With the widely known measures to prevent COVID-19, one more is added to the list. That is, the COVID-19 vaccines which (two vaccines-Covishield: AstraZeneca-Serum Institute of India and Covaxin Bharat Biotech Limited) [1] was authorized by the Drugs Controller General of India (DCGI) for emergency use in India. As people above 60 years and people with co-morbidities above 45 years were announced eligible for vaccination in India, we conducted a survey among our out-patients to know the number of people vaccinated and the reasons for not taking it.
This is a cross-sectional, single-centred survey. People with diabetes who attended the out-patient department of MV Hospital for Diabetes, Chennai between March 31, 2021, to April 9, 2021, & were above 18 years of age were approached for this survey and included after receiving their consent [Institutional Ethical Committee approval (IEC/N-008/03/2021)].
A total of 214 participants were surveyed & their mean age were 55.01 years (Mean & Standard deviation (M&SD) = 55.01 ± 11.64; min-25 years & max-83 years). Mean duration of diabetes were 11.15 years (M&SD = 11.15 ± 9.56) (Table 1 ).
Table 1.
Results of the survey.
| S·NO. | VARIABLE | NUMBER (%) |
|---|---|---|
| 1. | Total participants | 214 |
| 2. | Gender | |
| Male | 112 (52.3%) | |
| Female | 102 (47.7%) | |
| 3. | Age (M±SD) | 55.01 ± 11.64 |
| 4. | Duration of diabetes (M±SD) | 11.15 ± 9.56 |
| 5. | Hypertension | 78 (36.4%) |
| 6. | Cardiac illness | 32 (15%) |
| 7. | Renal impairment | 26 (12.1%) |
| 8. | Previous history of COVID-19 infection | 16 (7.5%) |
| 9. | Treatment received for COVID-19(n=16) | |
| Out-patient | 7 (43.7%) | |
| Admission | 9 (56.3%) | |
| 10. | Received COVD-19 vaccine | 46 (21.5%) |
| 11. | Vaccination details | |
| Covaxin | 18 (8.4%) | |
| Covishield | 28 (13%) | |
| 12. | Received vaccine in(n=46) | |
| Government hospital | 31 (67.4%) | |
| Private | 15 (32.6%) | |
| 13. | Dose received | |
| One dose | 37 (17.2%) | |
| Two doses | 9 (4.2%) | |
| 14. | Reason for not taking the vaccination(n=168) | |
|
46 (27.4%) | |
|
35 (20.8%) | |
|
45 (26.8%) | |
|
11 (6.5%) | |
|
4 (2.4%) | |
|
32 (19%) | |
|
18 (10.7%) | |
|
1 (0.6%) | |
|
6 (3.6%) | |
|
1 (0.6%) | |
|
1 (0.6%) | |
| 15. |
|
|
|
13 (37.1%) | |
|
21 (60%) | |
|
1 (2.9%) |
M±SD-Mean±Standard deviation
A total of 7.5% of the study participants had previous history of COVID-19 in which 43.7% participants were treated in outpatient and others as in-patient.
Among the study participants, 21.5% (n=46) had taken at least one dose of the COVID-19 vaccine. Approximately 17% (n=37) of the study participants had received only one dose. Both the doses were received by 9 participants (4.2%). Among the vaccinated study participants, 18 participants had received Covaxin, and 28 participants had received Covishield. The majority of the vaccinated study participants received their vaccine at a government facility nearby (n=31, 67.4%).
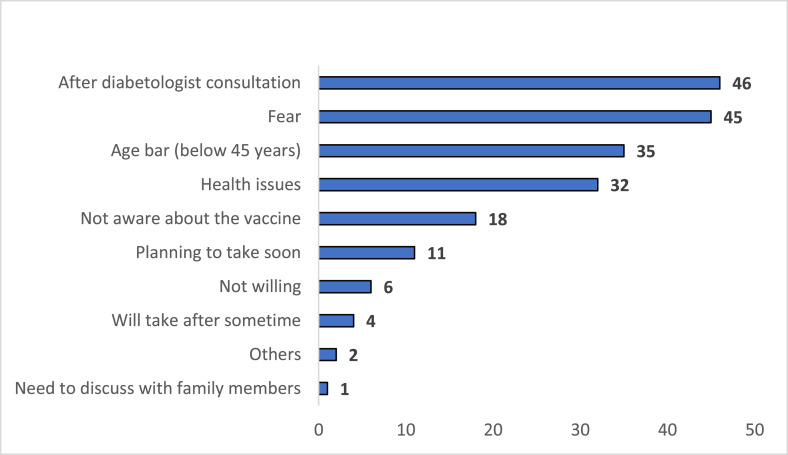
Most of the study participants who have not taken their vaccine mentioned they would take the vaccine after their diabetologist's advice (n = 46, 27.4%). The second reason for not taking the vaccine was fear (n = 45, 26.8%). The other reasons includes:
-
➢
Age bar (n = 35, 20.8%)
-
➢
Health issues (Eg. under gynecologist treatment, due to comorbid conditions like diabetes, hypertension) after physician advice (n = 32, 19%)
-
➢
Not aware of the vaccine (n = 18, 10.7%)
-
➢
Will take soon (n = 11, 6.5%)
-
➢
Not willing (n = 6, 3.6%)
-
➢
Others (n = 2, 1.2%) (Fig. 1 ).
Fig. 1.
Reasons for not taken the COVID-19 vaccine.
Vaccine hesitations arised from the day of the announcement of the development of vaccines against COVID-19 worldwide. Various reasons for this includes the efficacy of the vaccines, safety, duration of protection, health literacy, misinformation, lack of trust, need for additional information and cost of the vaccines [[2], [3], [4], [5]] as per the studies conducted across countries.
Physicians play a crucial role in this education & awareness as the people mostly rely on them for decision making and to break the hesitance.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.dsx.2021.102190.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.MoHFW-Frequently asked questions. https://www.mohfw.gov.in/covid_vaccination/vaccination/faqs.html Dated: 16th April 2021.
- 2.Montagni I., Ouazzani-Touhami K., Mebarki A., et al. Acceptance of a Covid-19 vaccine is associated with ability to detect fake news and health literacy [published online ahead of print, 2021 Mar 9] J Public Health (Oxf) 2021 doi: 10.1093/pubmed/fdab028. fdab028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Al-Qerem W.A., Jarab A.S. COVID-19 vaccination acceptance and its associated factors among a middle eastern population. Front Public Health. 2021;9:632914. doi: 10.3389/fpubh.2021.632914. Published 2021 Feb 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fisher K.A., Bloomstone S.J., Walder J., Crawford S., Fouayzi H., Mazor K.M. Attitudes toward a potential SARS-CoV-2 vaccine : a survey of U.S. Adults. Ann Intern Med. 2020;173(12):964–973. doi: 10.7326/M20-3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.COVID-19 communication stratergy. https://www.mohfw.gov.in/pdf/Covid19CommunicationStrategy2020.pdf Dated: 17th April 2021. MoHFW.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.