Abstract
Very rare cases of thrombosis associated with thrombocytopenia have occurred following the vaccination with AstraZeneca COVID‐19 vaccine. The aim of this concise review is to summarize the current knowledge on the epidemiologic and pathogenic mechanisms of this syndrome named vaccine‐associated immune thrombosis and thrombocytopenia (VITT). A practical patient management section will also be dealt with using information available from national and international scientific societies as well as expert panels. A literature search on the VITT syndrome was carried out in PubMed using appropriate MeSH headings. Overall, 40 VITT cases have been reported. Continuous pharmacovigilance monitoring is needed to collect more data on the real incidence and the pathogenesis of VITT syndrome. Such information will also help us to optimize the management this rare but often clinically severe thrombotic condition associated with COVID‐19 vaccination.
Keywords: AstraZeneca vaccine, cerebral venous thrombosis, heparin, thrombocytopenia
1. INTRODUCTION
Coronavirus disease 2019 (COVID‐19) is a still ongoing pandemic caused by the severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), originating in Wuhan, China, at the end of 2019 and disseminating all over the world causing an unprecedented sanitary, social and economic crisis. 1 As of April 20, 2021, the infection has already affected more than 130 million people and caused more than 3 million deaths worldwide, according to World Health Organization (WHO). 2 More than a year after the pandemic outbreak, very few therapeutic weapons have been shown to be able to fight COVID‐19, the most relevant including steroids and antibody‐based therapies. 3 , 4 , 5 The main progress of pharmaceutical research has been undoubtedly the development of COVID‐19 vaccines, available in Europe only 9 months after the first case of SARS‐CoV‐2 infection. 6 Currently, four COVID‐19 vaccines have been authorized in Europe by the European Medicine Agency (EMA): two using mRNA technology (Comirnaty by BioNTech Manufacturing GmbH and COVID‐19 Vaccine Moderna by Moderna Biotech Spain, SL) and two using adenovirus vector‐based technology (Vaxzevria by AstraZeneca AB and COVID‐19 Vaccine Janssen by Janssen‐Cilag International NV). 7 Considering the public health emergency, EMA accelerated the procedures for COVID‐19 vaccine evaluation, releasing a conditional marketing authorization and guaranteeing at the same time a continuous and rigorous safety monitoring through the European pharmacovigilance system. 8 While the post‐authorization surveillance performed by national and international health authorities did not show particular safety concerns following the administration of more than 700 million of COVID‐19 vaccine doses worldwide, very rare cases of thrombosis at unusual sites, mostly cerebral or sinus vein thrombosis (CVT), associated with thrombocytopenia have been reported shortly after the administration of the AstraZeneca (ChAdOx1 nCov‐19) COVID‐19 vaccine. 9
This paper summarizes the current knowledge on this rare but severe condition, named Vaccine‐associated Immune Thrombosis and Thrombocytopenia (VITT) syndrome, focusing in particular on epidemiologic, pathogenic, clinical, diagnostic, and therapeutic issues.
2. SEARCH STRATEGY
As a search literature strategy, the Medline and PubMed electronic database was searched for publications on VITT syndrome between November 2020 and April 2021 using English language as a restriction. The Medical Subject Heading (MeSH) and key words used were: “COVID‐19 vaccine,” “AstraZeneca,” ”Vaxzevria,” “ChAdOx1 nCoV‐19 vaccine,” “SARS‐CoV‐2,” “coronavirus,” “thrombosis,” “cerebral sinus vein thrombosis,” “unusual sites,” “pulmonary embolism,” “immune thrombocytopenia,” “heparin‐induced thrombocytopenia,” “thrombotic thrombocytopenia,” “adverse reaction.” We also screened the reference lists of the most relevant review articles and other publications for additional studies not captured in our initial literature search.
