Abstract
Aim
The pandemic has generated the need for COVID-19 patients to be treated as best as possible; however, the effect of these treatments on glycemic control has not yet been taken into account. This article aims to determine whether the daily variation of glucose is influenced by the use of corticosteroids in COVID-19 patients treated in Lima-Peru.
Methodology
A prospective cohort study was undertook, in which glucose was measured four times a day in 53 patients hospitalized due to COVID-19. These values were associated with the use of corticosteroids and adjusted for other socio-educational variables, all by means of PA-GEE models.
Results
Nested multivariate analysis of daily glucose variation found that those using corticosteroids increased the daily average glucose as well as the first and last glucose measurements, this is, at 6am and 10pm, respectively (all p-values <0.026). An increase in glucose levels was also observed in those with diabetes (all p-values <0.001). In contrast, we found that there was a decrease in the last glucose measurement of the day in obese patients (p-value = 0.044).
Conclusions
The patients who used corticosteroids for the treatment of COVID-19 increased the average glucose per day, especially in the first and last measurement.
Keywords: Dexamethasone, Type 2 diabetes mellitus, COVID-19, Blood glucose, Hyperglycemia, Glycemic variability
Introduction
The COVID-19 epidemic in Peru has one of the highest incidence and mortality rates worldwide [1], higher in people with previous pathologies, such as hypertension, obesity and type 2 diabetes mellitus (T2DM) [2,3].
The RECOVERY study demonstrated that the use of dexamethasone reduces mortality in one fifth of COVID-19 patients requiring oxygen therapy and in one third of patients requiring mechanical ventilation [4], through a possible decrease in the inflammatory cascade associated with the infection [5]. However, corticosteroid therapy increases insulin resistance, the endogenous glucose production and the effect of counterregulatory hormones [6]. In addition, it has been reported that, in hospitalized patients with diabetes, the administration of corticosteroids produces an exacerbation of glycemic excursions [7,8] and generates sustained hyperglycemia [9]. On the other hand, stress mechanisms occur during hospitalization, which worsen glucose control in diabetic patients.
The association between hyperglycemia and adverse clinical outcomes in critical and non-critical patients is well established. In patients with diabetes, adequate glucose control reduces hospitalization days, multi-organ failure and mortality [10]. In this context, clinicians face great challenges in the therapy of hospitalized patients with COVID-19 and diabetes, since they receive dexamethasone on the one hand, with the aim of reducing inflammation, and on the other hand, they must achieve adequate glycemic control [[11], [12], [13]]. The present study seeks to determine whether daily glucose variation is influenced by the use of corticosteroids in patients treated for COVID-19 in Lima-Perú.
Materials and methods
Study design and patient population
A prospective cohort study was conducted, in which daily capillary glucose was monitored four times, in 53 patients hospitalized due to SARS-COV-2 infection and who had received, as part of the treatment, dexamethasone during their hospitalization in COVID-19 wards of the National Hospital Arzobispo Loayza between July 1 and August 31, 2020. The study was approved by the Ethics Committee and the Teaching and Research Office of the National Hospital Arzobispo Loayza.
Procedures and variables
The following data were collected: age, gender, weight and height, diabetes diagnosis, admission glucose, hemoglobin, hematocrit, glutamic oxaloacetic transaminase (GOT), glutamic pyruvic transaminase (GPT), creatinine, CRP and glycated hemoglobin (only for patients with T2DM). During dexamethasone administration, capillary glycemic was monitored four times a day (6 h, 12 h, 17 h and 22 h) and monitoring continued until 72 h after dexamethasone discontinuation. Those patients who presented with hyperglycemia were prescribed insulin treatment. All this information was inserted into a data collection form (generated ad hoc for this purpose); then, a first quality control of the data was carried out (by another researcher), after which the information was passed to the STATA statistical program (version 11.1), where a second quality control was carried out (by the statistician). After that, we proceeded with the analysis.
Statistical analysis
For the construction of Table 1, Table 2 , the frequencies and percentages of the qualitative variables were obtained. For the description of the quantitative variables, they were evaluated with the Shapiro Wilk test and, according to the results, the best measure of central tendency and dispersion of each variable was obtained. For the construction of Table 2, Table 3, PA-GEE regression was used, taking into account the day of hospitalization as the variable time, the Gaussian family and the identity link function. With all these data, the coefficients, 95% confidence intervals (95%CI) and p-values were obtained (values less than 0.05 were considered statistically significant, which is part of the criterion to pass the multivariate statistics).
