There are limited treatment options for post allogeneic stem cell transplant (ASCT) relapse of hematological malignancies. Options include donor lymphocyte infusion (DLI), chemotherapy, second transplant, targeted therapies or combinations of these. DLI alone may not help due to time it takes for its graft versus leukemia (GVL) effects; it is effective only in about one-third patients [1]. Agents like nivolumab have been shown to induce GVL post ASCT without DLI [2, 3]. However, nivolumab is prohibitively expensive in India. Lenalidomide is an immunomodulator that induces effector T cells and has both anti-leukemia and lymphoma activity. It carries the advantage of being an oral drug and is inexpensive. We report outcomes of 3 patients with impending relapse post ASCT in whom lenalidomide was used for its immunomodulatory effects since no other treatment options were feasible.
Case 1
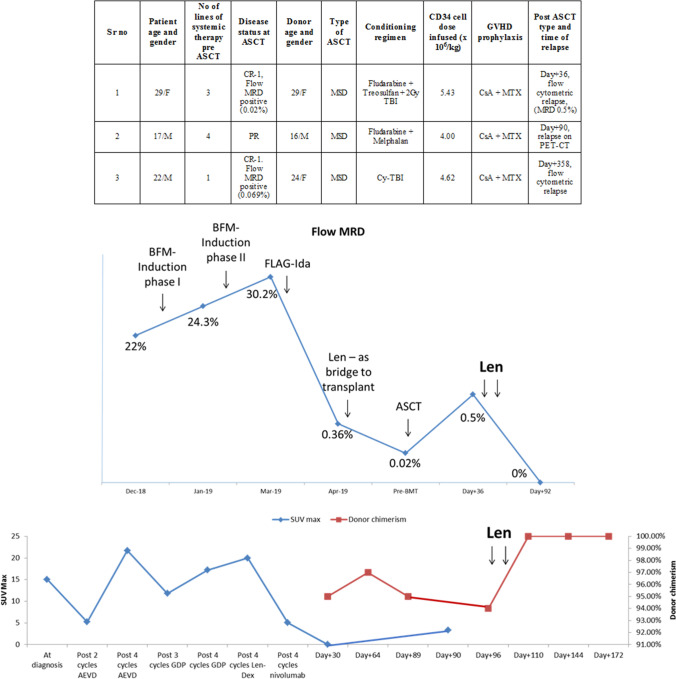
A 29 year old female with ulcerative colitis since past 15 years (treated with azathioprine for 13 years) developed secondary acute leukemia (mixed phenotype-T/Myeloid) with multiple cytogenetic abnormalities (TP 53 deletion, del 5q, ABL1 amplification and tri-tetrasomy of 7, 8, 11 and 21). Prior to ASCT, she had received multiple lines of therapy (Fig. 1, upper panel) including lenalidomide 10 mg daily as bridge to transplant (Fig. 1, middle panel); only toxicity was grade I skin rash. Post ASCT, MRD (day + 36) was 0.5% (Fig. 1, middle panel); cyclosporine was stopped and lenalidomide 10 mg/day started on day + 37. She developed fever and grade I skin rash with eosinophilia on day + 40. After excluding infectious causes, immune fever was suspected; lenalidomide was stopped on day + 42. Liver GVHD (Glucksberg grade I) was diagnosed on day + 52; this required prednisolone and mycophenolate. Although liver GVHD resolved, skin GVHD gradually evolved to chronic GVHD for which sirolimus was started on day + 79. Repeat bone marrow (day + 92) showed MRD 0%. She subsequently had extramedullary relapse on day + 190, which was treated with radiotherapy followed by 3 systemic therapies. At her last follow up (day + 405), she alive and disease free.
Fig. 1.
