Abstract
BACKGROUND
In hepatocellular carcinoma (HCC), detection and treatment prior to growth beyond 2 cm are important as a larger tumor size is more frequently associated with microvascular invasion and/or satellites. In the surveillance of very small HCC nodules (≤ 2 cm in maximum diameter, Barcelona clinical stage 0), we demonstrated that the tumor markers alpha-fetoprotein and PIVKA-Ⅱ are not so useful. Therefore, we must survey with imaging modalities. The superiority of magnetic resonance imaging (MRI) over ultrasound (US) to detect HCC was confirmed in many studies. Although enhanced MRI is now performed to accurately diagnose HCC, in conventional clinical practice for HCC surveillance in liver diseases, unenhanced MRI is widely performed throughout the world. While, MRI has made marked improvements in recent years.
AIM
To make a comparison of unenhanced MRI and US in detecting very small HCC that was examined in the last ten years in patients in whom MRI and US examinations were performed nearly simultaneously.
METHODS
In 394 patients with very small HCC nodules, those who underwent MRI and US at nearly the same time (on the same day whenever possible or at least within 14 days of one another) at the first diagnosis of HCC were selected. The detection rate of HCC with unenhanced MRI was investigated and compared with that of unenhanced US.
RESULTS
The sensitivity of unenhanced MRI for detecting very small HCC was 95.1% (97/102, 95% confidence interval: 90.9-99.3) and that of unenhanced US was 69.6% (71/102, 95% confidence interval: 60.7-78.5). The sensitivity of unenhanced MRI for detecting very small HCC was significantly higher than that of unenhanced US (P < 0.001). Regarding the location of HCC in the liver in patients in whom detection by US was unsuccessful, S7-8 was identified in 51.7%.
CONCLUSION
Currently, unenhanced MRI is a very useful tool for the surveillance of very small HCC in conventional clinical follow-up practice.
Keywords: Comparison of magnetic resonance imaging and ultrasound, Surveillance of very small hepatocellular carcinoma, Magnetic resonance imaging, Ultrasound, Unenhanced magnetic resonance imaging
Core Tip: Recent technological development of magnetic resonance imaging (MRI) scanners has been excellent. The 3.0-tesla (T) MR scanner with a higher field strength has been increasingly used because improved lesion detection can be expected as a result of the increased signal-to-noise ratio, which is theoretically twice with 3.0-T compared with 1.5-T. Another important improvement in MRI is the practical use of diffusion-weighted imaging. In this study, a comparison of unenhanced MRI and ultrasound in detecting very small hepatocellular carcinoma (2 cm in maximum diameter) was made. The sensitivity of unenhanced MRI for detecting very small hepatocellular carcinoma was as high as 95.1% as compared with 69.6% of unenhanced ultrasound (P < 0.001).
INTRODUCTION
If hepatocellular carcinoma (HCC) tumors are growing up to more than 2 cm in diameter, they are often associated with microvascular invasion and/or satellites, which are major predictors of recurrence after initial effective treatments[1]. The same tendency was observed by Stravitz et al[2], who reported that the early detection of HCC improves the prognosis. Therefore, we must identify very small HCC nodules (≤ 2 cm in maximum diameter) in the surveillance of HCC.
Recently, we demonstrated that more than one third of patients with very small HCC nodules were dropped from surveillance using the tumor markers alpha-fetoprotein (AFP) and PIVKA-Ⅱ[3]. Therefore, we must survey patients with liver diseases using imaging modalities.
Surveillance of HCC in liver diseases, especially in liver cirrhosis, has been conducted by ultrasound (US) or magnetic resonance imaging (MRI) throughout the world.
Although US was performed more popularly than MRI in the surveillance of HCC, the superiority of MRI over US has been demonstrated in many studies since 2001-2003[4,5]. Although enhanced MRI is now performed for the accurate diagnosis of HCC[5-9], in conventional clinical practice for HCC surveillance in liver diseases, unenhanced MRI is widely performed throughout the world. On the other hand, MRI has made much progress in recent years.
In this study, a comparison of unenhanced MRI and US in surveying very small HCC was made. In order to conduct precise evaluation, we selected patients in whom MRI and US were performed at about the same time.
