Abstract
The application of single-cell analytic techniques to the study of stem/progenitor cell niches supports the emerging view that pancreatic cell lineages are in a state of flux between differentiation stages. For all their value, however, such analyses merely offer a snapshot of the cellular palette of the tissue at any given time point. Conclusions about potential developmental/regeneration paths are solely based on bioinformatics inferences. In this context, the advent of new techniques for the long-term culture and lineage tracing of human pancreatic slices (HPSs) offers a virtual window into the native organ and presents the field with a unique opportunity to serially resolve pancreatic regeneration dynamics at the single-cell level.
Keywords: Single-cell RNA sequencing, human pancreatic slices, pancreatic regeneration, beta cell neogenesis, pseudotime
Pancreatic plasticity: a still life drawing
The study of pancreatic regeneration has immediate implications for the development of therapies for diabetes. However, there are conflicting views regarding the ability of the pancreas to regenerate in adulthood. The general consensus is that, aside from a wave of perinatal β-cell mass expansion, the number of islets does not change significantly throughout life. In the absence of injury to the pancreatic tissue, steady-state endocrine cell turnover is greatly reduced. While endocrine plasticity (e.g, a-to-β cell conversion, β-cell de-differentation/transdifferentiation/ redifferentiation) has been documented, the extent to which such processes can contribute to functional regeneration is still the subject of heated debate and conflicting reports. Overall lack of clarity is compounded by known limitations of transgenic mice and the lack of models to study the human pancreas (reviewed in [1]).
Single-cell sequencing (see Glossary) offers a powerful approach to the investigation of tissue plasticity by looking at cellular heterogeneity. Single-cell transcriptomics (scRNAseq) is currently the most commonly used type of analysis, followed by genomics (scDNAseq) and epigenomics (scATAC-Seq, or single-cell assay for transposase-accessible chromatin using sequencing). Proteomics and metabolomics are likely to come to the front line of single-cell analyses in the near future, and the integration of single-cell -omics with other multilayer single-cell analyses performed on the same cell is also expected to become a primary research option [2]. An early example of this has been the linking of functional and transcriptional data by patch-clamp recordings from single endocrine cells, which are later sequenced [3].
The emergence of scRNAseq is mainly due to the lower cost of next-generation sequencing and the development of microfluidics methods, which enable the analysis of large numbers of single cells. Recent scRNAseq studies support the plasticity of the human pancreas, judging from their revelation of fate gradients (rather than set fates) in the adult organ. However, testing the hypothesis that such plasticity reflects regeneration forces at work is challenging because most single-cell analyses represent mere transcriptomic snapshots at the time of tissue collection. One exception is murine developmental studies, where syngeneic embryos can be harvested and single-cell sequenced at different times, thus generating a dynamic picture of organogenesis [4]. Adult mice, however, exhibit a broad range of individual responses, even when syngeneic and controlled for age and sex. If this problem is difficult to overcome in laboratory animals, in humans it is nearly unsurmountable. New tools have been recently applied to infer developmental/regenerative trajectories from scRNAseq datasets by creating a pseudotemporal ordering of cells, but they are not infallible. If the transcriptome of any given cell were a still picture of the front of a car, the algorithm may conclude that it is moving towards the observer based on observations such as the headlights being on, its position in the road, or even its blurriness compared to other vehicles parked nearby. However, if the picture of the cell were a ball in midair, in most cases it would be difficult for these algorithms to determine whether it is going up or down. In another example, if the transcriptome of any three cells were represented by the colors red, purple and blue, pseudotime tools would likely arrange them in this precise order, identifying the purple one as a transitional stage between the other two. Still, in the absence of real time, there is no evidence that such transitions are in fact occurring. At the end of the day, bioinformatics inferences are speculative and difficult to test experimentally. Sometimes, our knowledge of the biology of the studied phenomenon can be used to validate these predictions. For instance, development can occur in only one direction. However, in other instances biology is not so clear-cut. If scRNAseq unveiled a partially undifferentiated β-cell in the adult pancreas, would we conclude that it is differentiating from a progenitor, or de-differentiating from a mature cell? Alternatively, could it not just be a new type of cell that is not “heading” anywhere? Unlike embryonic development, there is no guiding biological knowledge. New approaches are needed to validate such predictions and fully exploit the power of single cell sequencing techniques.
Limitations of scRNAseq studies
For all its usefulness at dissecting tissue heterogeneity, scRNAseq is not devoid of shortcomings. Technical limitations include RNA detection sensitivity, read precision (variation while quantifying sequenced reads), quantification accuracy (a correlation between observed and actual gene abundance), and transcript coverage bias due to the length and type of sequenced transcript [5]. Suboptimal sequencing quality will lead to poor detection sensitivity. While increasing sequencing depth would circumvent this problem, this is not always feasible owing to economic considerations. Recent studies suggest that a sequencing depth of one read per cell per (expressed) gene strikes the best balance between experimental and financial standpoints [6]. After this target has been reached, it is more cost-effective and experimentally sensible to enhance power by increasing cell number rather than sequencing depth.
While having sufficient reads (sensitivity) is necessary, it is equally important to ensure that calculated reads reflect actual transcript abundance within cells. Experimental variation in cell dispersion and lysis, PCR efficiency, stochastic cDNA sampling during sequencing, and analysis methodology significantly contribute towards misrepresentation of transcript abundances [7].
Efficiency in generating cDNA is another technical limitation that may influence data reproducibility across scRNAseq experiments. Even though the addition of Unique Molecular Identifiers (UMIs) has reduced errors due to PCR efficiency [8], caution must be used when assigning gene level counts computationally, as transcripts with partially different sequences but same UMIs do not necessarily arise from different mRNA molecules [9]. New normalization tools, along with batch correction across multiple donors within large datasets, have contributed to the stabilization of transcript count data, correcting for technical noise and reducing variation due to cell handling and sequencing modality [10, 11].
Studying splicing in single cells is particularly challenging owing to the technical limitation of most 3’ and 5’-end scRNAseq technologies [12], where bimodal splicing (some cells have one spliced variant vs. others) is often observed in apparently homogenous cell populations. This is difficult to reconcile with the underlying biology, as any given cell should have all splice variants. A method to tackle this problem is the sequencing of entire transcripts. Significant advances over the past decade have led to increased transcript coverage [14], allowing for information on RNA splicing, isoform coverage and allelic expression. However, even with these strides in scRNAseq transcript coverage, bias in determining alternative splicing can appear due to large numbers of drop-outs (false positive reads) [15].
