Abstract
Purpose
To report a case of a sterile iris abscess associated with herpes zoster ophthalmicus (HZO).
Observations
A 69-year-old African American female presented to SUNY Downstate Medical Center complaining of left-sided eye pain for two weeks. The patient had a best-corrected visual acuity of 20/30 OD and 20/200 OS. On external exam, vesicles were noted on the left upper lid and tip of the nose. Anterior segment exam was notable for decreased sensation without epithelial defects. The patient had 2+ stromal edema with 3+ cell and flare. The iris was flat with 1+ nuclear sclerosis OU. The intraocular pressure (IOP) was 14 mmHg OD and 40 mmHg OS. The patient was diagnosed with HZO with secondary uveitic glaucoma.
At ten weeks, anterior segment inflammation resolved and IOP stabilized. However, an iris nodule was noted superior nasal which continued to enlarge by 16 weeks follow up. Iris ultrasound revealed a 3 × 3 mm elevated lesion with internal homogeneity suggestive of an abscess.
At five months, a dense, mature cataract developed. The patient underwent cataract extraction with sector iridectomy. Gram staining and cultures were negative for organisms but positive for polymorphic neutrophils. Histopathology revealed fibrosis, surface necrosis, and stromal infiltration with chronic inflammatory cells consistent with chronic iritis and a sterile abscess secondary to HZO.
Conclusions and Importance
HZO is associated with a range of ocular sequelae with acquired iris nodule only mentioned once in the literature. As the second documented case, our findings will add to the general fund of knowledge regarding iris lesions and HZO.
Keywords: Herpes Zoster ophthalmicus, Iris nodule, Sterile abscess
1. Introduction
Varicella Zoster Virus (VZV), also known as shingles, is a common disease treated by healthcare practitioners. About 30% of individuals will develop shingles at least once in their lifetime, with a majority of individuals being 65 or older.1 It most often presents as a painful, vesicular rash which follows the dermatome.
When zoster involves the ophthalmic division of the fifth cranial nerve (V1) it is known as herpes zoster ophthalmicus (HZO).2 Ocular involvement occurs in about 10–20% of HZO cases,2 typically preceded by dermatomal vesicles which involve the tip or side of the nose, known as Hutchinson's sign. Ocular findings most commonly include conjunctivitis with severe pain, episcleritis/scleritis, uveitis with increased intraocular pressure, keratitis, corneal scarring, ptosis, and less commonly retinal necrosis.3
Another rare potential sequelae of HZO includes iris nodules which has only been previously documented once in the literature.4 Thus we present the second case of an iris nodules associated with HZO.
2. Case presentation
A 69-year-old African American female presented to SUNY Downstate medical center complaining of left-sided eye pain for two weeks duration. The patient had a best-corrected visual acuity (BCVA) of 20/30 OD and 20/200 OS. On external exam, vesicles were noted on the left eyebrow, left upper lid, and the tip of the nose. Anterior segment exam was notable for decreased sensation without epithelial defects or dendritic patterns. The patient had 2+ stromal edema with 3+ cell and flare. The iris was flat with 1+ nuclear sclerosis OU. The intraocular pressure (IOP) was 14 mmHg OD and 40 mmHg OS. Fundus exam was unremarkable for both eyes.
The patient was diagnosed with HZO with secondary uveitic glaucoma. She was started on IOP-lowering medication and anti-inflammatory agents including timolol BID, acetazolamide 500mg BID, prednisolone 1% Q2H, and atropine 1% BID. The patient was seen three days later. Anterior segment exam revealed nodular scleritis over the superior limbal area (Fig. 1). Anterior segment inflammation and elevated IOP continued. Thus, previous treatment modalities were continued.
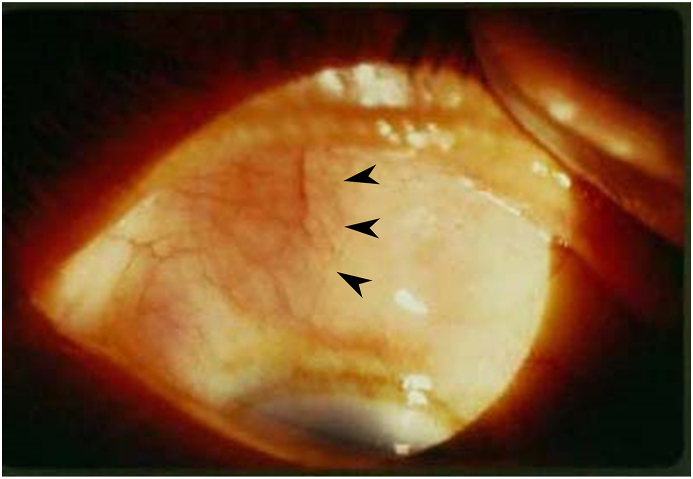
Fig. 1.