MATERIALS AND METHODS
Study population
This was a retrospective observational study that included 403 patients with small single HCC nodules (≤ 2 cm in maximum diameter, Barcelona clinical stage 0) who visited the following three hospitals and one clinic in Yokohama City for the first time between January 2008 and September 2020: Gastroenterological Center, Medical Center, Yokohama City University; Department of Gastroenterology, Yokohama Municipal Citizen's Hospital; Department of Gastroenterology, National Hospital Organization, Yokohama Medical Center; and Tarao’s Gastroenterological Clinic. Of the 403 patients with very small HCC, 102 were selected in whom MRI and US were conducted simultaneously (on the same day or at least within 14 days of one another) (Figure 1). In this series of the study, MRI and US were performed in unenhanced states because we wanted to study the usefulness to survey HCC in routine follow-up study. In the unenhanced MRI, a very small HCC usually appears as a dark spot in T1 image and light white spot in T2 image (see Figures 2-5). It is important that characteristics of both T1 and T2 images were present at the same time. In the US images, it usually appears as a dark round spot.
Figure 1.

Patient selection. HCC: Hepatocellular carcinoma; MRI: Magnetic resonance imaging; US: Ultrasound.
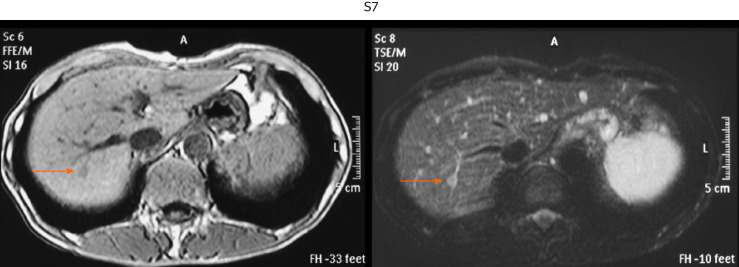
Figure 2.
Representative image of very small hepatocellular carcinoma by unenhanced magnetic resonance imaging. Hepatocellular carcinoma in S7 segment. T1-weighted image (left, light dark spot). T2-weighted image (right, light white spot).
Figure 5.
Representative image of very small hepatocellular carcinoma by unenhanced magnetic resonance imaging. Hepatocellular carcinoma in S8 segment. T1-weighted image (left, light dark spot). T2-weighted image (right, light white spot).
Figure 3.
Representative image of very small hepatocellular carcinoma by unenhanced magnetic resonance imaging. Hepatocellular carcinoma in S4 segment. T1-weighted image (left, light dark spot). T2-weighted image (right, light white spot).
Figure 4.
Representative image of very small hepatocellular carcinoma by unenhanced magnetic resonance imaging. Hepatocellular carcinoma in S3 segment. T1-weighted image (left, light dark spot). T2-weighted image (right, light white spot).
HCCs were diagnosed chiefly by dynamic computed tomography (CT) and abdominal angiography, which showed early enhancement and early washout. This work was performed in accordance with the Declaration of Helsinki.
Previously diagnosed HCC was excluded from the protocol. This study was performed after approval by the respective institutional review boards.
The patients were classified according to the etiologies of liver diseases (Table 1).
Table 1.
Background of hepatocellular carcinoma patients (≤ 2 cm in diameter) who underwent unenhanced magnetic resonance imaging and unenhanced ultrasound simultaneously
|
Background of patients
|
|
| Number of patients | 102 |
| Age (yr) | 72.4 ± 9.6 |
| Sex (%) | |
| Male | 52 (51.0) |
| Female | 50 (49.0) |
| Etiology (%) | |
| HBV | 13 (12.9) |
| HCV | 61 (60.3) |
| Alcohol | 14 (13.9) |
| NBNC | 7 (6.9) |
| Autoimmune | 2 (2.0) |
| NASH | 2 (2.0) |
| PBC | 1 (1.0) |
| Others | 2 (2.0) |
HBV: Hepatitis B virus; HCV: Hepatitis C virus; NBNC: Non-B non-C; NASH: Nonalcoholic steatohepatitis; PBC: Primary biliary cirrhosis.
