Abstract
The risk of rectal toxicity during and after prostate cancer radiation therapy is common to all treatment regimens. Hydrogel rectal spacers are increasingly being used to mitigate this risk and to facilitate dose-escalation, but also may infiltrate the rectal wall, with unclear clinical implication. We present a case of significant infiltration associated with severe late rectal injury (grade 4) and further grade 3 to 4 sequelae (recto-urethral fistula and associated osteomyelitis requiring exenteration) after high-dose stereotactic body radiation therapy for localized prostate cancer. The injury's temporal pattern associated with the expected timing of gel dissolution and displacement of infiltrated rectal layers potentially toward high dose regions together suggest a contributing role of the infiltration to the injury. In light of the rapid increase of hydrogel rectal spacer utilization, we review the case's evolution, concerning imaging findings, and associated literature and make suggestions regarding treatment planning and endoscopic assessment in the setting of infiltration or expected injury.
Introduction
Radiation therapy (RT) for prostate cancer remains associated with a dose-dependent and dose-limiting risk of rectal toxicity.1 Contemporary dose-escalated RT regimens,2, 3, 4, 5 have improved biochemical control at the cost of higher rectal toxicity. Temporary hydrogel spacer placement between the prostate and rectum is an increasingly used method to reduce such risk, with a randomized control trial demonstrating decreased grade 1 to 2 rectal toxicity and improved bowel domain patient-reported quality of life scores.6 Commercially available products are typically absorbed within 3 to 6 months after placement, performed by transrectal ultrasound-guided transperineal approach.7 Although placement is generally well tolerated,8 postapproval surveillance of unexpected and potentially related injuries remains important to ensuring patient safety. To this end, we present a case of suspected contribution of significant hydrogel spacer gel rectal wall infiltration to a high-grade rectal injury and subsequent grade 3 to 4 urethral/infectious sequelae in a man undergoing high-dose RT for prostate cancer.
Case Presentation
A 62-year-old man received a diagnosis of intermediate risk prostate cancer (cT1cN0M0, prostate-specific antigen 5.9 ng/mL, ISUP grade group 2). Biopsy was performed of 3 magnetic resonance imaging (MRI)-defined lesions by MRI in-bore transrectal biopsy method (8/9 cores positive from 3 lesions, range 15%-100% positive with maximum pattern 4 of 30%) without complication 3 months before therapy. He had no history of abdominopelvic surgical procedures, repeated prostate biopsies, major comorbidities, ongoing anticoagulant use or inflammatory bowel disease. He elected treatment with ultrahypofractionated RT using high-dose stereotactic ablative radiation therapy (SAbR) without androgen deprivation therapy.
In preparation, he underwent transrectal ultrasound-guided transperineal placement of a commercial hydrogel spacer (SpaceOAR, Boston Scientific) and gold fiducials under moderate sedation, as described.9 Both credentialed Urologists and Radiation Oncologists perform this procedure at our institution, and this procedure was performed by the treating radiation oncologist, who at the time had performed >200 such procedures. Visualization of needle placement was performed under both sagittal/axial views in a “dual” view mode. There was no apparent procedural complication or symptoms after the transient discomfort of the actual procedure. Computed tomography (CT)/MRI simulation was used to delineate prostate, standard organs at risk and spacer gel distribution. MRI included T1- and T2-weighted sequences with fast spoiled gradient sequence particularly for fiducial recognition to aid fusion to CT. Simulation procedures included enema for rectal emptying, bladder filling, vacuum mold from arms (up) to mid-thigh, and indexed stereotactic frame. Prostate volume was expanded 3 mm to create the planning target volume. MRI appeared to indicate adequate displacement of rectum from prostate, ranging from 1.2 to 1.5 cm along the craniocaudal axis of the prostate. In retrospect, however, significant anterior rectal wall infiltration was present. In particular, an area of deeper infiltration with “delamination” or discontinuity of the muscularis propria was identified (Fig. 1). SAbR was prescribed to an escalated dose of 45 Gy in 5 fractions, delivered on nonconsecutive days over 3 weeks using volumetric modulated arc therapy, after treatment delivery and institutional constraints based upon data from successive phase 2 to 3 trials of SAbR at our institution,10,11 including a trial specifically focused on use of rectal spacer and modified rectal constraints to reduce associated toxicity. Notably, rectal dosimetry (V24 Gy was ~33% of rectal circumference vs 50% limit, V39 Gy was ~10% of rectal circumference vs 35% limit, max dose to 0.035 mL volume was 43.8 Gy) was well within these limits.12, 13, 14, 15 Moreover, the rectal dosimetry met constraints of an ongoing collaborative group trial of SAbR NRG GU005 (NCT03367702) for all but the volumetric maximum constraint, as shown in Table 1. However, doses to the spacer gel in the adjacent area of infiltration ranged 20.7 to 48.6 Gy, creating the potential that poorly visualized layer of muscle wall may yet have received higher doses than anticipated.
