Summary
COVID-19 vaccination using mRNA technology began at the end of 2020 in several countries, approximately 9 months after the WHO declared the new coronavirus a pandemic, and began in Japan at the end of February 2021. Several studies have reported FDG avidity in enlarged axillary lymph nodes as a specific feature of FDG-PET/CT imaging after COVID-19 vaccination. A major concern is that this finding could lead to a misdiagnosis in patients with various types of malignancy. We review the impact of COVID-19 vaccination on the management of patients scheduled for FDG-PET/CT in the setting of nationwide mass vaccination.
Keywords: COVID-19, FDG, PET/CT, vaccine
Introduction
The coronavirus disease 2019 (COVID-19) pandemic, caused by SARS-CoV-2, has affected many countries worldwide (1). As of 10 June 2021, a total of 174,488,358 cases and 3,759,138 deaths have been reported (2). The development of COVID-19 vaccines has played a key role in protecting people from COVID-19 (3), as shown by the substantial early reductions in SARS-CoV-2 infection and symptomatic COVID-19 rates recorded following administration of the first dose of the vaccine (4). In general, vaccine development takes approximately 15 years (5-6), therefore the speed of development of COVID-19 vaccine within one year, and the proven efficacy of vaccines against SARS-CoV-2 are undoubtedly scientific breakthroughs. The two major mRNA-based vaccines, produced by Moderna and Pfizer/BioNTech, are 94-95% effective and there have been no critical safety concerns to date (7,8).
[18F]-2-fluoro-2-deoxyglucose positron emission tomography (FDG-PET)/computed tomography (CT) is useful for accurate tumor staging in various types of cancer and for monitoring the response to cancer therapy. Accurate nodal staging is one of the advantages of FDG-PET/CT, and is recommended for this purpose in several clinical guidelines. In contrast, nonspecific or inflammatory-related FDG uptake in the lymph nodes has been a limitation of FDG-PET/CT (9). We have previously reported the FDG-PET/CT findings of patients with COVID-19 as increased FDG uptake in lung lesions with segmental ground-glass densities and plaques, in normal-sized or slightly enlarged lymph nodes, and in the bone marrow and spleen (10).
Several recent reports have announced the specific findings of intense FDG uptake in axillary, supraclavicular and cervical lymph nodes on FDG-PET/ CT following COVID-19 vaccination based on mRNA biotechnology. Since lymphadenopathy after vaccination has been reported with several other types of available vaccines (11-13), the FDG-PET/CT imaging feature following COVID-19 vaccination could be predictable. However, the impact of the FDG-PET/CT following COVID-19 vaccination was higher than we had expected. Therefore, we present here the features of FDG-PET/CT imaging and conduct a review of the literature regarding the management of patients who underwent FDG-PET/ CT after COVID-19 vaccination.
Incidence of lymph node swelling in patient with COVID-19 infection
Among patients with COVID-19 infection, lymph node enlargement is seen on CT in less than 1% (14). These lymph nodes are generally small, nonspecific, and regular in shape. Enlargement of the lymph nodes is not significant, and FDG uptake in mediastinal and supraclavicular lymph nodes is frequently seen in patients with COVID-19 (10). Swelling of lymph nodes indicates immunoreactions that are activated by inflammatory cells. In the immune response to viral infections, the number of monocytes in lymphoid tissue increases, leading to increased FDG uptake (15).
However, several studies in patients with COVID-19 have reported negative FDG uptake in mediastinal and supraclavicular lymph nodes which may occur in the minimally invasive and early stages of the disease (16). Therefore, the immune response is weak or almost absent in the early stage of the disease and becomes more active over time. In addition, the reduction of FDG uptake in lymph nodes may indicate normalization of a hyperactive immune response in the body.
