Abstract
Background and purpose
Concurrent chemoradiation (cCRT) and durvalumab is standard therapy for patients with unresectable stage III non-small-cell lung cancers (NSCLC). Data is limited on outcomes with this regimen outside of clinical trials. Local-regional control rates remain undefined.
Materials and methods
We reviewed patients with stage III unresectable NSCLCs treated between November 2017 and February 2019 with cCRT and ≥1 dose of durvalumab. We examined 12-month progression-free-survival (PFS), overall-survival (OS), toxicities, and the incidence and pattern of local-regional and metastatic failures.
Results
Sixty-two patients (median follow-up 12 months) with median age of 66 years of which 73% had stage IIIB (n=33) or IIIC (n=12) disease started durvalumab a median of 1.5 months from the end of cCRT and were treated with a median of 8 months of durvalumab. Common reasons for stopping durvalumab included disease progression (32%, 20/62) and toxicity (24%, 15/62). The estimated 12-month PFS and OS were 65% (95% CI: 51–79%) and 85% (95% CI: 75 – 95%), respectively. The cumulative 12-month incidence of local-regional and distant failures were 18% (95% CI: 5.9 – 30%) and 30% (95% CI: 16.3 – 44.5%), respectively. Among patients with distant metastatic disease (n=17), 47% had oligometastatic disease. High tumor mutation burden (≥ 8.8 mt/Mb) or PD-L1 (≥ 1% or PD-L1 ≥ 50%) did not predict improved PFS.
Conclusions
Outcomes with cCRT and durvalumab in practice align with the PACIFIC trial. A substantial minority of patients are candidates for metastasis-directed therapies at progression. Local regional outcomes appear improved to historical data of cCRT alone.
Keywords: non-small cell, concurrent chemoradiation, durvalumab, local-regional control, metastasis-directed therapies
Introduction:
Concurrent chemoradiation (cCRT) followed by durvalumab in patients with stage III unresectable non-small cell lung cancers (NSCLCs) significantly improves survival outcomes and is now a standard of care 1, 2. In the PACIFIC trial, adding durvalumab resulted in an 11% absolute 2-year overall survival (OS) benefit and more than tripled the median progression-free survival (PFS; 17 vs 6 months) compared to chemoradiation alone 1. These improved outcomes were noted with a similar safety profile and without a detriment to quality of life measures as compared to cCRT alone 1, 3,4. However, outside of this trial, there is little known on the outcomes of patients treated per the PACIFIC regimen. Furthermore, local-regional control outcomes among patients treated on the PACIFIC protocol are poorly understood and complicated by the limited details on the radiation treatment among patients in the PACIFIC trial.
Local-regional control (LRC) is independently associated with long-term survival and remains a challenge in patients with stage III NSCLCs treated with chemoradiation5. Data prior to the PACIFIC trial have found nearly a third of patients to have local-regional relapse within one year and therefore radiation dose-escalation strategies have been explored to improve outcomes 5–8. However, the impact of adding durvalumab after chemoradiation on LRC remains undefined to date. Additionally, phase II data suggest a role for metastasis-directed therapies in oligometastatic NSCLCs to improve disease outcomes 9, 10, however the proportion of patients with progression on durvalumab eligible for metastasis-directed therapies is unknown. To examine this, we reviewed our institutional experience with patients with stage III NSCLCs treated with cCRT and durvalumab to report the largest series assessing real-world clinical outcomes and failure patterns and to determine LRC and the potential role of metastasis-directed therapies in patients treated with the PACIFIC regimen.
Materials and Methods:
Patients and Treatment:
We analyzed patients with American Joint Committee on Cancer (AJCC) 8th edition stage III NSCLCs treated at our institution between November 2017 and February 2019 who received curative intent cCRT, at least one dose of durvalumab, and a minimum of 6 months follow-up11. Before treatment, patients underwent a physical examination, computed tomography (CT) scan of the chest, abdomen and pelvis and/or whole-body fluorine-18 fluorodeoxyglucose positron emission tomography (PET), and magnetic resonance imaging (MRI) of the head. The radiation prescription dose ranged from 54Gy to 66Gy and was delivered in 2 Gy fractions using intensity-modulated radiation therapy per institutional standards. Patients had a PET scan available for target delineation. Treatment planning included a 4-dimensional CT simulation, wherein the gross tumor volume was contoured on the free-breathing CT scan using guidance from the diagnostic PET. No elective nodal irradiation was used. An internal target volume margin was added based off respiratory motion, to which a 5–7 mm clinical target volume expansion was added to encompass microscopic disease, and a 5 mm planning target volume expansion was added to account for day-to-day setup uncertainty. Patients were treated with platinum-based chemotherapy concurrent with radiation. Patients without disease progression or persistent chemoradiation toxicity after the completion of chemoradiation, received durvalumab (10mg/kg) every two weeks for up to 12 months (26 doses). Imaging with chest CT was performed every 3 months, or more frequently as clinically indicated. Follow-up brain imaging among asymptomatic patients was not performed. All patients suspected of disease progression underwent PET/CT imaging and repeat MRI brain, and whenever feasible, biopsy.
