Abstract
Targeted in ovo green light (GL) photostimulation during the last days of broiler egg incubation increases embryonic expression of the somatotropic axis, similar to in ovo green light photostimulation from embryonic day (ED) 0 to the end of incubation. The aim of this study was to examine the effect of selected in ovo GL photostimulation periods on post-hatch broiler growth. Four hundred twenty fertile broiler eggs were divided into 7 treatment groups: the first incubated in the dark (standard conditions) as a negative control; the second incubated under monochromatic GL from ED0-ED20 (positive control); the third group incubated under monochromatic GL light from ED15-ED20; the fourth, fifth and sixth groups were incubated under monochromatic GL on ED16, ED17, and ED18, respectively; and the seventh group was incubated under monochromatic GL from ED18-ED20. All illumination was provided intermittently using LED lamps. After hatch, all chicks were transferred to a controlled room under standard rearing conditions. The group incubated under green light from ED18 until hatch showed similar results to the positive control group in body weights, as well as breast muscle weights (as % of body weights), and an elevation in the somatotropic axis activity during the experiment. We suggest that broiler embryos can be exposed to in ovo GL photostimulation from ED18 until hatch (hatching period), and still exhibit the same performance as obtained by photostimulation from d 0 of incubation.
Key words: broiler, rearing, green light photostimulation, somatotropic axis
INTRODUCTION
Targeted green light (GL) photostimulation is a well-known management treatment for elevating different production processes, such as growth, meat yield and reproduction (Rozenboim et al., 1999a, 1999b, 2004; Olanrewaju et al., 2006; Zhang et al., 2012; Borille et al., 2013). Previous studies conducted in our lab have shown that broilers reared under GL photostimulation had increased growth rate and breast muscle weights (Rozenboim et al., 1999a,b; Rozenboim et al., 2004). Furthermore, embryonic green light photostimulation (in ovo) elevated embryonic body and breast muscle weights (Rozenboim et al., 2004) and elevated the body and breast muscle weights of broilers after hatch (Rozenboim et al., 2004). In ovo GL photostimulation affects development and growth of muscles, through its effects on proliferation and differentiation of satellite cells, and its effects on several myogenic proteins, such as myogenin, myostatin, and MyoD expression (Halevy et al., 2006a, 2016b; Zhang et al., 2014a).
The somatotropic axis, which is known to affect development and growth, is one of the major endocrine mechanisms studied regarding the effects of targeted in ovo GL photostimulation. The somatotropic axis begins with the hypothalamic growth hormone releasing hormone (GHRH). GHRH is released from hypothalamic neurons and travels through the portal vessels to the pituitary, where it increases both production of the growth hormone, and together with somatostatin and thyrotropin releasing hormone, regulates the secretion of growth hormone (GH) from the pituitary (Mayo, 1992; McMurtry et al., 1997; Porter et al., 2006; Kim, 2010). The production and secretion of GH elevate during embryonic development, reaching peak levels in the early post-hatch period, followed by a decline in plasma hormone levels (Buyse and Decuypere, 1999; Kim, 2010). GH can be found not only in the pituitary, but also in other tissues, such as the ovaries, testes, osteoblasts, thymus, spleen, the bursa of Fabricius, and more (Luna et al., 2008, Marisela Ahumada-Solórzano et al., 2012). GH has important effects on several tissues, such as decreasing lipolysis (Rosebrough et al., 1991), proliferation and differentiation of muscles cells (Halevy et al., 2006b), and growth of broilers, and GH levels are positively correlated to growth rate (Vasilatos-Younken and Scanes, 1991). The effects of GH are mediated by the growth hormone receptor (GHR), found in several tissues in broilers, such as the muscles, adipose, liver and more (Mao et al., 1998; Dishon et al., 2018). The effects of GH can be direct or indirect through the synthesis of insulin-like growth factor I (IGF-1) in the liver and muscles (Kanacki et al., 2012; Dishon et al., 2018). It was shown that in the post-hatch period a positive correlation between the GH and IGF-1 was detected and that plasma IGF-1 levels were closely related to plasma GH levels (McMurtry et al., 1997). IGF-1 has an important role in broiler's metabolism (such as carbohydrates, lipids and proteins) (McMurtry et al., 1997).
It was shown in several studies, including our previous papers, that in ovo GL photostimulation elevate the expression and secretion of different components of the somatotropic axis (Halevy et al., 1998; Zhang et al., 2014a, 2014b; Dishon et al., 2017; Dishon et al., 2018). In our previous paper (Dishon et al., 2021), we discovered that in ovo GL photostimulation of broiler embryos from embryonic day (ED) 18 until hatching (hatching period), showed similar elevation in somatotropic axis activity, manifested by increased plasma GH levels and hypothalamic GHRH and liver GHR and IGF-1 mRNA levels on the day of hatching. The aim of this study was to examine the effect of selected in ovo GL photostimulation periods on post-hatch broiler growth and somatotropic axis activity.
MATERIALS AND METHODS
Animals
All procedures in this experiment were approved by the Animal Care Committee of the Hebrew University of Jerusalem (research number AG-07-10346-2). A total of 420 fertile broiler (cobb 500) equal weight eggs (63± 3 g) were obtained from a 36-wk-old broiler breeder flock (Brown Hatchery, Hod Hasharon, Israel). Eggs were incubated inside a Petersime 9600 incubator (Petersime, Zulte, Belgium), under standard conditions until ED18 (37.8°C and 56% RH). On ED7 eggs were candled and infertile eggs were removed. On ED18 eggs were transferred to hatching baskets according to in ovo treatment groups and returned to the incubator, with changes to temperature and RH according to the Cobb hatchery management guide. After hatching, chicks were tagged by wing bands and transferred to environmental and light controlled rooms (with 8.4 chicks per square meter) under white light (day light, 0.1 W/m2 by LED lamps). All chicks from all the treatment groups were housed together in the same experimental room. All chicks were reared on pine wood shaving litter with ad libitium provision of feed ([Tadmir group, Bet Shemesh, Israel], feeding program according to Cobb broiler management guide, Cobb-Vantress, 2018) and water, and the rooms were heated to 33°C (with a decrease of 2°C every week).
