Abstracts
The proportion of CD34 + CD38 − CD123 + leukemia stem cells (LSCs) at diagnosis of Acute Myeloid Leukemia (AML) correlated with induction remission (IR), relapse free survival and overall survival in few studies. Prospectively bone marrows of AML patients were immunophenotyped for CD34 + CD38 − CD123 + LSCs at baseline using sequential gating, relevant clinical and laboratory data collected and clinical outcomes were studied.The patients (n = 47) were risk stratified as favorable risk, intermediate risk and adverse risk. The percent of LSCs at baseline in favorable risk group (mean = 13.06%) was significantly less than the adverse (mean = 34.8%, p = 0.027) and the intermediate risk group (mean = 53.2%, p = 0.001). On further analysis, 12 patients attaining IR in intermediate risk group had significantly less LSCs than 15 in non-IR group (mean = 21.18%; range 3–85.6% vs mean = 73.85%; range 12.1–97.9%, p = 0.0002). Of all 47 patients, the proportion of LSCs at baseline was significantly less in those achieving IR (p = 0.024) and correlated with time to response (TTR) (rs = 0.432). Thus to conclude, the proportion of CD34 + CD38 − CD123 + LSCs at diagnosis is less in the favorable than the intermediate and adverse risk groups and is an emerging novel marker for predicting remission in the prognostically diverse intermediate risk group.
Keywords: Leukemia stem cells, Aml, Prognosis, Induction remission, Relapse
Introduction
Acute Myeloid Leukemia (AML) is a genetically diverse disease with variable prognosis. The ELN risk stratification system 2017 has stratified AML cases in favorable, intermediate and adverse risk groups on the basis of cytogenetics and molecular profile [1]. However, the intermediate group is prognostically a very diverse group. Leukemia Stem Cells (LSCs) first described by Dick et al. are leukemia initiating cells with properties of self-renewal and differentiation [2]. Recent studies elucidate that CD34 + CD38 − CD123 + LSCs are chemoresistant and increased proportion of LSCs in the marrow portends induction failure and relapse, hence, can be useful for prognostication of AML [3–5].
The aim of our study was to study if the proportion of CD34 + CD38 − CD123 + LSCs at diagnosis in AML patients was different in the favorable, intermediate and the adverse risk groups stratified as per ELN 2017.We also intended to study if percent of CD34 + CD38 − CD123 + LSCs at baseline correlates with attainment of induction remission (IR) in all patients especially the prognostically heterogeneous intermediate risk group, time taken to attain remission (TTR) and relapse.
Material and Methods
This was a prospective analytical study was carried out in the hematology department of a tertiary care center from May 2017 to November 2019. A total of 47 consecutive AML patients with intention to treat were included in the study. Patients of Acute Promyelocytic Leukemia were excluded from the study as the treatment and risk stratification for these patients is different from other AMLs.
Immunophenotyping of Acute Leukemia Patients
Flow Cytometry with Acute Leukemia panel was done by multi-parametric 6-colour flow cytometry using the markers-CD45, CD13, CD33, CD10, CD19, CD117, CD34, HLA-DR, CD7, cMPO, cCD79a, cCD3, Tdt, CD64, CD11c/CD5. The technique of ‘stain-lyse-wash’ was done for membranous antigens and ‘lyse-perm-stain-wash’ for cytoplasmic markers. An additional tube comprising CD45, CD34, CD38 and CD123 for leukemia stem cells was put in patients diagnosed with AML.
Preparation of the Stained Tube and Unstained Tube for LSCs
50 µL of bone marrow aspirate was taken in 5 mL polystyrene round bottom tube (12 × 75 mm) and 50 µL normal saline was added to make the volume to 100 µL. 3.5 µL each of CD45-PerCPCy5.5 (clone H-130, Bio Legend), CD34-PECy7 (clone 581, BioLegend), CD38-APC (clone 952) and CD123-FITC (clone 6H6-BioLegend) were added to the tube and incubated in the dark for 30 min. After incubation, 2 mL lyse fluid was added and sample vortexed and kept in the dark for 10 min. After this, the sample was centrifuged for 5 min at 1500 rpm and the supernatant discarded. After this, 2 mL sheath fluid was added to the residue in the tube and the sample centrifuged again at 1500 rpm for 5 min without any incubation. The supernatant was again discarded and the residue resuspended in 0.5 mL sheath fluid and acquired.
The unstained tube was similarly prepared except that only 3.5 µL CD45 was added in the unstained tube instead of other antibodies in the stained tube.