Analysis:
We collected age, sex, stage, smoking history, histology, Eastern Cooperative Oncology Group (ECOG) performance status, programmed death-ligand 1 (PD-L1) expression, EGFR, KRAS, STK11, and KEAP1 mutational status, tumor mutational burden (TMB), time to durvalumab start from end of radiotherapy, and chemotherapy drugs received. We assessed for association with progression-free survival (PFS) using Cox proportional hazards modeling. Baseline characteristics were associated with PFS using univariate Cox proportional hazards modelling. PD-L1 was evaluated as a categorical variable, with PD-L1 positivity defined as ≥1% expression. PD-L1 immunohistochemistry was evaluated using the E1L3N antibody (Cell Signaling Technology, Danvers, MA), which has been validated against a 22C3 kit performed in a commercial laboratory with comparable results 12. Patients with available material and consent underwent targeted next generation sequencing (NGS) with our institutional platform, MSK-IMPACT™ (Integrated Mutation Profiling of Actionable Cancer Targets) 13, 14. TMB was calculated as the total number of somatic nonsynonymous mutations normalized to the total number of megabases sequenced and was reported as mutations/megabase (mt/Mb). TMB was evaluated as a categorical variable based on the median TMB of the entire cohort.
PFS was defined as time from durvalumab initiation to any disease-progression or death. Overall survival was defined as the time from durvalumab initiation to death from any cause. Kaplan-Meier analysis was used to determine 12-month survival outcomes and 95% confidence intervals (95% CI). Pneumonitis and toxicities leading to the discontinuation of durvalumab were graded using Common Terminology Criteria for Adverse Events (CTCAE) version 5.0. The clinical course and chest CT imaging of patients with pneumonitis were also assessed. A time-dependent univariate Cox proportional hazards regression model was analyzed to determine if patients who discontinued durvalumab due to toxicity had inferior PFS. Radiation treatment plans and dose distributions were reviewed in all patients with thoracic progression to categorize local-regional failure. Local-regional failure was classified as “in-field” if disease progression occurred within the 90% isodose volume, “marginal” if within or adjacent to ≥50% isodose volume, and “out-of-field” if it occurred as regional nodal failure outside of the ≥50% isodose volume. Distant failure was defined as metastatic disease progression per AJCC 8th edition staging. Local-regional failure and distant failure incidence rates were defined from the time of durvalumab start to disease progression. An additional measure of local-regional failure was defined from the start of cCRT to disease progression to allow for comparison with historical data prior to the PACIFIC trial. Patients were considered potential candidates for comprehensive ablative therapy at relapse if they had oligometastatic disease based solely on imaging criteria including: no progression of the primary tumor or nodal disease treated with radiation, no pleural effusion, and ≤5 discrete sites of disease 10. This study approved by the institutional review board and performed in accordance with the United States Common Rule. All statistical computations were performed using SAS Software Version 9.4 (The SAS Institute, Cary, NC).
Results:
We identified 83 consecutive patients with stage III NSCLCs treated with definitive-intent cCRT. Sixty-two (75%) of these patients then received durvalumab and were included in this analysis. The remaining 21 individuals did not receive durvalumab, most typically due to rapid distant disease-progression (n=9) or persistent cCRT toxicity (n=10) 15. The median follow-up from the start of durvalumab was 12 months (range: 6 – 20 months). Median patient age was 66 years, 58% (n=36) were male, 97% (n=60) were ever smokers, 73% (n=45) had stage IIIB or IIIC disease, 53% (n=33) were ECOG 0 and 58% (n=36) had tumors with adenocarcinoma histology (Table 1). PD-L1 was available in 81% (n=50) and TMB in 69% (n=43) (Table 1).