Light Management
The eggs were divided into 7 treatment groups (each light treatment with 60 fertile embryos, which were considered an experimental unit). All GL photostimulations were conducted by Light-emitting diode (LED), as described in our previous papers, with cardboard providing a separation between the different light treatment groups inside the hatchery (Dishon et al., 2017; Dishon et al., 2018), with 560 nm, intensity of 0.1 W/m2 at eggshell level, with intervals of 15 min light/15 min dark to avoid overheating the eggs (Rozenboim et al., 2004). Each treatment group was separated by cardboard to eliminate light transfer between the groups (after measuring no interference to air flow and changes in egg temperatures). The first group was incubated under dark conditions (Dark, negative control). The second group was incubated under GL photostimulation from ED0 until hatch (GD0-20, positive control). The third group was incubated under GL photostimulation from ED15 until hatch (GD15-20). The fourth group was incubated under GL photostimulation on ED16 (GD16). The fifth group was incubated under GL photostimulation on ED17 (GD17). The sixth group was incubated under GL photostimulation on ED18 (GD18). The seventh group was incubated under GL photostimulation from ED18 until hatch (GD18-20).
Post-Hatch Experimental Procedures
Body Weights
Body weight was recorded on d 0 (day of hatch), d 3, 7, 13, 20, 28 and 35 of age.
Blood Sampling and Tissue Sampling
Blood and tissue samples were collected on d 0, 5 and 35 d of age. Heparinized blood samples were drawn from the jugular vein (10 chicks of every treatment group). Birds were then euthanized by cervical dislocation. Body, breast muscle, abdominal fat and liver weights were recorded, and samples from the hypothalamus, liver, and breast muscle were taken and placed in liquid nitrogen, and afterwards stored at −80°C until mRNA expression analysis (as described in Dishon et al., 2017; Dishon et al., 2018).
Hormone Analysis
Competitive ELISA assay was conducted in order to measure plasma GH levels, using the corresponding biotinylated tracer, as described previously (Dishon et al., 2017).
RNA Extraction and Real-Time Polymerase Chain Reaction (Real-Time PCR)
The frozen tissue samples were homogenized by HG-300 homogenizer, and total RNA was extracted using RNAzol RT reagent, according to the manufacturer's protocol (GeneCopoeia, Rockville, MD). The RNA extraction protocol, as well as the cNDA production and real time PCR protocols was as described in our previous papers (Dishon et al., 2017; Dishon et al., 2021). A list of primers is found in Table 1.
Table 1.
Primers used in real-time PCR reactions.
| Gene | Primers | Product length | GenBank accession no. |
|---|---|---|---|
| GAPDH | F: GGCACGCCATCACTATC | 61 bp | NM_204305.1 |
| R: CCTGCATCTGCCCATTT | |||
| β-Actin | F: CCGCAAATGCTTCTAAACCG | 101 bp | NM_205518.1 |
| R: AAAGCCATGCCAATCTCGTC | |||
| GHRH | F: GGCAAACGGCTCAGAAACAG | 140 bp | NM_001040464.1 |
| R: AGCATGGCTCCCAAGAAGTC | |||
| GHR | F: GCGTGTTCAGGAGCAAAGCT | 121 bp | NM_001001293.1 |
| R: TGGGACAGGCATTTCCATACTT | |||
| IGF | F: GCTTTTGTGATTTCTTGAAGGTGAA | 195 bp | NM_001004384.2 |
| R: CATACCCTGTAGGCTTACTGAAGTA |
Statistical Analysis
Body, muscles, liver and abdominal adipose tissue weights, plasma GH levels, as well as mRNA expression levels data were analyzed by a two-way ANOVA model with post-hatch age, in ovo light treatment group, and their interaction as fixed effects. There was no significant interaction between the treatment group and the age of the broilers. Therefore, the tables and figures show least square means (±SEM) for each light treatment on a specific post hatch growth day. Tukey-Kramer HSD test for post-hoc testing of the differences between treatments' least squares means was used. All statistical analyses were conducted with JMP software Ver. 15 (SAS Institute).
RESULTS
Hatching rates form fertile eggs were similar in all treatment groups (88%-89%). In addition, a similar hatching window was observed in all groups at 478–482 (incubation hours) of hatching time.
No significant interaction was found between post-hatch age and in ovo light treatment group, throughout the experiment.
Body Weights
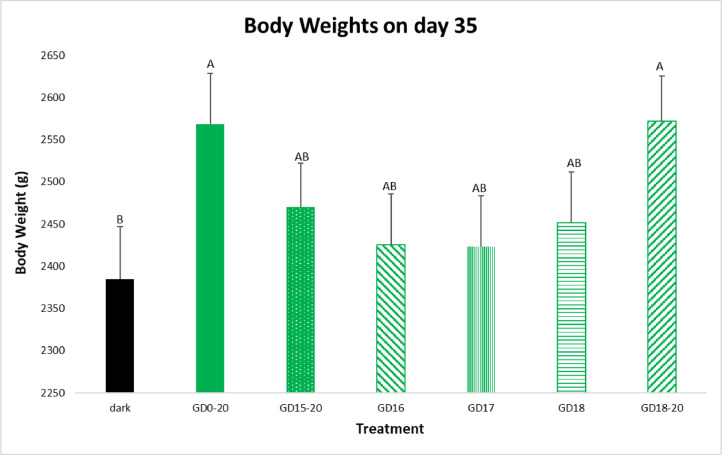
Broiler BW increased from 44.6 g on d 0, to between 2,384 and 2,571 g (depending on treatment group) on d 35 (Figure 1). During the rearing of the birds, we found that from d 3 until d 13, and then from d 28 until d 35, BW of GD18-20 group were significantly higher than those of the negative control, with no significant difference from the positive control group (GD0-20). Furthermore, both the GD15-20 group, as well as the GD16 group had similar results to the positive control during the rearing period. The GD17 and GD18 groups had similar weights to the negative control (Dark) until 13 d of age, but on 35 d of age had higher weights than the negative control group. On d 20 of the experiment, there were no significant differences between the treatment groups and the dark group (Table 2).