Table 1.
General characteristics of the study population.
| General characteristics | n (%) |
|---|---|
| Gender | |
| Male | 30 (56,6) |
| Female | 23 (43,4) |
| Agea (years old) | 57 (52–67) |
| Diabetes | |
| Yes | 27 (50,9) |
| No | 26 (49,1) |
| BMIa (kg/m2) | 29,1 (25,6–31,3) |
| Obesity | |
| Yes | 22 (41,5) |
| No | 31 (58,5) |
| Laboratory tests at hospital admission | |
| Serum glucosea (mg/dl) | 140 (118–243) |
| Hemoglobina (gr/dl) | 13.7 (12.9–14.6) |
| GOTa (U/L) | 34 (24–51) |
| GPTa (U/L) | 45 (28–68) |
| CRPa (mg/dl) | 11.1 (4.8–20.4) |
| Serum creatininea (mg/dl) | 0.75 (0.62–0.93) |
| HBA1ca (%) (N = 27) | 9.7 (7.1–12) |
Median and interquartile ranges, Hba1c: in diabetic patients.
Table 2.
Bivariate analysis of glucose variation as hospitalization days passed according to the use of corticosteroids in patients with COVID-19.
| Daily variation of glucose | Basal value | Bivariate analysis | P value |
|---|---|---|---|
| Glucose at 6 a.m. | 134,1 (113,7/154,6) | 23,9 (5,7/42,1) | 0.010 |
| Glucose at 12 p.m. | 144,8 (119,8/169,9) | 10,9 (−12,5/34,3) | 0.363 |
| Glucose at 5 p.m. | 195,7 (159,3/232,1) | −5,8 (−31,5/20,0) | 0.659 |
| Glucose at 10 p.m. | 153,5 (123,5/183,6) | 46,5 (20,3/72,6) | <0.001 |
| Daily average variation | 165,5 (141,5/189,5) | 14,9 (1,7/28,1) | 0.026 |
| Complete daily average variation | 142,5 (111,5/173,6) | 25,5 (−0,7/51,7) | 0.056 |
The coefficients (left), 95% confidence intervals (within parentheses) and p-values (right) were obtained with PA-GEE regression, taking into account the day of hospitalization as the variable time, the Gaussian family and the identity link function.
Table 3.
Nested multivariate analysis of glucose variation according to the use of corticosteroids in COVID-19 patients.
| Associated variables | Glucosa at 6am | Glucose at 10pm | Average glucose |
|---|---|---|---|
| Constant (basal) | 92,9 (77,5/108,4) <0,001 | 105,2 (73,5/136,8) <0,001 | 113,4 (100,1/126,8) <0,001 |
| Use of corticosteroids | +23,5 (+5,8/+41,2) <0,001 | +44,4 (+17,0/+71,7) 0,001 | +15,3 (+2,0/+28,7) <0,025 |
| Having diabetes | +81,3 (+53,4/+109,2) <0,001 | +118,4 (+87,0/+149,8) <0,001 | +101,6 (+70,5/+132,8) <0,001 |
| Obesity | Excluded | −34,9 (−68,8/−1,0) 0,044 | Excluded |
The coefficients (left), 95% confidence intervals (within parentheses) and p-values (right) were obtained with PA-GEE regression, taking into account day of hospitalization as the variable time, the Gaussian family and the identity link function. Nested models were used in each case (taking into account the p-value as the first step for decision making and the Wald test as the second).
RESULTS
A total of 352 glucose measurements were obtained from the 53 patients. Of the patients, 56.6% were male, the median age was 57 (interquartile range: 52–67 years), 50.9% had diabetes and 58.5% did not have obesity. In the diabetic patients, the median BMI was 29.1 (interquartile range: 25.6–31.3). The other general characteristics are shown in Table 1.