Incidence of lymph node swelling following COVID-19 vaccination
A public notification from the Centers for Disease Control and Prevention (CDC) indicated that the incidence of lymphadenopathy after the Pfizer-BioNTech COVID-19 vaccination was imbalanced, with 58 more cases recorded in a vaccine group (n = 64) compared with a placebo group (n = 6). Lymphadenopathy occurred in the arm and neck regions and was reported within 2-4 days after vaccination. The average duration of lymphadenopathy was approximately 10 days. However, lymph node swelling was defined as an unsolicited adverse event in this clinical trial (17). It appears that the size and number of lymphadenopathy was relatively severe as being able to identify with palpation and/or visual inspection. In a Moderna clinical trial with a cohort aged 18-64 years, axillary swelling or tenderness was regarded as a solicited adverse event that occurred in 11.6% of patients after the first vaccination and 16.0% after the second vaccination, which was higher than the incidence in placebos (5.0% and 4.3%, respectively) (17). This reaction was less common in subjects aged ≥ 65 years, occurring in 8.4% of this group after the second dose (8,17). These two mRNA vaccines appear to stimulate immune activity to a greater degree than do than vaccines based on traditional biotechnologies (18).
Features of FDG-PET/CT imaging after COVID-19 vaccination
After vaccination, lymph node size varies from normal to moderately increased with thickening of the cortex and fatty hilum, suggesting benign lesions. However, lymph nodes can show abnormal size and loss of fatty hilum shortly after vaccination, which could be considered to indicate malignancy. Therefore, it is important to carefully manage the timing of FDG-PET/ CT examination with respect to the date of vaccination.
FDG uptake has been identified in normal-sized to moderately enlarged axillary supraclavicular and cervical area nodes following intramuscular vaccination in the ipsilateral deltoid (19-32) (Figure 1). Nodal FDG uptake tends to occur within 7 days of vaccination and generally disappears by 12-14 days (12). However, it can remain for 4-6 weeks (19) or 7-10 weeks (23) after the injection. Lymph nodes on the injected side are mostly affected, but contralateral lymph nodes can also show FDG uptake. Therefore, the FDG-PET/CT findings can lead to misdiagnosis in the evaluation of malignancy and inflammatory disease, particularly with regard to breast cancer, melanoma, lymphoma, and sarcoidosis (31).
Figure 1.
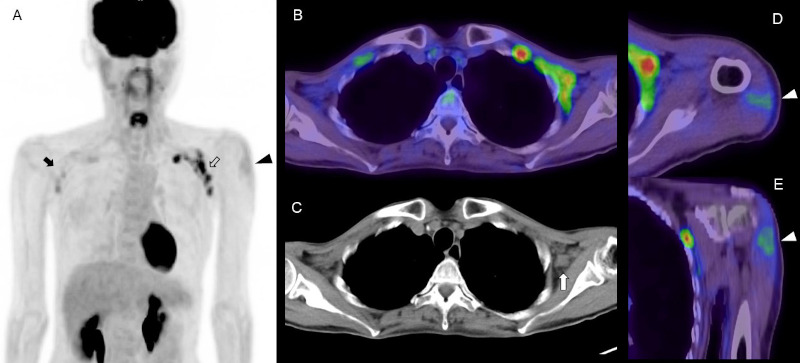
FDG PET/CT images in a woman in her 50s with abdominal malignancy at 4 days after COVID-19 vaccination. (A) MIP image, (B) axial fusion image, (C) CT image, (D) axial fusion image of deltoid muscle, (E) coronal fusion image of deltoid muscle. Increased FDG uptake is seen in the left axilla with supraclavicular lymphadenopathy (open arrow). There is slight FDG uptake in the right axillary and supraclavicular areas (arrow). The CT image shows enlarged lymph nodes with loss of fatty hilum in the left axillary and supraclavicular areas (white arrow). Intense FDG uptake in the left deltoid muscle indicates the site of COVID-19 vaccine injection (arrowhead). FDG uptake in the spleen was almost equal to that in the liver, which might have been caused by the vaccination.