Biological limitations of scRNAseq are dictated by the condition of the tissue at isolation and its subsequent culture period. Procedure length is particularly critical [16]. In addition to time, cells adapt to culture and alter their transcriptional signature (see next section). Extended cell culture impacts the outcome of single-cell transcriptomics in cultured cells [17], which is particularly true of pancreatic islets [18].
Finally, there are limitations derived from the experimental design. For instance, numerous studies in this field have focused on the sequencing of islet preparations, claimed to be representative of the entire organ [19]. This results in the overrepresentation of small populations of endocrine cells and the sequencing of too few acinar and ductal cells, limiting the investigator’s ability to study exocrine-endocrine interactions and processes such as regeneration. Recent efforts have aimed at correcting this disparity by targeting underrepresented populations for scRNAseq [20, 21] and subsequently integrating these new datasets with previous ones [20].
scRNA analyses of the human pancreas
The pancreas is one of the most analyzed organs at the single cell level. While many studies were performed initially in mice, the anatomical, physiological and functional differences between murine and human pancreata have spurred a wealth of newer analyses focused exclusively on the latter. Their main objective has been the investigation of the complexity and diversity of the endocrine compartment (particularly β-cells) in health and pathological conditions. β-cell heterogeneity had been observed before (reviewed in [22]), but the development of scRNAseq techniques provided the means to easily reveal it within cell populations that were previously hard to stratify. scRNAseq has also helped discover new markers of pancreatic cell populations and optimize islet isolation methods [23]. The first studies used manually processed samples, with a low cell yield and limited detection of low-expression genes [24]. The subsequent development of droplet-based analytical platforms enabled the isolation and scRNAseq of thousands of cells [25]. In general, these studies described the cell composition of the human pancreas and revealed heterogeneity within specific cell populations in health and disease [19–21, 23, 26–34] (Table 1).
TABLE 1.
Representative single-cell transcriptomic analyses conducted on human pancreatic tissue.
| Cell/ Tissue analyzed | Reference | Conclusion | |
|---|---|---|---|
| ENDOCRINE PANCREAS, NO DIABETES | scRNAseq analysis of human islets isolated with different treatments, including fixation and cryopreservation. | Bonnycastle et al. (2020) [23] | Differences in cell proportions and gene expression signatures according to the treatment. |
| scRNAseq of human adult islet preparations. | Muraro et al. (2016) [19] | Gene expression signatures and specific cell-surface markers of α- and β-cells. | |
| scRNAseq of adult islets from 4 human donors and two mouse strains. | Baron et al. (2016) [26] | Heterogeneity of stress in β-cells, activation of pancreatic stellate cells and functional ductal subpopulations. | |
| Single-cell transcriptomics of islets from human adult healthy donors. | Domínguez Gutiérrez et al. (2018) [27] | Molecular features defining the function of pancreatic ε cells, focusing on transcription factors and cell surface markers. | |
| scRNAseq analysis to assess the effects of aging in human pancreas cells from eight donors spanning six decades of life. | Enge et al. (2017) [28] | The analysis detected stochastic age-related errors and that cells from older donors have increased transcriptional noise. Also, endocrine pancreas cells displayed an oxidative stress-related mutational signature. | |
| ENDOCRINE PANCREAS, DIABETES | scRNAseq of islets from children, healthy adults, and T1D and T2D donors. | Wang et al. (2016) [29] | α- and β-cells from children exhibit less defined gene signatures than those in adults, and similar expression profiles of α- and β-cells in children/T2D. |
| Single-cell transcriptomics of pancreatic islet cells of T1D, autoantibody-positive, and healthy donors. | Fasolino et al. (2021) [BioRxiv] | A “hybrid”cluster (~5% of all cells) with an expression mixture of of canonical markers associated with β, α, ductal, and acinar cells. High expression of MHC Class II pathway genes in ductal cells of T1D donors, confirmed by CyTOF, in situ imaging mass cytometry, and immunofluorescence analysis. | |
| scRNAseq from human islets from healthy and T2D donors | Xin et al. (2019) [30] | Cell-type-specific genes and pathways and 245 genes affected by T2D. | |
| Single-cell analysis of transcriptomes of human islets cells from healthy and T2D donors. | Segerstolpe et al. (2016) [31] | Specific genetic programs for endocrine and exocrine cells, including δ, γ, ε, and stellate cells. δ cells expressed receptors indicating a role in integrating paracrine and systemic metabolic signals. | |
| scRNA-seq analysis of human islet cells from healthy and T2D donors. | Lawlor et al. (2017) [32] | Identification of distinct α, β, γ, and PP/γ cell-type signatures. δ cells express receptors that coordinate systemic signals from leptin, ghrelin, and dopamine pathways. Discovery of genes differentially expressed between T2D and healthy controls in α, β and γ, cells not detected in whole islet analyses. | |
| NON-ENDOCRINE PANCREAS | scRNA of ALK3-sorted human pancreatic cells | Qadir et al., 2020 [20] | Identification of potential ducto-acinar and ducto-endocrine differentiation axes and dissection of progenitor cell niche. In vivo pancreatic multilineage differentiation upon transplantation |
| Single-nucleus RNA-sequencing using snap-frozen biopsies of pancreas of human nondiabetic, human neonatal pancreatic (1 dayold) and pancreatitis samples. | Tosti et al. (2020) [21] | Single-nucleus RNAseq generation of a comprehensive atlas of human pancreatic cells, including epithelial and non-epithelial cells, and identification of 3 distinct acinar cell types, likely regulating inflammatory processes of the pancreas. | |
| FETAL PANCREAS | scRNA-seq analysis of human fetal pancreatic epithelial cells isolated by FACS. | Ramond et al. (2018) [33] | Transcriptome analysis of human fetal pancreata at 9 weeks of development, revealing a large degree of conservation among vertebrates in key genes driving changes that occur at different steps of differentiation. |
| scRNAseq of human fetal pancreatic cells from 15.4 weeks of gestational age (WGA). | Villani et al. (2019) [34] | scRNAseq identification of a cluster of SOX9+/PTF1A+ cells and expression of two genes related to multipotent progenitor cells (MPC) in human fetal 15.4 WGA, a stage representative of the tip progenitor cells in the third trimester. | |
| DIFFERENTIATION | scRNAseq of human cells at different stages of in vitro differentiation. | Weng et al. (2020) [36] | Single-cell transcriptomic analysis reconstructs a lineage tree of the entire differentiation process from human embryonic stem cells to β-like cells. Identification of ‘switch genes’ at the branching point between endocrine and non-endocrine cells. |
| scRNA-seq analysis of human cells undergoing in vitro β-cell differentiation. | Veres et al. (2019) [35] | Perspective of human stem cell differentiation into pancreatic endocrine cells. Study of β-cells, α-like polyhormonal cells, and cells that resemble pancreatic exocrine and enterochromaffin cells. |