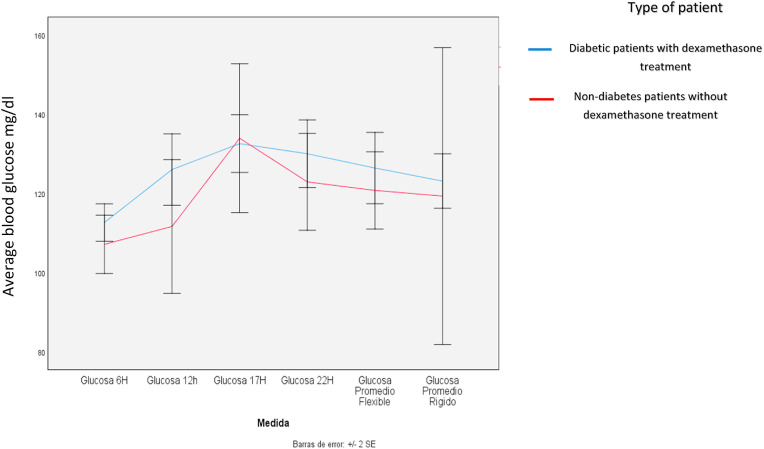
When bivariate analysis was performed, it was found that those receiving corticosteroids presented with a daily variation in glucose at 6am (basal glucose increased by 23.9 mg/dl; 95% CI: 5.7–42.1; p-value = 0.010), 10pm (glucose increased by 46.5 mg/dl with respect of the initial value; 95%CI: 20.3–72.6; p-value<0.001) and in the daily average (basal glucose increased by 14.9 mg/dl; 95%CI: 1.7–28.1; p-value = 0.026). The other glucose values were not significantly altered. Table 2 and Fig. 1 .
Fig. 1.
Glucose at 6:00- Glucose at 12:00-Glucose at 17:00-Glucose at 22:00 -flexible average glucose-rigid average glucose.
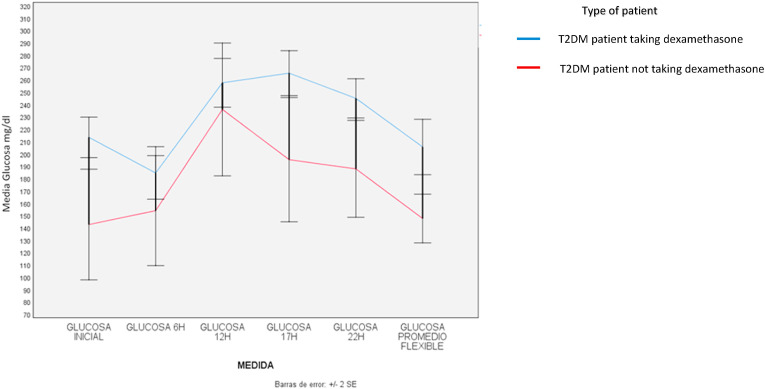
When nested multivariate analysis of daily glucose variation was performed, it was found that those using corticosteroids not only increased the daily average glucose, but also the first and last glucose measurement, that is at 6am and 10pm, respectively (all values p < 0.026). In patients who had diabetes, there was also an increase in the daily average glucose and in the 6am and 10pm glucose. This increase was much more marked than in non-diabetics. (all values p < 0.001). In the case of obese patients, there was a decrease in the last glucose measurement of the day (p-value = 0.044). Table 3 and Fig. 2 .
Fig. 2.
Initial glucose – Glucose at 6:00 - Glucose at 12:00- Glucose at 17:00- Glucose at 22:00 -flexible average glucose.
Discussion
In our findings, COVID-19 patients with a history of diabetes presented with hyperglycemia when being admitted to the hospital. Given the prescription of dexamethasone as part of the treatment during their hospitalization, it was evidenced that glucose values could double throughout the day (especially in the first and last measurements of the day); this was monitored with the glucose self-monitoring control profile. Similarly, non-diabetic patients receiving dexamethasone also increased their blood glucose levels, although to a lesser extent than diabetic patients.
Although the findings of the RECOVERY study [4] and a recent meta-analysis [14] support the indication of dexamethasone in patients with COVID-19 (requiring ventilation or oxygen requirement to reduce mortality); corticosteroid therapy with dexamethasone increases hyperglycemia [15]. Our findings suggest the personalized risk/benefit assessment of this drug in patients with diabetes and COVID-19.
In addition, corticosteroid therapy in patients with COVID-19 may precipitate the onset of diabetes in previously predisposed individuals with “de novo diabetes " [16]. These findings are extremely important, because in the current pandemic there are many cases that are complicated by having diabetes, and many of these patients use corticosteroid therapy.
It is reported that the values can double, this in a patient who has values of 120 mg/dL could mean the reaching of values of more than 200 mg/dL; also, if a patient has 200 or 300 of glycemia, added to an inadequate treatment, it could reach 400 or 600 mg/dL, respectively. This could be very serious if it cannot be adequately controlled. That is why clinical guidelines for the management of hospitalized patients with diabetes recommend the use of insulin therapy. In patients who do not require intensive care, the indication of a basal-bolus scheme for the management of hyperglycemia is suggested [10,17]. All this should be taken into account by the physicians and services of each health institution that is managing patients with diabetes and COVID-19, evaluating their own reality, generating their own management guidelines (based on those that already exist and the results of new research), as well as gradually adapting them.