Table 1:
Baseline Characteristics
| Patients (n=62)* | |
|---|---|
| Sex | |
| Female | 42% (n=26) |
| Male | 58% (n=36) |
| Age (years) | |
| Median (Range) | 66 (49–86) |
| Performance Status | |
| ECOG 0 | 53% (n=33) |
| ECOG 1 | 47% (n=29) |
| Smoking History | |
| Yes | 97% (n=60) |
| Histology | |
| Adenocarcinoma | 58% (n=36) |
| Squamous Cell | 31% (n=19) |
| Other | 11% (n=7) |
| AJCC 8th Edition Stage | |
| IIIA | 27% (n=17) |
| IIIB | 53% (n=33) |
| IIIC | 19% (n=12) |
| T-Stage | |
| T1/T0 | 26% (n=16) |
| T2 | 23% (n=14) |
| T3 | 26% (n=16) |
| T4 | 26% (n=16) |
| N-Stage | |
| N0 | 2% (n=1) |
| N1 | 5% (n=3) |
| N2 | 47% (n=29) |
| N3 | 47% (n=29) |
| EGFR mutational status | |
| Negative | 81% (n=50) |
| Positive | 2% (n=1) |
| Unavailable | 18% (n=11) |
| PD-L1 Expression (n=50) | |
| < 1% | 34% (n=17) |
| ≥ 1%–49 | 30% (n=15) |
| ≥ 50% | 36% (n=18) |
| KRAS mutational status (n=43) | |
| Negative | 58% (n=25) |
| Positive | 42% (n=18) |
| STK11 mutational status (n=43) | |
| Negative | 81% (n=35) |
| Positive | 19% (n=8) |
| KEAP mutational status (n=43) | |
| Negative | 77% (n=33) |
| Positive | 23% (n=10) |
Patient characteristics are from time of initiation of radiation therapy
Note: due to rounding, not all percentages sum to exactly 100%
Abbreviations: ECOG, Eastern Cooperative Oncology Group; PD-L1, programmed death-ligand 1
Patients (n=62) were treated to a median of 60Gy and received concurrent platinum plus pemetrexed (52%, n=62), paclitaxel (37%, n= 23), or etoposide (11%, n=7). In total, 68%% (n=42) received carboplatin and 32% (n=20) received cisplatin. Patients started their first cycle of durvalumab a median of 1.5 months from the end of radiation (range 0.3 – 7.7 months). Patients were treated with median of 18 of a planned 24 doses (IQR: 4 –24) of durvalumab over a median of 8 months (IQR: 2.2 – 12 months) and 35% (n=22) of patients completed 12 months of therapy.
Among evaluable patients, 24% (n=15) discontinued durvalumab due to treatment-related adverse events a median of 1.4 months after their first infusion (IQR: 0.5 – 4.4 months). Patients who discontinued durvalumab due to adverse events were treated with a median of 3 cycles of durvalumab (range: 1–17 cycles). The most common toxicity leading to discontinuation was pneumonitis with 11 cases of grade 2 and one grade 3. Other toxicities leading to durvalumab discontinuation included myositis (n=1), neuropathy (n=1), and arthralgias/rash (n=1). There were no grade 4 or 5 toxicities.