Anterior segment color photo demonstrating a nodular superior nasal scleritis. Aspiration of the limbal vesicle revealed purulent discharge. Gram staining and cultures were negative for organisms but positive for polymorphic neutrophils (PMNs). (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Five-week follow-up revealed growth of a limbal nodule at the 12 o'clock position. Aspiration of the limbal vesicle revealed purulent discharge. Gram staining and cultures were negative for organisms but positive for polymorphic neutrophils (PMNs).
At ten weeks, anterior segment inflammation resolved following a slow tapper of topical prednisolone along with stabilization of IOP. However, an iris nodule was noted superior nasal, which continued to enlarge by 16 weeks follow up. A workup for sarcoidosis and metastatic cancer was negative. A fast-growing iris melanoma versus an abscess was suspected. Iris ultrasound was performed, revealing a 3 × 3 mm elevated iris lesion within the anterior chamber. The lesion appeared solid with internal homogeneity suggestive of an abscess. The lesion did not extend to the ciliary body.
At five months, 360-degree posterior synechiae was noted with iris bombe. The iris mass partially regressed but a dense, mature cataract developed (Fig. 2). The patient underwent cataract extraction with sector iridectomy at eight months from presentation. During iridectomy, a pocket of loculated, yellowish purulent fluid was observed. Gram staining and cultures were negative for organisms but positive for PMNs. Pathology of excised iris mass revealed perivascular collagenization, an area of surface necrosis with neutrophils, and stromal infiltration with chronic inflammatory cells, plasma cells, and lymphocytes (Fig. 3). The pathology report was consistent with chronic iritis and a sterile iris abscess from a previous HZO infection. Follow up in the subsequent years documented no recurrence of iris nodules.
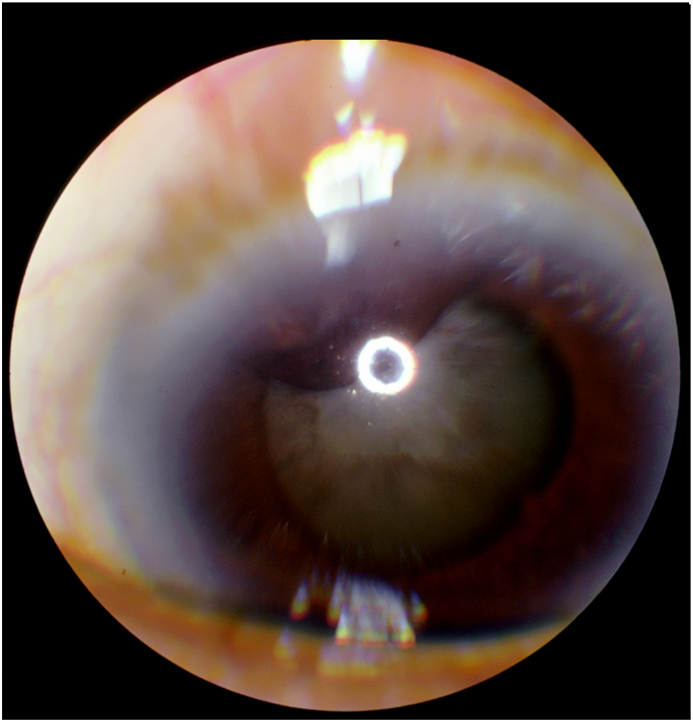
Fig. 2.
