Abstract
The conditioning regimens used for the allo-HSCT include either myeloablative conditioning (MAC) or reduced intensity conditioning (RIC) regimens based on the age, performance status and co-morbidities. Studies comparing the survival outcomes of RIC and MAC allo-HSCT in AML and MDS patients have reported contradictory results. We therefore retrospectively analyzed our data of AML and MDS patients who received MAC and RIC allo-HSCT at our center and compared the long term outcome of the two conditioning regimens. One hundred twenty six consecutive patients were evaluated, 32 (25.4%) underwent MAC allo-HSCT and 94 (74.6%) underwent RIC allo-HSCT. The most common MAC regimen used was busulfan plus cyclophosphamide and the most common RIC regimen used was fludarabine plus melphalan. The median age was higher in RIC group (44 years, range 4–75 years) compared to MAC group (31 yrs, range 6–51 yrs, p = 0.001). There was no significant difference in terms of overall survival (p = 0.498), relapse-free survival (p = 0.791) and non-relapse mortality (p = 0.366) between the two groups. In multivariate analysis, only chronic graft-versus-host disease resulted in decreased risk of relapse and improved overall survival irrespective of the conditioning regimens used.
Keywords: Allogeneic stem cell transplant, Reduced intensity conditioning, Myeloablative conditioning, Overall survival, GVHD
Introduction
Allogeneic hematopoietic stem cell transplantation (allo-HSCT) is a potentially curative treatment for acute myeloid leukemia (AML) and myelodysplastic syndrome (MDS). Traditionally, myeloablative conditioning (MAC) has been the standard conditioning regimen for AML patients in need of allo-HSCT. MAC is, however, associated with a high risk of toxicity and nonrelapse mortality (NRM), especially among elderly patients and patients with co-morbidities. This led to the exploration of reduced-intensity conditioning (RIC) regimens. Multiple variables have been shown to effect the outcome of allo-HSCT including recipient age, disease status at the time of allo-HSCT, donor type, risk category of AML/MDS patient, co-morbidity status and type of conditioning regimen used [1]. The RIC regimen has extended the approach of allo-HSCT in AML/MDS to include patients who are not eligible for standard allo-HSCT because of their advanced age and/or co-morbidities [2]. Few randomized clinical trials have compared the survival outcomes between MAC and RIC in AML/MDS patients for allo-HSCT [3, 4] and there are still controversies as to which is the best conditioning regimen for a given patient. Furthermore, several previous studies comparing the survival outcomes of RIC and MAC allo-HSCT in AML patients have reported contradictory results [2–4]. Thus, we retrospectively compared long-term outcomes of AML and MDS patients who received MAC and RIC regimens for allo-HSCT at our Bone Marrow Transplant centre.
Patients and Methods
One hundred twenty six consecutive patients, who underwent allo-HSCT for AML/MDS from August 2010 to May 2020, were evaluated retrospectively using hospital information system and analyzing the data from medical records. Informed consent was taken from all patients and the study was approved by Hospital Ethical Committee and Institutional Review Board (in accordance with Declaration of Helsinki). The transplants were conducted in HEPA-filtered rooms. The indications for selecting the RIC regimen were as follows: inadequate liver, kidney, or cardiac functions (defined as serum transaminase levels > 3 times the upper limit of normal reference value, total bilirubin > 2 mg/dl, creatinine clearance < 60 mL/min, left ventricular ejection fraction < 50%); ECOG performance status; prior or concurrent severe infections; and the physician’s discretion depending upon the age and tolerance of previous induction/consolidation courses. Young patients who had severe Gram negative sepsis or pneumonia requiring intensive care management during induction therapy were preferably considered for RIC allo-HSCT.
Stem cells were obtained from HLA-matched related and unrelated donors. Peripheral blood stem cells were used for all patients who underwent allo-HSCT. Donor peripheral blood stem cells were mobilized by granulocyte colony-stimulating factor. All patients received antimicrobial prophylaxis including fluconazole, acyclovir and co-trimoxazole and treatment of febrile neutropenia as per hospital policy. Engraftment was defined by absolute neutrophil count more than 500/µl for 3 consecutive days and platelet counts more than 20,000/µl for 7 days after last platelet transfusion. After discharge the patients were regularly followed up in Out-patient clinics. Patients with haploidentical transplant were excluded to limit the heterogeneity of the study population.