3. THE BURDEN OF THE DISEASE
The incidence of VITT appears to be between 1 in 125 000 and 1 in 1 million of vaccinated cases. 10 As of March 29th, 2021, a total of 19 cases of CVT had been reported in Germany to the Paul Ehrlich Institute following approximately 2.2 million AstraZeneca COVID‐19 vaccine doses administered. 11 Up to April 7th, 2021, 79 reports of thrombosis with thrombocytopenia (44 CVT and 35 thromboses in other major veins), out of a total of 20.2 million doses (incidence rate 1:250 000) of COVID‐19 vaccine AstraZeneca administered by that date, have been recorded in United Kingdom by the Medicines and Healthcare products Regulatory Agency (MHRA). 12 Five suspected thrombosis cases out of 400 000 injections and 23 cases out of 2.7 million injections have been recorded in The Netherlands and France, respectively. The incidence of VITT in Italy has not yet been established: A total of 11 suspected VITT cases, out of a total of 2.2 million AstraZeneca COVID‐19 vaccine injected doses, are currently under investigation by the Italian health regulatory agency (Agenzia Italiana del Farmaco, AIFA). 13 The EMA'S Pharmacovigilance Risk Assessment Committee (PRAC) performed periodic reviews of the safety issues of COVID‐19 vaccines. As of April 4th, 2021, a total of 222 CVT cases were reported to the European drug safety database (EudraVigilance) out of 34 million AstraZeneca vaccinated people, with an estimated incidence of 1:150 000. 14 Although EMA, in a statement dated April 7th, 2021, suggested a possible link between unusual blood clots with low blood platelets and AstraZeneca COVID‐19 vaccine, it concluded that the overall benefits far outweigh the risks of side effects. 9 Nevertheless, PRAC continued to monitor AstraZeneca COVID‐19 vaccine safety and effectiveness as for all other vaccines. 9 Although EMA did not restrict the use of AstraZeneca COVID‐19 vaccine to particular categories of subjects, many European and non‐European countries made decisions on their own: the Danish Health and Medicine authority decided to temporarily (3 weeks) suspend AstraZeneca vaccine, The Netherlands and Germany administered the vaccine only to subjects over 60 years of age, France and Canada only to over 55 years of age and Sweden and Finland only to over 65 years of age. The Italian AIFA left the prescription of the AstraZeneca vaccine free suggesting, however, a preferential use over the age of 60. In any case, the situation is constantly evolving, depending on the number of VITT cases reported in relation to the number of vaccinations, and it is likely that at the time of publication it has changed again.
4. PATHOGENESIS
While thrombotic events in VITT are preferentially, but not exclusively, located in intracranial veins, the concomitant thrombocytopenia suggests an immunological cause of the hypercoagulative tendency. 15 The pathogenic mechanism, which has been recently clarified by the German Greifswald Working Group under the leadership of Andreas Greinacher, resembles that of heparin‐induced thrombocytopenia (HIT), 10 a prothrombotic disorder provoked by IgG‐specific antibodies that recognize multimolecular complexes between the cationic platelet‐factor 4 (PF4) and the anionic heparin and cause platelet activation through the FcγRIIA receptor. 16 , 17 However, the fact which renders unique this condition is that it occurs in COVID‐19‐vaccinated patients who did not receive any heparin treatment during their life. Thus, an anionic substance other than heparin must be involved to explain the genesis of this prothrombotic syndrome which has been called ‘spontaneous’ or autoimmune HIT. 15 , 17 , 18 It has been, indeed, recently recognized that triggers other than heparin can cause a prothrombotic disorder with clinical and laboratory features that strongly resemble HIT. These include certain highly sulfated and highly negatively charged oligosaccharides, such as pentosan polysulfate, hypersulfated chondroitin sulfate and related molecules. 19 , 20 , 21 In addition to exposure to polyanionic medications, a HIT‐like phenomenon has also been observed after knee‐replacement surgery and bacterial or viral and infections. 21 , 22 , 23 , 24 These latter findings are in agreement with the antimicrobial, beyond procoagulant, suggested activity of PF4. 25 , 26 In response to microorganisms, indeed, activated platelets release PF4 contributes to the recruitment of neutrophils and facilitates neutrophil exocytosis to release myeloperoxidase and lysozyme. 27 In addition, PF4 binds directly to bacteria thus creating a neoantigen which is targeted by anti‐PF4/heparin or anti‐PF4/polyanion antibodies to form immune complexes that play a key role in antibacterial host defense. 26 A similar mechanism could also be implicated in the antiviral response to SARS‐CoV‐2 infection, considering that non‐platelet‐activating and platelet‐activating anti‐PF4/heparin antibodies have been detected by several investigators in COVID‐19 patients. 28 , 29 , 30
Regarding the cases of VITT syndrome observed after AstraZeneca COVID‐19 vaccine administration, anti‐PF4 antibodies might be elicited by the inflammatory stimulus of the vaccination or by the vaccine itself, which cross‐reacts with PF4 and platelets. With this regards, Greinacher and colleagues found an enhanced reactivity of VITT patients' serum with platelets in the presence of AstraZeneca COVID‐19 vaccine, 11 suggesting that interactions between the vaccine and platelets or between the vaccine and PF4 could play a role in pathogenesis. The adenovirus used as a vector in AstraZeneca vaccine could be involved in this pathway: It is well known, indeed, that adenovirus has a strong affinity for PF4 and can cause platelet activation. 31 , 32
Finally, different polymorphisms in exon 4 of the FcγRIIA region could be implicated in the modulation of the immune‐complex‐dependent thrombotic risk in VITT patients. 33 Further experimental studies are required to elucidate the exact mechanism through which AstraZeneca COVID‐19 vaccine triggers the generation of anti‐PF4 autoantibodies.