Among the 12 patients who discontinued durvalumab due to pneumonitis, symptom onset was a median of 1.5 months after the first durvalumab infusion (range: 0.5 – 7.8 months), and 3.2 months from the end of cCRT (range: 2.3 – 8.8 months). Chest CT findings in 10 patients included ground-glass opacities and consolidations mostly limited to the radiation field, whereas 2 patients had more diffuse CT imaging findings. All 12 patients with pneumonitis symptoms were treated with high-dose corticosteroids with an improvement in symptoms.
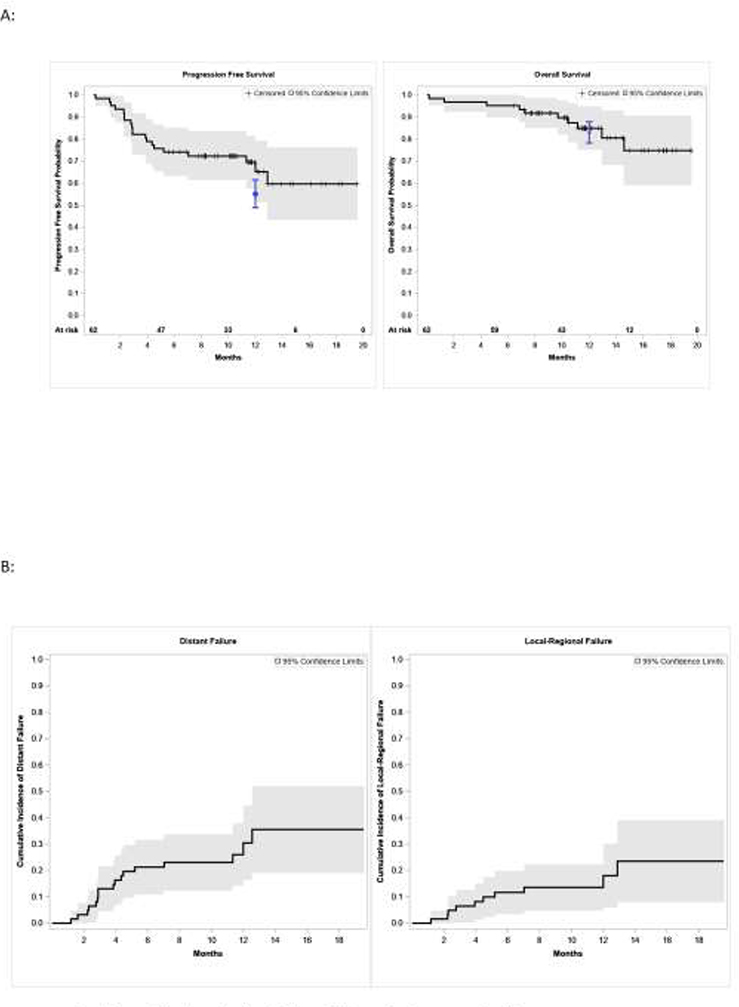
The 12-month PFS was 65% (95% CI: 51 – 79%) and the 12-month OS was 85% (95% CI: 75 – 94.8%) (Figure 1A and B). The 12-month incidence of local-regional and distant failure defined from the start of durvalumab initiation were 18% (95% CI: 5.9 – 30%) and 30% (95% CI: 16.3 – 44.5%), respectively (Figure 1C, Figure 1D). Age, ECOG performance status, stage, time to durvalumab start from end of cCRT, and receipt of either pemetrexed, paclitaxel or etoposide did not predict for poor PFS on univariate analysis. Molecular characteristics includingTMB ≥ 8.8 mt/Mb, PD-L1 ≥ 1% or PD-L1 ≥ 50% did not predict poorer PFS (Table 2). Patients who discontinued durvalumab due to toxicity did not have inferior PFS (hazards ratio: 1.4, 95% CI: 0.45 – 4.10, p=0.59)
Figure 1.
Outcomes and failures in patients treated with concurrent chemoradiation and durvalumab. (A) Progression-free survival and overall survival, and (B) incidence of local-regional and distant failure as calculated from durvalumab initiation. The 12-month PFS was 65% (95% CI: 51–79%) and the 12-month OS was 85% (95% CI: 75–95%). In blue, the respective 12-month PFS and OS estimates and 95% CI of patients treated in the PACIFIC study is shown (1). The numbers of patients at risk are noted above the x-axis in the survival curves. Shading represents that 95% confidence intervals.
Figure 2.
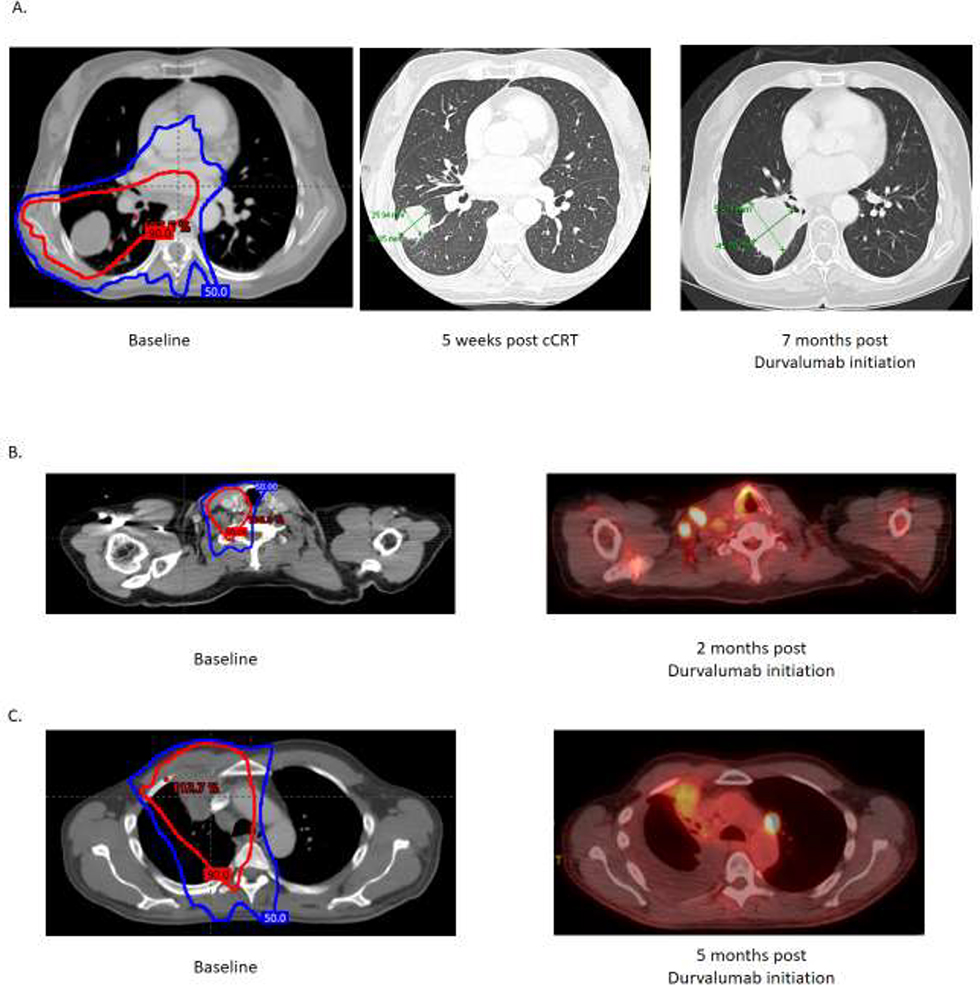
Representative local-regional disease failure patterns: A) in-field progression of primary tumor within 90% dose volume B) marginal progression of supraclavicular adenopathy adjacent to 50% dose volume C) out-of-field progression of subaortic adenopathy outside 50% dose volume. The 50% radiation dose volume (blue) and 90% dose volume (red) are shown.