Sample Acquisition and Analysis for LSCs
One lakh events were acquired in the stained tube and 30,000 in the unstained tube. In the unstained tube, the events which were CD45 dim in the blast window on the CD45-SSC plot were gated and the quadrants set according to their auto-fluorescence.
In the stained tube, first the doublets were excluded on the FSC-H vs FSC-A plot and then all nucletaed cells were gated on the CD45 versus SSC plot. The CD34 positive events were then gated on the SSC vs CD34 plot then they were backgated on the CD45 versus SSC plot to ensure that they come from the progenitor/ blast region. The CD34positive and CD38 negative events were then selected on the CD34 versus CD38 plot and then sequential gating was done on the SSC or CD38 versus CD123 plot to assess the percentage of events positive for CD123 in the CD34 positive CD38 negative population which corresponded to our LSCs. The LSCs were expressed as a percentage of blasts.
The quadrants for the LSCs were set according to the unstained tube and bone marrow aspirates of 7 Immune Thrombocytopenia patients to delineate the area in which the normal progenitors lie. The cut-off for CD38 and CD123 expression was fixed at > 0.1% after analyzing them and the same window was applied in the LSC analysis of AML patients.
Correlation of Proportion of LSCs with Clinical and Hematological Profile
Relevant data was collected on the clinical profile, laboratory parameters, cytogenetics and molecular profile of these patients and these patients were followed up for IR, TTR and relapse. All the patients in our study received standard induction (3 + 7) comprising Daunorubicin (60 mg/m2 for 3 days) and Cytarabine (100 mg/m2 for 7 days). The consolidation therapy was high dose cytarabine at dose ranging from 1.5gm/m2 to 3gm/m2 twice a day on days 1,3 and 5. The number of consolidations ranged from 1 to 3 depending on the patient's co-morbidities and risk group.
Induction Remission was defined as attainment of Complete Remission/Complete Remission with incomplete count recovery (CR/CRi). Time to Remission was defined as the time taken to attain CR/CRi. CR was defined as attainment of morphological CR (i.e. transfusion independence, Absolute neutrophil count > 1000/µL, platelets > 100,000/µL, bone marrow blasts < 5% without Auer rods in an aspirate with spicules and absence of extra-medullary disease), cytogenetic and molecular CR when applicable. CRi was defined as < 5% bone marrow blasts with either Absolute neutrophil count < 1000/µL or platelets < 100,000/µL and absence of transfusion requirement. TTR takes into account the responsiveness of the disease to chemotherapy as patients who did not show decrease in blast count on day 14 bone marrow aspirate smears after first induction (3 + 7) were given either re- induction in form of a repeat 3 + 7 or High Dose Ara-Cytarabine (HIDAC). Relapse was defined as recurrence of disease after CR characterized by > 5% blasts on peripheral blood or bone marrow and/or extramedullary disease. Early relapse was defined as relapse occurring within 18 months of attaining remission and relapses occurring thereafter were late relapses. We compared the proportion of LSCs in these 47 patients with the ELN 2017 risk group and clinical outcome in terms of Induction Remission (IR), Time to Response (TTR) and relapse.
Statistical Analysis
Statistical analysis was done using SPSS software version 21. Quantitative variables were compared using Independent t-test/Mann–Whitney Test (when the data sets were not normally distributed) between the 2 groups and ANOVA/Kruskal Wallis test between more than 2 groups. Qualitative variables were correlated using Fisher’s Exact test. Spearman rank correlation coefficient was used to assess the association of various parameters. A p value of < 0.05 was considered statistically significant.
Results
Patient Characteristics
The age of the study population ranged from 4 to 66 years with the mean age being 28 years. The median age of our study population was 27 years. Majority of our patients were males (27/47) with a male to female ratio of 1.4:1. The list of cytogenetic and molecular abnormalities in our patient is summarized as in Table 1.
Table 1.