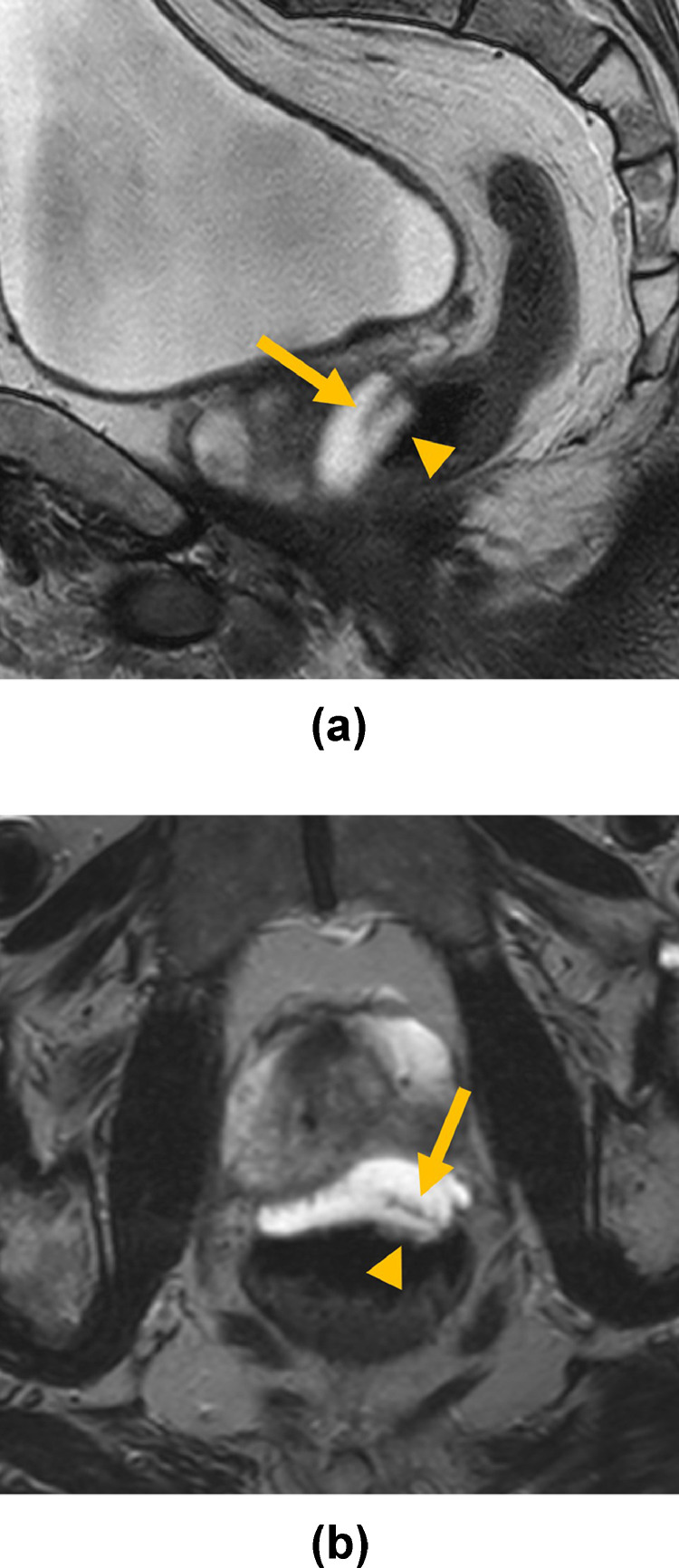
Fig. 1.
Sagittal (A) and axial (B) T2-weighted treatment planning magnetic resonance imaging show infiltration of hydrogel within the rectal wall with delamination and discontinuity of the muscularis propria (arrows) resulting in accumulation of spacer material in the submucosa (arrowheads).
Table 1.