Table 2:
Univariate Analysis of Predictors for Progression-Free Survival
| Variable | HR (95% CI) | p-value | |
|---|---|---|---|
| Age | 1.01 (0.96–1.06) | 0.70 | |
| Sex | 0.76 | ||
| Male | Ref. | ||
| Female | 0.87 (0.36–2.13) | ||
| ECOG performance status | 0.62 | ||
| 0 | Ref. | ||
| 1 | 1.25 (0.52–3.01) | ||
| Stage | 0.30 | ||
| IIIB | Ref. | ||
| IIIA | 0.38 (0.11–1.35) | ||
| IIIC | 1.02 (0.36–2.92) | ||
| N-Stage | 0.80 | ||
| N0-N2 | Ref. | ||
| N3 | 1.12 (0.46–2.69) | ||
| T-Stage | 0.33 | ||
| T0-T2 | Ref. | ||
| T3-T4 | 1.55 (0.63–3.81) | ||
| Programmed death-ligand 1 (PD-L1) expression | 0.38 | ||
| < 1% | Ref. | ||
| ≥ 1% | 0.64 (0.24–1.72) | ||
| TMB (mt/Mb) | 0.34 | ||
| < 8.8 | Ref. | ||
| ≥ 8.8 | 1.69 (0.58–4.90) | ||
| Time to Durvalumab | 0.68 (0.40–1.17) | 0.17 | |
| Grade ≥ 2 Pneumonitis | 0.84 | ||
| No | Ref. | ||
| Yes | 0.89 (0.30–2.67) | ||
The incidence of local-regional failure at 12-months defined from the start of cCRT was 14% (95% CI: 4.7 – 22.3%). Among the 10 patients with local-regional failure, five patients had in-field failures, four patients had marginal failures and one patient had out-of-field failure. Representative disease failure patterns with comparison to radiation volumes are shown in Figure 2. Among the patients with in-field failures (n=5), three had progression of their primary tumor, and the two remaining patients had progression of nodal disease within the 100% isodose volume. Among patients with local-regional failures (n=10), 9 also had distant metastatic disease progression at the time of local-regional failure (Table 3).
Table 3:
Disease Progression Patterns and Candidacy for Metastasis-Directed Ablative Therapy
| Patient | Brain | Thorax | Abdominal | Osseous | Local-Regional Failure? | Distant Metastasis? | Ablative Candidate* |
|---|---|---|---|---|---|---|---|
| 1 | N | Y | N | N | Marginal | N | Y |
| 2 | Y | Y | N | Y | In-Field | Y | N |
| 3 | N | Y | N | N | Marginal | Y | Y |
| 4 | N | Y | N | N | Out-of-Field | Y | N |
| 5 | N | Y | N | N | Marginal | Y | Y |
| 6 | N | Y | Y | N | In-Field | Y | N |
| 7 | N | Y | N | Y | In-Field | Y | N |
| 8 | N | Y | N | N | In-Field | Y | N |
| 9 | Y | Y | Y | N | In-Field | Y | N |
| 10 | N | Y | N | N | Marginal | Y | Y |
| 11 | Y | N | N | N | N | Y | Y |
| 12 | N | Y | N | N | N | Y | Y |
| 13 | N | N | Y | N | N | Y | Y |
| 14 | N | Y | Y | N | N | Y | N |
| 15 | N | Y | Y | N | N | Y | Y |
| 16 | Y | N | N | N | N | Y | Y |
| 17 | N | Y | N | N | N | Y | N |
| 18 | N | Y | N | N | N | Y | N |
Candidacy for Ablative Therapy: ≤5 discrete sites of disease, no progression of treated primary tumor, no pleural effusion
In total, 27% (17/62) of patients had distant metastatic disease as their first disease progression event; 7 (41%) of these patients had metastases limited only to the thorax (pleural nodules [n=1], malignant pleural effusion [n=4], and contralateral lung disease [n=4]). Most patients with metastatic progression also had a concurrent intrathoracic recurrence (88%, n=15) at first progression. Eight patients (47%) with metastatic disease would have been potential candidates for comprehensive ablative therapy based on imaging analysis (Table 3).