In the United Kingdom, a meeting of experts on the management of hyperglycemia in patients with diabetes and COVID-19 who receive dexamethasone, promotes the use of insulin therapy schemes with greater insulin requirements and strict glycemic control. Similar recommendations are suggested by another panel of experts [16,18,19]. However, we still do not have clinical trials to support decision-making [20]. Therefore, it is hoped that future research will aim to evaluate this situation in controlled populations, with differentiated management with corticosteroids in diabetic and non-diabetic patients suffering from COVID-19 to ratify that these alterations exist on a daily basis, and what would be the best management for this situation, proposing different forms of treatment.
One of our limitations in the study is not having glycated hemoglobin A1c when the patients were admitted, which could unmask undiagnosed diabetes. Another of our limitations in the context of clinical management is the limited number of glucose controls for decision making. In addition, we have limitations of retrospective and non-experimental studies themselves, which do not allow us to have all the variables or randomization. Lastly, the dose and timing of administration of corticosteroids has not been taken into account, these are important factors affecting blood glucose values. In spite of all these limitations, the results are extremely important, since they show a major problem in the management of patients with diabetes and COVID-19 (which may currently number over millions worldwide), hence, it is expected that future research will be able to carry out this type of investigation.
Based on the above, it is concluded that those patients who used corticosteroids for the treatment of COVID-19 increased the average glucose per day, especially in the first and last measurement. There was also an increase in these glucose levels in those who had diabetes and those who were obese had a decrease in the last glucose level of the day. In obese patients the reduced glucose values after corticosteroids are unexpected and needs to be demonstrated in futures researches.
Funding and duality of interest
The study was self-funded by the authors. F.E. is a medical scientific liaison for SANOFI Peru. The rest of the co-authors report no relevant conflicts of interest related to this work.
Declaration of competing interest
The authors declare that there are no conflicts of interest with this article.
References
- 1.de Salud Ministerio. 2020. Análisis Epidemiológico de la Situación Actual de COVID-19 en el Perú, basado en la información de la Vigilancia Epidemiológica y la Investigación de Campo [Internet]https://www.dge.gob.pe/portal/docs/tools/coronavirus/analisiscoronavirus080520.pdf Lima. [cited 2020 Oct 19]. Available from: [Google Scholar]
- 2.Mejía F MC, Cornejo E, Morello E, Vásquez S, Alave J, et al. Características clínicas y factores asociados a mortalidad en pacientes adultos hospitalizados por COVID-19 en un hospital público de Lima, Perú. [Internet]. SciELO Preprints; [cited 2020 Nov 10]. Available from: 10.1590/SciELOPreprints.858. [DOI]
- 3.Munayco C., Chowell G., Tariq A., Undurraga E.A., Mizumoto K. Risk of death by age and gender from CoVID-19 in Peru, March-May, 2020. Aging. 2020 Jul 21;12(14):13869–13881. doi: 10.18632/aging.103687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.RECOVERY Collaborative Group. Horby P., Lim W.S., Emberson J.R., Mafham M., Bell J.L., Linsell L., Staplin N., Brightling C., Ustianowski A., Elmahi E., Prudon B., Green C., Felton T., Chadwick D., Rege K., Fegan C., Chappell L.C., Faust S.N., Jaki T., Jeffery K., Montgomery A., Rowan K., Juszczak E., Baillie J.K., Haynes R., Landray M.J. Dexamethasone in hospitalized patients with covid-19. N Engl J Med. 2021 Feb 25;384(8):693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meyerowitz E.A., Sen P., Schoenfeld S.R., Neilan T.G., Frigault M.J., Stone J.H., et al. Immunomodulation as Treatment for Severe COVID-19: a systematic review of current modalities and future directions. Clin Infect Dis. 2021 Jun 15;72(12):e1130–e1143. doi: 10.1093/cid/ciaa1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tamez-Pérez H.E., Quintanilla-Flores D.L., Rodríguez-Gutiérrez R., González-González J.G., Tamez-Peña A.L. Steroid hyperglycemia: prevalence, early detection and therapeutic recommendations: a narrative review. World J Diabetes. 2015;6(8):1073–1081. doi: 10.4239/wjd.v6.i8.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burt M.G., Roberts G.W., Aguilar-Loza N.R., Frith P., Stranks S.N. Continuous monitoring of circadian glycemic patterns in patients receiving prednisolone for COPD. J Clin Endocrinol Metab. 2011;96(6):1789–1796. doi: 10.1210/jc.2010-2729. [DOI] [PubMed] [Google Scholar]