HCC detection
The diagnosis of HCC was confirmed by US, MRI, CT, enhanced dynamic CT, and abdominal angiography. All patients underwent abdominal angiography to confirm the single nodules. The maximum diameter of the HCC nodules was scaled by US or MRI.
Helical dynamic CT and abdominal angiography were performed in almost all patients except those with hypersensitivity to iodine and advanced kidney disease. In the helical dynamic CT, an intravenous bolus injection of contrast material and sequential scanning were performed, and an intense homogenous arterial phase (early enhancement) and early washout in the venous phase were considered to be characteristic of HCC[10-12]. Abdominal angiography was also performed to exclude the benign nodular lesions and exclude HCC patients with macrovascular invasion. Of course, the characteristic features of very small HCC in unenhanced MRI as mentioned above were taken into account.
Patients with macrovascular invasion or extrahepatic metastasis were excluded. In patients undergoing hepatectomy, the final decision on HCC was made by pathological diagnosis, and cases of benign nodules were excluded.
Statistical analysis
We calculated the detection rate and its 95% confidence interval (CI) for each method. We then compared the detection rates between MRI and US using McNemar's test.
RESULTS
The sensitivity of unenhanced MRI for detecting very small HCC (≤ 2 cm in diameter) was 95.1% [97/102, 95%CI: 90.9-99.3] and that of unenhanced US was 69.6% (71/102, 95%CI: 60.7-78.5) (P < 0.001).
Table 2 shows the location of the HCC in the liver of patients in whom detection by US was unsuccessful. S7-8 was the site in 51.7% of these patients. Thus, HCC lesions in S7-8 may be difficult to identify by US. Representative images of four cases of very small HCC (A, B, C, and D) by unenhanced MRI are shown in Figures 2-5. In all the four cases, HCC was confirmed using hepatectomized specimens.
Table 2.
Location of hepatocellular carcinoma in the liver in patients for whom detection by ultrasound was unsuccessful
|
Location in the liver
|
Number of patients (%)
|
| S1-4 | 6 (20.7) |
| S5-6 | 8 (27.6) |
| S7-8 | 15 (51.7) |
Moreover, the treatment methods for 102 HCC patients are shown in Table 3.
Table 3.
Treatment methods for hepatocellular carcinoma in 102 very small hepatocellular carcinoma patients
|
Therapy
|
Number of treated patients
|
| Hepatectomy | 19 |
| RFA | 58 |
| TACE | 14 |
| TACE + RFA | 2 |
| TAI | 1 |
| Chemotherapy | 2 |
| BSC | 3 |
| Others | 3 |
BSC: Best supportive care; RFA: Radiofrequency ablation; TACE: Transcatheter arterial chemoembolization; TAI: Transcatheter arterial infusion.
DISCUSSION
For the surveillance of very small HCC, US was hitherto performed worldwide. However, in recent years, the superiority of MRI over US to detect very small HCC has been reported in many articles.
Colli et al[4] conducted a systemic review on this issue, and found that the pooled estimate of 14 US studies was 60.5% (95%CI: 44-76) for sensitivity[13-25], and that of 9 MRI studies was 80.6% (95%CI: 70-91) for sensitivity[9,23,24,26-31]. The difference in sensitivity between US and MRI may be due to the fact that MRI is less influenced by the operator's technique, patient's body type, and location of HCC lesions.
More recently, in 2017, Kim et al[5] compared MRI and US in a cohort of 407 patients with cirrhosis who underwent 1100 surveillance examinations, and found that MRI had a sensitivity of 83.7% (95%CI: 69.7-92.2) for early HCC detection, which was significantly higher than that of US (25.6%, 95%CI: 14.8-49.4).
We demonstrated in this study that 95% of cases with very small HCC can be detected by unenhanced MRI. This figure is very high compared with previous reports published between 2001 and 2003 concerning the sensitivity of unenhanced MRI for detecting very small HCC. Table 4 shows the reported sensitivity of unenhanced MRI for detecting very small HCC between 2001 and 2003 when MRI used 1.5-tesla (T) imaging. The average sensitivity in that period was 60.3% (95%CI: 52.2-68.4)[25,27,28,30,31].
Table 4.