Cytogenetic abnormalities in the 47 acute myeloid leukemia patients
| Number of patients | Cytogenetics | Molecular abnormalities |
|---|---|---|
| 4 | 8:21 | 2- c-kit positive |
| 1 | Inv 16 | - |
| 3 | Normal | NPM1 mutated FLT3-ITDwt# |
| 2 | Normal | Biallelic CEBPA mut* |
| 16 | Normal | Normal |
| 11 | Normal | NPM1 mutated with FLT3-ITDmut−hi** |
| 2 | -7 | Normal |
| 1 | Inv 7 | Normal |
| 3 | t (5:11) | |
| t(11:19) | - | |
| t (11:17) | ||
| 1 | del 5q | - |
| 2 | Complex cytogenetics | - |
| 1 | t (9:22) | BCR-ABL1 (p210) |
# FLT3-ITD wild type, * Biallelic CEBPA mutated, ** FLT3-ITD mutated allelic ratio ≥ 0.5
Leukemia Stem Cells
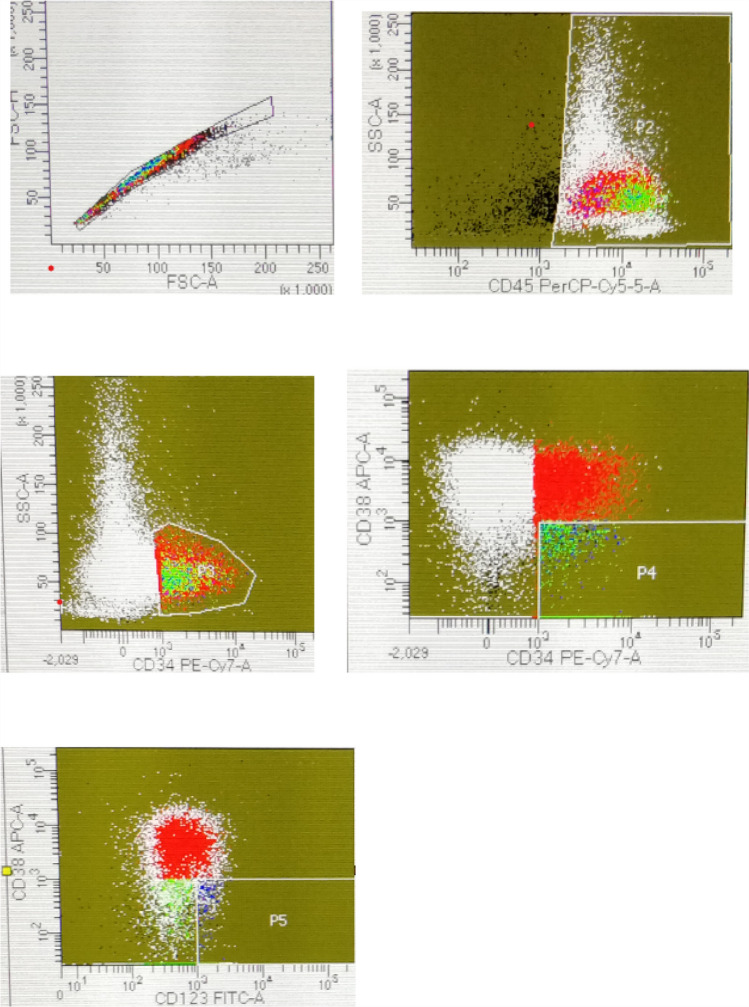
The LSCs were calculated as percentage of blasts at baseline (prior to induction chemotherapy) and ranged from 0.8 to 87.9% of all blasts. In these 47 patients, 28 patients achieved induction remission and 19 patients either did not achieve induction remission or died after receiving induction prior to attaining remission. Figure 1 shows dot-plot in a NPM1 positive patient who attained induction remission.
Fig. 1.