Conditioning Regimens and Graft-Versus-Host Disease Prophylaxis
Conditioning intensity was defined as per Consensus CIBMTR criteria [5]: MAC regimens were defined by busulfan (Bu) dose > 6.4 mg/kg IV, whereas RIC regimens were defined by Bu dose ≤ 6.4 mg/kg IV and melphalan (Mel) ≤ 150 mg/m2 IV. The various RIC conditioning regimens used were FluMel (Flu 30 mg/m2 IV for 5 days and Mel 140 mg/m2 IV for 1 day), FluBu (Flu 30 mg/m2 IV for 5 days and Bu 3.2 mg/kg/day IV for 2 days), Flu/Ara-C/Idarubicin/Mel (Flu 30 mg/m2 IV for 5 days, cytarabine 2gm/m2 IV for 5 days, Idarubicin 8 mg/m2 IV for 3 days and Mel 140 mg/m2 IV for 1 day), FluCy (Flu 30 mg/m2 IV for 5 days and Cy 60 mg/kg IV for 2 days). MAC regimen included BuCy (Bu 3.2 mg/kg/day IV for 4 days and Cy 60 mg/kg IV for 2 days).
All patients received standard cyclosporine A (CsA) and methotrexate therapy for graft-versus-host disease (GVHD) prophylaxis. For RIC, GVHD prophylaxis was methotrexate 5 mg/m2 IV on day + 1, + 3, + 6 and cyclosporine 1.5 mg/kg IV twice daily as three-hours infusion from Day-2. For MAC, methotrexate 15 mg/m2 was given on day + 1 followed by 10 mg/m2 on day + 3, + 6, + 11 followed by leucovorin rescue 15 mg/m2 IV 24 h after methotrexate and cyclosporine 1.5 mg/kg IV twice daily as three-hours infusion from Day-2. Cyclosporine was switched to oral form with trough cyclosporine levels maintained between 150–300 ng/ml. Tapering of immune suppression was initiated at 3 months after allo-HSCT in the absence of acute or chronic GVHD, with the aim of stopping it by approximately 6 months after HSCT.
Study End Points and Statistical Analysis
This is a retrospective descriptive study comparing outcomes after RIC and MAC allo-HSCT using related and unrelated donors for patients with AML or MDS. The primary end point of the study was overall survival (OS). OS was defined as the time from transplantation to death from any cause or last follow-up. Surviving patients were censored at last follow-up. Secondary end points included relapse free survival (RFS), non-relapse mortality (NRM) and cumulative incidence of relapse and acute and chronic GVHD. RFS was defined as time from transplantation to either relapse or death from any cause. NRM was defined as death from any cause in remission. Acute and chronic GVHD were graded according to the related consensus criteria [6].
SPSS 25 (IBM Corp., USA) was used to perform statistical analyses. Statistical comparisons were made using chi-square tests for categorical data. Survival analyses were made using the Kaplan–Meier test. Multivariate analyses of predictors of survival were performed using the Cox regression test and p value < 0.05 was considered to indicate statistical significance.
Results
Out of 126 patients, 94 underwent RIC allo-HSCT and 32 underwent MAC allo-HSCT. The median age was higher in RIC group (44 yrs, range 4–75 years) compared to MAC group (31 years, range 6–51 years, p = 0.001) (Table 1). There were two matched unrelated donor (MUD) transplants in each group. The indications of transplant in AML were CR1 in intermediate risk (normal karyotype, mutated NPM without FLT3 or wild type NPM without FLT3) and high risk (del 5, del 7, del 17 or complex karyotype) and CR2 and subsequent relapses. Thirty seven patients were in intermediate risk and 16 patients had high risk AML (del 5 and del 7–1 patient each, complex karyotype-4 patients and FLT 3 positive-10 patients). Nineteen patients with MDS with IPSS intermediate-risk 2 or higher underwent transplant. Patients who received the MAC regimen had better ECOG performance status than patients who received the RIC regimen. The most common RIC regimen used was fludarabine (Flu)/Mel in 69/96 (71.8%), followed by FluBu in 24/96 (25%) patients. Five patients received Flu/Ara-C/Idarubicin/Mel, 2 of these patients had full blown disease (blasts more than 20% at the time of relapse and 3 patients were not in complete remission (CR) and had blasts between 6 and 10%. Two patients received FluCy. Among MAC regimen all patients received busulfan and cyclophosphamide (BuCy).