Reported sensitivity of unenhanced magnetic resonance imaging to detect very small hepatocellular carcinomas (≤ 2 cm in diameter) between 2001 and 2003
|
Ref.
|
Sensitivity (%)
|
| Krinsky et al[27], 2001 | 7/15 (46.7) |
| de Lédinghen et al[28], 2002 | 33/54 (61.1) |
| Libbrecht et al[25], 2002 | 7/10 (70.0) |
| Bhartia et al[30], 2003 | 15/21 (71.4) |
| Burrel et al[31], 2003 | 23/41 (56.1) |
| Pooled estimates | 85/141 (60.3) |
| 95%CI: 52.2-68.4 |
CI: Confidence interval.
The reasons why this marked improvement appeared in the sensitivity of unenhanced MRI with regard to detecting very small HCC must be considered.
First of all, MRI has made marked progress in its ability in recent years. Recent technological development of MRI scanners has allowed high-quality multiphasic imaging of the entire liver. Since 2003-2005, the 3.0-T magnetic resonance (MR) scanner with a higher field strength has been increasingly used because improved lesion detection can be expected as a result of the increased signal-to-noise ratio (SNR), which is theoretically twice the SNR at 1.5-T[32,33]. Indeed, it was demonstrated that 3.0-T images were superior to 1.5 T images for detecting hepatic metastases[34]. Previous misdiagnoses of HCC on MRI maybe have been due to poor patient compliance, especially the inability to suspend respiration. These problems can be resolved by the new advancements mentioned above to develop faster and motion-robust sequences.
Another important improvement of MRI is the practical use of diffusion-weighted imaging. Indeed, it was demonstrated that the sensitivity of detecting pancreatic cancer rose with the use of diffusion-weighted imaging[35].
On the other hand, the sensitivity of unenhanced US in our study for detecting very small HCC was 69.6%, which was nearly the same as those in previous reports[9,22,24,26-29]. One of the reasons for the inferiority of US may be the location of HCC in the liver. A lesion located at S7-8 (the most frequent HCC lesion in the liver) may be difficult to identify by US.
Our present study indicates the importance of unenhanced MRI in detecting very small HCC, because more than one third of these patients were dropped from surveillance by tumor markers AFP and PVKA-II. However, there are two limitations of unenhanced MRI. First, it is more expensive than US. Second, in case of very tiny HCC (3-5 mm), it is difficult to find HCC by unenhanced MRI.
CONCLUSION
Considering the above-mentioned facts, unenhanced MRI is a very useful tool for detecting very small HCC in the conventional follow-up of patients with liver diseases, especially liver cirrhosis.
ARTICLE HIGHLIGHTS
Research background
Nowadays advancement of magnetic resonance imaging (MRI) has markedly improved the quality of liver imaging. We believe that a high-speed scan and diffusion-weighted imaging are two major factors that have contributed to the improved detection of hepatocellular carcinomas (HCCs). In early MRI, a respiration artifact was the most troublesome factor deteriorating the quality of images of the liver. A high-speed scan brought by the conversion from 1.5-tesla (T) to 3.0-T facilitates whole-liver MRI while patients hold their breath. Breath-holding scans reduce motion and misregistration artifacts, and create high-quality liver images. In addition, the practical use of diffusion-weighted imaging has contributed to the detection of cell-rich lesions. Tumors are proper objects of these sequences. There is a report (or several reports) that the sensitivity of detecting pancreatic cancer rose with the use of diffusion-weighted imaging. We believe that the same can be applied to detect HCC. Currently, dynamic MRI with contrast media is considered the standard procedure to diagnose HCC. However, with improved images, non-contrasted liver MRI is still a useful modality to detect HCCs.
Research motivation
Previous reports in 2001-2003 stated that the sensitivity of unenhanced MRI to detect very small HCC (≤ 2 cm in diameter) was about 60%. Since then, there have been few reports on the sensitivity to detect very small HCC, especially in recent years.
Research objectives
Surveillance of HCC in liver diseases, especially in liver cirrhosis, has been conducted by ultrasound (US) or MRI throughout the world. Although US was performed more popularly than MRI in the surveillance of HCC, the superiority of MRI over US has been demonstrated in many studies since 2001-2003. Although enhanced MRI is now performed for the accurate diagnosis of HCC, in conventional clinical practice for HCC surveillance in liver diseases, unenhanced MRI is widely performed throughout the world. On the other hand, MRI has made marked improvements in recent years. In this study, a comparison of unenhanced MRI and US in detecting very small HCC was made. In order to conduct precise evaluation, we selected patients in whom MRI and US were performed at about the same time (on the same day whenever possible or at least within 14 d of one another).