Cohen et al. categorized vaccine-associated hypermetabolic lymphadenopathy (VAHL) according to the intensity and area of FDG uptake in axial lymph nodes as follows: grade 1, mild FDG-uptake intensity (SUVmax < 2.2); grade 2, moderate FDG-uptake intensity (2.2 ≤ SUVmax < 4); grade 3, high FDG-uptake intensity (SUVmax ≥ 4) in normal-size nodes; and grade 4, high FDG-uptake intensity (SUVmax ≥ 4) in enlarged nodes. The incidence of VAHL was 36.5% among all subjects who were vaccinated, but was significantly higher after the 2nd vaccination (45.8%) compared with the 1st vaccination (26.3%). Node size, FDG uptake in axial lymph nodes beyond level 1, and the site of vaccination were all more prominent after the 2nd vaccination. After the first vaccination, the incidence of VAHL was higher at 6-12 days after vaccination compared with that in the first 5 days and at 13 days. After the second vaccination, the incidence and grade of VAHL were highest in the first 6 days, decreased gradually over time, and were significantly low at more than 20 days after vaccination. VAHL was recognized in 29% of vaccinated patients at 3 weeks after the second vaccination, but only 7% had grade 3 or 4 VAHL. After the first vaccination, there was a higher incidence and higher grade of VAHL in subjects aged ≤ 62 years than in others; whereas after the second vaccination, there was a higher incidence and higher grade of VAHL in subjects aged ≤ 64 years than in others (26). Regarding FDG uptake in lymph nodes, greater uptake was associated with second vaccination; in contrast, lower uptake was associated with older age, immunosuppressive treatment, and hematologic disease (30). The incidences of FDG uptake in lymph nodes were reported as higher for the Moderna vaccine than the Pfizer vaccine (32).
FDG uptake in small axillary lymph nodes is a common feature after vaccination against influenza (11-13,16,33) and other diseases (34). Considering that the reaction to the COVID-19 vaccine is more severe and of longer duration than that to the influenza vaccine, the FDG-PET/CT imaging findings may represent the nature of mRNA biotechnology vaccines for increased immunogenicity compared with traditional vaccines.
Intramuscular injection into the deltoid muscle has been recommended for administration of the COVID-19 vaccine (35), and it has been reported that the injected muscle shows increased FDG uptake shortly after injection. The factor of occurring increased FDG uptake in the deltoid muscle following COVID-19 mRNA vaccination was the number of days between the last vaccination and second vaccination (30).
In Japan, the influenza vaccine is injected into subcutaneous tissue, most commonly in the upper arm. In our experience, the site of influenza injection can be confirmed as a relatively small and slight FDG uptake shortly after administration of the vaccine (Figure 2). In contrast, following administration of the COVID-19 vaccine, moderate FDG uptake is confirmed as a broad area indicating a high degree of inflammatory change in the deltoid muscle. However, it is unclear whether this uptake is related to pain and swelling at the injection site.
Figure 2.
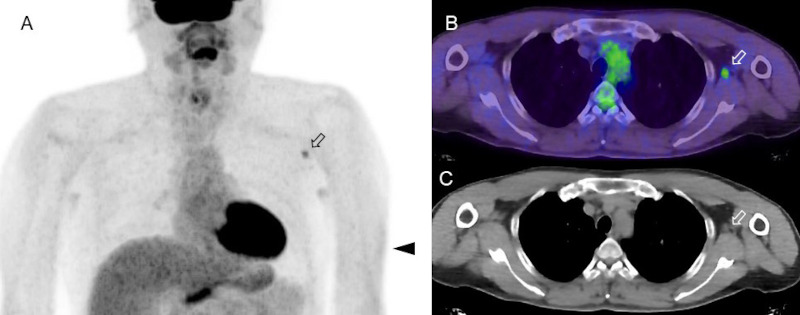
FDG-PET/CT images in a subject who received an influenza vaccination 4 days before the imaging examination. (A) MIP image, (B) axial fusion image, (C) CT image. Focal FDG uptake is seen in an axillary lymph node, which was slightly enlarged on CT image. Slight FDG uptake in the left arm indicates the site of COVID-19 vaccine injection at subcutaneous tissue (arrowhead). There are slight and symmetrical FDG uptake in the bilateral axillary area due to non-specific uptake for sweat glands.