5. CLINICAL AND DIAGNOSTIC ASPECTS
The onset of moderate to severe thrombocytopenia and thrombotic complications at unusual sites, particularly CVT, beginning approximately 1‐2 weeks after vaccination against SARS‐CoV‐2 with AstraZeneca is strongly suggestive for VITT. 15
To date, 40 cases of VITT have been published 34 , 35 , 36 , 37 and their main characteristics are summarized in Table 1. Sixty‐seven percent (27/40) of patients were females with a female/male ratio of 2.1. The median age was 40.5 years, ranging between 21 and 77 years. According to these literature data, the thrombotic complications occurred after a median of 10 days (range 5‐24 days) from the first dose of AstraZeneca COVID‐19 vaccine. In the majority of the cases (27/39, 69.2%), the site of thrombosis was the cerebral veins, while splanchnic vein thrombosis and pulmonary embolism were present in 17.9% (7/39) and 23.1% (9/39) of cases, respectively. The median platelet count was 23,000/mm3 ranging from 7000 to 113 000 mm3. A severe thrombocytopenia, defined as a platelet count below 25 000/mm3, was present in 52.6% (20/38) of the evaluable cases. 38 Notably, a hormonal contraception or treatment was present in 25.9% (7/27) of women, while a congenital or thrombophilic status was detected in 7 (17.9%) cases.
TABLE 1.
Summary of VITT cases reported in literature
| First Author | Case no. | Sex | Age (y) | Days after vaccination | Site of thrombosis | Platelet count (mm3) | Thrombotic risk factors | Anti‐PF4 ab testing b | Treatment | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|
| Greinacher 34 | 1 | Female | 49 | 5 | CVT, SVT, PE, AT | 13 000 | – a | Positive | Heparin | Died |
| 2 | Female | 35 | 6 | PE | 107 000 | – | Positive | LMWH | Alive | |
| 3 | Female | 48 | 9 | CVT | 60 000 | – | Positive | NA | NA | |
| 4 | Female | 35 | 7 | CVT | 9000 | – | Positive | Heparin | Died | |
| 5 | Female | 43 | 13 | CVT, SVT, PE, DVT right intraventricular | 23 000 | FVL, ACL‐Ab | Positive | Heparin | Alive | |
| 6 | Female | 22 | 7 | CVT | 75 000 | – | NA | NA | Alive | |
| 7 | Female | 36 | 8 | CVT | 29 000 | – | NA | Heparin | Alive | |
| 8 | Female | 46 | 8 | CVT | 16 000 | – | Positive | ‐ | Died | |
| 9 | Female | 24 | 16 | CVT | 13 000 | – | Positive | ‐ | Died | |
| 10 | Male | NA | 11 | CVT, SVT | 8000 | – | Positive | ‐ | Died | |
| 11 | Male | NA | 12 | NA | NA | NA | Positive | ‐ | Died | |
| Schultz 35 | 1 | Female | 37 | 8 | CVT | 22 000 | Hormonal contraception | Positive | LMWH, PLTS | Died |
| 2 | Female | 42 | 10 | CVT | 14 000 | Hormonal contraception | Positive | LMWH, PLTS, steroids, IVIG | Died | |
| 3 | Male | 32 | 7 | SVT | 10 000 | – | Positive | LMWH, PLTS, steroids, IVIG | Alive | |
| 4 | Female | 39 | 10 | CVT | 70 000 | – | Positive | LMWH, steroids, IVIG | Alive | |
| 5 | Female | 54 | 7 | CVT | 19 000 | Hormonal replacement tp | Positive | Heparin, PLTS, steroids, IVIG | Died | |
| Franchini 36 | 1 | Male | 50 | 7 | CVT | 15 000 | MTHFR het | Positive | PLTS | Died |
| Scully 37 | 1 | Female | 30 | 13 | CVT, SVT, PE, II | 27 000 | – c | Positive | NA | Alive |
| 2 | Female | 55 | 6 | SVT, AT | 11 000 | – | Positive | NA | Died | |
| 3 | Female | 26 | 12 | CVT | 64 000 | – | Positive | NA | Alive | |