Upper panel shows the demographic and donor details and transplant related details of the 3 patients in a tabular format, middle panel shows the flow cytometric MRD of patient 1 (along with the treatments received) and lower panel shows the serial SUVmax (blue line) and donor chimerism (red line) of patient 2 at various time points. MRD measurable residual disease, Cy Cyclophosphamide, TBI Total Body Irradiation, CsA Cyclosporine, MTX Methotrexate, Len Lenalidomide (color figure online)
Case 2
A 17 year-old male with primary progressive Hodgkin lymphoma underwent MSD ASCT after failing 4 lines of systemic therapy (Fig. 1, upper panel). Pre ASCT, he had received lenalidomide (10/mg/day) with weekly dexamethasone (as 3rd line systemic therapy), but without attaining complete remission (Fig. 1, bottom panel). Post ASCT, PET CT (day + 30) showed complete remission. Subsequent PET CT (day + 90) was suggestive of disease relapse. Cyclosporine was stopped (day + 90). Donor chimerism dropped to 94% (day + 96). He had not developed any GVHD till then. Lenalidomide 10 mg/day was started from day + 104. On day + 107, he developed acute gut GVHD; lenalidomide was stopped on day + 108. Methylprednisolone was started from day + 109. Donor chimerism improved to 100% donor (day + 110). He however succumbed to steroid refractory GVHD on day + 177.
Case 3
A 22 year old male with high risk pre-B ALL underwent ASCT in CR-1. Flow MRD was negative on day + 30 and day + 85. He did not develop any GVHD. Cyclosporine was stopped on day + 189. Bone marrow (day + 356) showed MRD of 0.001%. Repeat bone marrow (day + 385) showed persisting MRD (0.001%). Lenalidomide 10 mg/day was started from day + 421. Grade I liver GVHD was diagnosed on day + 427, which resolved spontaneously. He is currently being continued on lenalidomide. At his last follow up (1 year from start of lenalidomide) he is in complete remission with no active GVHD.
The above cases show that lenalidomide is a potent immunomodulator which alone can induce GVL. However, it does carry an increased risk of GVHD [4, 5]. Lenalidomide may be a useful option, particularly when DLI / other therapies are not feasible. Larger prospective studies are warranted to further explore lenalidomide for this indication.
Conflict of interest
The authors declare that there is no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Vaezi M, Zokaasadi M, Shahsavari Pour S, Kasaeian A, Nikbakht M, Kamranzadeh Fumani H, et al. The role of donor leukocyte infusions in the treatment of relapsed acute leukemia after allogeneic stem cell transplantation: a retrospective analysis. Int J Hematol Oncol Stem Cell Res. 2018;12(3):185–191. [PMC free article] [PubMed] [Google Scholar]
- 2.Albring J, Inselmann S, Sauer T, Schliemann C, Altvater B, Kailayangiri S, et al. PD-1 checkpoint blockade in patients with relapsed AML after allogeneic stem cell transplantation. Bone Marrow Transpl. 2017;52:317–320. doi: 10.1038/bmt.2016.274. [DOI] [PubMed] [Google Scholar]
- 3.Herbaux C, Gauthier J, Brice P, Drumez E, Ysebaert L, Doyen H, et al. Efficacy and tolerability of nivolumab after allogeneic transplantation for relapsed hodgkins lymphoma. Blood. 2017;129:2471–2478. doi: 10.1182/blood-2016-11-749556. [DOI] [PubMed] [Google Scholar]
- 4.Wolschke C, Stübig T, Hegenbart U, et al. Post allograft lenalidomide induces strong NK cell-mediated antimyeloma activity and risk for Tcell-mediated GvHD: results from a phase I/II dose-finding study. Exp Hematol. 2013;41:134–142. doi: 10.1016/j.exphem.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 5.Sockel K, Bornhaeuser M, Mischak-Weissinger E, et al. Lenalidomide maintenance after allogeneic HSCT seems to trigger acute graft-versus-host disease in patients with high-risk MDS or AML and del(5q): results of the LENAMAINT trial. Haematologica. 2012;97(9):e34–35. doi: 10.3324/haematol.2012.067629. [DOI] [PMC free article] [PubMed] [Google Scholar]