- 8.O'Connell R.S., Clinger B.N., Donahue E.E., Celi F.S., Golladay G.J. Dexamethasone and postoperative hyperglycemia in diabetics undergoing elective hip or knee arthroplasty: a case control study in 238 patients. Patient Saf Surg. 2018;12:30. doi: 10.1186/s13037-018-0178-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nyenwe E., James D., Wan J., Dagogo-Jack S. Glycemic response to oral dexamethasone predicts incident prediabetes in normoglycemic subjects with parental diabetes. Journal of the Endocrine Society. 2020;4(11):bvaa137. doi: 10.1210/jendso/bvaa137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pasquel F.J., Fayfman M., Umpierrez G.E. Debate on insulin vs non-insulin use in the hospital setting-is it time to revise the guidelines for the management of inpatient diabetes? Curr Diabetes Rep. 2019;19(9):65. doi: 10.1007/s11892-019-1184-8. [DOI] [PubMed] [Google Scholar]
- 11.Waterer G.W., Rello J. Steroids and COVID-19: we need a precision approach, not one size fits all. Infectious diseases and therapy. 2020:1–5. doi: 10.1007/s40121-020-00338-x. [DOI] [PubMed] [Google Scholar]
- 12.Alessi J., de Oliveira G.B., Schaan B.D., Telo G.H. Dexamethasone in the era of COVID-19: friend or foe? An essay on the effects of dexamethasone and the potential risks of its inadvertent use in patients with diabetes. Diabetol Metab Syndrome. 2020;12:80. doi: 10.1186/s13098-020-00583-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ruiz de Adana M.S., Colomo N., Maldonado-Araque C., Fontalba M.I., Linares F., García-Torres F., et al. Randomized clinical trial of the efficacy and safety of insulin glargine vs. NPH insulin as basal insulin for the treatment of glucocorticoid induced hyperglycemia using continuous glucose monitoring in hospitalized patients with type 2 diabetes and respiratory disease. Diabetes Res Clin Pract. 2015;110(2):158–165. doi: 10.1016/j.diabres.2015.09.015. [DOI] [PubMed] [Google Scholar]
- 14.Sterne J.A.C., Murthy S., Diaz J.V., Slutsky A.S., Villar J., Angus D.C., et al. Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: a meta-analysis. Jama. 2020;324(13):1330–1341. doi: 10.1001/jama.2020.17023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Herbst R.A., Telford O.T., Hunting J., Bullock W.M., Manning E., Hong B.D., et al. The effects of perioperative dexamethasone on glycemic control and postoperative outcomes. Endocr Pract : official journal of the American College of Endocrinology and the American Association of Clinical Endocrinologists. 2020;26(2):218–225. doi: 10.4158/EP-2019-0252. [DOI] [PubMed] [Google Scholar]
- 16.Rayman G., Lumb A.N., Kennon B., Cottrell C., Nagi D., Page E. Dexamethasone therapy in COVID-19 patients: implications and guidance for the management of blood glucose in people with and without diabetes. 2021;38(1) doi: 10.1111/dme.14378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Umpierrez G.E., Hellman R., Korytkowski M.T., Kosiborod M., Maynard G.A., Montori V.M., et al. Management of hyperglycemia in hospitalized patients in non-critical care setting: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2012;97(1):16–38. doi: 10.1210/jc.2011-2098. [DOI] [PubMed] [Google Scholar]
- 18.Singh A.K., Khunti K. Assessment of risk, severity, mortality, glycemic control and antidiabetic agents in patients with diabetes and COVID-19: a narrative review. Diabetes Res Clin Pract. 2020;165:108266. doi: 10.1016/j.diabres.2020.108266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bornstein S.R., Rubino F., Khunti K., Mingrone G., Hopkins D., Birkenfeld A.L., et al. Practical recommendations for the management of diabetes in patients with COVID-19. The lancet Diabetes & endocrinology. 2020;8(6):546–550. doi: 10.1016/S2213-8587(20)30152-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bloomgarden Z. Does glycemic control affect outcome of COVID-19? J Diabetes. 2020;12(12):868–869. doi: 10.1111/1753-0407.13116. [DOI] [PMC free article] [PubMed] [Google Scholar]