Research methods
Out of the 403 patients with very small HCC nodules (≤ 2 cm in maximal diameter), 102 who underwent unenhanced MRI and US at nearly the same time (on the same day whenever possible or at least within 14 d of one another) at the first diagnosis of HCC were selected. The detection rate of HCC by unenhanced MRI was studied in comparison with unenhanced US.
Research results
We found that the sensitivity of unenhanced MRI for detecting very small HCC was as high as 95.1%, as compared with 69.6% by unenhanced US (P < 0.001).
Research conclusions
Currently, unenhanced MRI is a very important imaging modality for picking up very small HCC in usual clinical practice.
Research perspectives
As in this study, the marked superiority of unenhanced MRI to detect very small HCC as compared with unenhanced US was confirmed, and it may be desirable to perform routine surveillance of HCC in liver diseases by unenhanced MRI.
Footnotes
Institutional review board statement: The study was reviewed and approved by the Ethics Committee of Yokohama Municipal Citizen's Hospital Institutional Review Board (Approval No. 21-02-01).
Informed consent statement: This study was performed after approval by the respective institutional review boards. The study used anonymous clinical data that were obtained after each patient agreed to treatment by written consent.
Conflict-of-interest statement: There are no conflicts of interest to disclose.
Manuscript source: Invited manuscript
Corresponding Author's Membership in Professional Societies: The Japanese Society of Gastroenterology.
Peer-review started: February 4, 2021
First decision: February 24, 2021
Article in press: May 20, 2021
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Elsayed M, Yang L S-Editor: Gao CC L-Editor: Wang TQ P-Editor: Wang LL
Contributor Information
Kazuo Tarao, Tarao's Gastroenterological Clinic, Yokohama 241-0821, Japan. nrg18449@nifty.com.
Akito Nozaki, Gastroenterological Center, Yokohama City University Medical Center, Yokohama 232-0024, Japan.
Hirokazu Komatsu, Department of Gastroenterology, Yokohama Municipal Citizen's Hospital, Yokohama 240-0855, Japan.
Tatsuji Komatsu, Department of Clinical Research, National Hospital Organization, Yokohama Medical Center, Yokohama 245-8575, Japan.
Masataka Taguri, Department of Data Science, Yokohama City University, Yokohama 236-0004, Japan.
Katsuaki Tanaka, Department of Gastroenterology, Hadano Red Cross Hospital, Hadano City 257-0017, Japan.
Testuo Yoshida, Department of Radiology, Ashigarakami Hospital, Yokohama 258-0003, Japan.
Hideki Koyasu, Department of Radiology, Koyasu Clinic, Yokohama 241-0821, Japan.
Makoto Chuma, Gastroenterological Center, Yokohama City University Medical Center, Yokohama 232-0024, Japan.
Kazushi Numata, Gastroenterological Center, Yokohama City University Medical Center, Yokohama 232-0024, Japan.
Shin Maeda, Department of Gastroenterology, Yokohama City University Graduate School of Medicine, Yokohama 236-0004, Japan.
Data sharing statement
No additional data are available.