Table 1.
Patient characteristics
| Parameter | RIC | MAC | p value |
|---|---|---|---|
| No | 94 | 32 | – |
| Age, years (median) (range) | 44 (4–75) | 31 (6–51) | 0.001 |
| Sex, Male (%) | 61 (64.9%) | 19 (59.4%) | – |
| Female | 33 (35.1%) | 13 (40.6%) | – |
| ECOG performance status | 0–2 | 0–1 | – |
| Disease | |||
| AML | 79 (84%) | 28 (87.5%) | – |
| MDS | 15 (16%) | 4 (12.5%) | – |
| CD34 cell count (X106 cells/kg) mean ± SD | 6.36 ± 3.60 | 7.02 ± 3.02 | – |
| CMV status recipient (R) to donor (D) | |||
| R + D + | 87 (92.6%) | 29 (90.6%) | – |
| R + D − | 5 (5.3%) | 1 (3.1%) | – |
| R − D + | 2 (2.1%) | 2 (6.3%) | – |
| Disease status during transplantation in AML | |||
| CR1 | 39 (41.5%) | 14 (43.8%) | – |
| ≥ CR2 (%) | 35 (37.2%) | 14 (43.8%) | – |
| Active disease (%) | 5 (5.3%) | – | – |
Survival Outcomes
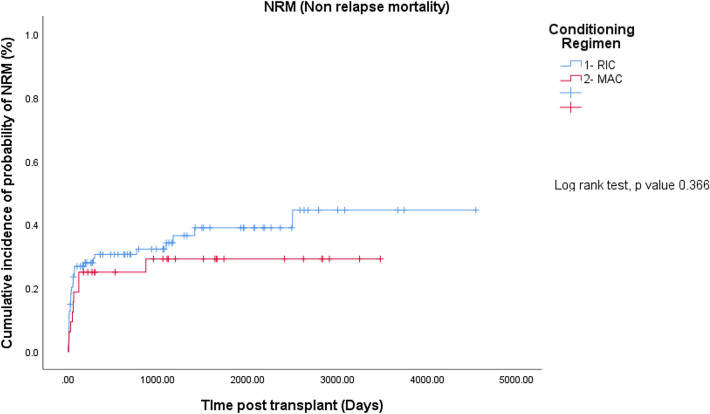
The median follow-up for all patients was 648 days (range 10–4542 days). The median follow-up of patients in RIC group was 590 days (range 10–4542 days). The 3 year OS, RFS and NRM was 58.5%, 53.2% and 33%, respectively. The median follow-up for the MAC group was 1004 days (29–3479 days). The 3 year OS, RFS and NRM was 59.4%, 53.1% and 28.1%, respectively. There was no significant difference in terms of OS (p = 0.498), RFS (p = 0.791) and NRM (p = 0.366) between the two groups (Figs. 1 and 2). Cytomegalovirus seropositivity status of the patients and donors were similar between the two groups receiving RIC and MAC regimens. The major causes of death were infections, relapses and GVHD (Table 1).
Fig. 1.
The overall survival for RIC and MAC regimens
Fig. 2.
The Relapse-free survival for RIC and MAC regimens
In multivariate analysis adjusted for relevant variables (Tables 2 and 3), the conditioning regimens did not significantly influence the outcome of OS, EFS and relapse. However, those patients who developed chronic GVHD irrespective of conditioning regimen had lower risk of relapse (HR 3.83, CI 1.031–14.231, p = 0.045) and borderline significant improvement in OS (HR 1.749, CI 1.00–3.057, p = 0.05).
Table 2.