With a focus on the study of pancreatic regeneration, our team has sequenced the transcriptome of single ALK3bright+-sorted ductal cells, a fraction that harbors Bone Morphogenetic Protein (BMP) signaling-responsive, multipotent progenitor-like cells within major ducts [20]. This analysis unveiled multiple subpopulations along two axes, one encompassing a gradient of ductal differentiation stages (including two progenitor-like populations, centroacinar, and mature ductal cells), and another with seemingly “transitional” phenotypes between ductal and acinar tissue. A third potential ducto-endocrine axis was revealed upon integration of the ALK3bright+ dataset with other single-cell whole-pancreas transcriptomes, which showed transcriptomic continuity between the progenitor-like populations and islet endocrine cells (a prediction subsequently validated in a transplantation model). These results have been partially confirmed by scRNAseq of the adult murine ductal tree (bioRxiv: https://doi.org/10.1101/2020.10.12.336784), where analogous subpopulations were identified. Even more recently, scRNAseq, combined with CyTOF and in situ imaging mass cytometry, revealed the presence of both canonical and “hybrid” cells expressing a mixture of endocrine and exocrine genes. Such cells were found in non-diabetic, autoantibody-positive and type 1 diabetes donors (bioRxiv: https://doi.org/10.1101/2021.01.28.428598).
The closest approximation thus far to longitudinally studying the human pancreas at the single cell level has been the analysis of human islet development [33, 34]. This research leveraged a combination of RNAseq and scRNAseq of pancreatic endocrine cells, sorted at specific time points during embryonic development, to chart differentiation paths. scRNAseq has also proven useful to understand the in vitro differentiation of human embryonic stem cells into islet-like cells [35, 36]. Finally, the development of single-nucleus RNA sequencing (sNuc-RNAseq) has broadened the range of single-cell applications by enabling the analysis of archived human tissues [21]. Although sNuc-RNAseq has generally shown concordance between single-cell and single-nucleus transcriptome profiles in mice [37], difficulties at identifying dynamic cellular substrates have been reported due to the depletion of some genes in nuclei compared to whole cells [38].
Dissociation and culture of pancreatic tissue
If we wanted to dissect human regeneration in vitro, the simplest approach would be to culture pancreatic cells, expose them to regenerative stimuli, and resolve single-cell population changes as a function of time. However, the pancreas has traditionally been a difficult organ from which to culture cells –or, at least, populations that are representative of the native constitution of the organ. While progress has been made in obtaining viable single-cell suspensions from murine pancreata, no equivalent success has been reported using human samples. One problem is the islet-centric nature of most of the research conducted on human donor tissue. Islet isolation is done by injecting a collagenase-based solution through the major duct, followed by mechanical dissociation. The rigors of isolation affect the various cell types of the pancreas in different ways. Our team has reported, for example, that the non-endocrine fraction resulting from collagenase digestion is highly enriched in ductal (~51%) at the expense of acinar (~36%) cell types [39], which is in fact a reversal of the native 10% ductal/85% acinar histological composition [40]. Notwithstanding this, ductal cells located in the wall of the major pancreatic duct bear the brunt of the direct injection of collagenase, and the ensuing cell suspensions may therefore be depleted of them [41]. To all practical effects, even “still life” single-cell analyses of the human pancreas are likely to suffer from representation biases due to the very method used for the procurement of the cells.
Even in the hypothetical case that dissociated populations replicated truthfully the native cellular composition of the pancreas, their culture and serial harvesting for scRNAseq would create additional bias. Culturing pancreatic cells is invariably associated with artifacts that further change the tissue’s cellular makeup. Some are the result of the intrinsic adaptability of different cell types to cultureware. This is known, for example, to disproportionately favor the adhesion of fibroblastic/mesenchymal over epithelial cell types. Other pervasive alterations, such as epithelial-to-mesenchymal transition (EMT), occur inexorably over time and can only be slowed down [39]. The loss of natural cell-to-cell interactions, the destruction of the complex ultrastructure of the extracellular matrix, and the disintegration of native progenitor cell niches, are all inherent to the placement of dissociated pancreatic cells in culture. Simply put, conventional culture is incompatible with single-cell applications designed to study dynamic phenomena with any meaningful degree of biological accuracy.
For the pseudotime being…
Given the difficulty to develop in vitro models that represent biological time, advances in bioinformatics have offered the alternative of pseudotime. Several algorithms allow for the ordering of cells based on the determination of cellular fate, particularly during organogenesis [35, 42]. The same techniques can be applied to cells harvested at different time points, allowing for their temporal organization based on their transcriptional dynamics [43] or the ratio of unspliced vs. spliced RNA species [44, 45]. More recently, these tools have been applied to cells taken at singular time points, which has offered intriguing insights into differentiation. However, this application comes with its own caveats, as discussed before [46]. Directionality (e.g., differentiation vs. de-differentiation) is usually inferred based at best on biological assumptions, and at worst on investigator bias. At the end of the day, the greatest limitation is the lack of multimodal data to correlate genomic-transcriptomic-proteomic data. Upcoming high-throughput studies of the human epigenome and proteome is expected to allow for integration across multimodal datasets, increasing the validity of trajectory analyses [47].
Pancreatic slices bring back the time
The recent refinement of organotypic culturing techniques may break the standstill in our quest to resolve time in single-cell analyses of pancreatic regeneration, which neither pseudotime nor conventional cell culture can do. The first attempt at generating organotypic sections of the pancreas was described in a 1950 report on amylase secretion from pigeon pancreatic slices [48]. Many studies until the mid-nineties further explored this model in the context of basic molecular biology and biochemical assays, using species as diverse as rat, mouse, chick, dog, rabbit, cow, cat or cod. The technical advance of agarose stabilization [49] represented a qualitative leap in our ability to maintain the cytoarchitecture of the organ in vitro, and is the basis of current slicing standards. The recent adaptation of slicing to human pancreatic samples took place as a result of an initiative sponsored by the Network for Pancreatic Donors with Diabetes (nPOD).