Comparison of rectal dose constraints in NRG GU005 to dosimetric values achieved in the presented treatment
| Rectal dose volume | NRG GU005 (Gy) | Case (Gy) |
|---|---|---|
| Point Dose | <38.06 | 43.9 |
| D3cc | <34.4 | 32.2 |
| D10% | <29 | 21.2 |
| D20% | <29 | 17.4 |
| D50% | <18.13 | 2.0 |
Representative dosimetry and dose volume histogram are demonstrated in Fig. 2. Standard procedures including use of rectal enema, bladder filling, and frame-based stereotactic setup using fiducial and soft tissue anatomy guidance by matching on-board cone beam CT to reference CT image were used. No abnormalities were noted during plan quality assurance or intrafraction imaging at the time and in repeated later review. The patient tolerated treatment well without gastrointestinal complaints and return to baseline urinary function by 6 weeks on selective alpha-blocker (tamsulosin).
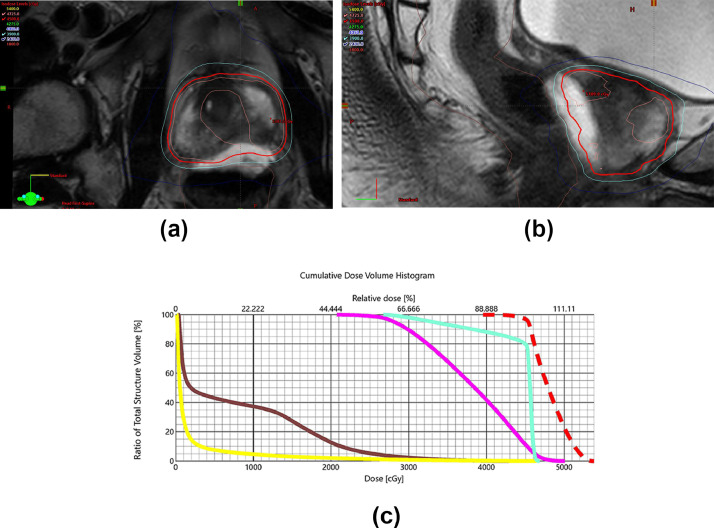
Fig. 2.
Clockwise from top left: (A) shows axial dose distribution on fused magnetic resonance imaging with sparing of rectal wall, <35% of circumference receiving 39 Gy (cyan) and <50% of circumference receiving 24 Gy (blue). (B) Shows similar rectal wall sparing in sagittal view. (C) Shows dose volume histogram with doses to the planning target volume (red), urethra (green), spacer infiltration (magenta), rectum (brown), and bladder (yellow).
Starting ~5 months after RT completion, the patient reported worsening urinary symptoms, perineal fullness and occasional blood on wiping after bowel movements attributed initially to hemorrhoids. Cystoscopic examination was negative for signs of urethral or bladder neck injury. During the next month, he subsequently developed anal pain and mucous discharge with some bowel movements, unresponsive to hydrocortisone suppository, and methylprednisolone dose pack for suspected radiation proctitis. Digital rectal examination noted anterior rectal wall tenderness with nodularity in expected area of prostate but without blood. Before endoscopic assessment could be pursued electively, he experienced acute worsening of rectal bleeding, prompting hospital admission outside our institution. Sigmoidoscopy revealed a large anterior wall ulceration (Fig. 3) corresponding with the previously seen area of spacer infiltration, and diverting colostomy was pursued at now 6 months after RT. Biopsies were taken of the ulcerated edge, consistent on pathology with colitis without malignancy. Notably the endoscopy did not show diffuse radiation changes of the adjacent rectum. Thereafter, he underwent hyperbaric oxygen treatments (50 dives) during the next 2 months, with resolution of rectal bleeding and pain.