Increased FDG uptake can also be observed in the spleen after COVID-19 vaccination (25); although not mentioned specifically by the author, this finding could be observed in several of the FDG-PET/CT images in that study. In addition to FDG imaging, specific PET uptake has also been observed in 68Ga-DOTA-TATE, 11C-and 18F-choline imaging (30,36,37), but less frequently with 68Ga- and 18F-PSMA (41).
Brewer et al. proposed lymph node swelling as a potential biomarker for successful vaccination in the DepoVax-based COVID-19 vaccine (38). However, further observation is necessary to clarify the relation between the antibody production and incidence of FDG-uptake after the COVID-19 vaccination.
Patient scheduling for FDG-PET/CT after COVID-19 vaccination
Physicians should take into account the specific imaging features of FDG-PET/CT and other imaging examinations following COVID-19 vaccination. When imaging studies are planned for the management of patients with COVID-19, information regarding the timing of COVID-19 vaccinations should be available for these patients. A preliminary patient interview by imaging unit staff regarding prior vaccination may be an effective method for ensuring that examinations are scheduled appropriately. Sharing this information with the radiologist would contribute to increasing the accuracy of diagnosis.
To reduce false positive findings, FDG-PET/ CT imaging should ideally be scheduled for either immediately after or 4-6 weeks after vaccination. However, immediately after vaccination, patient may request to reschedule the FDG-PET/CT test due to the common side effects (fever, nausea, chills, headache, tiredness, etc.) after getting a COVID-19 vaccine. Enlarged lymph nodes that are observed in the axillary, supraclavicular, or cervical areas with laterality can confuse the diagnosis (particularly in breast cancer, melanoma, lymphoma and any malignancy which have high possibility of invasion around these sites). Therefore, the vaccine should preferably be administered in the contralateral arm to the side of disease.
Acknowledgements
We thank all the staff in National Center for Global Health and Medicine who struggled with COVID-19. We also thank Kaori Saito, Daisuke Horikawa, Masashi Hayano, Megumi Fujii, Kakeru Iso, Kazuhiko Nakajima, Hironori Kajiwara, and Futoshi Matsunaga for contributing to the PET/CT examination.
Funding:
None.
Conflict of Interest
The authors have no conflicts of interest to disclose.
References
- 1. Mitsuya H, Kokudo N. Sustaining containment of COVID-19: global sharing for pandemic response. Glob Health Med. 2020; 2:53-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. JOHNS HOPKINS CORONAVIRUS RESOURCE CENTER. https://coronavirus.jhu.edu/map.html (last updated at 6/10/2021). (accessed June 21, 2021).
- 3. Douglas D, Richman. COVID-19 v a c c ines : implementation, limitations and opportunities. Glob Health Med. 2021; 3:1-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Amit S, Regev-Yochay G, Afek A, Kreiss Y, Leshem E. Early rate reductions of SARS-CoV-2 infection and COVID-19 in BNT162b2 vaccine recipients. Lancet. 2021; 397:875-877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Le TT, Andreadakis Z, Kumar A, Gómez Román R, Tollefsen S, Saville M, Mayhew S. The COVID-19 vaccine development landscape. Nat Rev Drug Discov; 2020: 19:305-306. [DOI] [PubMed] [Google Scholar]