Comparison of key parameters following RIC and MAC regimens
| Parameter | RIC | MAC | p value |
|---|---|---|---|
| GvHD | |||
| Acute GvHD (grade I) | 6 | 2 | – |
| Acute GvHD (II–IV) | 26 (27.7%) | 12 (37.5%) | 0.29 |
| Chronic GvHD (Extensive) | 7 | 1 | – |
| Chronic GvHD (limited) | 21 | 6 | – |
| Chronic GvHD (Total) | 28 (29.8%) | 7 (21.9%) | 0.38 |
| Causes of death | |||
| Relapse | 8 (8.5%) | 4 (12.5%) | – |
| Infection | 28 (29.8%) | 4 (12.5%) | – |
| GvHD | 6 (6.4%) | 5 (15.6%) | – |
| OS | 52 (55.3%) | 19 (59.4%) | – |
| Relapse | 13 (13.8%) | 6 (18.8%) | – |
| NRM | 33 (35.1%) | 9 (28.1%) | – |
Table 3.
Multivariate analysis of overall survival, relapse free survival and relapse
| Variables | OS | RFS | Relapse | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | p value | HR | 95% CI | p value | HR | 95% CI | P value | ||
| Conditioning regimen | RIC | |||||||||
| MAC | 0.71 | 0.34–1.46 | 0.35 | 0.74 | 0.34–1.59 | 0.45 | 0.43 | 0.12–1.49 | 0.18 | |
| CD34 cell count (× 106 cells/kg) mean ± SD | 0.98 | 0.91–1.05 | 0.55 | 1.03 | 0.95–1.13 | 0.39 | 0.99 | 0.84–1.17 | 0.98 | |
| Acute GvHD | 0.55 | 0.28–1.07 | 0.07 | 0.55 | 0.28–1.10 | 0.09 | 0.39 | 0.12–1.28 | 0.12 | |
| Chronic GvHD | 1.74 | 1.00–3.05 | 0.05 | 1.21 | 0.66–2.21 | 0.52 | 3.83 | 1.03–14.23 | 0.04 | |
Acute and Chronic GVHD
Acute GVHD grade II–IV developed in 26 patients in the RIC group (27.7%) and 12 patients in the MAC group (37.5%) whereas chronic GVHD developed in 28 patients in RIC group (29.8%) and 7 patients in the MAC group (21.9%). There was no statistically significant difference between the RIC and MAC patients in terms of acute (p = 0.295) and chronic GVHD (p = 0.388).
Non-Relapse Mortality
Non-relapse mortality (NRM) was not different between the RIC and MAC patients (35.1% vs. 28.1%, p = 0.366) (Fig. 3). The major causes of NRM were infections (29.8% vs. 12.55%) and GVHD (6.4% vs. 15.6%).
Fig. 3.
Cumulative incidence of non-relapse mortality for RIC and MAC regimens
Discussion
Allogeneic HSCT is the standard treatment for patients with intermediate to high risk AML and intermediate-2 and poor risk MDS patients. MAC is the preferred regimen for allo-HSCT for young adults without co-morbidities, however in elderly patients and patients with co-morbidities, MAC regimens results in unacceptable mortality. Since graft versus leukemia (GVL) effect has been shown to lower the risk of relapse, the GVL based therapy can be better implemented in these group of patients by delivering reduced intensity regimens so that regimen related mortality can be reduced. Based on this principle RIC regimens have been developed. However, RIC regimens have been shown to be associated with higher relapse rate compared to MAC and therefore effect on the OS is still debated [4, 7]. Retrospective studies comparing MAC with RIC in patients with AML or MDS suggested that RIC was associated with increased relapse but reduced NRM, resulting in similar OS [4, 7]. Luger et al. [8], have also shown similar outcomes using MAC and RIC preparative regimens for AML or MDS. Goker et al. [9], showed that in comparison to the RIC regimen, the MAC regimen was associated with lower OS and progression-free survival (PFS). Additionally, RIC regimen was associated with a lower acute GVHD but a higher rate of chronic GVHD [9]. We compared the two types of conditioning regimens in our cohort of patients and found no significant difference in OS and RFS between the two groups. The NRM and the acute and chronic GVHD between the two groups were also not different. Chronic GVHD has been shown to correlate with a lower relapse rate and better PFS. In a study by Valcárcel et al. [10], the development of chronic GVHD was the major factor associated with lower relapse incidence and improved PFS and OS in AML and MDS patients undergoing RIC allo-HSCT. In our study also, chronic GVHD was the only variable that decreased the relapse risk and increased the OS in multivariate analysis, suggesting that development of chronic GVHD rather than the type of conditioning regimen used, improves the survival.