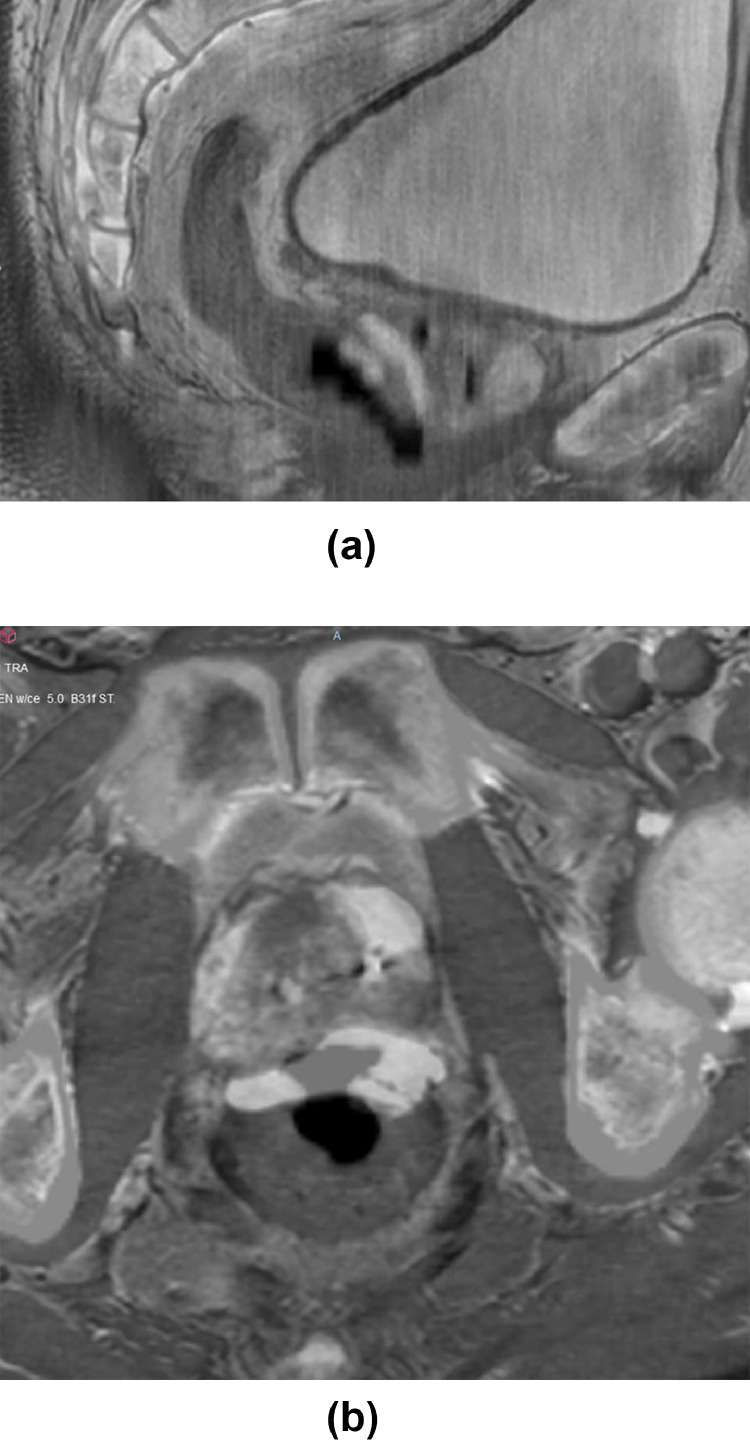
Fig. 3.
Sagittal (A) and axial (B) follow-up contrast-enhanced computed tomography images at 7 months posttreatment blended with pretreatment magnetic resonance imaging depict a large defect in the lower anterior rectal wall corresponding to the abnormal area shown in Fig. 1A. This results in free communication between the rectal lumen and the air-filled rectoprostatic space, in keeping with the wall defect identified on sigmoidoscopy (C).
At 9 months after RT completion, the patient developed urine per rectum, related to a large recto-urethral fistula, as shown on retrograde urethrogam (Fig. 4). Despite receiving suprapubic tube in attempt to temporize for flap repair, he developed osteomyelitis and soft tissue abscess, eventually requiring abdomino-perineal resection, cystoprostatectomy, and ileal conduit urinary diversion, now at 15 months from RT. Pathology demonstrated prostate adenocarcinoma with marked radiation therapy changes confined to the prostate and at the prostatic urethra margin, rectourethral fistula tract, and unremarkable rectal mucosa. Due to these complications and corrective procedures, the patient has required routine physical therapy and had to take leave from work for several months, with associated substantial effects on his quality of life. Despite this, he has made remarkable progress. As of last follow-up, he has undetectable prostate-specific antigen, is without evidence of infection or further sequelae, and has even returned to work full time.