Unlike islet isolation, which uses enzymatic dissociation to disintegrate the very fabric of extracellular proteins that keeps the pancreas together, slicing is a purely mechanical method that leads to minimal disruption of the extracellular matrix beyond the immediate cell layers around the sectioning plane. This maintains not only the native proportions of the different cell types that make up the organ, but also the cell-to-cell interactions and the physical microenvironment of islets, acini, and ducts. Slicing preserves the islet vascular networks, and their function can now be studied within the native pancreatic environment [50, 51]. The enhanced anatomical features of slices generated in this manner has enabled research of ever-increasing complexity, including the dynamic study of islet physiology [52]), patch-clamp [53] and calcium imaging [54]; the functional analysis of islet pericytes [55] and macrophages [56]; ductal [57] and acinar [58] cell biology and function; and, in general, the exploration of the nuanced interactions between islets, blood vessels [59], neural networks [60], and the immune system [61] (figure 1). Research on these areas has been recently expanded to encompass samples from type 1 and 2 diabetes patients [62–64]), as well as pancreatic ductal adenocarcinoma [61, 65], which has opened the door to the study of pancreatic disease in an in vitro setting closely resembling the real organ. Importantly, viable/functional human slices can be shipped from centralized procurement centers, which has made this valuable model available to laboratories around the world.
Figure 1. Described applications of pancreatic slices.
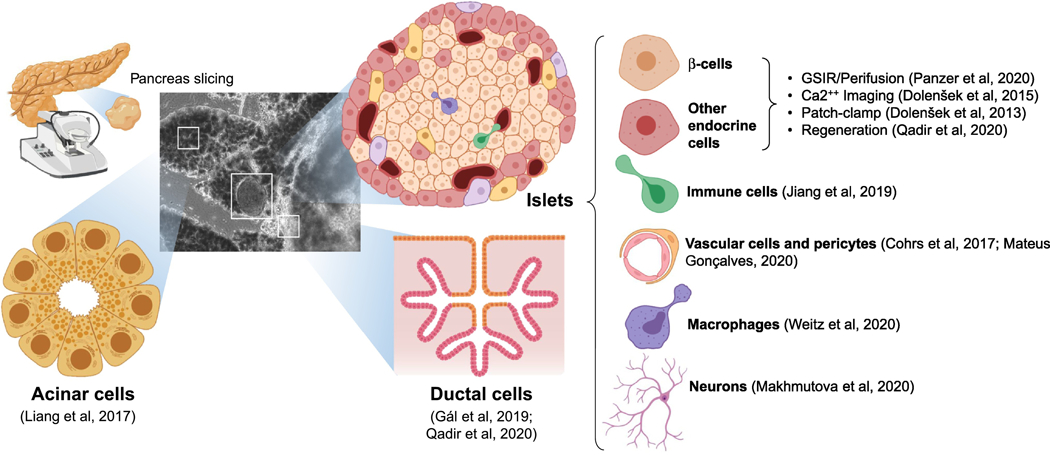
Recent refinements on pancreatic slicing techniques have enabled the execution of live studies on each of the main cell types found in the native pancreas (acinar, ductal, endocrine, endothelial, immune, neuronal) in their proper anatomical context. Representative references are shown next to each approach.
For all the benefits of slices [60, 66] vs. cell lines [67, 68], conventional cell cultures of the pancreas [39], isolated/stem cell-derived islets [69–75] and organoids [76] (see Table 2), this model is not without limitations. Unlike the murine pancreas, human pancreatic tissue blocks can only be embedded in, but not perfused with low melting point agarose. This makes them less stable and difficult to cut. Fibrotic or fatty tissue make sectioning even more challenging, so not every sample is amenable to slicing. The possibility of self-digestion is also an important consideration, albeit one that can be at least partially abated by the addition of protease inhibitors [64, 77]. Finally, due to the lack of blood circulation, oxygen diffusion becomes limiting within hours of their placement in culture. Core anoxia is conducive to their rapid degradation, a switch from oxidative phosphorylation to glycolysis, progressive loss of endocrine phenotypes, and an overall decline in viability and function [64]. This is why most of the studies described above have been “acute”, i.e., short-term and concluded within hours of generating/receiving the slices. However, research on regeneration would require slices to be alive and functional for extended periods. Thus, we reasoned that the provision of adequate oxygenation would be critical to overcome these problems. Culturing human pancreatic slices (HPSs) atop air-permeable membranes [73] proved this hypothesis correct, as evidenced by their near-intact functionality, high viability and complete histological preservation of exocrine and endocrine markers for at least 10 days. One of the most important benefits of the enhanced oxygenation is that slices cultured in this manner metabolize glucose in a manner that resembles that of the organ, i.e., chiefly through oxidative phosphorylation. Slices kept in control conditions, in contrast, rapidly switch to ATP-inefficient glycolysis and are forced to increase many-fold the rate of glucose uptake to meet energy demands [64]. The importance of having an in vitro model that faithfully replicates the glucose utilization profile of the pancreas cannot be understated, as data obtained from metabolically aberrant models may not reflect the biology of the native organ.
TABLE 2. Comparison of features/limitations between different in vitro models of pancreatic tissue.