Dot-plots in a patient showing 7.5% blasts which are CD34 + CD38−CD123 cells who achieved induction remission. Patient was NPM1 positive
LSCs in the ELN 2017 Stratified Risk Groups
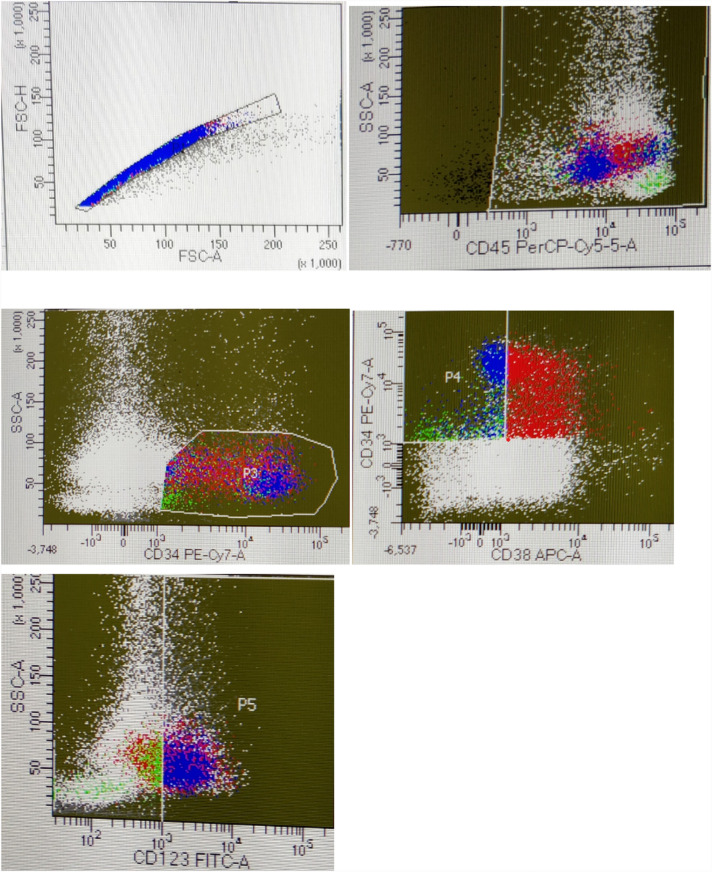
The 47 patients were risk stratified into 3 groups according to ELN 2017 risk stratification system as follows–favorable risk-10, intermediate risk-27 and adverse risk-10. The mean percentage of LSCs as percent of blasts in these 3 groups were–favorable risk = mean +− SD 13.06% + 5.35, intermediate risk group = mean +− SD 53.2% + 35.82 and adverse risk = mean +− SD 34.8% + 28.13. The mean percent of LSCs in the favorable risk group was significantly less than the adverse risk group (p = 0.027) and the intermediate risk (p = 0.001). The mean proportion of LSCs in the intermediate risk group was more than the adverse risk,however, the difference was not statistically significant (p = 0.152). Figure 2 shows dot-plots in an intermediate risk patient with 73.7% CD34 + CD38 − CD123 + LSCs as percent of blasts.
Fig. 2.
Dot-plots in a patient showing 73.7% blasts which are CD34 + CD38−CD123+ cells. patient was intermediate risk
In the intermediate risk group, 12 of 27 patients attained induction remission and 15 did not attain induction remission. The percentage of LSCs in the group which attained remission (mean +– SD 21.18% + 25.06, range 3–85.6%) was significantly less (p = 0.0002) than the intermediate risk group without remission (mean +– SD 73.85% + 29.8, range 12.1–97.9%).
Correlation of LSCs with Induction Remission
Of 47 patients, the proportion of LSCs in the 19 patients who did not attain remission was significantly more (mean +– SD 63.57% + 35.06, range 12.1–95.1%) than the 28 patients who attained induction remission (mean +– SD 19.25% + 18.06, range 12.1–85.6%).The difference in the mean percentage of LSCs as percentage of blasts in the 2 groups was statistically significant. (p < 0.001).
Correlation of LSCs with Time to Remission
The Time to remission in patients who attained induction remission ranged from 22–42 days with a median of 29 days. In the group which attained induction remission, there was statistically significant correlation between the percentage of LSCs and the time to remission (Spearman's correlation coefficient rs = 0.432 and p = 0.024).
Correlation of LSCs with Relapse
Out of the 28 patients who achieved induction remission, 5 patients relapsed and all were early relapses. The percentage of LSCs in the group which relapsed (range 0.8–32.4%; mean17.4%) and group which did not relapse (range 3–58.7%; mean 16.6%) had no significant difference (p value = 0.596). Table 2 summarizes the correlation of LSCs as percent of blasts with ELN 2017 risk group and clinical outcome in our patients.
Table 2.
Summary of the correlation of leukemia stem cells with ELN risk group 2017 and clinical outcome in our study
| S No | Clinical characteristic/outcome | Mean LSC (SD) as percent of blasts | p value |
|---|---|---|---|
| 1 | ELN 2017 risk group | ||
| Favorable risk vs | 13.06% (5.35) | ||
| a. Intermediate risk | 53.2% (35.82) | 0.001 | |
| b. Adverse risk | 34.8% (28.13) | 0.027 | |
| 2 | Induction remission | 27.5% (18.06) | 0.001 |
| No induction remission | 65.7% (35.06) | ||
| 3 | Time to remission | - | 0.024 |
| 4 | Relapse | 17.4% | 0.596 |
| No relapse | 16.6% | ||
| 5 | Intermediate risk group | ||
| a. Induction remission | 21.18% (25.06) | 0.0002 | |
| b. No induction remission | 73.85% (29.8) |
Discussion
The proportion of CD34 + CD38 − CD123 + LSCs at diagnosis has been found to correlate with induction remission, relapse free survival and overall survival in various studies [6–8]. However, not much data is available on the differences in the proportion of CD34 + CD38 − CD123 + LSCs in patients with favorable cytogenetics and molecular profile versus the adverse and intermediate cytogenetics and molecular risk groups.