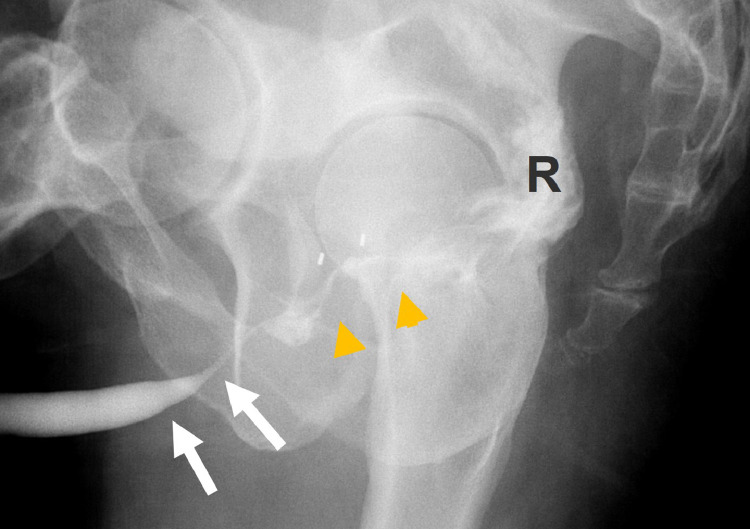
Fig. 4.
Oblique lateral radiograph obtained during retrograde urethrogram reveals normal penile and bulbar segments of the anterior urethra (white arrows) but fistulous communication (yellow arrowheads) between the membranous urethra and the rectum (R). Abbreviations: P = prostate; R = rectal wall.
Discussion
The rapid adoption of rectal hydrogel spacer placement ahead of RT for prostate cancer reflects increasing focus on quality of life in localized prostate cancer RT. Nonetheless, as with any device, postapproval experience may reveal rare complications that inform practice. In our case, we observed a proctitis event whose unusual severity, timing and context are concerning for an injury contributed to if not partially precipitated by the substantial rectal infiltration of the hydrogel spacer itself.
Several factors suggest this contribution rather than typical RT related injury mechanisms alone. First, the timing of rapid and new symptom onset at ~5 months after spacer gel insertion approximates the expected time of its resorption, whereas severe late radiation proctitis typically occurs later and is often preceded by acute/subacute symptoms,16 particularly in the setting of high dose SBRT related injuries in our prior trial experience.11,17 Classically, acute proctitis develops within 3 months of treatment, although late proctitis typically develops >9 months after treatment and is commonly associated with prior acute symptoms.16,17 Second, while infrequent but severe rectal toxicity has been observed with high dose SAbR in early studies without spacer gel, as reported by our group,10,15 the patient's rectal dosimetry fell well within institutional constraints developed based on those experiences15 and largely met constraints of lower SAbR dose regimens (Table 1). Notably, in our own institution's prospective13 and retrospective11 experiences because initiation of rectal hydrogel spacer placement with high dose SAbR across all risk groups (n = 250), no other single case of grade >2 rectal toxicity has been noted.11 Third, rectal wall infiltration in retrospect was of substantial depth and resulted in an area of delamination of deep rectal wall muscle layers, best approximated on sagittal sequences.
Infiltration of the rectal wall without apparent consequence was noted in 6% of cases in the randomized prospective study of conventionally fractionated RT leading to rectal hydrogel spacer approval.18 However, the effect of more severe infiltration and related ischemic stress on the rectal wall in the setting of increasingly used intensified and hypofractionated regimens remains unassessed by such data. Indeed, our elected institutional SAbR dose (45 Gy in 5 fractions) exceeds that endorsed by other practitioners (36.25-40 Gy in 5 fractions)19 and relies upon the demonstrated ability of rectal hydrogel spacer to facilitate rectal doses normally achieved by those latter regimens.11 However, in the setting of delamination of muscle layers, we speculate that portions of vascular supply containing rectal wall may be displaced toward the high dose regions, creating the potential for an occult ischemic injury revealed upon dissolution of the hydrogel. This possibility would argue for conservative organ-at-risk delineation of rectum to include gel in areas of infiltration. Lastly, although we have predicated our dosing upon the argument that lower SAbR doses approximate contemporary conventionally fractionated RT disease control in randomized comparison,20 which are inferior to the outcomes of RT combined with brachytherapy boost,21 the recently published Focal Lesion Ablative Microboost in ProstatE Cancer (FLAME) trial22 suggests that selective dose-escalation to MRI-defined lesions may offer flexibility for reducing dose along suspected rectal infiltration areas when concern arises.
Regardless of mechanism, a recent report from the Food and Drug Administration manufacturer and user facility device experience (MAUDE) database and other available case reports describe a low number of severe injuries associated with use of spacer gel, as summarized in Table 2,23, 24, 25, 26 suggesting a rare injury potential exists. Notably, many of the reported injuries occurred as a result of direct perforation of the rectal mucosa with symptoms presenting earlier than in our case; thus, and direct rectal mucosa disruption was possible as a direct injury mechanism, we do not have the support of symptom timing or direct endoscopy to confirm this is what occurred here. Similar injury risk at low rates has also been observed for analogous rectal spacer balloons, whose rigidity was hypothesized to be more commonly associated with acute injuries.27 The present case thus was accordingly reported as another potentially associated event in MAUDE.23
Table 2.