From left to right, cell lines (e.g., EndoC-βH1), dissociated pancreatic tissue (e.g., human non-endocrine pancreatic tissue, hNEPT), isolated islets/stem cell (SC)-derived β-cells, adult pancreatic organoids, human pancreatic slices (cultured in enhanced oxygenation conditions) and the native pancreas are compared side by side. Select representative references are provided in parentheses.
| Cell lines | Dissociated pancreas | Islets and SC-β cells | Organoids | HPSs | Pancreas | ||
|---|---|---|---|---|---|---|---|
| 3D Tissue Organization | NO | NO | YES | YES | YES | YES | |
| Niche preservation | ECM | NO | NO | NO (disrupted) [70] | YES (artificial) [76] | YES | YES |
| Endocrine/exocrine | NO | YES [39] | NO | YES | YES | YES | |
| Blood vessels/pericytes | NO | NO | NO (or rapidly lost) [69] | NO | YES [50] | YES | |
| Immune cells | NO | NO (or rapidly lost) | NO (or rapidly lost) [71] | NO | YES [66] | YES | |
| Neurons | NO | NO (or rapidly lost) | NO (or rapidly lost) [72] | NO | YES [60] | YES | |
| Native cell-to-cell interaction | NO | NO | NO | NO | YES | YES | |
| Blood circulation | NO | NO | NO | NO | NO | YES | |
| Functional/metabolic changes and culture-induced artifacts | Significant biological changes vs. native tissue [67,68] |
|
|
Potential partial self-digestion [64] | NONE | ||
This method, coupled with adenoviral transduction, enabled lineage-tracing studies in a native-like organotypic setting for the real-time monitoring of β-cell neogenesis in response to a known regeneration stimulus [64]. Unlike previous studies where conclusions from lineage-tracing in vitro may have been confounded by all the limitations inherent to conventional culture, here the appearance of new insulin-producing cells could be tracked to the very anatomical region of the pancreas where progenitors had been previously identified, namely, the ductal tree [41].
Concluding remarks and future perspectives
Arguably, the long-term culture of HPSs is the final piece needed to realize the full potential of single-cell transcriptomics for the study of human pancreatic regeneration. The combination of long-term organotypic culture with lineage-tracing in vitro and sequential scRNAseq offers a wealth of new research possibilities (see Outstanding Questions). For instance, now we can conduct longitudinal single-cell sequencing of slices from the same donor at multiple time points, thus mapping cell fate changes in response to regeneration triggers in a dynamic fashion (key figure, A). This would allow us to chart –not just infer– true cellular trajectories in a manner that was simply not possible prior to the development of these tools. We could also lineage-trace the appearance of any cell of interest (e.g., β- or α- cells), sort transitional events by fluorescence patterns [e.g., ductal cells becoming β-cells (key figure, B), or α-cells transdifferentiating into β-cells] and conduct scRNAseq of such populations to reveal the precise transcriptomic signatures of single cells caught in the act of changing fates. No “single frame” single-cell analysis would allow for the unequivocal identification of transitional cells as such. Similar lineage-tracing approaches could also be used to visualize de-differentiation events in real-time, should they happen in response to specific stressors in HPSs. Conducting all these studies on slices from diabetic patients would add another layer of significance to this research. Of note, the pancreas is not the only organ that can be sectioned into live organotypic slices. Other tissues such as brain [78], liver [79], thyroid [80] and even tongue [81] have been subjected to slicing for physiological and pathological studies. The multi-organ pursuit of all these possibilities to the fullest extent afforded by these novel methods will likely revolutionize the design of human regeneration research.
Key figure. Prospective applications of long-term cultured slices for the temporal study of pancreatic regeneration.
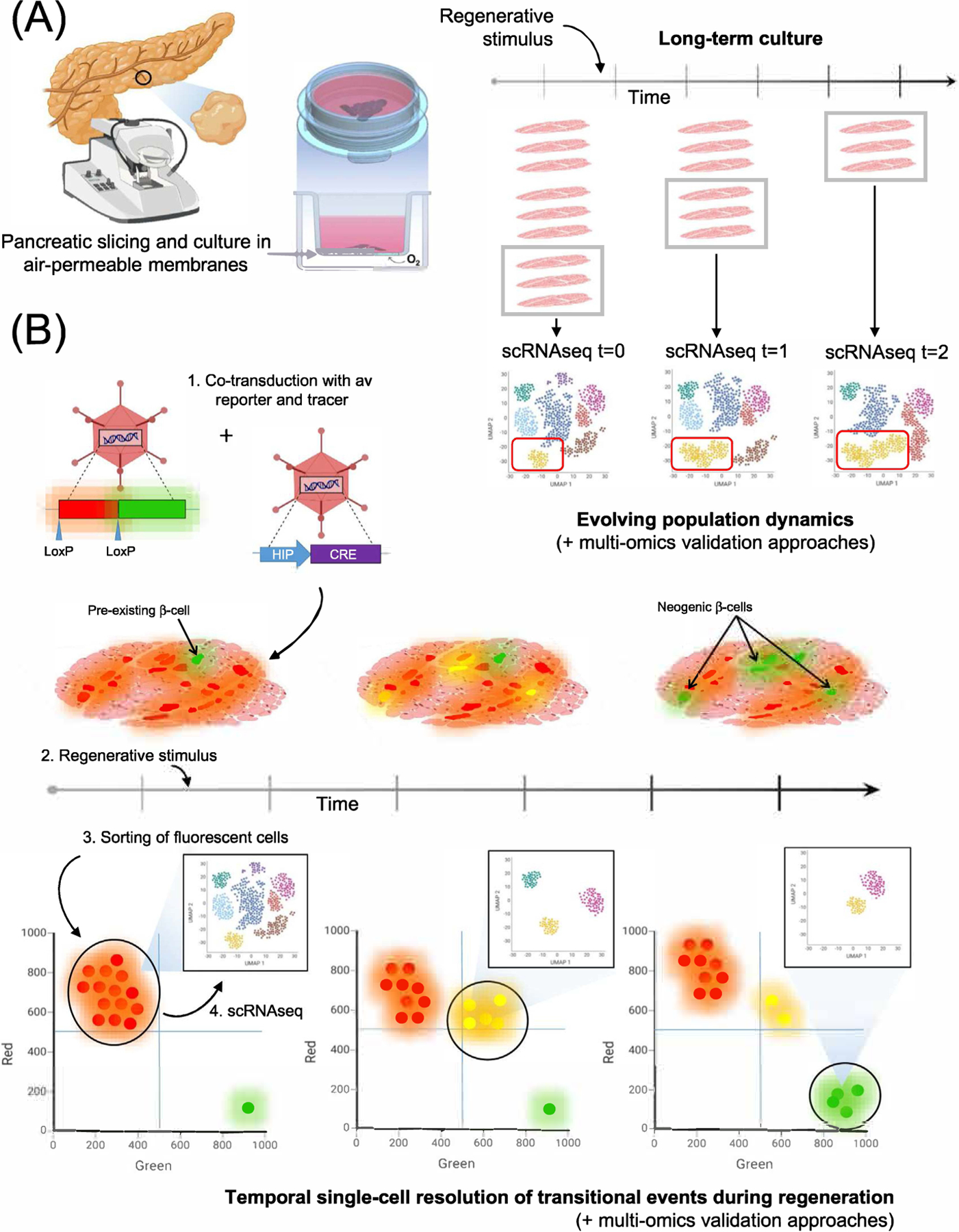
(A) Same-donor serial HPSs can be exposed to a regeneration stimulus (e.g., BMP-7). Conducting scRNAseq of dissociated slices at different time points is expected to yield true temporal information about population dynamics, including, potentially, fate trajectories leading to β-cell neogenesis. (B) Lineage-tracing approach to dissect regeneration at the single-cell level. Co-transduction of HPSs with adenoviruses carrying a loxP-Red-loxP-Green reporter and a Human Insulin Promoter (HIP)-driven Cre results in green labeling of pre-existing β-cells and red tagging of all other transduced cells. Dissociation, fluorescent sorting and scRNAseq of each fraction would resolve the various sub-populations of transduced non-endocrine cells (red) and insulin-producing cells (green) (shown only for red cells in the panel). Upon addition of a regenerative stimulus, some non-endocrine cells (red) will progressively become green through an intermediate red + green (yellow) stage, as described in [64]. A second sampling, dissociation, fluorescence sorting and scRNAseq of slices treated in this manner would allow for the single-cell transcriptomic profiling of the red + green fraction, consisting of cells at different stages of fate-switching. Neogenic β-cells (green) would appear after the resolution of the above transitional events, days after the original labeling of pre-existing β-cells. Dissociation/sorting/scRNAseq of green cells at this stage would identify the transcriptomic signature of neogenic β-cells, and reveal potential differences with pre-existing ones. Charting regeneration pathways at the single-cell level is an expected outcome of the temporal integration of datasets obtained at each time point. Additional multi-omics approaches and other confirmatory assays should be used for confirmation of these findings.
Acknowledgments
We thank Dr. Dagmar Klein and Ms. Silvia Alvarez for their critical reading of our manuscript. The corresponding authors were funded by the Diabetes Research Institute Foundation (DRIF), the Inserra family, the Fred and Mabel R. Parks Foundation, the Tonkinson Foundation, ADA grant #1-19-ICTS-078 and NIH grant U01DK120393.