Discussion:
We report the largest series of patients with stage III unresectable NSCLC treated with cCRT and durvalumab outside of the PACIFIC trial. Our data suggest the improvement in survival noted in the PACIFIC trial held true with comparable 12-month outcomes. We also provide the first assessment of LRC in patients treated with chemoradiation and durvalumab that suggests a notable impact of durvalumab on improving LRC compared to historical data of cCRT alone. Additionally, we found that nearly half of the patients with recurrence may be candidates for metastasis-directed therapy at first progression.
The 12-month PFS and OS estimates in our cohort (65% and 85%, respectively) compared favorably to that seen in PACIFIC (56% and 83%, respectively) 1. These outcomes are improved when compared to historical data from our institution in patients with stage III NSCLCs treated with definitive chemoradiation alone 16. Patient age, sex, ECOG performance status, stage, time to initiation of durvalumab, or PD-L1 expression level were not associated with PFS in our series. The tolerance of durvalumab in our patients appears mostly consistent with the PACIFIC trial data; however, more patients in discontinued durvalumab due to toxicity. We did not find high-grade ≥ 4 CTCAE toxicities in patients, consistent with the <5% grade 5 toxicity rate reported in the PACIFIC trial. Similar to the PACIFIC trial, the most common cause for treatment-related discontinuation of durvalumab was pneumonitis. More patients in this report (24%) discontinued durvalumab due to treatment-related toxicity than in the PACIFIC trial (15%). However, more patients in their report were of older age and therefore potentially frailer compared to the rigorously screened PACIFIC cohort. Most patients were also stage IIIB and IIIC, which often require larger radiation volumes and therefore could have impacted our incidence of pneumonitis. However, we did not find patients who discontinued durvalumab due to treatment-related toxicity to have inferior PFS, potentially suggesting a benefit of durvalumab therapy even with early discontinuation.
Half of patients treated with cCRT without durvalumab have local-regional relapse within 2 years 17. Local-regional control is a critical outcome, independently associated with overall survival in stage III NSCLCs 5. Given that adding durvalumab improved the objective response rate of intrathoracic disease in the PACIFIC trial, a benefit to LRC would be anticipated 18. Prior data have found 12-month local-regional progression to be between 24 – 37% among patients treated with cCRT alone 5, 19, 20. Therefore, the 14% local-regional progression rate at 12-months seen in our patients suggests that adding durvalumab markedly improves LRC. Interestingly, the LRC outcomes we found in this report compare favorably to the 12-month results seen in recent phase II radiation dose-escalation trials using advanced radiation planning or high-dose proton beam radiotherapy 6, 7. Local-regional failure at 12 months has been estimated to be nearly 20% using either mid-treatment PET/CT to target dose-escalation up to 86Gy or with high-dose (74Gy) proton beam therapy 6, 7. Therefore, given the LRC achieved with 60Gy cCRT and durvalumab, the role of radiation dose-escalation in the era of the PACIFIC regimen requires further investigation.
A recent analysis of the PACIFIC trial found most patients with progression had limited sites of extrathoracic disease, suggesting a potential role for ablative, oligometastases-directed therapies 21. Phase II data in patients with non-disseminated NSCLCs with primary tumor control have found metastasis-directed therapies to significantly improve disease outcomes 10. Data on the use of stereotactic-body radiation therapy (SBRT) among patients with advanced NSCLCs with progression on pembrolizumab found a subset of patients have prolonged disease control after metastasis-directed SBRT 22. Based on imaging criteria alone, we found that 47% of patients with metastatic disease progression would have been candidates for metastasis-directed ablative therapies, supporting future evaluations of such ablative strategies in patients with progression on the PACIFIC regimen. However, the most common relapse pattern was disseminated disease, again highlighting the need for better systemic therapies 23–25.
This series was collected at a single tertiary cancer center. The results presented here, however, are consistent with the PACIFIC trial despite the fact our cohort is older and has a more advanced stage population than the PACIFIC trial population. PD-L1, TNB and NGS results were not associated with outcomes, however, these results were not available for all patients. Additionally, further studies are warranted assessing clinical outcomes among patients that receive comprehensive ablative therapies at first progression to better determine the value of this approach. Furthermore, although this is the largest series of patients with stage III unresectable NSCLC treated with cCRT and durvalumab outside of the PACIFIC trial, and the first analysis to define LRC in patients treated with consolidative durvalumab, our patient numbers are still somewhat limited, and further analysis is encouraged.