References
- 1.Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012;379:1245–1255. doi: 10.1016/S0140-6736(11)61347-0. [DOI] [PubMed] [Google Scholar]
- 2.Stravitz RT, Heuman DM, Chand N, Sterling RK, Shiffman ML, Luketic VA, Sanyal AJ, Habib A, Mihas AA, Giles HC, Maluf DG, Cotterell AH, Posner MP, Fisher RA. Surveillance for hepatocellular carcinoma in patients with cirrhosis improves outcome. Am J Med. 2008;121:119–126. doi: 10.1016/j.amjmed.2007.09.020. [DOI] [PubMed] [Google Scholar]
- 3.Tarao K, Nozaki A, Komatsu H, Komatsu T, Taguri M, Tanaka K, Chuma M, Numata K, Maeda S. Real impact of tumor marker AFP and PIVKA-II in detecting very small hepatocellular carcinoma (≤ 2 cm, Barcelona stage 0) - assessment with large number of cases. World J Hepatol. 2020;12:1046–1054. doi: 10.4254/wjh.v12.i11.1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Colli A, Fraquelli M, Casazza G, Massironi S, Colucci A, Conte D, Duca P. Accuracy of ultrasonography, spiral CT, magnetic resonance, and alpha-fetoprotein in diagnosing hepatocellular carcinoma: a systematic review. Am J Gastroenterol. 2006;101:513–523. doi: 10.1111/j.1572-0241.2006.00467.x. [DOI] [PubMed] [Google Scholar]
- 5.Kim SY, An J, Lim YS, Han S, Lee JY, Byun JH, Won HJ, Lee SJ, Lee HC, Lee YS. MRI With Liver-Specific Contrast for Surveillance of Patients With Cirrhosis at High Risk of Hepatocellular Carcinoma. JAMA Oncol. 2017;3:456–463. doi: 10.1001/jamaoncol.2016.3147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Motosugi U, Ichikawa T, Morisaka H, Sou H, Muhi A, Kimura K, Sano K, Araki T. Detection of pancreatic carcinoma and liver metastases with gadoxetic acid-enhanced MR imaging: comparison with contrast-enhanced multi-detector row CT. Radiology. 2011;260:446–453. doi: 10.1148/radiol.11103548. [DOI] [PubMed] [Google Scholar]
- 7.Di Martino M, Marin D, Guerrisi A, Baski M, Galati F, Rossi M, Brozzetti S, Masciangelo R, Passariello R, Catalano C. Intraindividual comparison of gadoxetate disodium-enhanced MR imaging and 64-section multidetector CT in the Detection of hepatocellular carcinoma in patients with cirrhosis. Radiology. 2010;256:806–816. doi: 10.1148/radiol.10091334. [DOI] [PubMed] [Google Scholar]
- 8.Park MJ, Kim YK, Lee MH, Lee JH. Validation of diagnostic criteria using gadoxetic acid-enhanced and diffusion-weighted MR imaging for small hepatocellular carcinoma (<= 2.0 cm) in patients with hepatitis-induced liver cirrhosis. Acta Radiol. 2013;54:127–136. doi: 10.1258/ar.2012.120262. [DOI] [PubMed] [Google Scholar]
- 9.Rode A, Bancel B, Douek P, Chevallier M, Vilgrain V, Picaud G, Henry L, Berger F, Bizollon T, Gaudin JL, Ducerf C. Small nodule detection in cirrhotic livers: evaluation with US, spiral CT, and MRI and correlation with pathologic examination of explanted liver. J Comput Assist Tomogr. 2001;25:327–336. doi: 10.1097/00004728-200105000-00001. [DOI] [PubMed] [Google Scholar]
- 10.Hayashi M, Matsui O, Ueda K, Kawamori Y, Kadoya M, Yoshikawa J, Gabata T, Takashima T, Nonomura A, Nakanuma Y. Correlation between the blood supply and grade of malignancy of hepatocellular nodules associated with liver cirrhosis: evaluation by CT during intraarterial injection of contrast medium. AJR Am J Roentgenol. 1999;172:969–976. doi: 10.2214/ajr.172.4.10587130. [DOI] [PubMed] [Google Scholar]
- 11.Ueda K, Terada T, Nakanuma Y, Matsui O. Vascular supply in adenomatous hyperplasia of the liver and hepatocellular carcinoma: a morphometric study. Hum Pathol. 1992;23:619–626. doi: 10.1016/0046-8177(92)90316-u. [DOI] [PubMed] [Google Scholar]