GLOSSARY BOX
- Single-cell sequencing
Set of experimental approaches and analytical techniques based on the use of next-generation sequencing (NGS), intended to generate sequence information (DNA, RNA, etc.) from individual cells in a sample. Taken together, these approaches are designed to provide a better understanding of the heterogeneity, functions and interactions of single cells in their tissue context. Single-cell RNA sequencing (scRNAseq) provides the transcriptional profiling of thousands of cells. This is arguably the most widely used single-cell sequencing analysis
- RNA detection sensitivity
The probability that one molecule of RNA within a cell will be converted into cDNA and subsequently sequenced. As capture sensitivity is almost impossible to calculate and remains a theoretical estimate, it is indirectly inferred by “depth of sequencing coverage”. However, coverage is usually calculated as sequencing depth (number of times a transcript read is to be sampled) per cell
- Pseudotime
This concept was originally introduced by the creators of the Monocle algorithm suite as a measure of how cells transition between states or through time. In single-cell studies, cells from any given population may be distributed along differentiation gradients. Pseudotime is an abstract unit of progress through those gradients, so that the outcome is a relative ordering of individual cells along an inferred trajectory. When applied to scRNAseq datasets captured at a single time point, this approach makes a series of assumptions about the directionality of cellular trajectories –which may or may not even exist if the studied tissue is of adult origin. However, despite its limitations, pseudotemporal analyses offer an alternative to the collection of time series data, which is challenging in the context of the study of pancreatic regeneration
- Organotypic
Refers to the culture of complex tissues or organs without cell/extracellular matrix dissociation. Organotypic cultures preserve the cytoarchitecture of the tissue and most of its cellular interactions. Methods to minimize the effect of oxygen transfer limitations include the use of air-liquid interfaces and, more recently, air-permeable membranes
- Pancreatic slices
Sections (typically 120 μm-thick) generated with a vibratome from low-melting point agarose (LMP)-injected (murine) or embedded (human) blocks of pancreatic tissue. LMP agarose hardens and stabilizes the tissue, so that the resulting sections exhibit a native-like cytoarchitecture and conservation of cell-to-cell interactions. This technical advance has recently popularized pancreatic slices as a first-rate organotypic tool to study the physiology, immunology and regenerative abilities of the pancreas
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Dominguez-Bendala J et al. (2019) Pancreatic Progenitors: There and Back Again. Trends Endocrinol Metab 30 (1), 4–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kashima Y et al. (2020) Single-cell sequencing techniques from individual to multiomics analyses. Exp Mol Med 52 (9), 1419–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Camunas-Soler J et al. (2020) Patch-Seq Links Single-Cell Transcriptomes to Human Islet Dysfunction in Diabetes. Cell Metabolism 31 (5), 1017–1031.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bastidas-Ponce A et al. (2019) Comprehensive single cell mRNA profiling reveals a detailed roadmap for pancreatic endocrinogenesis. Development 146 (12). [DOI] [PubMed] [Google Scholar]
- 5.Ziegenhain C et al. (2017) Comparative analysis of single-cell RNA sequencing methods. Molecular cell 65 (4), 631–643.e4. [DOI] [PubMed] [Google Scholar]
- 6.Zhang MJ et al. (2020) Determining sequencing depth in a single-cell RNA-seq experiment. Nature communications 11 (1), 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Denisenko E et al. (2020) Systematic assessment of tissue dissociation and storage biases in single-cell and single-nucleus RNA-seq workflows. Genome biology 21, 1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Islam S et al. (2014) Quantitative single-cell RNA-seq with unique molecular identifiers. Nature methods 11 (2), 163. [DOI] [PubMed] [Google Scholar]
- 9.Sena JA et al. (2018) Unique Molecular Identifiers reveal a novel sequencing artefact with implications for RNA-Seq based gene expression analysis. Scientific reports 8 (1), 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hafemeister C and Satija R (2019) Normalization and variance stabilization of single-cell RNA-seq data using regularized negative binomial regression. Genome biology 20 (1), 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haghverdi L et al. (2018) Batch effects in single-cell RNA-sequencing data are corrected by matching mutual nearest neighbors. Nature biotechnology 36 (5), 421–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Najar CFBA et al. (2020) Coverage-dependent bias creates the appearance of binary splicing in single cells. Elife 9, e54603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Westoby J et al. (2018) Simulation-based benchmarking of isoform quantification in single-cell RNA-seq. Genome biology 19 (1), 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hagemann-Jensen M et al. (2020) Single-cell RNA counting at allele and isoform resolution using Smart-seq3. Nature Biotechnology 38 (6), 708–714. [DOI] [PubMed] [Google Scholar]
