and wrote the manuscript.
Data Availability
The data that support the findings of this study are available from the corresponding author [ST], upon reasonable request.
Compliance with Ethical Standards
Conflict of interest
None of the authors have anything to disclose.
Ethics approval
The study was approved by the Institutional Review Board (Silver, Research and Ethics Committee) of the Christian Medical College, Vellore vide ref no. IRB Min. No. 12727 [Retrospective] dated 25.03.2020.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hunger SP, Mullighan CG. Acute Lymphoblastic Leukemia in Children. N Engl J Med. 2015;373(16):1541–1552. doi: 10.1056/NEJMra1400972. [DOI] [PubMed] [Google Scholar]
- 2.Pui C-H, Yang JJ, Hunger SP, Pieters R, Schrappe M, Biondi A, et al. Childhood acute lymphoblastic leukemia: progress through collaboration. J Clin Oncol Off J Am Soc Clin Oncol. 2015;33(27):2938–2948. doi: 10.1200/JCO.2014.59.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Athale UH, Gibson PJ, Bradley NM, Malkin DM, Hitzler J, POGO MRD Working Group (2016) Minimal residual disease and childhood leukemia: standard of care recommendations from the pediatric oncology group of ontario MRD working group. Pediatr Blood Cancer 63(6):973–82 [DOI] [PubMed]
- 4.Vora A, Goulden N, Wade R, Mitchell C, Hancock J, Hough R, et al. Treatment reduction for children and young adults with low-risk acute lymphoblastic leukaemia defined by minimal residual disease (UKALL 2003): a randomised controlled trial. Lancet Oncol. 2013;14(3):199–209. doi: 10.1016/S1470-2045(12)70600-9. [DOI] [PubMed] [Google Scholar]
- 5.Vora A, Goulden N, Mitchell C, Hancock J, Hough R, Rowntree C, et al. Augmented post-remission therapy for a minimal residual disease-defined high-risk subgroup of children and young people with clinical standard-risk and intermediate-risk acute lymphoblastic leukaemia (UKALL 2003): a randomised controlled trial. Lancet Oncol. 2014;15(8):809–818. doi: 10.1016/S1470-2045(14)70243-8. [DOI] [PubMed] [Google Scholar]
- 6.Patkar N, Alex AA, Bargavi B, Ahmed R, Abraham A, George B, et al. Standardizing minimal residual disease by flow cytometry for precursor B lineage acute lymphoblastic leukemia in a developing country. Cytom B Clin Cytom. 2012;82(4):252–258. doi: 10.1002/cyto.b.21017. [DOI] [PubMed] [Google Scholar]
- 7.Trehan A, Bansal D, Varma N, Vora A. Improving outcome of acute lymphoblastic leukemia with a simplified protocol: report from a tertiary care center in north India. Pediatr Blood Cancer. 2017 doi: 10.1002/pbc.26281. [DOI] [PubMed] [Google Scholar]
- 8.Gupta S, Antillon FA, Bonilla M, Fu L, Howard SC, Ribeiro RC, et al. Treatment-related mortality in children with acute lymphoblastic leukemia in Central America. Cancer. 2011;117(20):4788–4795. doi: 10.1002/cncr.26107. [DOI] [PubMed] [Google Scholar]
- 9.Totadri S, Trehan A, Kaur A, Bansal D. Effect of socio-economic status & proximity of patient residence to hospital on survival in childhood acute lymphoblastic leukaemia. Indian J Med Res. 2019;149(1):26–33. doi: 10.4103/ijmr.IJMR_579_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bhojwani D, Yang JJ, Pui C-H. Biology of childhood acute lymphoblastic leukemia. Pediatr Clin North Am. 2015;62(1):47–60. doi: 10.1016/j.pcl.2014.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pui C-H, Evans WE. Treatment of acute lymphoblastic leukemia. N Engl J Med. 2006;354(2):166–178. doi: 10.1056/NEJMra052603. [DOI] [PubMed] [Google Scholar]
- 12.Reiter A, Schrappe M, Ludwig WD, Hiddemann W, Sauter S, Henze G, et al. Chemotherapy in 998 unselected childhood acute lymphoblastic leukemia patients. Results and conclusions of the multicenter trial ALL-BFM 86. Blood. 1994;84(9):3122–3133. doi: 10.1182/blood.V84.9.3122.3122. [DOI] [PubMed] [Google Scholar]
- 13.Manabe A, Ohara A, Hasegawa D, Koh K, Saito T, Kiyokawa N, et al. Significance of the complete clearance of peripheral blasts after 7 days of prednisolone treatment in children with acute lymphoblastic leukemia: the Tokyo Children’s Cancer Study Group Study L99–15. Haematologica. 2008;93(8):1155–1160. doi: 10.3324/haematol.12365. [DOI] [PubMed] [Google Scholar]
- 14.Yetgin S, Cetin M. The dose related effect of steroids on blast reduction rate and event free survival in children with acute lymphoblastic leukemia. Leuk Lymphoma. 2003;44(3):489–495. doi: 10.1080/1042819021000055048. [DOI] [PubMed] [Google Scholar]
- 15.Lauten M, Möricke A, Beier R, Zimmermann M, Stanulla M, Meissner B, et al. Prediction of outcome by early bone marrow response in childhood acute lymphoblastic leukemia treated in the ALL-BFM 95 trial: differential effects in precursor B-cell and T-cell leukemia. Haematologica. 2012;97(7):1048–1056. doi: 10.3324/haematol.2011.047613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schultz KR, Pullen DJ, Sather HN, Shuster JJ, Devidas M, Borowitz MJ, et al. Risk- and response-based classification of childhood B-precursor acute lymphoblastic leukemia: a combined analysis of prognostic markers from the Pediatric Oncology Group (POG) and Children’s Cancer Group (CCG) Blood. 2007;109(3):926–935. doi: 10.1182/blood-2006-01-024729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stary J, Zimmermann M, Campbell M, Castillo L, Dibar E, Donska S, et al. Intensive chemotherapy for childhood acute lymphoblastic leukemia: results of the randomized intercontinental trial ALL IC-BFM 2002. J Clin Oncol Off J Am Soc Clin Oncol. 2014;32(3):174–184. doi: 10.1200/JCO.2013.48.6522. [DOI] [PubMed] [Google Scholar]
- 18.Conter V, Bartram CR, Valsecchi MG, Schrauder A, Panzer-Grümayer R, Möricke A, et al. Molecular response to treatment redefines all prognostic factors in children and adolescents with B-cell precursor acute lymphoblastic leukemia: results in 3184 patients of the AIEOP-BFM ALL 2000 study. Blood. 2010;115(16):3206–3214. doi: 10.1182/blood-2009-10-248146. [DOI] [PubMed] [Google Scholar]
- 19.Borowitz MJ, Wood BL, Devidas M, Loh ML, Raetz EA, Larsen E, et al (2013) Assessment of end induction minimal residual disease (MRD) in childhood B precursor acute lymphoblastic leukemia (ALL) to eliminate the need for day 14 marrow examination: A Children’s Oncology Group study .J Clin Oncol (2013 ASCO Annual Meeting Abstracts) 31(20Suppl;10001).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author [ST], upon reasonable request.