| 4 | Female | 52 | 10 | CVT, PE, II | 31 000 | – | Positive | NA | Died | |
| 5 | Male | 38 | 14 | PE | 16 000 | – | Positive | NA | Died | |
| 6 | Female | 49 | 15 | CVT | 14 000 | – | Positive | NA | Alive | |
| 7 | Male | 25 | 9 | CVT | 19 000 | – | Positive | NA | Died | |
| 8 | Male | 32 | 19 | CVT | 87 000 | – | Positive | NA | Alive | |
| 9 | Female | 35 | 9 | CVT | 65 000 | – | Positive | NA | Alive | |
| 10 | Male | 77 | 8 | PE | NA | – | Positive | NA | Alive | |
| 11 | Male | 66 | 12 | DVT | 34 000 | – | ± d | NA | Alive | |
| 12 | Male | 34 | 14 | CVT | 23 000 | – | Positive | NA | Alive | |
| 13 | Male | 54 | 10 | SVT, MI | 71 000 | – | Positive | NA | Died | |
| 14 | Female | 71 | 14 | No thrombosis | 17 000 | – | Positive | NA | Alive | |
| 15 | Female | 22 | 10 | CVT | 100 000 | – | Positive | NA | Died | |
| 16 | Female | 39 | 10 | MCAI | 57 000 | – | Positive | NA | Alive | |
| 17 | Female | 70 | 17 | DVT, PE | 28 000 | – | Positive | NA | Alive | |
| 18 | Male | 21 | 10 | MCAI | 113 000 | – | Positive | NA | Alive | |
| 19 | Female | 46 | 14 | CVT | 7000 | – | Positive | NA | Alive | |
| 20 | Female | 32 | 12 | CVT | 98 000 | – | Positive | NA | Died | |
| 21 | Male | 48 | 14 | CVT | 16 000 | – | Positive | NA | Alive | |
| 22 | Female | 49 | 24 | PE | 61 000 | – | Positive | NA | Alive | |
| 23 | Female | 46 | 10 | CVT | 36 000 | – | Negative | NA | Alive |
Abbreviations: ab, antibody; ACL‐Ab, anticardiolipin antibodies; AT, aortic thrombosis; CVT, cerebral vein thrombosis; DVT, deep vein thrombosis; FVL, factor V Leiden; II, intestinal infarct; IVIG, intravenous immunoglobulin; LMWH, low molecular weight heparin; MCAI, middle cerebral artery infarct; MI, myocardial infarction; MTHFR het, heterozygosity for methylene tetrahydrofolate reductase C677T polymorphism; NA, data not available; PE, pulmonary embolism; PLTS, platelet concentrates; SVT, splanchnic vein thrombosis; tp, therapy.
Three out of the nine women were under hormonal contraception.
Testing for anti‐PF4 antibodies performed by means of enzyme‐linked immunosorbent assay (ELISA).
One out of the 14 women were under oral contraceptive pill. Tests for thrombophilia were negative. A test for lupus anticoagulant was positive in 5 of the 10 patients for whom results were available.
Equivocal result.
Many patients (10/15, 66.7%) received heparin (unfractionated or low molecular weight) as treatment for thrombosis, while only a minority (4/15, 26.7%) was treated with immunomodulatory/immunosuppressive agents (ie, steroids and intravenous immunoglobulin). A 41.0% (16/39) mortality rate was reported.
Regarding the laboratory aspects, apart from thrombocytopenia, the diagnostic hallmark is the positivity of the HIT enzyme‐linked immunosorbent assay (ELISA), which identifies antibodies to PF4 and needs to be confirmed by a functional heparin‐induced platelet activation assay (HIPA) or serotonin‐release assay (SRA). 15 The current available data demonstrate that ELISA HIT assays have the most appropriate sensitivity for all pathophysiologically relevant anti‐PF4 antibodies. Other HIT assay methods have shown a low sensitivity. 15 Ninety‐five percent (36/38) of valuable VITT cases reported so far resulted positive for ELISA HIT assay (Table 1). Figure 1 reports the diagnostic algorithm of VITT syndrome.