Summary of high-grade complications associated with hydrogel spacer in the treatment of prostate cancer
| Date of report | Grade | Description | Notes |
|---|---|---|---|
| 12/5/1422 | 3 | Ulceration treated conservatively | Received LDR brachytherapy, symptoms began 1 month after placement |
| 1/19/1620 | 3 | Proctitis requiring colostomy | Symptoms started 6 months after placement |
| 10/24/1720 | 3 | Rectourethral fistula requiring colostomy | High-risk posterior cancer with extraprostatic extension, stepper not used |
| 4/1/1820 | 3 | Rectourethral fistula requiring colostomy | Two patients with HDR treatment, fistula “several months” after placement |
| 6/4/1820 | 3 | Perirectal fistula requiring surgery | Spacer eroded through rectal wall before radiation, poor rectal preparation |
| 9/29/1820 | 3 | Ulceration after rectal wall injection | Radiation not given |
| 1/2/1920 | 3 | Rectourethral fistula requiring colostomy | Rectal wall infiltration was present |
| 2/21/1920 | 3 | Rectourethral fistula requiring colostomy | Rectal muscle infiltration seen |
| 4/8/1923 | 3 | Rectal ulcer resolved with HBO therapy | High dose IMRT, acute ulceration associated with pseudolumen abutting rectal wall |
| Present report | 4 | Rectourethral fistula and associated osteomyelitis ultimately requiring exenteration | Anterior rectal wall infiltration was present, high dose SAbR delivered, symptoms began 5 months after treatment, rectal ulcer was instrumented, symptoms initially improved with HBO but later developed fistula, abscess, and osteomyelitis |
Abbreviations: HDR = high dose rate; IMRT = intensity-modulated radiation therapy; LDR = low dose rate; SabR = stereotactic ablative radiation therapy.
In regard to the ensuing recto-urethral fistula, its occurrence by timing and location is best ascribed to a mechanism of direct exposure of recently irradiated prostate and adjacent membranous urethra to a major rectal ulcer. Moreover, it must be noted that the biopsy at time of sigmoidoscopy evaluation of the rectal injury may have contributed, as suggested in prior experiences.28
In summary, we believe this case reflects an extremely rare potential for rectal hydrogel spacer itself to contribute to major rectal injury, when observed to deeply infiltrate the rectal wall ahead of dose-escalated prostate RT. We report this case to raise awareness of this complication and provide suggested guidance on steps to recognize and intervene upon such infiltration. First, we highlight the importance of careful hydrodissection technique, with care to avoid insertion of the needle into the peri-rectal fat and to abort cases where confident placement is not assured. Second, infiltration of the anterior rectal wall at simulation imaging, particularly with MRI, should be evaluated ideally in collaboration with a MRI radiologist in cases of concern, specifically as regards the presence of deep muscle layer delamination. In such cases, we would propose endoscopic evaluation for evidence of submucosal injury that may argue for delay of RT until the gel dissolves or confirmation after RT that gel dissolution has occurred without later injury. We have instituted this approach because this case without any subsequent rectal injuries observed. Third, we propose to delineate the area of spacer infiltration as part of the rectal wall organ at risk for treatment planning to avoid the potential for treating separated outer layers of rectal wall unintentionally. Finally, given the low incidence of rectal toxicity in those with hydrogel spacer, the occurrence of unusual rectal symptoms or at unusual timing should trigger direct endoscopic evaluation to rule out this rare event.
Conclusions
We describe a case of high-grade toxicity potentially contributed to by deep hydrogel spacer rectal wall infiltration in the context of dose-escalated RT. We suggest concrete steps for its early identification and monitoring, particularly when rectal wall infiltration is associated with delamination of muscle layers. Although the occurrence of this toxicity appears very low, attention to these details may further improve confidence in improving the therapeutic ratio of prostate RT in the long term.
Footnotes
Sources of support: This work had no specific funding.
Disclosures: Dr Desai: Boston Scientific clinical trial funding and consulting. Dr Timmerman: Boston Scientific departmental grant support and participation in strategy meeting. Dr McLaughlin: Augmenix/Boston Scientific departmental grant support, travel reimbursement. Varian travel reimbursement. Dr Hannan: Reports grant funding from Prometheus Laboratories.