Figure 1.
Broiler BW on 35 d of age. Broilers incubated in the dark as a negative control (dark) or under monochromatic green light from different days and periods of incubation to day of hatch (GD0-20, GD15-20, GD16, GD17, GD18, and GD18-20). Data are presented as means ± SEM. Means of the groups on a specific day with different letters differ significantly (P < 0.05).
Table 2.
Broiler body weights on 0, 3, 7, 13, 20 and 28 d of age, of broilers incubated in the dark as a negative control (dark) or under monochromatic green light from different days of incubation to day of hatch (GD0-20, GD15-20, GD16, GD17, GD18, and GD18-20).
| TreatmentDay | Dark | GD0-20 | GD15-20 | GD16 | GD17 | GD18 | GD18-20 |
|---|---|---|---|---|---|---|---|
| 0 | 44.5AB | 44.3AB | 43.7B | 45.6A | 45.1A | 44.7AB | 44.4AB |
| 3 | 75.4C | 78.0ABC | 79.95AB | 78.65ABC | 76.7BC | 75.3C | 81.2A |
| 7 | 164.8B | 167.4AB | 177.8A | 168.1AB | 163.2B | 161.7B | 177.2A |
| 13 | 384.9B | 394.6AB | 403.2AB | 392.9AB | 388.1B | 379.4B | 419.6A |
| 20 | 897.0A | 898.2A | 916.3A | 903.1A | 885.3A | 869.45A | 907.4A |
| 28 | 1585.1B | 1634.2AB | 1650.4AB | 1604.8AB | 1594.7B | 1603.6AB | 1673.7A |
Data are presented as means of the groups on a specific day, with different letters indicating significantly different means (P < 0.05).
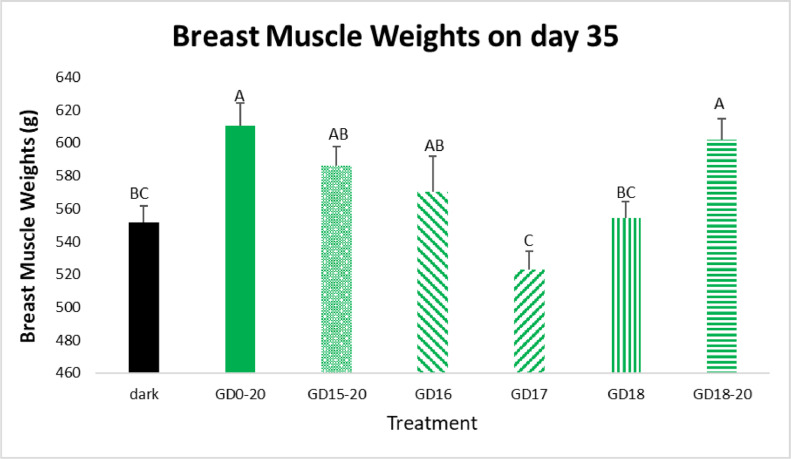
No significant differences between the in ovo light treatment groups were found for liver and abdominal adipose tissue weights. For the breast muscle weights, we found on day of hatching that the GD18-20 group had a similar weight to the GD0-20 group and a significantly higher breast muscle weight compared to the Dark group, while all other treatment groups had weights between the negative and positive control. On d 5 we found that the GD15-20 and GD18-20 groups had a significantly higher muscle weights compared to the Dark group, while all other treatment groups had higher weights than the Dark group, but with no significant differences. On d 35 (Figure 2), the GD18-20 group had similar breast muscle weights to the GD0-20 group and significantly higher weights than the Dark group, while the GD15-21 and GD16 groups had breast muscle weights between the GD0-20 and Dark group. The GD18 group had similar results as the dark group, while the GD17 group had lower breast muscle weights than the dark group, and the GD18 group had lower breast muscle weights than the Dark group (Table 3).
Figure 2.
Broiler breast muscle weights on 35 d of age. Broilers incubated in the dark as a negative control (dark) or under monochromatic green light from different days and periods of incubation to day of hatch (GD0-20, GD15-20, GD16, GD17, GD18, and GD18-20). Data are presented as means ± SEM. Means of the groups on a specific day with different letters differ significantly (P < 0.05).
Table 3.
Broiler breast muscle weights on 0 and 5 d of age, of broilers incubated in the dark as a negative control (dark) or under monochromatic green light from different days of incubation to day of hatch (GD0-20, GD15-20, GD16, GD17, GD18, and GD18-20).
| Treatmentday | Dark | GD0-20 | GD15-20 | GD16 | GD17 | GD18 | GD18-20 |
|---|---|---|---|---|---|---|---|
| 0 | 0.66B | 0.75A | 0.71AB | 0.69AB | 0.73AB | 0.69AB | 0.74A |
| 5 | 5.78B | 6.54AB | 6.89A | 6.49AB | 6.33AB | 6.32AB | 6.65A |
Data are presented as means of the groups on a specific day, with different letters indicating significantly different means (P < 0.05).