- 6. Wibawa T. COVID-19 vaccine research and development: ethical issues. Trop Med Int Health. 2021; 26:14-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Polack FP, Thomas SJ, Kitchin N, et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med. 2020; 383:2603-2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Baden LR, El Sahly HM, Essink B, et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N Engl J Med. 2021; 384:403-416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gambhir SS. Molecular imaging of cancer with positron emission tomography. Nat Rev Cancer. 2002; 2:683-693. [DOI] [PubMed] [Google Scholar]
- 10. Minamimoto R, Hotta M, Ishikane M, Inagaki T. FDG-PET/CT images of COVID-19: a comprehensive review. Glob Health Med. 2020; 2:221-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Thomassen A, Lerberg Nielsen A, Gerke O, Johansen A, Petersen H. Duration of 18F-FDG avidity in lymph nodes after pandemic H1N1v and seasonal influenza vaccination. Eur J Nucl Med Mol Imaging. 2011; 38:894-898. [DOI] [PubMed] [Google Scholar]
- 12. Panagiotidis E, Exarhos D, Housianakou, Bournazos A, Datseris I. FDG uptake in axillary lymph nodes after vaccination against pandemic (H1N1). Eur Radiol. 2010; 20:1251-1253. [DOI] [PubMed] [Google Scholar]
- 13. Burger IA, Husmann L, Hany TF, Schmid DT, Schaefer NG. Incidence and intensity of F-18 FDG uptake after vaccination with H1N1 vaccine. Clin Nucl Med. 2011; 36:848-853. [DOI] [PubMed] [Google Scholar]
- 14. Xu X, Yu C, Qu J, et al. Imaging and clinical features of patients with 2019 novel coronavirus SARS-CoV-2. Eur J Nucl Med Mol Imaging. 2020; 47:1275-1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Deng Y, Lei L, Chen Y, Zhang W. The potential added value of FDG PET/CT for COVID-19 pneumonia. Eur J Nucl Med Mol Imaging. 2020; 47:1634-1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kirienko M, Padovano B, Serafini G, Marchianò A, Gronchi A, Seregni E, Alessi A. CT, [18F]FDG-PET/CT and clinical findings before and during early Covid-19 onset in a patient affected by vascular tumour. Eur J Nucl Med Mol Imaging. 2020; 47:1769-1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Centers for Disease Control and Prevention, Local Reactions, Systemic Reactions, Adverse Events, and Serious Adverse Events: Pfizer-BioNTech COVID-19 Vaccine. https://www.cdc.gov/vaccines/covid-19/info-by-product/pfizer/reactogenicity.html (accessed June 21, 2021).
- 18. Pardi N, Hogan M, Porter F, Weissman D. mRNA vaccines − a new era in vaccinology. Nat Rev Drug Discov. 2018; 17:261-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. McIntosh LJ, Rosen MP, Mittal K, Whalen GF, Bathini VG, Ali T, Edmiston KL, Walsh WV, Gerber JM. Coordination and optimization of FDG PET/CT and COVID-19 vaccination; Lessons learned in the early stages of mass vaccination. Cancer Treat Rev. 2021; 98:102220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Barrière J, Bondouy M. COVID arm and PET/FDG imaging. Bull Cancer. 2021; 108:668-669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ulaner GA, Giuliano P. 18F-FDG-Avid Lymph Nodes After COVID-19 Vaccination on 18F-FDG PET/CT. Clin Nucl Med. 2021; 46:433-434. [DOI] [PubMed] [Google Scholar]
- 22. Bernstine H, Priss M, Anati T, Turko O, Gorenberg M, Steinmetz AP, Groshar D. Axillary Lymph Nodes Hypermetabolism After BNT162b2 mRNA COVID-19 Vaccination in Cancer Patients Undergoing 18F-FDG PET/CT: A Cohort Study. Clin Nucl Med. 2021; 46:396-401. [DOI] [PubMed] [Google Scholar]