Scott et al. [4], in a randomized study, compared RIC and MAC regimens in patients with AML or MDS in remission. In their study there was excess relapse in the RIC arm and in the AML subgroup, lower OS, concluding that MAC conditioning should be the standard of care for fit patients with AML or MDS [4]. However, in our cohort RIC was not associated with increased relapse or lower OS compared to MAC and chronic GVHD mitigated relapse probably by mediating GVL effect and improved survival irrespective of type of conditioning regimens used. In a similar cohort of patients, Ganapule et al., have reported improved OS and event free survival with RIC compared to MAC in AML patients [11]. Median age of patients in their study was 34 years, similar to that of our patient population.
RIC regimens are supposed to result in less GVHD compared to MAC regimens, however, studies have found similar risk of GVHD between the MAC and RIC regimens [12, 13]. We also found no difference in the risk of acute and chronic GVHD between the two groups. The major causes of death in our cohort were infections, GVHD and relapse and these variables were not different between the MAC and RIC groups. Relapse incidence was supposed to be lower with the MAC regimen but was similar in the two groups probably because since GVHD was similar in the two groups, the GVL effect was also presumed to be similar in the two groups and since the risk of relapse decreased with chronic GVHD, the development of chronic GVHD per se (which is an indirect indicator of graft-vs-leukemia effect) was the most important mechanism for relapse prevention irrespective of MAC or RIC regimen. NRM is supposed to be more with MAC but since patients undergoing MAC conditioning had better performance status and younger age compared to those receiving RIC, the NRM was not higher in the MAC group and these patients were able to tolerate intensive conditioning. Moreover, the incidence of GVHD and infection were also similar between the two groups, thereby leading to similar NRM rates.
Elderly patients and those with co-morbidities should be offered RIC allo-HSCT as it can lead long-term cure [14]. The drawback of our study, In addition to being a retrospective, was less number of patients, however, results of our study are consistent with those reported in the literature [4, 7, 8].
Conclusion
RIC regimens are feasible in patients with older age or co-morbidities and result in similar survival as MAC regimens in younger patients. Patients who develop chronic GVHD have lower risk of relapse and improved overall survival irrespective of type of conditioning regimen used. Elderly patients and patients with poor performance status who cannot be considered for MAC should be offered RIC to achieve the above target.
Acknowledgements
We are thankful to Ms Saheli and Ms Anubha for collecting and formatting the data.
Funding
There is no financial support for this study.
Compliance with Ethical Standards
Conflict of interest
The authors declare no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Wahid SFA. Indications and outcomes of reduced-toxicity hematopoietic stem cell transplantation in adult patients with hematological malignancies. Int J Hematol. 2013;97(5):581–598. doi: 10.1007/s12185-013-1313-0. [DOI] [PubMed] [Google Scholar]
- 2.Horwitz ME. Reduced intensity versus myeloablative allogeneic stem cell transplantation for the treatment of acute myeloid leukemia, myelodysplastic syndrome and acute lymphoid leukemia. Curr Opin Oncol. 2011;23(2):197–202. doi: 10.1097/CCO.0b013e328342b82a. [DOI] [PubMed] [Google Scholar]
- 3.Bornhäuser M, Kienast J, Trenschel R, Burchert A, Hegenbart U, Stadler M, et al. Reduced-intensity conditioning versus standard conditioning before allogeneic haemopoietic cell transplantation in patients with acute myeloid leukaemia in first complete remission: a prospective, open-label randomised phase 3 trial. Lancet Oncol. 2012;13(10):1035–1044. doi: 10.1016/S1470-2045(12)70349-2. [DOI] [PubMed] [Google Scholar]