- 12.Roncalli M, Roz E, Coggi G, Di Rocco MG, Bossi P, Minola E, Gambacorta M, Borzio M. The vascular profile of regenerative and dysplastic nodules of the cirrhotic liver: implications for diagnosis and classification. Hepatology. 1999;30:1174–1178. doi: 10.1002/hep.510300507. [DOI] [PubMed] [Google Scholar]
- 13.Okazaki N, Yoshida T, Yoshino M, Matue H. Screening of patients with chronic liver disease for hepatocellular carcinoma by ultrasonography. Clin Oncol. 1984;10:241–246. [PubMed] [Google Scholar]
- 14.Maringhini A, Cottone M, Sciarrino E, Marcenò MP, La Seta F, Rinaldi F, Pagliaro L. Ultrasonographic and radionuclide detection of hepatocellular carcinoma in cirrhotics with low alpha-fetoprotein levels. Cancer. 1984;54:2924–2926. doi: 10.1002/1097-0142(19841215)54:12<2924::aid-cncr2820541218>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 15.Kobayashi K, Sugimoto T, Makino H, Kumagai M, Unoura M, Tanaka N, Kato Y, Hattori N. Screening methods for early detection of hepatocellular carcinoma. Hepatology. 1985;5:1100–1105. doi: 10.1002/hep.1840050607. [DOI] [PubMed] [Google Scholar]
- 16.Tanaka S, Kitamura T, Ohshima A, Umeda K, Okuda S, Ohtani T, Tatsuta M, Yamamoto K. Diagnostic accuracy of ultrasonography for hepatocellular carcinoma. Cancer. 1986;58:344–347. doi: 10.1002/1097-0142(19860715)58:2<344::aid-cncr2820580224>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 17.Dodd GD 3rd, Miller WJ, Baron RL, Skolnick ML, Campbell WL. Detection of malignant tumors in end-stage cirrhotic livers: efficacy of sonography as a screening technique. AJR Am J Roentgenol. 1992;159:727–733. doi: 10.2214/ajr.159.4.1326883. [DOI] [PubMed] [Google Scholar]
- 18.Saada J, Bhattacharya S, Dhillon AP, Dick R, Burroughs AK, Rolles K, Davidson BR. Detection of small hepatocellular carcinomas in cirrhotic livers using iodised oil computed tomography. Gut. 1997;41:404–407. doi: 10.1136/gut.41.3.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chalasani N, Horlander JC Sr, Said A, Hoen H, Kopecky KK, Stockberger SM Jr, Manam R, Kwo PY, Lumeng L. Screening for hepatocellular carcinoma in patients with advanced cirrhosis. Am J Gastroenterol. 1999;94:2988–2993. doi: 10.1111/j.1572-0241.1999.01448.x. [DOI] [PubMed] [Google Scholar]
- 20.Gambarin-Gelwan M, Wolf DC, Shapiro R, Schwartz ME, Min AD. Sensitivity of commonly available screening tests in detecting hepatocellular carcinoma in cirrhotic patients undergoing liver transplantation. Am J Gastroenterol. 2000;95:1535–1538. doi: 10.1111/j.1572-0241.2000.02091.x. [DOI] [PubMed] [Google Scholar]
- 21.Kim CK, Lim JH, Lee WJ. Detection of hepatocellular carcinomas and dysplastic nodules in cirrhotic liver: accuracy of ultrasonography in transplant patients. J Ultrasound Med. 2001;20:99–104. doi: 10.7863/jum.2001.20.2.99. [DOI] [PubMed] [Google Scholar]
- 22.Bennett GL, Krinsky GA, Abitbol RJ, Kim SY, Theise ND, Teperman LW. Sonographic detection of hepatocellular carcinoma and dysplastic nodules in cirrhosis: correlation of pretransplantation sonography and liver explant pathology in 200 patients. AJR Am J Roentgenol. 2002;179:75–80. doi: 10.2214/ajr.179.1.1790075. [DOI] [PubMed] [Google Scholar]
- 23.Teefey SA, Hildeboldt CC, Dehdashti F, Siegel BA, Peters MG, Heiken JP, Brown JJ, McFarland EG, Middleton WD, Balfe DM, Ritter JH. Detection of primary hepatic malignancy in liver transplant candidates: prospective comparison of CT, MR imaging, US, and PET. Radiology. 2003;226:533–542. doi: 10.1148/radiol.2262011980. [DOI] [PubMed] [Google Scholar]