- 23. Eshet Y, Tau N, Alhoubani Y, Kanana N, Domachevsky L, Eifer M. Prevalence of Increased FDG PET/CT Axillary Lymph Node Uptake Beyond 6 Weeks after mRNA COVID-19 Vaccination. Radiology. 2021; doi: 10.1148/radiol.2021210886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Eifer M, Tau N, Alhoubani Y, Kanana N, Domachevsky L, Shams J, Keret N, Gorfine M, Eshet Y. Covid-19 mRNA Vaccination: Age and Immune Status and its Association with Axillary Lymph Node PET/CT Uptake. J Nucl Med. 2021; jnumed.121.262194. doi: 10.2967/ jnumed.121.262194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Seban RD, Champion L, Deleval N, Richard C, Provost C. Immune Response Visualized In Vivo by [18F]-FDG PET/CT after COVID-19 Vaccine. Diagnostics (Basel). 2021; 11:676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cohen D, Krauthammer SH, Wolf I, Even-Sapir E. Hypermetabolic lymphadenopathy following administration of BNT162b2 mRNA Covid-19 vaccine: incidence assessed by [18F]FDG PET-CT and relevance to study interpretation. Eur J Nucl Med Mol Imaging. 2021; 48:1854-1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Johnson BJ, Van Abel K, Ma D, Johnson DR. FDG Avid Axillary Lymph Nodes After COVID-19 Vaccination. J Nucl Med. 2021; jnumed.121.262108. doi: 10.2967/ jnumed.121.262108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Avner M, Orevi M, Caplan N, Popovtzer A, Lotem M, Cohen JE. COVID-19 vaccine as a cause for unilateral lymphadenopathy detected by 18F-FDG PET/CT in a patient affected by melanoma. Eur J Nucl Med Mol Imaging. 2021; 1-2. doi: 10.1007/s00259-021-05278-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nawwar AA, Searle J, Hagan I, Lyburn ID. COVID-19 vaccination induced axillary nodal uptake on [18F]FDG PET/CT. Eur J Nucl Med Mol Imaging. 2021; 1-2. doi: 10.1007/s00259-021-05274-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Eifer M, Eshet Y. Imaging of COVID-19 Vaccination at FDG PET/CT. Radiology. 2021; 299:E248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. McIntosh LJ, Bankier AA, Vijayaraghavan GR, Licho R, Rosen MP. COVID-19 Vaccination-Related Uptake on FDG PET/CT: An Emerging Dilemma and Suggestions for Management. AJR Am J Roentgenol. 2021; doi: 10.2214/AJR.21.25728. [DOI] [PubMed] [Google Scholar]
- 32. Adin ME, Isufi E, Kulon M, Pucar D. Association of COVID-19 mRNA Vaccine With Ipsilateral Axillary Lymph Node Reactivity on Imaging. JAMA Oncol. 2021; e211794. doi: 10.1001/jamaoncol.2021.1794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mingos M, Howard S, Giacalone N, Kozono D, Jacene H. Systemic Immune Response to Vaccination on FDG-PET/CT. Nucl Med Mol Imaging. 2016; 50:358-361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Coates EE, Costner PJ, Nason MC, Herrin DM, Conant S, Herscovitch P, Sarwar UN, Holman L, Mitchell J, Yamshchikov G, Koup RA, Graham BS, Millo CM, Ledgerwood JE; VRC 900 Study Team. Lymph node activation by PET/CT following vaccination with licensed vaccines for human papilloma viruses. Clin Nucl Med. 2017; 42:329-334. [DOI] [PubMed] [Google Scholar]
- 35. COVID-19 vaccine FAQs for healthcare professionals. Centers for Disease Control and Prevention. https://www.cdc.gov/vaccines/covid-19/hcp/faq.html (accessed June 21, 2021).
- 36. Schroeder DG, Jang S, Johnson DR, Takahashi H, Navin PJ, Broski SM, Thorpe MP, Johnson GB, Young JR. Frequency and Characteristics of Nodal and Deltoid FDG and 11C-Choline Uptake on PET Imaging Performed After COVID-19 Vaccination. AJR Am J Roentgenol. 2021; doi: 10.2214/AJR.21.25928. [DOI] [PubMed] [Google Scholar]
- 37. Nawwar AA, Searle J, Singh R, Lyburn ID. Oxford- AstraZeneca COVID-19 vaccination induced lymphadenopathy on [18F]Choline PET/CT-not only an FDG finding. Eur J Nucl Med Mol Imaging. 2021; 4:1-2. doi: 10.1007/s00259-021-05279-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Brewer KD, DeBay DR, Dude I, Davis C, Lake K, Parsons C, Rajagopalan R, Weir G, Stanford MM, Mansour M, Bowen CV. Using lymph node swelling as a potential biomarker for successful vaccination. Oncotarget. 2016; 735655-35669. [DOI] [PMC free article] [PubMed] [Google Scholar]