In conclusion, this assessment of durvalumab after cCRT in stage III NSCLC found disease outcomes consistent with data from the PACIFIC trial, found the regimen led to favorable local-regional control and in addition quantified the potential role of metastasis-directed therapies in patients with recurrent disease. Further studies are needed to better identify predictors of response to the PACIFIC regimen and elucidate strategies to combine and intensify therapies to improve outcomes for patients with unresectable stage III NSCLCs.
Highlights.
Disease control and toxicity outcomes with concurrent chemoradiation (cCRT) and durvalumab in clinical practice appear consistent with the outcomes described in the PACIFIC trial.
Patients treated with cCRT and durvalumab appear to have improved local-regional control compared to historical data of patients treated with cCRT alone.
Nearly half of patients appear to have oligometastatic disease at first progression and may be candidates for metastasis-directed therapies.
Funding:
This work was supported by the National Institutes of Health [grant number P30 CA008748].
Declaration of Competing Interest:
Jamie E. Chaft: Reports consulting and research funds from BMS, Genentech, AZ, Merck. Annemarie F. Shepard: Reports travel reimbursement and consulting from ASCO.
Charles B. Simone II: Reports that he has received honorarium from Varian Medical Systems.
Andreas Rimner: Reports consulting with AstraZeneca, Varian Medical Systems, Merck, Cybrexa, MoreHealth, research grants from AstraZeneca, Varian Medical Systems, Boehringer Ingelheim, Pfizer, Merck, and travel reimbursement from Philips/Elekta.
Charles Rudin: Reports consulting/advisory roles with AbbVie, Amgen, Ascentage, Astra Zeneca, BMS, Celgene, Daiichi Sankyo, Genentech/Roche, J&J, Loxo, Pharmamar, Vavotek, Harpoon, Bridge Medicines, and research funding from AbbVie, Daiichi Sankyo, Pfizer, Viralytics, Merck
Abraham J. Wu: Reports research grants from CivaTech Oncology, personal fees from AstraZeneca, and travel support from AlphaTau Medical.
Mathew D. Hellmann: Reports personal fees from Genentech, grants, personal fees and non-financial support from Bristol Myers Squibb, personal fees from Merck, personal fees and non-financial support from AstraZeneca, personal fees from Mirati, personal fees from Syndax, personal fees and equity from Shattuck Labs, personal fees and equity from Immunai, personal fees from Nektar, personal fees from Blueprint Medicines. In addition, Dr. Hellmann has a pending patent Determinants of Cancer Response to Immunotherapy by PD-1 Blockade (PCT/US2015/062208) licensed to PGDx.
Daniel R. Gomez: Reports honoraria from Merck, BMS, AstraZeneca, Reflexion, Medscape, Vindico, US Oncology, and Varian. Reports research support from Merck, BMS, AstraZeneca, and Varian. Serves on advisory board for AstraZeneca.
Daphna Y. Gelblum: No COI to report.
Narek Shaverdian: No COI to report.
Stephanie M Lobaugh: No COI to report.
Zhigang Zhang: No COI to report.
Nancy Lee: Reports consultant/advisory board roles with Pfizer, Merck, Merck Serono, and research funding from Pfizer, Astra Zeneca.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.Antonia SJ, Villegas A, Daniel D, et al. Overall Survival with Durvalumab after Chemoradiotherapy in Stage III NSCLC. The New England journal of medicine 2018;379:2342–2350. [DOI] [PubMed] [Google Scholar]
- 2.Network NCC. Non-Small Cell Lung Cancer (Version 3. 2020). Available at https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf. Accessed February 16, 2020
- 3.Hui R, Özgüroglu M, Villegas A, et al. Patient-reported outcomes with durvalumab after chemoradiotherapy in stage III, unresectable non-small-cell lung cancer (PACIFIC): a randomised, controlled, phase 3 study. The Lancet Oncology. [DOI] [PubMed]
- 4.Hui R, Ozguroglu M, Villegas A, et al. Patient-reported outcomes with durvalumab after chemoradiotherapy in stage III, unresectable non-small-cell lung cancer (PACIFIC): a randomised, controlled, phase 3 study. The Lancet Oncology 2019;20:1670–1680. [DOI] [PubMed] [Google Scholar]