Plasma GH Levels
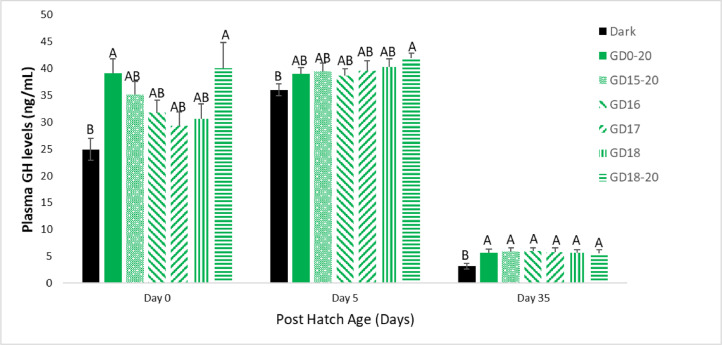
Plasma GH levels were elevated from d 0 to d 5 then declined up to d 35 (termination of the experiment). When examining the different levels of each treatment group, we found that the GD0-20 group had significantly higher levels on d 0 and d 35 than the Dark group. On d 5 the GD0-20 had higher plasma GH levels compared to the Dark group, but with no significant difference. All other in ovo light treatment groups had similar plasma GH levels to the GD0-20 group and higher than the Dark group, but with no significant difference. The GD18-20 group had significantly higher plasma GH levels compared to the Dark group throughout the experiment (Figure 3).
Figure 3.
Broiler plasma GH levels on d 0, 5, and 35. Broilers incubated in the dark as a negative control (dark) or under monochromatic green light from different days and periods of incubation to day of hatch (GD0-20, GD15-20, GD16, GD17, GD18, and GD18-20). Data are presented as means ± SEM. Means of the groups on a specific day with different letters differ significantly (P < 0.05).
Hypothalamic GHRH, Liver GHR, Liver IGF-1 and Muscle IGF-1 mRNA Gene Expression
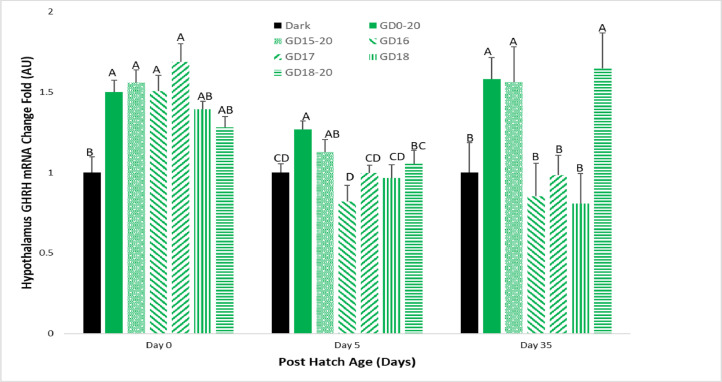
Hypothalamic GHRH mRNA expression on d 0 was elevated in all in ovo light treatment groups compared to the Dark group. On d 5 of the experiment, the GD0-20 group had significantly higher levels than the Dark group, with the GD15-20 group having similar expression to the GD0-20. The GD18-20 group had slightly lower results than the GD0-20 group and GD15-20 group, but higher than the Dark group. The GD17 and GD18 groups had similar expression to the Dark group, and GD16 had the lowest expression. On d 35 of the experiment, the GD0-20 group, as well as the GD15-20 and GD18-20 groups, had significantly higher expression than the Dark group. The GD16, GD17 and GD18 treatment groups had similar results to the Dark group (Figure 4).
Figure 4.
Broiler hypothalamic GHRH mRNA expression levels on d 0, 5, and 35. Broilers incubated in the dark as a negative control (dark) or under monochromatic green light from different days and periods of incubation to day of hatch (GD0-20, GD15-20, GD16, GD17, GD18, and GD18-20). Data are presented as means ± SEM. Means of the groups on a specific day with different letters differ significantly (P < 0.05).
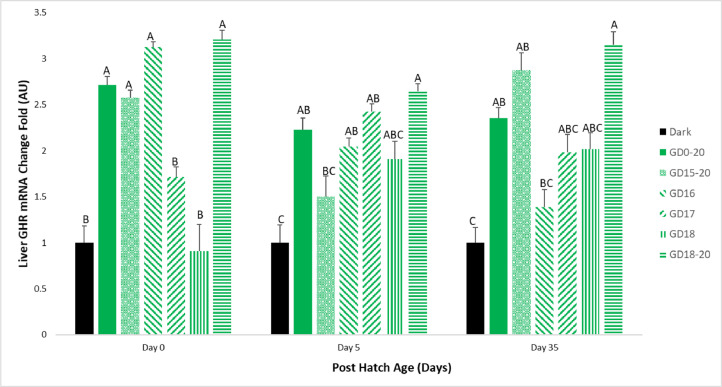
Liver GHR mRNA levels on d 0 were significantly elevated in the GD0-20, GD15-20, GD16 and GD18-20 groups, when compared to the Dark group. The GD17 and GD18 groups had similar results as the Dark group. On d 5 of the experiment, the GD0-20, GD16, GD17 and GD18-20 groups had significantly higher GHR mRNA levels than the Dark group. The GD15-20 and GD18 groups had higher levels than the Dark group but lower than the GD0-20 group. On d 35, the GD18-20 group, as well as GD15-20 group, had similar GHR mRNA levels to the GD0-20 group, and significantly higher levels than the Dark group. The GD16, GD17 and GD18 groups had higher expression than the Dark group but lower than the GD0-20 group (Figure 5).
Figure 5.
Broiler liver GHR mRNA expression levels on d 0, 5, and 35. Broilers incubated in the dark as a negative control (dark) or under monochromatic green light from different days and periods of incubation to day of hatch (GD0-20, GD15-20, GD16, GD17, GD18, and GD18-20). Data are presented as means ± SEM. Means of the groups on a specific day with different letters differ significantly (P < 0.05).