All data generated and analyzed during this study are included in this published article (and its supplementary information files).
References
- 1.Hamdy FC, Donovan JL, Lane JA. 10-year outcomes after monitoring, surgery, or radiotherapy for localized prostate cancer. N Engl J Med. 2016;375:1415–1424. doi: 10.1056/NEJMoa1606220. [DOI] [PubMed] [Google Scholar]
- 2.Dearnaley D, Syndikus I, Mossop H. Conventional versus hypofractionated high-dose intensity-modulated radiotherapy for prostate cancer: 5-year outcomes of the randomised, non-inferiority, phase 3 CHHiP trial. Lancet Oncol. 2016;17:1047–1060. doi: 10.1016/S1470-2045(16)30102-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee WR, Dignam JJ, Amin MB. Randomized phase III noninferiority study comparing two radiotherapy fractionation schedules in patients with low-risk prostate cancer. J Clin Oncol. 2016;34:2325–2332. doi: 10.1200/JCO.2016.67.0448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rodda S, Tyldesley S, Morris WJ. ASCENDE-RT: An analysis of treatment-related morbidity for a randomized trial comparing a low-dose-rate brachytherapy boost with a dose-escalated external beam boost for high- and intermediate-risk prostate cancer. Int J Radiat Oncol Biol Phys. 2017;98:286–295. doi: 10.1016/j.ijrobp.2017.01.008. [DOI] [PubMed] [Google Scholar]
- 5.Michalski JM, Moughan J, Purdy J. Effect of standard vs dose-escalated radiation therapy for patients with intermediate-risk prostate cancer: The NRG Oncology RTOG 0126 randomized clinical trial. JAMA Oncol. 2018;4 doi: 10.1001/jamaoncol.2018.0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hamstra DA, Mariados N, Sylvester J. Continued benefit to rectal separation for prostate radiation therapy: Final results of a phase III trial. Int J Radiat Oncol Biol Phys. 2017;97:976–985. doi: 10.1016/j.ijrobp.2016.12.024. [DOI] [PubMed] [Google Scholar]
- 7.Hatiboglu G, Pinkawa M, Vallee JP, Hadaschik B, Hohenfellner M. Application technique: Placement of a prostate-rectum spacer in men undergoing prostate radiation therapy. BJU Int. 2012;110(11 Pt B):E647–E652. doi: 10.1111/j.1464-410X.2012.11373.x. [DOI] [PubMed] [Google Scholar]
- 8.Pinkawa M, Klotz J, Djukic V. Learning curve in the application of a hydrogel spacer to protect the rectal wall during radiotherapy of localized prostate cancer. Urology. 2013;82:963–968. doi: 10.1016/j.urology.2013.07.014. [DOI] [PubMed] [Google Scholar]
- 9.Montoya J, Gross E, Karsh L. How I do it: Hydrogel spacer placement in men scheduled to undergo prostate radiotherapy. Can J Urol. 2018;25:9288–9293. [PubMed] [Google Scholar]
- 10.Hannan R, Tumati V, Xie XJ. Stereotactic body radiation therapy for low and intermediate risk prostate cancer-Results from a multi-institutional clinical trial. Eur J Cancer. 2016;59:142–151. doi: 10.1016/j.ejca.2016.02.014. [DOI] [PubMed] [Google Scholar]
- 11.Chen L, Gao A, Bhavani S, et al. Safety and outcome of stereotactic body radiation therapy (SBRT) with rectal hydrogel spacer for prostate cancer. J Clin Oncol. 38 (Suppl_76).