FIGURE 1.
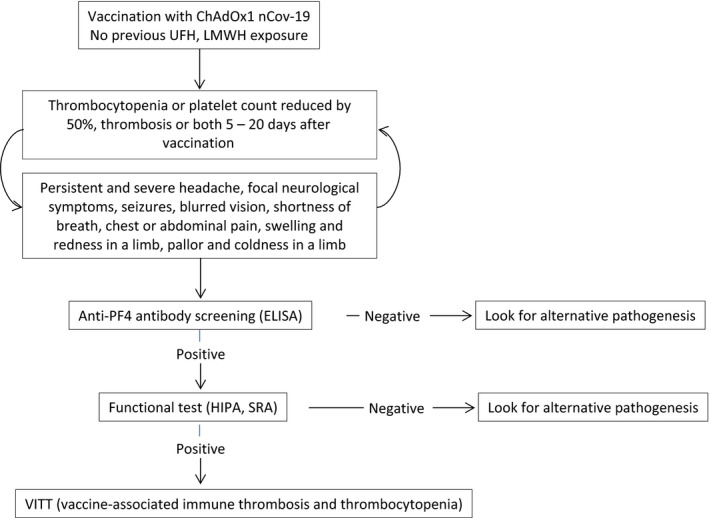
Diagnostic algorithm of VITT syndrome. UFH, unfractionated heparin; LMWH: low molecular weight heparin; ELISA, enzyme‐linked immunosorbent assay; HIPA, heparin‐induced platelet activation assay; SRA, serotonin‐release assay
6. MANAGEMENT OF VITT SYNDROME
Summarizing the data reported in Table 1, it can be assumed that VITT is a very severe condition, which arises most frequently in young female individuals 1‐2 weeks after the first dose of AstraZeneca COVID‐19 vaccine and is almost invariably characterized by severe thrombocytopenia and CVT with a fatality rate higher than 60%.
Following the first VITT reports, several national and international scientific societies and panel of experts released some recommendations on the management of patients with suspected VITT syndrome from diagnostic to treatment aspects. 15 , 39 , 40 , 41 , 42 , 43 On the basis of such contributions, the results of the literature data and our personal experience, we report these issues on the practical management of patients with suspected and confirmed VITT:
Individuals with persisting symptoms (>3 days), including severe headache, dizziness, focal neurological symptoms, visual disturbances, shortness of breath, chest, abdominal or extremities pain, arising 5‐20 days following AstraZeneca COVID‐19 vaccination should be urgently investigated through laboratory tests (ie, complete blood count, blood smear, D‐dimer and fibrinogen levels, activated partial thromboplastin time, and prothrombin time) and imaging‐based screening (ie, depending on symptoms, cranial magnetic resonance imaging, and ultrasound or computed tomography of the chest/abdomen).
In case of thrombosis and/or thrombocytopenia (< 150,000/mm3), a screening enzyme immunoassay for HIT, based on the immunological detection of antibodies against PF4, is mandatory. In case of positivity for ELISA HIT assay, a HIPA or SRA should be performed as functional confirmatory test for VITT.
Until VITT is ruled out, anticoagulation with unfractionated heparin (UFH) or low molecular weight heparin (LMWH) should be avoided and alternative HIT‐compatible anticoagulants (ie, fondaparinux, danaparoid, and argatroban) should be administered. We also suggest the use of direct oral anticoagulants (DOACs), which have been shown to be safe for the treatment of HIT and do not require initial heparin therapy, for anticoagulation treatment of presumptive and confirmed VITT cases. 10 The administration of DOACs, however, may be difficult in critical patients often hospitalized in Intensive care Units. Platelet concentrates should not be transfused.
In patients with confirmed VITT, administration of high‐dose intravenous immunoglobulin (IVIG, 1 g/kg daily for 2 consecutive days) or dexamethasone (40 mg days for 4 days) may be useful to interrupt the prothrombotic mechanism. In addition, Bruton tyrosine kinase inhibitors, which are able to block the FCγRIIA receptor‐mediated platelet activation, have been proposed as alternative treatment option for VITT. 44
7. CONCLUSION
The VITT syndrome is a very rare but often life‐threatening thrombotic condition associated with ChAdOx1 nCoV‐19 vaccine. On the basis of the few cases reported, it seems that young females, particularly those under hormonal contraception, are those at highest risk of developing this vaccine‐related adverse reaction. However, this finding could be an artifact due to the fact that this vaccine was registered until recently only for individuals under 55 years of age and that a consistent proportion of women take anti‐conception pill during childbearing age. The situation is, however, rapidly changing with daily updates from national and international health authorities.