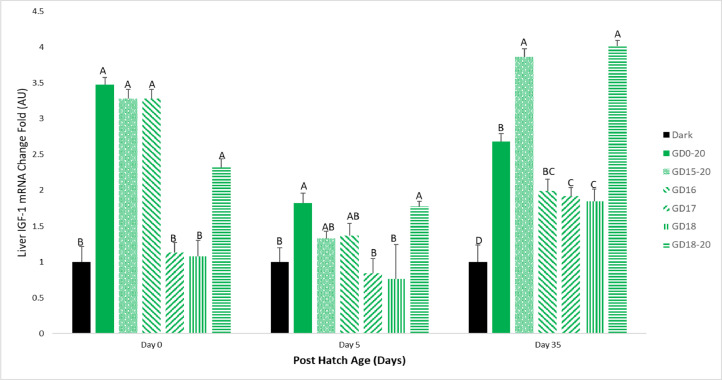
Liver IGF-1 gene expression on day 0 of the experiment was significantly elevated in the GD0-20, GD15-20, GD16 and GD18-20 groups, compared to the Dark group. The GD17 and GD18 groups had similar results to the Dark group. On d 5 of the experiment, the GD18-20 group had similar results to the GD0-20 group, and significantly higher levels than the Dark group. The GD15-20 and GD16 groups had IGF-1 mRNA levels between the GD0-20 and Dark groups, and the GD 17 and GD18 groups had similar results to the Dark group. On d 35 of the experiment, all light treatment groups had significantly higher results than the Dark group, with the GD15-20 and GD18-20 having the highest IGF-1 mRNA levels (Figure 6).
Figure 6.
Broiler liver IGF-1 mRNA expression levels on d 0, 5, and 35. Broilers incubated in the dark as a negative control (dark) or under monochromatic green light from different days and periods of incubation to day of hatch (GD0-20, GD15-20, GD16, GD17, GD18, and GD18-20). Data are presented as means ± SEM. Means of the groups on a specific day with different letters differ significantly (P < 0.05).
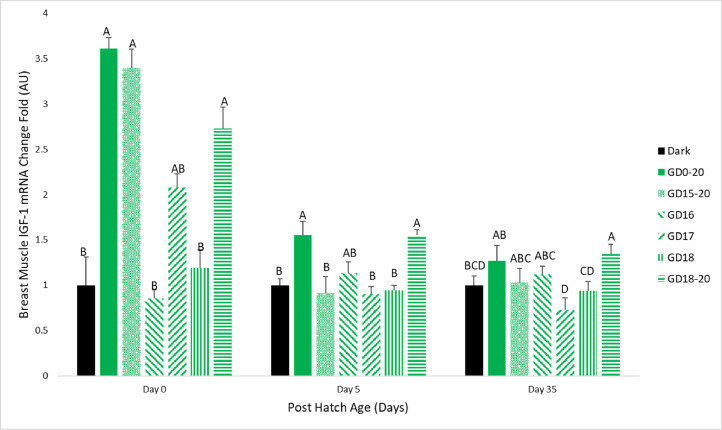
Breast muscle IGF-1 gene expression on d 0 of the experiment was significantly elevated in the GD15-20 and GD18-20 groups, compared to the negative control, and similar to the positive control. The GD17 group had results higher than the negative control but lower than the positive control. The GD16 and GD18 groups had similar results to the negative control. On d 5 of the study, the GD18-20 group had significantly higher expression when compared to the negative control, and similar to the positive control. The GD16 group had results between the negative and positive control expressions, and all other treatment groups had similar results to the negative control. On d 35 of the experiment, the GD18-20 group had significantly higher muscle IGF-1 gene expression, compared to the negative control, and similar to the positive control. The GD15-20 and GD16 groups had levels between the positive and negative control. The GD17 and GD18 groups had lower results than the negative control (Figure 7).
Figure 7.
Broiler breast muscle IGF-1 mRNA expression levels on d 0, 5, and 35. Broilers incubated in the dark as a negative control (dark) or under monochromatic green light from different days and periods of incubation to day of hatch (GD0-20, GD15-20, GD16, GD17, GD18, and GD18-20). Data are presented as means ± SEM. Means of the groups on a specific day with different letters differ significantly (P < 0.05).
DISCUSSION
In ovo green light photostimulation from embryonic d 18 until hatch significantly elevated broiler growth, breast muscle weight, hypothalamic GHRH, liver GHR and IGF-1 mRNA, breast muscle IGF-1 mRNA, and plasma GH levels. This elevation was similar to the positive control group (in ovo green light photostimulation from embryonic d 0 until hatch). These results are in accordance with, and can serve as an extension to, a previous study in our laboratory, which showed that in ovo GL photostimulation during the whole incubation period (similar to the positive control in the current study) elevated both body weight and breast muscle weights in broilers, even on d 35 post hatch (Rozenboim et al., 2013). The results in this study can also serve as a continuation of both Zhang et al. (2014b), who showed that in ovo GL photostimulation increased plasma GH levels until d 5 post-hatch, as well as our previous studies (Dishon et al., 2018; Dishon et al., 2021), because those studies showed that in ovo GL photostimulation between ED18 and ED20 (hatching day) caused an elevation in the somatotropic axis activity during the embryonic stages, while the present study shows that this elevation of the somatotropic axis continues well after the end of the embryonic stages, as well as increases the productivity of the broilers.
This study shows that the positive effect of in ovo GL photostimulation during the late embryonic, pre-hatching period (GD18-20 group) has on the expression of the somatotropic axis is not limited to the embryonic stages and until hatching, or even to the first week of rearing, but continue even until the end of the broiler rearing period. These last days of incubation are considered a critical stage or period in the development of the chicken embryo (De Oliveira et al., 2008). The chicken embryo goes through 3 major critical periods, or periods of distinctive physiological changes (De Oliveira et al., 2008; Tong et al., 2013). The first critical stage is called the establishment of germ, or the early critical stage. The second stage is the completion of the embryo. The last critical stage is called the emergence period, or the perinatal period, and occurs during the last week of the incubation (Romanoff, 1949; De Oliveira et al., 2008). Each of these periods has distinctive different changes in the broiler embryo. The last critical period is identified with growth of the embryo's organs, and development and maturation of several physiological and metabolic systems (Tong et al., 2013), such as the transition to pulmonary respiration (De Oliveira et al., 2008), development of hearing to allow response to external sounds, as well as vocalization (Tong et al., 2013), maturation of the functionality of pituitary-adrenal axis (Decuypere and Bruggeman, 2005), elevation of glucocorticoids (Decuypere and Bruggeman, 2007), maturation of the thyrotropic and somatotropic axes (Ellestad, et al., 2011), elevation of plasma GH levels (Zhang et al., 2014b; Dishon et al., 2018), elevation in liver metabolism pathways, such as gluconeogenesis, glycolysis, glycogenesis, glycogenolysis, pentose phosphate and more, in order to maintain energy homeostasis during hatching and until feeding of the hatchling (De Oliveira et al., 2008), and an increase in the brush border enzymes of the small intestines (Uni et al., 2003).