- 12.Boike TP, Lotan Y, Cho LC. Phase I dose-escalation study of stereotactic body radiation therapy for low- and intermediate-risk prostate cancer. J Clin Oncol. 2011;29:2020–2026. doi: 10.1200/JCO.2010.31.4377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Folkert MR, Zelefsky MJ, Hannan R. Multi-institutional phase 2 trial of high-dose stereotactic body radiation therapy with temporary hydrogel spacer for low- and intermediate-risk prostate cancer. International Journal of Radiation Oncology, Biology, Physics. 2017;99:1319–1320. [Google Scholar]
- 14.Kumar S, Perlman E, Harris CA, Raffeld M, Tsokos M. Myogenin is a specific marker for rhabdomyosarcoma: an immunohistochemical study in paraffin-embedded tissues. Mod Pathol. 2000;13:988–993. doi: 10.1038/modpathol.3880179. [DOI] [PubMed] [Google Scholar]
- 15.Kim DW, Straka C, Cho LC, Timmerman RD. Stereotactic body radiation therapy for prostate cancer: Review of experience of a multicenter phase I/II dose-escalation study. Front Oncol. 2014;4:319. doi: 10.3389/fonc.2014.00319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gilinsky NH, Burns DG, Barbezat GO, Levin W, Myers HS, Marks IN. The natural history of radiation-induced proctosigmoiditis: An analysis of 88 patients. Q J Med. 1983;52:40–53. [PubMed] [Google Scholar]
- 17.Schultheiss TE, Lee WR, Hunt MA. Late GI and GU complications in the treatment of prostate cancer. Int J Radiat Oncol Biol Phys. 1997;37:3–11. doi: 10.1016/s0360-3016(96)00468-3. [DOI] [PubMed] [Google Scholar]
- 18.Fischer-Valuck BW, Chundury A, Gay H, Bosch W, Michalski J. Hydrogel spacer distribution within the perirectal space in patients undergoing radiotherapy for prostate cancer: Impact of spacer symmetry on rectal dose reduction and the clinical consequences of hydrogel infiltration into the rectal wall. Pract Radiat Oncol. 2017;7:195–202. doi: 10.1016/j.prro.2016.10.004. [DOI] [PubMed] [Google Scholar]
- 19.National Comprehensive Cancer Network. Bone Cancer (Version 2.2021). Available at:http://www.nccn.org/professionals/physician_gls/pdf/bone.pdf. Accessed February 17, 2021.
- 20.Widmark A, Gunnlaugsson A, Beckman L. Ultra-hypofractionated versus conventionally fractionated radiotherapy for prostate cancer: 5-year outcomes of the HYPO-RT-PC randomised, non-inferiority, phase 3 trial. Lancet. 2019;394:385–395. doi: 10.1016/S0140-6736(19)31131-6. [DOI] [PubMed] [Google Scholar]
- 21.Morris WJ, Tyldesley S, Rodda S. Androgen Suppression Combined with Elective Nodal and Dose Escalated Radiation Therapy (the ASCENDE-RT Trial): An analysis of survival endpoints for a randomized trial comparing a low-dose-rate brachytherapy boost to a dose-escalated external beam boost for high- and intermediate-risk prostate cancer. Int J Radiat Oncol Biol Phys. 2017;98:275–285. doi: 10.1016/j.ijrobp.2016.11.026. [DOI] [PubMed] [Google Scholar]
- 22.Kerkmeijer LGW, Groen VH, Pos FJ. Focal boost to the intraprostatic tumor in external beam radiotherapy for patients with localized prostate cancer: Results from the FLAME randomized phase III trial. J Clin Oncol. 2021;39:787–796. doi: 10.1200/JCO.20.02873. [DOI] [PubMed] [Google Scholar]
- 23.Administration USFaD. MAUDE: Manufacturer and user facility device experience. Secondary MAUDE: Manufacturer and user facility device experience April 30, 2020. Available at: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfmaude/search.cfm. Accessed April 30, 2020.
- 24.Aminsharifi A, Kotamarti S, Silver D, Schulman A. Major complications and adverse events related to the injection of the spaceoar hydrogel system before radiotherapy for prostate cancer: Review of the manufacturer and user facility device experience database. J Endourol. 2019;33:868–871. doi: 10.1089/end.2019.0431. [DOI] [PubMed] [Google Scholar]
- 25.Teh AY, Ko HT, Barr G, Woo HH. Rectal ulcer associated with SpaceOAR hydrogel insertion during prostate brachytherapy. BMJ Case Rep. 2014;2014 doi: 10.1136/bcr-2014-206931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dinh Tru-Khang T, Schade George R, Liao Jay J. A case of rectal ulcer during intensity modulated radiotherapy for prostate cancer using hydrogel spacer. Urology Practice. 2020;7:158–161. doi: 10.1097/UPJ.0000000000000071. [DOI] [PubMed] [Google Scholar]
- 27.Schörghofer A, Drerup M, Kunit T. Rectum-spacer related acute toxicity: Endoscopy results of 403 prostate cancer patients after implantation of gel or balloon spacers. Radiat Oncol. 2019;14:47. doi: 10.1186/s13014-019-1248-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Phan J, Swanson DA, Levy LB. Late rectal complications after prostate brachytherapy for localized prostate cancer: Incidence and management. Cancer. 2009;115:1827–1839. doi: 10.1002/cncr.24223. [DOI] [PubMed] [Google Scholar]