The current therapeutic management of confirmed VITT is based on treatment with IVIG and non‐heparin anticoagulants. Further surveillance safety data in parallel with clinical and experimental studies are needed to definitely assess the incidence of VITT, its pathogenesis, and optimal management. Additional research is also required to identify, if possible, those individuals at increased risk of developing VITT syndrome in order to prevent this fearsome complication.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
AUTHOR CONTRIBUTIONS
MF involved in study conception and design. All authors are involved in data analysis, interpretation, and manuscript writing, editing, and approval.
Franchini M, Liumbruno GM, Pezzo M. COVID‐19 vaccine‐associated immune thrombosis and thrombocytopenia (VITT): Diagnostic and therapeutic recommendations for a new syndrome. Eur J Haematol. 2021;107:173–180. 10.1111/ejh.13665
DATA AVAILABILITY STATEMENT
Not applicable.
REFERENCES
- 1. Mahase E. Covid‐19: WHO declares pandemic because of “alarming levels” of spread, severity, and inaction. BMJ. 2020;368:m1036. [DOI] [PubMed] [Google Scholar]
- 2. World Health Organization (WHO) . Coronavirus disease (COVID‐19). https://www.who.int/emergencies/diseases/novel‐coronavirus‐2019. Accessed April 20, 2021.
- 3. Franchini M, Liumbruno GM. Convalescent plasma for the treatment of severe COVID‐19. Biologics. 2021;15:31‐38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. WHO Rapid Evidence Appraisal for COVID‐19 Therapies (REACT) Working Group , Sterne JAC, Murthy S, et al. Association between administration of systemic corticosteroids and mortality among critically Ill patients with COVID‐19: a meta‐analysis. JAMA. 2020;324:1330‐1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Weinreich DM, Sivapalasingam S, Norton T, et al. Trial investigators. REGN‐COV2, a neutralizing antibody cocktail, in outpatients with Covid‐19. N Engl J Med. 2021;384(3):238‐251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rawat K, Kumari P, Saha L. COVID‐19 vaccine: A recent update in pipeline vaccines, their design and development strategies. Eur J Pharmacol. 2021;892:173751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. European Medicine Agency (EMA) . Treatment and vaccines for COVID‐19. https://www.ema.europa.eu/en/human‐regulatory/overview/public‐health‐threats/coronavirus‐disease‐covid‐19/treatments‐vaccines‐covid‐19. Accessed April 19, 2021.
- 8. European Medicine Agency (EMA) . Conditional marketing authorization. https://www.ema.europa.eu/en/human‐regulatory/marketing‐authorisation/conditional‐marketing‐authorisation. Accessed April 19, 2021.
- 9. European Medicine Agency (EMA) . AstraZeneca's COVID‐19 vaccine: EMA finds possible link to very rare cases of unusual blood clots with low blood platelets. https://www.ema.europa.eu/en/news/astrazenecas‐covid‐19‐vaccine‐ema‐finds‐possible‐link‐very‐rare‐cases‐unusual‐blood‐clots‐low‐blood. Accessed April 19, 2021.
- 10. Pai M, Schull M, Razak F, et al. Vaccine‐induced prothrombotic immune thrombocytopenia VIPIT following AstraZeneca COVID‐19 vaccination: interim guidance for healthcare professionals in emergency department and inpatient settings. Ontario, Canada: Science Briefs of the Ontario COVID‐19 Science Advisory Table; 2021;1(21). 10.47326/ocsat.2021.02.21.1.0 [DOI] [Google Scholar]
- 11. GTH . Updated GTH statement on vaccination with AstraZeneca COVID‐19 vaccine, as of April 1, 2021. https://gth‐online.org/wp‐content/uploads/2021/04/GTH‐Statement‐AstraZeneca_englisch_4‐1‐2021.pdf. Accessed April 16, 2021.
- 12. Medicines and Healthcare products Regulatory Agency (MHRA) . Coronavirus vaccine. Summary of the yellow card reporting. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/975888/Coronavirus_vaccine_‐_summary_of_Yellow_Card_reporting_21.03.21_.pdf. Accessed April 19, 2021.