- 15.Qiu P (2020) Embracing the dropouts in single-cell RNA-seq analysis. Nature communications 11 (1), 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Massoni-Badosa R et al. (2020) Sampling time-dependent artifacts in single-cell genomics studies. Genome Biology 21 (1), 112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Januszyk M et al. (2015) Evaluating the Effect of Cell Culture on Gene Expression in Primary Tissue Samples Using Microfluidic-Based Single Cell Transcriptional Analysis. Microarrays (Basel, Switzerland) 4 (4), 540–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mawla AM and Huising MO (2019) Navigating the Depths and Avoiding the Shallows of Pancreatic Islet Cell Transcriptomes. Diabetes 68 (7), 1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Muraro MJ et al. (2016) A Single-Cell Transcriptome Atlas of the Human Pancreas. Cell Syst 3 (4), 385–394 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qadir MMF et al. (2020) Single-cell resolution analysis of the human pancreatic ductal progenitor cell niche. Proceedings of the National Academy of Sciences 117 (20), 10876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tosti L et al. (2020) Single-Nucleus and In Situ RNA-Sequencing Reveal Cell Topographies in the Human Pancreas. Gastroenterology. [DOI] [PubMed]
- 22.Avrahami D et al. (2017) Beta cell heterogeneity: an evolving concept. Diabetologia 60 (8), 1363–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bonnycastle LL et al. (2020) Single-cell transcriptomics from human pancreatic islets: sample preparation matters. Biol Methods Protoc 5 (1), bpz019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li J et al. (2016) Single-cell transcriptomes reveal characteristic features of human pancreatic islet cell types. EMBO Rep 17 (2), 178–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Salomon R et al. (2019) Droplet-based single cell RNAseq tools: a practical guide. Lab Chip 19 (10), 1706–1727. [DOI] [PubMed] [Google Scholar]
- 26.Baron M et al. (2016) A Single-Cell Transcriptomic Map of the Human and Mouse Pancreas Reveals Inter- and Intra-cell Population Structure. Cell Syst 3 (4), 346–360 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dominguez Gutierrez G et al. (2018) Gene Signature of the Human Pancreatic epsilon Cell. Endocrinology 159 (12), 4023–4032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Enge M et al. (2017) Single-Cell Analysis of Human Pancreas Reveals Transcriptional Signatures of Aging and Somatic Mutation Patterns. Cell 171 (2), 321–330 e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang YJ et al. (2016) Single-Cell Transcriptomics of the Human Endocrine Pancreas. Diabetes 65 (10), 3028–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xin Y et al. (2019) Single-cell RNA Sequencing and Analysis of Human Pancreatic Islets. J Vis Exp (149). [DOI] [PubMed]
- 31.Segerstolpe A et al. (2016) Single-Cell Transcriptome Profiling of Human Pancreatic Isletsin Health and Type 2 Diabetes. Cell Metab. [DOI] [PMC free article] [PubMed]
- 32.Lawlor N et al. (2017) Single-cell transcriptomes identify human islet cell signatures and reveal cell-type-specific expression changes in type 2 diabetes. Genome Res 27 (2), 208–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ramond C et al. (2018) Understanding human fetal pancreas development using subpopulation sorting, RNA sequencing and single-cell profiling. Development 145 (16). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Villani V et al. (2019) SOX9+/PTF1A+ Cells Define the Tip Progenitor Cells of the Human Fetal Pancreas of the Second Trimester. Stem Cells Transl Med 8 (12), 1249–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Veres A et al. (2019) Charting cellular identity during human in vitro beta-cell differentiation. Nature 569 (7756), 368–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weng C et al. (2020) Single-cell lineage analysis reveals extensive multimodal transcriptional control during directed beta-cell differentiation. Nat Metab 2 (12), 1443–1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Habib N et al. (2017) Massively parallel single-nucleus RNA-seq with DroNc-seq. Nat Methods 14 (10), 955–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thrupp N et al. (2020) Single-Nucleus RNA-Seq Is Not Suitable for Detection of Microglial Activation Genes in Humans. Cell Rep 32 (13), 108189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Klein D et al. (2015) BMP-7 induces adult human pancreatic exocrine-to-endocrine conversion. Diabetes 64, 4123–4134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brockman D (1998) Histology and fine structure, Malden MA: Blackwell Science. [Google Scholar]
- 41.Qadir MMF et al. (2018) P2RY1/ALK3-Expressing Cells within the Adult Human Exocrine Pancreas Are BMP-7 Expandable and Exhibit Progenitor-like Characteristics. Cell Rep 22 (9), 2408–2420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cao J et al. (2019) The single-cell transcriptional landscape of mammalian organogenesis. Nature 566 (7745), 496–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Trapnell C et al. (2014) The dynamics and regulators of cell fate decisions are revealed by pseudotemporal ordering of single cells. Nature Biotechnology 32 (4), 381–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.La Manno G et al. (2018) RNA velocity of single cells. Nature 560 (7719), 494–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bergen V et al. (2020) Generalizing RNA velocity to transient cell states through dynamical modeling. Nat Biotechnol 38 (12), 1408–1414. [DOI] [PubMed] [Google Scholar]
- 46.Weinreb C et al. (2018) Fundamental limits on dynamic inference from single-cell snapshots. Proceedings of the National Academy of Sciences 115 (10), E2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stuart T and Satija R (2019) Integrative single-cell analysis. Nature Reviews Genetics 20 (5), 257–272. [DOI] [PubMed] [Google Scholar]
- 48.Hokin LE (1950) The synthesis and secretion of amylase by pigeon pancreas slices in vitro. Biochem J 47 (4), xlvi–ii. [PubMed] [Google Scholar]
- 49.Speier S and Rupnik M (2003) A novel approach to in situ characterization of pancreatic beta-cells. Pflugers Arch 446 (5), 553–8. [DOI] [PubMed] [Google Scholar]