Taken together our current findings that in ovo GL photostimulation during the last critical period of the broiler's incubation increased growth and somatotropic axis expression, we suggest that the last incubation days might be considered a photostimulation critical period for broiler somatotropic axis activity. These findings are important, because they confirm that the positive effect of in ovo GL photostimulation during the hatching process (from the time the eggs are transferred to hatching trays and until hatch), will remain during the entire growth period, and will give results similar to GL photostimulation during the entire incubation period. Furthermore, it is possible that the in ovo GL photostimulation effect on plasma GH levels during the last critical stage of incubation (as shown in Dishon et al., 2021), may affect the hormonal imprinting process, by affecting the responsiveness and quantity of the GH receptors, as found during last stages of incubation and hatching (Dishon et al., 2021) as well as during the post-hatch period in this experiment. According to Csaba (1986), hormonal imprinting processes occur when a hormone affects its receptors either in quantity, responsiveness, or both. According to Csaba (2011), hormonal imprinting means that during the developmental stages, a hormone (or related structures) affects the receptor binding capacity, which can affect the responsiveness to the hormone for life (Csaba, 2011). The imprinting first occurs during ontogenetic development, but can occur in any organ or cell that continues to develop during any time in life (Csaba, 2011). It is stated that faulty hormonal imprinting (such as exposure to a structurally related foreign molecule) may give rise to a faulty imprinting by diminishing the quantity or responsiveness of the receptors as well as cause lifelong morphological and biochemical consequences (Csaba, 1986; Csaba, 2011). An example of this is the exposure of perinatal rats to diethyl-stilbestrol, which causes a significant decrease in the binding capacity of estrogen receptors during adulthood (Csaba, 2011). Another example of hormonal imprinting was given by Rozenboim et al. (1990). During the experiment, they discovered that heavy breed male chicks were resistant to the slimming effect of testosterone. However, they found that embryonic exposure to androgen sensitizes the heavy breed male chicks to the anti-fattening effect of the testosterone, which suggests that hormonal imprinting may be involved in the determination of the slimming effect of testosterone on heavy breed male chicks (Rozenboim et al., 1990). Consistent with the notion of hormonal imprinting, the critical period for GL photostimulation of the somatotropic axis was determined to be between ED18 and ED20, the period during chicken development when the somatotropic axis matures (Ellestad, et al., 2011).
One of the questions arising from these results is whether the changes we discovered may be considered epigenetic alterations\adaptations? Epigenetics refers to the regulation of gene expression with no changes to DNA sequence (Jiang et al., 2016). Epigenetic adaptation or modification is defined by a prenatal manipulation that generates a physiological memory, which in turn facilitates a better adaptation during the postnatal period (Yahav, 2009; Vinoth et al., 2018). According to Guerrero-Bosagna (2018), a variety of traits can be affected by environmental interaction, through epigenetics. Thus, external changes which affect the pre- and postnatal periods can have enormous effects on the adult phenotype (Guerrero-Bosagna et al., 2018). According to Tzschentke and Halle (2009) changes in incubation temperatures during critical periods of the incubation, such as the end of the incubation, may induce prenatal epigenetic temperature adaptation, which will result in adaptation to different environmental temperature during the post hatching development (Tzschentke and Halle, 2009). Furthermore, Zhu el al. (2020), showed that in ovo feeding with vitamin C on ED11, affected several parameters during the post-hatch period, such as the average daily feed intake, average daily gain and feed conversion ratio, as well as increased expression of enzymes related to DNA methylation and reduced the expression of enzymes related to DNA demethylation. They contributed these results to nutria-epigenetics (which studies the effects of a specific nutrient during a physiological phase on the regulation of gene expression through epigenetic modifications) (Zhu el al., 2020). Similar to those studies, and others like them, we found that in ovo photostimulation during the last days of the incubation (GD18-20 group), as well as during the entire embryogenesis period (GD 0-20 group), elevated somatotropic axis gene expression during the post-hatch period (even 35 d post hatch), as well as affected the growth and production of the broilers throughout this period of time. Therefore, we suggest it may be possible that the in ovo GL photostimulation, especially during the entire embryogenesis, or during the last critical stage of incubation, causes an epigenetic adaptation for somatotropic axis expression, which in turn affects the performance of the broilers.
However, further studies are required in order to examine and understand whether there are epigenetic modifications during these changes, as well as to examine whether these changes\adaptations can carry on to future generations. Moreover, the in ovo GL photostimulation between ED18 and hatching can become a commercial tool to increase performance in broilers, as it requires GL LEDs only during the hatching process, and increases performance as well as photostimulation from ED0 to hatching.
DISCLOSURES
There are no conflicts of interest in the experiment or the paper.