In the 47 patients of AML, percentageof CD34 + CD38 − CD123 + LSCs rangedbetween 3–97.2% with a mean of 50.6% which is higher than the mean of 22.25% (range 1–46.2%) obtained by Zahran et al. in their study [6]. In the study by Han et al., the frequency of CD123 + cells within CD34 + CD38 − CD99 + LSCs was significantly higher in AML samples at diagnosis (median 78.1%, range 38.4–96.3%) [9] which is higher than the median LSC of our study. This variability could be explained due to genetic/molecular profile of the included patients.
We found significantly lower proportion of LSCs in the favorable risk group than the adverse risk (p = 0.027) and the intermediate risk (p = 0.001) group. However, the mean proportion of LSCs in the intermediate risk group was more than the adverse risk, although, the difference was not statistically significant (p = 0.152). Higher proportion of LSCs in adverse risk group was also seen in the study by Han et al. where they found that a higher proportion of CD123 positive cells on CD34 + stem/progenitor cells correlated with adverse cytogenetics (p = 0.034) [9].The proportion of CD34 + CD38 − CD123 + LSCs at diagnosis was more in the adverse risk group than the favorable risk group which could define its chemoresistance. This could be a plausible explanation for the poor outcomes in the adverse risk group. We found lower proportion of CD34 + CD38 − CD123 + cells in NPM1 mutated patients. This is similar to the study by Han et al. where they observed that higher proportion of CD123 positive cells on CD34 + stem/progenitor cells was associated with NPM wild type (p = 0.003) [9].
In the intermediate risk group, the mean proportion of LSCs at baseline was higher than the adverse risk group. This could be because the intermediate risk group is genetically as well as prognostically very diverse. However, we observed that the proportion of CD34 + CD38 − CD123 + LSCs at baseline in the intermediate risk patients who attained induction remission was significantly lower than the patients who did not attain remission (p = 0.028). This observation is supported by previous studies who found significantly lower LSCs in AML patients in remission than those not in remission [6, 7, 10]. So, LSCs can be a valuable predictor of prognosis in intermediate risk patients who have normal cytogenetics and molecular profile. This is a wastebasket category in the ELN 2017 risk stratification system for AML which requires NGS for better prognostication of these patients [1].However, a simple flow cytometric based quantification of CD34 + CD38 − CD123 + LSCs at baseline in these patients can help in prognosticating these patients and guide their management.
We analyzed the difference in the mean percentage of LSCs in the patients who attained induction remission and those who did not attain remission. We found that patients who achieved induction remission had significantly lower proportion of CD34 + CD38−CD123 + LSCs than the non-IR group (p < 0.001). Similar observations were made by Zahran et al. in their study in which CD34 + CD38low/ − CD123 + LSCs were significantly lower in AML patients with complete remission than those without complete response (p < 0.001) [6]. Hwang et al. observed higher level of CD123 expression on LSCs in the non-complete response group and relapsed group, but the differences between them and patients who attained CR was not statistically significant [7]. Our results were in concordance with Jin et al. who observed that the increased levels of CD123 + LSCs in AML patients were known to be associated with a high proportion of blasts and a low CR rate [10]. We however did not find any statistically significant correlation between percentage of blasts at diagnosis in the bone marrow and proportion of CD34 + CD38 − CD123 + LSCs at baseline.
We did not observe any statistically significant difference in the mean LSC as percentage of blasts in the group who relapsed and who did not (p = 0.596). Though the difference was not statistically significant, the proportion of LSCs at baseline was higher in the patients who relapsed. This is similar to observations of Hwang et al. who found a higher proportion of LSCs in the relapse group than in non-relapsing patients, however, no significant relationship was evident between LSC proportion and relapse rate [7]. Zahran et al. however found a statistically significant negative correlation between CD34 + CD38 − CD123 + LSCs at baseline and RFS (p = 0.006) [6].