- 4.Scott BL, Pasquini MC, Logan BR, Wu J, Devine SM, Porter DL, et al. Myeloablative versus reduced-intensity hematopoietic cell transplantation for acute myeloid leukemia and myelodysplastic syndromes. J Clin Oncol. 2017;35(11):1154–1161. doi: 10.1200/JCO.2016.70.7091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bacigalupo A, Ballen K, Rizzo D, Giralt S, Lazarus H, Ho V, et al. Defining the intensity of conditioning regimens: working definitions. Biol Blood Marrow Transpl. 2009;15(12):1628–1633. doi: 10.1016/j.bbmt.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Filipovich AH, Weisdorf D, Pavletic S, Socie G, Wingard JR, Lee SJ, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. diagnosis and staging working group report. Biol Blood Marrow Transpl. 2005;11(12):945–956. doi: 10.1016/j.bbmt.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 7.Aoudjhane M, Labopin M, Gorin NC, Shimoni A, Ruutu T, Kolb H-J, et al. Comparative outcome of reduced intensity and myeloablative conditioning regimen in HLA identical sibling allogeneic haematopoietic stem cell transplantation for patients older than 50 years of age with acute myeloblastic leukaemia: a retrospective survey from the Acute Leukemia Working Party (ALWP) of the European group for Blood and Marrow Transplantation (EBMT) Leukemia. 2005;19(12):2304–2312. doi: 10.1038/sj.leu.2403967. [DOI] [PubMed] [Google Scholar]
- 8.Luger SM, Ringdén O, Zhang M-J, Pérez WS, Bishop MR, Bornhauser M, et al. Similar outcomes using myeloablative vs reduced-intensity allogeneic transplant preparative regimens for AML or MDS. Bone Marrow Transpl. 2012;47(2):203–211. doi: 10.1038/bmt.2011.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goker H, Ozdemir E, Uz B, Buyukasik Y, Turgut M, Serefhanoglu S, et al. Comparative outcome of reduced intensity and myeloablative conditioning regimen in HLA identical sibling allogeneic hematopoietic stem cell transplantation for acute leukemia patients: a single center experience. Transfus Apher Sci. 2013;49(3):590–599. doi: 10.1016/j.transci.2013.07.030. [DOI] [PubMed] [Google Scholar]
- 10.Valcárcel D, Martino R, Caballero D, Martin J, Ferra C, Nieto JB, et al. Sustained remissions of high-risk acute myeloid leukemia and myelodysplastic syndrome after reduced-intensity conditioning allogeneic hematopoietic transplantation: chronic graft-versus-host disease is the strongest factor improving survival. J Clin Oncol. 2008;26(4):577–584. doi: 10.1200/JCO.2007.11.1641. [DOI] [PubMed] [Google Scholar]
- 11.Ganapule A, Nemani S, Korula A, Lakshmi KM, Abraham A, et al. Allogeneic stem cell transplant for acute myeloid leukemia: evolution of an effective strategy in India. J Glob Oncol. 2017;3(6):773–781. doi: 10.1200/JGO.2016.006650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abdul Wahid SF, Ismail N-A, Mohd-Idris M-R, Jamaluddin FW, Tumian N, Sze-Wei EY, et al. Comparison of reduced-intensity and myeloablative conditioning regimens for allogeneic hematopoietic stem cell transplantation in patients with acute myeloid leukemia and acute lymphoblastic leukemia: a meta-analysis. Stem Cells Dev. 2014;23(21):2535–2552. doi: 10.1089/scd.2014.0123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kröger N, Iacobelli S, Franke G-N, Platzbecker U, Uddin R, Hübel K, et al. Dose- reduced versus standard conditioning followed by allogeneic stem-cell transplantation for patients with myelodysplastic syndrome: a prospective randomized phase III study of the EBMT (RICMAC Trial) J Clin Oncol. 2017;35(19):2157–2164. doi: 10.1200/JCO.2016.70.7349. [DOI] [PubMed] [Google Scholar]
- 14.Sharma SK, Choudhary D, Kaul E, Kharya G, Khandelwal V, Kothari S, et al. Hematopoietic stem cell transplant in elderly patients: Experience from a tertiary care centre in Northern India. Indian J Hematol Blood Transfus. 2016;32:205–207. doi: 10.1007/s12288-015-0624-0. [DOI] [PMC free article] [PubMed] [Google Scholar]