- 5.Machtay M, Paulus R, Moughan J, et al. Defining local-regional control and its importance in locally advanced non-small cell lung carcinoma. J Thorac Oncol 2012;7:716–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kong F-M, Ten Haken RK, Schipper M, et al. Effect of Midtreatment PET/CT-Adapted Radiation Therapy With Concurrent Chemotherapy in Patients With Locally Advanced Non–Small-Cell Lung Cancer: A Phase 2 Clinical Trial. JAMA oncology 2017;3:1358–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang JY, Verma V, Li M, et al. Proton Beam Radiotherapy and Concurrent Chemotherapy for Unresectable Stage III Non–Small Cell Lung Cancer: Final Results of a Phase 2 Study. JAMA oncology 2017;3:e172032–e172032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bradley JD, Paulus R, Komaki R, et al. Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-celllungcancer(RTOG 0617): a randomised, two-by-two factorial phase 3 study. The Lancet Oncology 2015;16:187–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gomez DR, Tang C, Zhang J, et al. Local Consolidative Therapy Vs. Maintenance Therapy or Observation for Patients With Oligometastatic Non-Small-Cell Lung Cancer: Long-Term Results of a Multi-Institutional, Phase II, Randomized Study. Journal of clinical oncology: official journal of the American Society of Clinical Oncology 2019;37:1558–1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Palma DA, Olson R, Harrow S, et al. Stereotactic ablative radiotherapy versus standard of care palliative treatment in patients with oligometastatic cancers (SABR-COMET): a randomised, phase 2, open-label trial. Lancet (London, England) 2019;393:2051–2058. [DOI] [PubMed] [Google Scholar]
- 11.Edge SB, Byrd DR, Carducci MA, et al. AJCC cancer staging manual. Springer; New York; 2010. [Google Scholar]
- 12.Gaule P, Smithy JW, Toki M, et al. A Quantitative Comparison of Antibodies to Programmed Cell Death 1 Ligand 1. JAMA oncology 2017;3:256–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheng DT, Mitchell TN, Zehir A, et al. Memorial Sloan Kettering-Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT): A Hybridization Capture-Based Next-Generation Sequencing Clinical Assay for Solid Tumor Molecular Oncology. The Journal of molecular diagnostics: JMD 2015;17:251–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zehir A, Benayed R, Shah RH, et al. Mutational landscape of metastatic cancer revealed from prospective clinical sequencing of 10,000 patients. Nature medicine 2017;23:703–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shaverdian N, Offin MD, Rimner A, et al. Utilization and factors precluding the initiation of consolidative durvalumab in unresectable stage III non-small cell lung cancer. Radiotherapy and Oncology 2020;144:101–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shepherd AF, Leeman JE, Wild A, et al. A Comparison of Trimodality Therapy Versus Definitive Concurrent Chemoradiation in Patients With Stage IIIA Non–small Cell Lung Cancer. International Journal of Radiation Oncology • Biology • Physics 2017;99:E495–E496. [Google Scholar]
- 17.Machtay M, Bae K, Movsas B, et al. Higher Biologically Effective Dose of Radiotherapy Is Associated With Improved Outcomes for Locally Advanced Non–Small Cell Lung Carcinoma Treated With Chemoradiation: An Analysis of the Radiation Therapy Oncology Group. International Journal of Radiation Oncology*Biology*Physics 2012;82:425–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Antonia SJ, Villegas A, Daniel D, et al. Durvalumab after Chemoradiotherapy in Stage III Non–Small-Cell Lung Cancer. New England Journal of Medicine 2017;377:1919–1929. [DOI] [PubMed] [Google Scholar]
- 19.Jaksic N, Chajon E, Bellec J, et al. Optimized radiotherapy to improve clinical outcomes for locally advanced lung cancer. Radiation Oncology 2018;13:147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bradley JD, Paulus R, Komaki R, et al. A randomized phase III comparison of standard-dose (60 Gy) versus high-dose (74 Gy) conformal chemoradiotherapy with or without cetuximab for stage III non-small cell lung cancer: Results on radiation dose in RTOG 0617. Journal of Clinical Oncology 2013;31:7501–7501. [Google Scholar]
- 21.Raben D, Rimner A, Senan S, et al. Patterns of Disease Progression with Durvalumab in Stage III Non-small Cell Lung Cancer (PACIFIC). International Journal of Radiation Oncology • Biology • Physics 2019;105:683. [Google Scholar]
- 22.Campbell AM, Cai WL, Burkhardt D, et al. Final Results of a Phase II Prospective Trial Evaluating the Combination of Stereotactic Body Radiotherapy (SBRT) with Concurrent Pembrolizumab in Patients with Metastatic Non-Small Cell Lung Cancer (NSCLC). International Journal of Radiation Oncology • Biology • Physics 2019;105:S36–S37. [Google Scholar]
- 23.Moding EJ, Liu Y, Nabet B, et al. ctDNA analysis for personalization of consolidation immunotherapy in localized non-small cell lung cancer. 2019;37:2547–2547. [Google Scholar]
- 24.Césaire M, Thariat J, Candéias SM, et al. Combining PARP inhibition, radiation, and immunotherapy: A possible strategy to improve the treatment of cancer? Int J Mol Sci 2018;19:3793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paz-Ares L, Garon EB, Ardizzoni A, et al. 1587TiPCANOPY phase III program: Three studies evaluating canakinumab in patients with non-small cell lung cancer (NSCLC). Annals of Oncology 2019;30. [Google Scholar]