REFERENCES
- Borille R., Garcia R.G., Royer A.F.B., Santana M.R., Colet S., Naas I.A., Caldara F.R., Almeida Paz I.C.L., Rosa E.S., Castilho V.A.R. The use of light-emitting diodes (LED) in commercial layer production. Braz. J. Poult. Sci. 2013;14:135–140. [Google Scholar]
- Buyse J., Decuypere E. The role of the somatotropic axis in the metabolism of the chicken. Domest. Anim. Endocrin. 1999;17:245–255. doi: 10.1016/s0739-7240(99)00041-7. [DOI] [PubMed] [Google Scholar]
- Cobb-Vantress. 2018. Broiler management guide. Accessed March 2019. https://www.cobb-vantress.com/assets/5c7576a214/Broiler-guide-R1.pdf
- Csaba G. Receptor ontogeny and hormonal imprinting. Experientia. 1986;42:750–759. doi: 10.1007/BF01941521. [DOI] [PubMed] [Google Scholar]
- Csaba G. The biological basis and clinical significance of hormonal imprinting, an epigenetic process. Clin. Epigenet. 2011;2:187–196. doi: 10.1007/s13148-011-0024-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Oliveira J.E., Uni Z., Ferket P.R. Important metabolic pathways in poultry embryos prior to hatch. World. Poult. Sci. J. 2008;64:488–499. [Google Scholar]
- Decuypere E., Bruggeman V. Endocrine aspects of development: new challenges for the control of incubation process. Worlds Poult. Sci. J. 2005;61:278–284. [Google Scholar]
- Decuypere E., Bruggeman V. The endocrine interface of environmental and egg factors affecting chick quality. Poult. Sci. 2007;86:1037–1042. doi: 10.1093/ps/86.5.1037. [DOI] [PubMed] [Google Scholar]
- Dishon L., Avital-Cohen N., Malamud D., Heiblum R., Druyan S., Porter T.E., Gumułka M., Rozenboim I. In ovo monochromatic green light photostimulation enhances embryonic somatotropic axis activity. Poult. Sci. 2017;96:1884–1890. doi: 10.3382/ps/pew489. [DOI] [PubMed] [Google Scholar]
- Dishon L., Avital-Cohen N., Zaguri S., Bartman J., Heiblum R., Druyan S., Porter T.E., Gumułka M., Rozenboim I. In-ovo green light photostimulation during different embryonic stages affect somatotropic axis. Poult. Sci. 2018;97:1998–2004. doi: 10.3382/ps/pey078. [DOI] [PubMed] [Google Scholar]
- Dishon L., Avital-Cohen N., Zaguri S., Bartman J., Heiblum R., Druyan S., Porter T.E., Gumułka M., Rozenboim I. In ovo green light photostimulation during the late incubation stage affects somatotropic axis activity. Poult. Sci. 2021;100:467–473. doi: 10.1016/j.psj.2020.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellestad L.E., Saliba J., Porter T.E. Ontogenic characterization of gene expression in the developing neuroendocrine system of the chick. Gen. Comp. Endocrinol. 2011;171:82–93. doi: 10.1016/j.ygcen.2010.12.006. [DOI] [PubMed] [Google Scholar]
- Guerrero-Bosagna C., Morisson M., Liaubet L., Bas Rodenburg T., De Haas E.N., Kost'a´ l L., Pitel F. Transgenerational epigenetic inheritance in birds. Environ. Epigenetics. 2018;4:1–8. doi: 10.1093/eep/dvy008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halevy O., Biran I., Rozenboim I. Various light source treatments affect body and skeletal muscle growth by affecting skeletal muscle satellite cell proliferation in broilers. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 1998;120:317–323. doi: 10.1016/s1095-6433(98)10032-6. [DOI] [PubMed] [Google Scholar]
- Halevy O., Piestun Y., Rozenboim I., Yablonka-Reuveni Z. In ovo exposure to monochromatic green light promotes skeletal muscle cell proliferation and affects myofiber growth in posthatch chicks. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006;290:1062–1070. doi: 10.1152/ajpregu.00378.2005. [DOI] [PubMed] [Google Scholar]
- Halevy O., Yahav S., Rozenboim I. Enhancement of meat production by environmental manipulations in embryo and young proilers. World. Poult. Sci. J. 2006;62:485–497. [Google Scholar]
- Jiang Y., Denbow C., Meiri N., Denbow D.M. Epigenetic-imprinting changes caused by neonatal fasting stress protect from future fasting stress. J. Neuroendocrinol. 2016;28:1–12. doi: 10.1111/jne.12333. [DOI] [PubMed] [Google Scholar]
- Kanački Z., Stojanović S., Ušćebrka G., Žikić D. The development pattern of IGF-1 (insulin-like growth factor-1) protein expression in breast muscle of broiler chickens. Biotechnol. Anim. Husbandry. 2012;28:797–805. [Google Scholar]
- Kim J.W. The endocrine regulation of chicken growth. Asian-Aust. J. Anim. Sci. 2010;23:1669–1676. [Google Scholar]
- Luna M., Rodrı´guez-Me´ndez A.J., Berumen L., Carranza M., Riesgo-Escovar J., Baudet M.L., Harvey S., Ara´mburo C. Immune growth hormone (GH): localization of GH and GH mRNA in the bursa of Fabricius. Dev. Comp. Immunol. 2008;32:1313–1325. doi: 10.1016/j.dci.2008.04.008. [DOI] [PubMed] [Google Scholar]
- Mao J.N.C., Burnside J., Postel-Vinay M.C., Pesek J.D., Chambers J.R., Cogburn L.A. Ontogeny of growth hormone receptor gene expression in tissue of growth-selected strains of broiler chickens. Endocrinology. 1998;156:67–75. doi: 10.1677/joe.0.1560067. [DOI] [PubMed] [Google Scholar]
- Marisela Ahumada-Solórzano S., Carranza M.E., Pedernera E., Rodríguez-Méndez A.J., Luna M., Arámburo C. Local expression and distribution of growth hormone and growth hormone receptor in the chicken ovary: effects of GH on steroidogenesis in cultured follicular granulosa cells. Gen. Comp. Endocrinol. 2012;175:297–310. doi: 10.1016/j.ygcen.2011.11.027. [DOI] [PubMed] [Google Scholar]