- 13. Agenzia Italiana del Farmaco (AIFA) . https://www.aifa.gov.it/content/segnalazioni‐reazioni‐avverse. Accessed April 16, 2021.
- 14. Eudravigilance – European database of suspected adverse reaction reports. https://www.adrreports.eu/en/index.html. Accessed April 16, 2021.
- 15. Oldenburg J, Klamroth R, Langer F, et al. Diagnosis and management of vaccine‐related thrombosis following AstraZeneca COVID‐19 vaccination: guidance statement from the GTH. Hamostaseologie. 2021. 10.1055/a-1469-7481 [DOI] [PubMed] [Google Scholar]
- 16. Marcucci R, Berteotti M, Gori AM, et al. Heparin induced thrombocytopenia: position paper from the Italian Society on Thrombosis and Haemostasis (SISET). Blood Transfus. 2021;19(1):14‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Greinacher A. Heparin‐induced thrombocytopenia. N Engl J Med. 2015;373:252‐261. [DOI] [PubMed] [Google Scholar]
- 18. Greinacher A, Selleng K, Warkentin TE. Autoimmune heparin‐induced thrombocytopenia. J Thromb Haemost. 2017;15:2099‐2114. [DOI] [PubMed] [Google Scholar]
- 19. Tardy‐Poncet B, Tardy B, Grelac F, et al. Pentosan polysulfate‐induced thrombocytopenia and thrombosis. Am J Hematol. 1994;45(3):252‐257. [DOI] [PubMed] [Google Scholar]
- 20. Rosenthal MA, Rischin D, McArthur G, et al. Treatment with the novel anti‐angiogenic agent PI‐88 is associated with immune‐mediated thrombocytopenia. Ann Oncol. 2002;13(5):770‐776. [DOI] [PubMed] [Google Scholar]
- 21. Goad KE, Horne MK, Gralnick HR. Pentosan‐induced thrombocytopenia: support for an immune complex mechanism. Br J Haem. 1994;88:803‐808. [DOI] [PubMed] [Google Scholar]
- 22. Jay RM, Warkentin TE. Fatal heparin‐induced thrombocytopenia (HIT) during warfarin thromboprophylaxis following orthopedic surgery: another example of 'spontaneous' HIT? J Thromb Haemost. 2008;6(9):1598‐1600. [DOI] [PubMed] [Google Scholar]
- 23. Hwang SR, Wang Y, Weil EL, Padmanabhan A, Warkentin TE, Pruthi RK. Cerebral venous sinus thrombosis associated with spontaneous heparin‐induced thrombocytopenia syndrome after total knee arthroplasty. Platelets. 2020;1‐5. 10.1080/09537104.2020.1828574 [DOI] [PubMed] [Google Scholar]
- 24. Warkentin TE, Makris M, Jay RM, Kelton JG. A spontaneous prothrombotic disorder resembling heparin‐induced thrombocytopenia. Am J Med. 2008;121:632‐636. [DOI] [PubMed] [Google Scholar]
- 25. Krauel K, Potschke C, Weber C, et al. Platelet factor 4 binds to bacteria, inducing antibodies cross‐reacting with the major antigen in heparin induced thrombocytopenia. Blood. 2011;117:1370‐1378. [DOI] [PubMed] [Google Scholar]
- 26. Krauel K, Weber C, Brandt S, et al. Platelet factor 4 binding to lipid A of Gram negative bacteria exposes PF4/heparin‐like epitopes. Blood. 2012;120:3345‐3352. [DOI] [PubMed] [Google Scholar]
- 27. Cai Z, Greene MI, Zhu Z, Zhang H. Structural features and PF4 functions that occur in heparin‐induced thrombocytopenia (HIT) complicated by COVID‐19. Antibodies (Basel). 2020;9(4):52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nazy I, Jevtic SD, Moore JC, et al. Platelet‐activating immune complexes identified in critically ill COVID‐19 patients suspected of heparin‐induced thrombocytopenia. J Thromb Haemost. 2021;19(5):1342‐1347. 10.1111/jth.15283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Brodard J, Kremer Hovinga JA, Fontana P, Studt JD, Gruel Y, Greinacher A. COVID‐19 patients often show high‐titer non‐platelet‐activating anti‐PF4/heparin IgG antibodies. J Thromb Haemost. 2021;19(5):1294‐1298. 10.1111/jth.15262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Warrior S, Behrens E, Gezer S, Venugopal P, Jain S. Heparin induced thrombocytopenia in patients with COVID‐19. Blood. 2020;136:17‐18. [Google Scholar]
