We attentively read the communication by Pozzi et al. about the first case reported of Good's syndrome diagnosed patient, affected by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, which had a fatal outcome [1]. Considering the few Good syndrome's cases reported in the literature and the unpredictable evolution of patients with antibody deficiency and coronavirus disease-2019 (COVID-19), we believe it could be of interest reporting our experience with a patient with a Good's syndrome and COVID-19.
Good's syndrome is a rare disease, described in 1954 by Robert Good et al. as a thymoma associated with hypogammaglobulinemia [2]. The pathophysiology is unknown, but there is probably a bone marrow defect, with the release of T and B cell maturation inhibitors. The thymus plays a major role in the regulation of the immune system. Immunological manifestations may be absent or produce severe immunodeficiency with or without associated autoimmune phenomena [3].
A positive diagnosis requires the existence of thymoma, with hypogammaglobulinemia, and absence or decrease of B cells in most cases [4]. To avoid further complications, replacement treatment with immunoglobulins is recommended since its prognosis is poor compared to common variable immunodeficiency.
The purpose of this note is to report a case of Good's syndrome who despite having a severe COVID-19 pneumonia had a favorable outcome in overt contrast to the aforementioned case [1] .
1. Case report
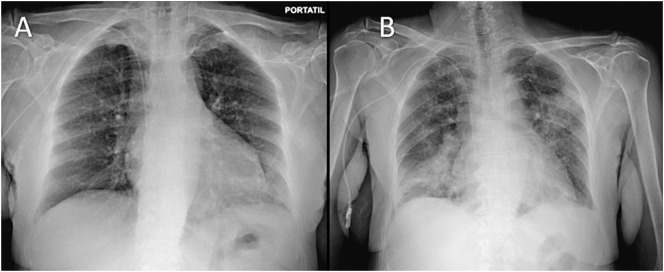
We present a 79-year-old woman with Good's syndrome diagnosed in the prior 4 months. She was under treatment with monthly subcutaneous immunoglobulin (Ig). The patient was admitted to our institution to perform a lymph node biopsy. During the COVID-19 pandemic, all hospitalized patients were routinely screened for SARS-CoV-2 by polymerase chain reaction (PCR) as protocol. Despite being asymptomatic, the PCR test was positive. Five days later, she started symptomatology consistent with SARS-CoV-2 infection (fever, cough, and headache). Initially, the chest X-ray did not show pneumonia (Fig. 1A), but hypoxemia was observed. Oxygen support with nasal cannula and dexamethasone 6 mg every 24 h were initiated. However, she progressively evolved to acute respiratory distress syndrome and bilateral pneumonia was observed in a chest X-ray (Fig. 1B) performed at day 12 since symptoms onset. Tocilizumab was administered as rescue treatment and she was admitted to the intensive care unit with high-flow oxygen therapy needs.
Fig. 1.
A) Normal chest X-ray at day 1. B) Chest X-ray at day 12 showing bilateral lung opacities.
Analytical tests during admission showed anemia (haemoglobin 7.8 g/dl, reticulocytes 0.2%), leukocyte, lymphocyte, and neutrophil count, 6310, 1260, and 4180 × 109/L, respectively, and bone marrow consistent with pure red cells aplasia. The inflammatory parameters were increased, and the highest levels observed were the following: C-reactive protein 12.5 (ref. range 0–0.5 mg/dl), interleukin-6503 pg/ml (ref. range 0–7) and ferritin 11.414 ng/ml (ref. range 20–150). Further, anti-acetylcholine receptor antibodies were positive but no clinical symptoms consistent with myasthenia gravis were observed.
Treatment with subcutaneous Ig was continued during the hospitalization for Good's syndrome. Ig at the diagnosis of Good's syndrome were IgG 190 mg/dl (ref. range: 700–1600), IgM <5 mg/dl (ref. range 40–230), IgA 26 mg/dl (ref. range 70–400), IgG1 196 mg/dl (ref. range 382–928), IgG2 88 mg/dl (ref. range 241–700), IgG3 41 mg/dl (ref. range 22–176), and IgG4 16 mg/dl (ref. range 3.9–86).