- Mayo K.E. Molecular cloning and expression of a pituitary-specific receptor for growth hormone-releasing hormone. Mol. Endocrinol. 1992;6:1734–1744. doi: 10.1210/mend.6.10.1333056. [DOI] [PubMed] [Google Scholar]
- McMurtry J.P., Francis G.L., Upton Z. Insulin-like growth factors in poultry. Domest. Anim. Endocrinol. 1997;14:199–229. doi: 10.1016/s0739-7240(97)00019-2. [DOI] [PubMed] [Google Scholar]
- Olanrewaju H.A., Thaxton J.P., Dozier W.A., III, Purswell J., Roush W.B., Branton S.L. A review of lighting programs for broiler production. Int. J. Poult. Sci. 2006;5:301–308. [Google Scholar]
- Porter T.E., Ellestad L.E., Fay A., Stewart J.L., Bossis I. Identification of the chicken growth hormone-releasing hormone receptor (GHRH-R) mRNA and gene: regulation of anterior pituitary GHRH-R mRNA levels by homologous and heterologous hormones. Endocrinology. 2006;147:2535–2543. doi: 10.1210/en.2005-1534. [DOI] [PubMed] [Google Scholar]
- Romanoff A.L. Critical periods and causes of death in avian embryonic development. The Auk. 1949;66:264–270. [Google Scholar]
- Rosebrough R., McMurtry J.P., Vasilatos-Younken R.V. Effect of pulsatile or continuous administration of pituitary- derived chicken growth hormone (p-cGH) on lipid metabolism in broiler pullets. Comp. Biochem. Physiol. 1991;99a:207–214. doi: 10.1016/0300-9629(91)90260-j. [DOI] [PubMed] [Google Scholar]
- Rozenboim I., Biran I., Uni Z., Halevy O. The involvement of monochromatic light in growth, development and endocrine parameters of broilers. Poult. Sci. 1999;78:135–138. doi: 10.1093/ps/78.1.135. [DOI] [PubMed] [Google Scholar]
- Rozenboim I., El Halawani M.E., Kashash Y., Piestun Y., Halevy O. The effect of monochromatic photostimulation on growth and development of broiler birds. Gen. Comp. Endocrinol. 2013;190:214–219. doi: 10.1016/j.ygcen.2013.06.027. [DOI] [PubMed] [Google Scholar]
- Rozenboim I., Piestun Y., Mobarkey N., Barak M., Hoyzman A., Halevy O. Monochromatic light stimuli during embryogenesis enhance embryo development and posthatch growth. Poult. Sci. 2004;83:1413–1419. doi: 10.1093/ps/83.8.1413. [DOI] [PubMed] [Google Scholar]
- Rozenboim I., Robinzon B., Ron B., Arnon E., Snapir N. The response of broilers' diposity to testosterone after embryonic exposure to androgen and tamoxifen. Br. Poult. Sci. 1990;31:645–650. doi: 10.1080/00071669008417295. [DOI] [PubMed] [Google Scholar]
- Rozenboim I., Robinzon B., Rosenstrauch A. Effect of light source and regimen on growing broilers. Br. Poult. Sci. 1999;40:452–457. doi: 10.1080/00071669987197. [DOI] [PubMed] [Google Scholar]
- Tong Q., Romanini C.E., Exadaktylos V., Bahr C., Berckmans D., Bergoug H., Eterradossi N., Roulston N., Verhelst R., McGonnell I.M., Demmers T. Embryonic development and the physiological factors that coordinate hatching in domestic chickens. Poult. Sci. 2013;92:620–628. doi: 10.3382/ps.2012-02509. [DOI] [PubMed] [Google Scholar]
- Tzschentke B., Halle I. Influence of temperature stimulation during the last 4 days of incubation on secondary sex ratio and later performance in male and female broiler chicks. Br. Poult. Sci. 2009;50:634–640. doi: 10.1080/00071660903186570. [DOI] [PubMed] [Google Scholar]
- Uni Z., Tako E., Gal-Garber O., Sklan D. Morphological, molecular, and functional changes in the chicken small intestine of the late-term embryo. Poult. Sci. 2003;82:1747–1754. doi: 10.1093/ps/82.11.1747. [DOI] [PubMed] [Google Scholar]
- Vasilatos-Younken R., Scanes C.G. Growth hormone and insulin-like growth factors in poultry growth: required, optimal, or ineffective? Poult. Sci. 1991;70:1764–1780. doi: 10.3382/ps.0701764. [DOI] [PubMed] [Google Scholar]
- Vinoth, A., T. Thirunalasundari, M. Shanmugam, A. Uthrakumar, S. Suji, and U. Rajkumar. 2018. Cell stress chaperones. 23:235–252. [DOI] [PMC free article] [PubMed]
- Yahav S. Alleviating heat stress in domestic fowl: different strategies. Worlds Poult. Sci. J. 2009;65:719–732. [Google Scholar]
- Zhang L., Wu S., Wang J., Qiao X., Yue H., Yao J., Zhang H., Qi G. Changes of plasma growth hormone, insulin-like growth factors-1, thyroid hormones, and testosterone concentrations in embryos and broiler chickens incubated under monochromatic green light. Ital. J. Anim. Sci. 2014;13:530–535. [Google Scholar]
- Zhang L., Zhang H.J., Qiao X., Yue H.Y., Wu S.G., Yao J.H., Qi G.H. Effect of monochromatic light stimuli during embryogenesis on muscular growth, chemical composition, and meat quality of breast muscle in male broilers. Poult. Sci. 2012;91:1026–1031. doi: 10.3382/ps.2011-01899. [DOI] [PubMed] [Google Scholar]
- Zhang L., Zhang H.J., Wang J., Wu S.G. Stimulation with monochromatic green light during incubation alters satellite cell mitotic activity and gene expression in relation to embryonic and posthatch muscle growth of broiler chickens. Animal. 2014;8:86–93. doi: 10.1017/S1751731113001882. [DOI] [PubMed] [Google Scholar]
- Zhu Y., Li S., Diam Y., Ren Z., Yang X., Yang X. Effects of in ovo feeding of itamin C on post-hatch performance, immune status and DNA methylation-related gene expression in broiler chickens. Br. J. Nutr. 2020;124:903–911. doi: 10.1017/S000711452000210X. [DOI] [PubMed] [Google Scholar]