Small sample size and limited follow up are shortcomings of our study. Further studies with larger sample size and longer follow up are needed for further analyzing the role of CD34 + CD38 − CD123 + LSCs in better prognosticating the intermediate risk group.
Conclusion
The LSCs have emerged as independent marker of prognosis in the AML patients and patients with higher proportion of CD34 + CD38 − CD123 + LSCs have lower rates of induction remission and longer time to remission in the patients who attain remission. Also, in the intermediate risk group, patients with higher proportion of CD34 + CD38 − CD123 + LSCs have lower rates of induction remission. Hence, in this large group of patients with normal cytogenetics and molecular workup, the proportion of CD34 + CD38 − CD123 + LSCs quantified by simple flow cytometry can serve as a valuable marker for prognosticating the patients and guiding treatment.
Data Availability
The data published in this study is original to the author and the raw data is available with the first author as she is the primary author of this study.
Compliance with Ethical Standards
Conflicts of interest
The authors declare that they have no competing interest.
Ethics
The study was approved by the Institute Ethics Committee (IEC).
Consent for Publication
All the authors consent to publish the article in Indexed journal. The article has not been sent to any other journal for publication at the same time.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Priyanka Mishra, Email: priyanka9581@gmail.com.
Seema Tyagi, Email: drseematyagi@hotmail.com.
Rahul Sharma, Email: rahulsharmabio@gmail.com.
Rohan Halder, Email: rohanhalder86@gmail.com.
Hara P. Pati, Email: harappati@yahoo.co.in
Renu Saxena, Email: renusaxena@outlook.com.
Manoranjan Mahapatra, Email: mrmahapatra@hotmail.com.
References
- 1.Döhner H, Estey E, Grimwade D, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2017;129(4):424–447. doi: 10.1182/blood-2016-08-733196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997;3:730–737. doi: 10.1038/nm0797-730. [DOI] [PubMed] [Google Scholar]
- 3.Vergez F, Green AS, Tamburini J, et al. High levels of CD34+ CD38low/−CD123+blasts are predictive of an adverse outcome in acute myeloid leukemia: a GroupeOuest-Est des LeucemiesAigues et Maladies du Sang (GOELAMS) study. Haematologica. 2011;96:1792–1798. doi: 10.3324/haematol.2011.047894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ishikawa F, Yoshida S, Saito Y, et al. Chemotherapy resistant human AML stem cells home to and engraft within the bone marrow endosteal region. Nat Biotechnol. 2007;25(11):1315–1321. doi: 10.1038/nbt1350. [DOI] [PubMed] [Google Scholar]
- 5.Al-Mawali A. Leukemic stem cells shows the way for novel target of acute myeloid leukemia therapy. J Stem Cell Res Ther. 2013;3:151. doi: 10.4172/2157-7633.1000151. [DOI] [Google Scholar]
- 6.Zahran AM, Aly SS, Rayan A, et al. Survival outcomes of CD34+CD38−LSCs and their expression of CD123 in adult AML patients. Oncotarget. 2018;9(75):34056–34065. doi: 10.18632/oncotarget.26118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hwang K, Park CJ, Jang S, et al. Flow cytometric quantification and immunophenotyping of leukemic stem cells in acute myeloid leukemia. Ann Hematol. 2012;91:1541–1546. doi: 10.1007/s00277-012-1501-7. [DOI] [PubMed] [Google Scholar]
- 8.Plesa A, Elhamri M, Clapisson G, et al. Higher percentage of CD34+CD38-cells detected by multiparameter flow cytometry from leukapheresis products predicts unsustained complete remission in acute myeloid leukemia. Leuk Lymphoma. 2015;56:622–629. doi: 10.3109/10428194.2014.927453. [DOI] [PubMed] [Google Scholar]
- 9.Han L, Jorgensen JL, Wang SA, et al. Leukemia stem cell marker CD123 (IL-3R alpha) predicts minimal residual disease and relapse, providing a valid target For SL-101 in acute myeloid leukemia with FLT3-ITD mutations. Blood. 2013;122:359. doi: 10.1182/blood.V122.21.359.359. [DOI] [Google Scholar]
- 10.Jin L, Lee EM, Ramshaw HS, et al. Monoclonal antibody-mediated targeting of CD123, IL-3 receptor alpha chain, eliminates human acute myeloid leukemia stem cells. Cell Stem Cell. 2009;5:31–42. doi: 10.1016/j.stem.2009.04.018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data published in this study is original to the author and the raw data is available with the first author as she is the primary author of this study.