Flow cytometry analysis showed that, in clear contrast with the previously reported case [1], our patient maintained B lymphocytes count within normal range. However, the differentiation program was largely interrupted, with presence of residual memory populations. Other immunophenotypic features included a marked increase of gamma delta T cells percentage, a slight inversion of the CD4/CD8 ratio, and a marked predominance of memory cells (see Table 1 ).
Table 1.
Immunological evaluation at diagnosis of Good's syndrome of the previous reported case by Pozzi et al. [1] and the current case.
| Case reported by Pozzi et al. [1] | Current case | Normal range for age (%) | |
|---|---|---|---|
| T cells (CD3+) | 90.8 | 76.8 | 58,1-80,1 |
| CD3+ CD4+ | 30.9 | 36.5 | 27,9-53,4 |
| CD4 Naive (CD45RA+ CCR7+) | 47.9 | 11.5 | 35,1-82,2 |
| CD4 Central Memory (CD45RA-CCR7+) | 35.3 | 14.2 | 26,2-67,1 |
| CD4 Efector Memory (CD45RA-CCR7-) | 30.7 | 69.0 | 10,7-44,3 |
| CD4 EMRA (CD45RA + CCR7-) | 5.9 | 6.8 | 0,6-6,5 |
| CD3+ CD8+ | 56 | 51.7 | 12,3-31,9 |
| CD8 Naive (CD45RA+ CCR7+) | 54.5 | 11.70 | 15,1-76,7 |
| CD8 Central Memory (CD45RA-CCR7+) | 5 | 2.2 | 1,3-15,4 |
| CD8 Efector Memory (CD45RA-CCR7-) | 17.2 | 64.7 | 6,1-40,1 |
| CD8 EMRA (CD45RA + CCR7-) | 23.4 | 23.80 | 6.8–46,7 |
| TCR gamma delta | 4.6 | 19.5 | 2–18,2 |
| B cells (CD19+) | 0 | 9.5 | 6,3-24,5 |
| NK cells (CD56+ CD3-) | 8.9 | 10.3 | 3,8-24,6 |
A PET – CT scan was performed during admission and evinced a 19 × 25 cm solid injury in the anterior mediastinum consistent with low-grade thymoma. Neither the lymph node biopsy nor the immunohistochemical study showed alterations suggestive of lymphoproliferative syndrome.
Regarding follow up tests for COVID-19, the PCR became negative and the SARS-CoV-2 IgG spike antibodies (SARS-CoV-2 TrimericS IgG®; Diasorin Inc) turned positive at day 16 from symptoms onset. Cellular immunity studied by QuantiFERON® (SARS-CoV-2 Starter Set; Qiagen) which was negative at day 7, tested positive at day 30.
The patient was discharged after 22 days from hospital admission. She was asymptomatic, in good general condition, and her baseline O2 saturation was 98%.
2. Discussion
To our knowledge this is the first reported case of a patient with Good's syndrome and a severe COVID-19 who survived.
There is controversy as to how SARS-CoV-2 infection affects patients with antibody deficiency. Some publications did not observe an increased risk in patients with primary immunodeficiency disorder (PID) compared to the general population [5,6]. On the contrary, it has been hypothesized that PID could be a paradoxically protective factor against the SARS-CoV-2 infection [7]. A hyper-inflammation state has been suspected of playing a major role in the fatality of COVID-19 [8]. In this scenario the intrinsic lack of B cells could be considered as an advantage by preventing the development of inflammation. Since literature is scarce and contradictory [[5], [6], [7],9] longer data series are needed to elucidate the matter.
In the present case our patient presented a favorable outcome against the SARS-CoV-2 infection in contrast to the previous case reported [1]. We hypothesize that she maintained the presence of cells involved in humoral and cellular immunity throughout the infection. The presence of memory T cells observed in the immunophenotype and the positivity of QuantiFERON® test after infection could be an additional reason to explain her favorable evolution.
In conclusion, given there is an intrinsic immunity variability in patients with Good's syndrome, it results hard to predict their response to SARS-CoV-2 infection. However, the prior knowledge of B and T cell populations might be a useful tool in anticipating the course of the infectious disease.
Declaration of Competing Interest
The authors declare no conflict of interest.