- 31. Denard J, Rouillon J, Leger T, et al. AAV‐8 and AAV‐9 vectors cooperate with serum proteins differently than AAV‐1 and AAV‐6. Mol Ther Methods Clin Dev. 2018;10:291‐302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Stone D, Liu Y, Shayakhmetov D, Li Z‐Y, Ni S, Lieber A. Adenovirus‐platelet interaction in blood causes virus sequestration to the reticuloendothelial system of the liver. J Virol. 2007;81:4866‐4871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rollin J, Pouplard C, Gruel Y. Risk factors for heparin‐induced thrombocytopenia: focus on Fcgamma receptors. Thromb Haemost. 2016;16:799‐805. [DOI] [PubMed] [Google Scholar]
- 34. Greinacher A, Thiele T, Warkentin TE, Weisser K, Kyrle PA, Eichinger S. Thrombotic thrombocytopenia after ChAdOx1 nCov‐19 vaccination. N Engl J Med. 2021. 10.1056/NEJMoa2104840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Schultz NH, Sørvoll IH, Michelsen AE, et al. Thrombosis and thrombocytopenia after ChAdOx1 nCoV‐19 vaccination. N Engl J Med. 2021. 10.1056/NEJMoa2104882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Franchini M, Testa S, Pezzo M, et al. Cerebral venous thrombosis and thrombocytopenia post‐ COVID‐19 vaccination. Thromb Res. 2021;202:182‐183. 10.1016/j.thromres.2021.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Scully M, Singh D, Lown R, et al. Pathologic antibodies to Platelet Factor 4 after ChAdOx1 nCoV‐19 vaccination. N Engl J Med. 2021. 10.1056/NEJMoa2105385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. US Department of Health and Human Services . Common Terminology Criteria for Adverse Events. Version 5.0 November 27, 2017. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcae_v5_quick_reference_5x7.pdf. Accessed April 16, 2021.
- 39. GPNI . Guidance produced from the Expert Haematology Panel (EHP) focused on syndrome of Thrombosis and Thrombocytopenia occurring after coronavirus vaccination. https://www.gpni.co.uk/guidance‐produced‐from‐the‐expert‐haematology‐panel‐ehp‐focussed‐on‐syndrome‐of‐thrombosis‐and‐thrombocytopenia‐occurring‐after‐coronavirus‐vaccination/. Accessed April 16, 2021.
- 40. Canadian Covid‐19 Science Advisory Table . Thrombosis Canada. Vaccine‐induced prothrombotic immune thrombocytopenia (VIPIT). https://thrombosiscanada.ca/wp‐uploads/uploads/2021/04/51.‐Vaccine‐induced‐prothrobotic‐immune‐thrombcytopenia_02April2021.pdf. Accessed April 16, 2021.
- 41. Groupe Français d'études sur l'Hémostase et la Thrombose (GFHT) . Thrombose Astra Zeneca: essayons de comprendre. https://medvasc.info/1183‐thrombose‐astra‐zeneca‐essayons‐de‐comprendre [medvasc.info]. Accessed April 16, 2021.
- 42. International Society on Thrombosis and Haemostasis (ISTH) . Statement from the ISTH on reports indicating blood clots associated with the AstraZeneca vaccine. https://www.isth.org/news/556057/ISTH‐Statement‐on‐AstraZeneca‐COVID‐19‐Vaccine‐and‐Thrombosis.htm. Accessed April 16, 2021.
- 43. Società Italiana per lo Studio dell'Emostasi e della Trombosi (SISET) . SISET circa le trombosi cerebrali e viscerali con trombocitopenia segnalate in soggetti in precedenza vaccinati con vaccino Vaxzevria (AstraZeneca). http://www.siset.org/2‐non‐categorizzato/344‐siset‐circa‐le‐trombosi‐cerebrali‐e‐viscerali‐con‐trombocitopenia‐segnalate‐in‐soggetti‐in‐precedenza‐vaccinati‐con‐vaccino‐vaxzevria‐astrazeneca. Accessed April 16, 2021.
- 44. von Hundelshausen P, Lorenz R, Siess W, Weber C. Vaccine‐induced immune thrombotic thrombocytopenia (VITT): targeting pathomechanisms with Bruton tyrosine kinase inhibitors. Thromb Haemost. 2021. 10.1055/a-1481-3039 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.