- 50.Mateus Goncalves L and Almaca J (2020) Functional Characterization of the Human Islet Microvasculature Using Living Pancreas Slices. Front Endocrinol (Lausanne) 11, 602519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Almaca J et al. (2018) The Pericyte of the Pancreatic Islet Regulates Capillary Diameter and Local Blood Flow. Cell Metab 27 (3), 630–644 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Huang YC et al. (2011) Unperturbed islet alpha-cell function examined in mouse pancreas tissue slices. J Physiol 589 (Pt 2), 395–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dolensek J et al. (2013) The relationship between membrane potential and calcium dynamics in glucose-stimulated beta cell syncytium in acute mouse pancreas tissue slices. PLoS One 8 (12), e82374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dolensek J et al. (2015) Membrane Potential and Calcium Dynamics in Beta Cells from Mouse Pancreas Tissue Slices: Theory, Experimentation, and Analysis. Sensors (Basel) 15 (11), 27393–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mateus Goncalves L et al. (2020) Islet pericytes convert into profibrotic myofibroblasts in a mouse model of islet vascular fibrosis. Diabetologia 63 (8), 1564–1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Weitz JR et al. (2020) Secretory Functions of Macrophages in the Human Pancreatic Islet Are Regulated by Endogenous Purinergic Signaling. Diabetes 69 (6), 1206–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gal E et al. (2019) A Novel in situ Approach to Studying Pancreatic Ducts in Mice. Front Physiol 10, 938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liang T et al. (2017) Ex vivo human pancreatic slice preparations offer a valuable model for studying pancreatic exocrine biology. J Biol Chem 292 (14), 5957–5969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cohrs CM et al. (2017) Vessel Network Architecture of Adult Human Islets Promotes Distinct Cell-Cell Interactions In Situ and Is Altered After Transplantation. Endocrinology 158 (5), 1373–1385. [DOI] [PubMed] [Google Scholar]
- 60.Makhmutova M et al. (2021) Pancreatic beta-Cells Communicate With Vagal Sensory Neurons. Gastroenterology 160 (3), 875–888 e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jiang X et al. (2017) Long-lived pancreatic ductal adenocarcinoma slice cultures enable precise study of the immune microenvironment. Oncoimmunology 6 (7), e1333210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cohrs CM et al. (2020) Dysfunction of Persisting beta Cells Is a Key Feature of Early Type 2 Diabetes Pathogenesis. Cell Rep 31 (1), 107469. [DOI] [PubMed] [Google Scholar]
- 63.Panzer JK et al. (2020) Pancreas tissue slices from organ donors enable in situ analysis of type 1 diabetes pathogenesis. JCI Insight 5 (8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Qadir MMF et al. (2020) Long-term culture of human pancreatic slices as a model to study real-time islet regeneration. Nat Commun 11 (1), 3265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lim CY et al. (2018) Organotypic slice cultures of pancreatic ductal adenocarcinoma preserve the tumor microenvironment and provide a platform for drug response. Pancreatology 18 (8), 913–927. [DOI] [PubMed] [Google Scholar]
- 66.Jiang X et al. (2019) Establishment of Slice Cultures as a Tool to Study the Cancer Immune Microenvironment. Methods Mol Biol 1884, 283–295. [DOI] [PubMed] [Google Scholar]
- 67.Gurgul-Convey E et al. (2016) Sensitivity profile of the human EndoC-betaH1 beta cell line to proinflammatory cytokines. Diabetologia 59 (10), 2125–33. [DOI] [PubMed] [Google Scholar]
- 68.Oleson BJ et al. (2015) Distinct differences in the responses of the human pancreatic beta-cell line EndoC-betaH1 and human islets to proinflammatory cytokines. Am J Physiol Regul Integr Comp Physiol 309 (5), R525–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Brissova M and Powers AC (2008) Revascularization of transplanted islets: can it be improved? Diabetes 57 (9), 2269–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Llacua LA et al. (2018) Extracellular matrix molecules and their potential contribution to the function of transplanted pancreatic islets. Diabetologia 61 (6), 1261–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lacy PE and Finke EH (1991) Activation of intraislet lymphoid cells causes destruction of islet cells. Am J Pathol 138 (5), 1183–90. [PMC free article] [PubMed] [Google Scholar]
- 72.Pour PM and Saruc M (2011) The pattern of neural elements in the islets of normal and diseased pancreas and in isolated islets. JOP 12 (4), 395–403. [PubMed] [Google Scholar]
- 73.Fraker CA et al. (2013) A physiological pattern of oxygenation using perfluorocarbon-based culture devices maximizes pancreatic islet viability and enhances beta-cell function. Cell Transplant 22 (9), 1723–33. [DOI] [PubMed] [Google Scholar]
- 74.Davis JC et al. (2020) Glucose Response by Stem Cell-Derived beta Cells In Vitro Is Inhibited by a Bottleneck in Glycolysis. Cell Rep 31 (6), 107623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dominguez-Bendala J et al. (2016) The Human Endocrine Pancreas: New Insights on Replacement and Regeneration. Trends Endocrinol Metab 27 (3), 153–162. [DOI] [PubMed] [Google Scholar]
- 76.Balak JRA et al. (2019) Organoids from the Human Fetal and Adult Pancreas. Curr Diab Rep 19 (12), 160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Marciniak A et al. (2014) Using pancreas tissue slices for in situ studies of islet of Langerhans and acinar cell biology. Nat Protoc 9 (12), 2809–22. [DOI] [PubMed] [Google Scholar]
- 78.Humpel C (2015) Organotypic brain slice cultures: A review. Neuroscience 305, 86–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Palma E et al. (2019) Precision-cut liver slices: a versatile tool to advance liver research. Hepatol Int 13 (1), 51–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shuman SJ et al. (1976) Exposure of thyroid slices to thyroid-stimulating hormone induces refractoriness of the cyclic AMP system to subsequent hormone stimulation. J Clin Invest 57 (5), 1132–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Caicedo A et al. (2002) Individual mouse taste cells respond to multiple chemical stimuli. J Physiol 544 (2), 501–9. [DOI] [PMC free article] [PubMed] [Google Scholar]