Capsule summary: This study describes a case of Good's syndrome who despite having a severe COVID-19 pneumonia had a favorable outcome in contrast to the previous published case.
References
- 1.Pozzi M.R., Baronio M., Janetti M.B., Gazzurelli L., Moratto D., Chiarini M., Plebani A., Lougaris V. Fatal SARS-CoV-2 infection in a male patient with Good’s syndrome. Clin. Immunol. 2021;223:108644. doi: 10.1016/j.clim.2020.108644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.RA G. Agammaglobulinaemia—a provocative experiment of nature. Bull. Univ. Minnesota. 1954:1–19. [Google Scholar]
- 3.Singh A., Jindal A.K., Joshi V., Anjani G., Rawat A. An updated review on phenocopies of primary immunodeficiency diseases. Genes Dis. 2020;7:12–25. doi: 10.1016/j.gendis.2019.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Primary immunodeficiency diseases report of an IUIS scientific committee. Clin. Exp. Immunol. 1999;118:1–28. doi: 10.1046/j.1365-2249.1999.00109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marcus N., Frizinsky S., Hagin D., Ovadia A., Hanna S., Farkash M., Maoz-Segal R., Agmon-Levin N., Broides A., Nahum A., Rosenberg E., Kuperman A.A., Dinur-Schejter Y., Berkun Y., Toker O., Goldberg S., Confino-Cohen R., Scheuerman O., Badarneh B., Epstein-Rigbi N., Etzioni A., Dalal I., Somech R. Minor clinical impact of COVID-19 pandemic on patients with primary immunodeficiency in Israel. Front. Immunol. 2021;11 doi: 10.3389/fimmu.2020.614086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meyts I., Bucciol G., Quinti I., Neven B., Fischer A., Seoane E., Lopez-Granados E., Gianelli C., Robles-Marhuenda A., Jeandel P.-Y., Paillard C., Sankaran V.G., Demirdag Y.Y., Lougaris V., Aiuti A., Plebani A., Milito C., Dalm V.A., Guevara-Hoyer K., Sánchez-Ramón S., Bezrodnik L., Barzaghi F., Gonzalez-Granado L.I., Hayman G.R., Uzel G., Mendonça L.O., Agostini C., Spadaro G., Badolato R., Soresina A., Vermeulen F., Bosteels C., Lambrecht B.N., Keller M., Mustillo P.J., Abraham R.S., Gupta S., Ozen A., Karakoc-Aydiner E., Baris S., Freeman A.F., Yamazaki-Nakashimada M., Scheffler-Mendoza S., Espinosa-Padilla S., Gennery A.R., Jolles S., Espinosa Y., Poli M.C., Fieschi C., Hauck F., Cunningham-Rundles C., Mahlaoui N., Warnatz K., Sullivan K.E., Tangye S.G. Coronavirus disease 2019 in patients with inborn errors of immunity: an international study. J. Allergy Clin. Immunol. 2021;147:520–531. doi: 10.1016/j.jaci.2020.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Babaha F., Rezaei N. Primary immunodeficiency diseases in COVID-19 pandemic: a predisposing or protective factor? Am. J. Med. Sci. 2020;360:740–741. doi: 10.1016/j.amjms.2020.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Channappanavar R., Perlman S. Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology. Semin. Immunopathol. 2017;39:529–539. doi: 10.1007/s00281-017-0629-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Delavari S., Abolhassani H., Abolnezhadian F., Babaha F., Iranparast S., Ahanchian H., Moazzen N., Nabavi M., Arshi S., Fallahpour M., Bemanian M.H., Shokri S., Momen T., Sadeghi-Shabestari M., Molatefi R., Shirkani A., Vosughimotlagh A., Safarirad M., Sharifzadeh M., Pashangzadeh S., Salami F., Shirmast P., Rezaei A., Shad T. Moeini, Mohraz M., Rezaei N., Hammarström L., Yazdani R., Aghamohamamdi A. Impact of SARS-CoV-2 pandemic on patients with primary immunodeficiency. J. Clin. Immunol. 2021;41:345–355. doi: 10.1007/s10875-020-00928-x. [DOI] [PMC free article] [PubMed] [Google Scholar]