- 24.Tong MJ, Blatt LM, Kao VW. Surveillance for hepatocellular carcinoma in patients with chronic viral hepatitis in the United States of America. J Gastroenterol Hepatol. 2001;16:553–559. doi: 10.1046/j.1440-1746.2001.02470.x. [DOI] [PubMed] [Google Scholar]
- 25.Libbrecht L, Bielen D, Verslype C, Vanbeckevoort D, Pirenne J, Nevens F, Desmet V, Roskams T. Focal lesions in cirrhotic explant livers: pathological evaluation and accuracy of pretransplantation imaging examinations. Liver Transpl. 2002;8:749–761. doi: 10.1053/jlts.2002.34922. [DOI] [PubMed] [Google Scholar]
- 26.Born M, Layer G, Kreft B, Schwarz N, Schild H. [MRI, CT and CT arterial portography in the diagnosis of malignant liver tumors in liver cirrhosis] Rofo. 1998;168:567–572. doi: 10.1055/s-2007-1015282. [DOI] [PubMed] [Google Scholar]
- 27.Krinsky GA, Lee VS, Theise ND, Weinreb JC, Rofsky NM, Diflo T, Teperman LW. Hepatocellular carcinoma and dysplastic nodules in patients with cirrhosis: prospective diagnosis with MR imaging and explantation correlation. Radiology. 2001;219:445–454. doi: 10.1148/radiology.219.2.r01ma40445. [DOI] [PubMed] [Google Scholar]
- 28.de Lédinghen V, Laharie D, Lecesne R, Le Bail B, Winnock M, Bernard PH, Saric J, Couzigou P, Balabaud C, Bioulac-Sage P, Drouillard J. Detection of nodules in liver cirrhosis: spiral computed tomography or magnetic resonance imaging? Eur J Gastroenterol Hepatol. 2002;14:159–165. doi: 10.1097/00042737-200202000-00010. [DOI] [PubMed] [Google Scholar]
- 29.Mori K, Scheidler J, Helmberger T, Holzknecht N, Schauer R, Schirren CA, Bittmann I, Dugas M, Reiser M. Detection of malignant hepatic lesions before orthotopic liver transplantation: accuracy of ferumoxides-enhanced MR imaging. AJR Am J Roentgenol. 2002;179:1045–1051. doi: 10.2214/ajr.179.4.1791045. [DOI] [PubMed] [Google Scholar]
- 30.Bhartia B, Ward J, Guthrie JA, Robinson PJ. Hepatocellular carcinoma in cirrhotic livers: double-contrast thin-section MR imaging with pathologic correlation of explanted tissue. AJR Am J Roentgenol. 2003;180:577–584. doi: 10.2214/ajr.180.3.1800577. [DOI] [PubMed] [Google Scholar]
- 31.Burrel M, Llovet JM, Ayuso C, Iglesias C, Sala M, Miquel R, Caralt T, Ayuso JR, Solé M, Sanchez M, Brú C, Bruix J Barcelona Clínic Liver Cancer Group. MRI angiography is superior to helical CT for detection of HCC prior to liver transplantation: an explant correlation. Hepatology. 2003;38:1034–1042. doi: 10.1053/jhep.2003.50409. [DOI] [PubMed] [Google Scholar]
- 32.Norris DG. High field human imaging. J Magn Reson Imaging. 2003;18:519–529. doi: 10.1002/jmri.10390. [DOI] [PubMed] [Google Scholar]
- 33.Schick F. Whole-body MRI at high field: technical limits and clinical potential. Eur Radiol. 2005;15:946–959. doi: 10.1007/s00330-005-2678-0. [DOI] [PubMed] [Google Scholar]
- 34.Sofue K, Tsurusaki M, Miyake M, Sakurada A, Arai Y, Sugimura K. Detection of hepatic metastases by superparamagnetic iron oxide-enhanced MR imaging: prospective comparison between 1.5-T and 3.0-T images in the same patients. Eur Radiol. 2010;20:2265–2273. doi: 10.1007/s00330-010-1798-3. [DOI] [PubMed] [Google Scholar]
- 35.Matsuki M, Inada Y, Nakai G, Tatsugami F, Tanikake M, Narabayashi I, Masuda D, Arisaka Y, Takaori K, Tanigawa N. Diffusion-weighed MR imaging of pancreatic carcinoma. Abdom Imaging. 2007;32:481–483. doi: 10.1007/s00261-007-9192-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No additional data are available.