Anterior segment color photo demonstrating a 3 × 3 mm superior nasal iris lesion associated with correctopia without a feeder vessel. Iris pigmentation are also present on the anterior lens capsule, depicting a mature cataract with 360° of posterior synechia and iris bombe. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
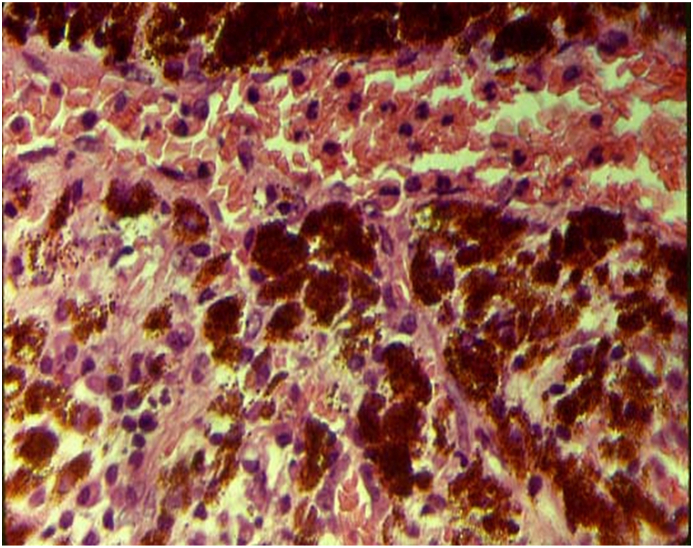
Fig. 3.
Histopathology revealed perivascular collagenization, an area of surface necrosis with neutrophils, and stromal infiltration with chronic inflammatory cells, plasma cells, and lymphocytes. Gram staining and cultures were negative for organisms but positive for polymorphic neutrophils. Findings were consistent with chronic iritis and a sterile iris abscess secondary to HZO.
3. Discussion
Iris lesions resulting from HZO infection are extremely rare, with only one previously documented case in the literature.4 Iris masses are typically classified into solid or cystic lesions, which most commonly affect Caucasian individuals.5
Solid iris tumors include melanocytic and nonmelanocytic lesions which encompasses both benign and malignant neoplasms. Melanocytic iris tumors are more common and include freckles, nevi, melanocytomas, Lisch nodules, and melanomas.6 Although rare, nonmelanocytic lesions include a wider variety of origin, including choristomatous, vascular, fibrous, neural, myogenic, epithelial, xanthomatous, metastatic, lymphoid, leukemic, and non-neoplastic simulators.6
Iris cysts are subsequently divided into two major categories, primary or secondary. Primary cysts involve the iris pigment epithelium (IPE) or iris stroma. IPE cysts are classified based on location, often described as pupillary margin, midzonal, peripheral, or posterior iris. IPE cysts can also dislodge, becoming free-floating cysts within the anterior chamber. Secondary or acquired cysts are typically iris stromal cysts which are classified based on three distinct types, including serous, pearl, and epithelial cysts.6,7 Acquired cysts form in response to tumors, inflammatory conditions (e.g., uveitis), parasitic infection, and trauma or can be drug-induced.6,7
Differentiation of solid versus acquired cystic lesions can be made with slit-lamp examination. Acquired serous cysts typically are lucent, fluid-filled structures with thin, smooth, well-defined borders. Occasionally, a septate, fluid-debris interface may be noted. Pearl cysts usually have dense, opaque walls within the iris stroma, containing inflammatory exudate, mucin, or keratin debris. Lastly, epithelial downgrowth cysts can manifest as sheets of cells as well as serous or pearl cysts.6,8
In comparison, solid masses have irregular, thick-walled borders which may be associated with sentinel vessels, ectropion uvea, corectopia, glaucoma, and cataract formation.6,8 The type of lesion can be further analyzed by ultrasound biomicroscopy (UBM) or anterior segment optical coherence tomography (AS-OCT). However, definitive diagnosis is made by histopathologic analysis.
Our patient developed an iris nodule five weeks after developing a uveitis secondary to HZO. The iris nodule continued to enlarge by 16 weeks follow up. The nodule appeared solid with irregular, thick-walled borders associated with corectopia. However, no sentinel vessels were noted. Iris ultrasound revealed a 3 × 3 mm elevated iris lesion with internal homogeneity suggestive of an abscess. The lesion did not extend to the ciliary body. Although the nodule partially regressed at 5 months, our patient developed 360 posterior synechia and a dense, mature cataract, significantly affecting her vision.
An iris nodule has been documented in response HZO uveitis.4 However, in their report, regression of the iris nodule occurred in the absence of reduced visual acuity, iris bombe, corneal decompensation, or cataract formation. Thus, treatment involved simple observation without a definitive biopsy. However, our patient developed a visually significant cataract and iris bombe, requiring surgical intervention. As a result, we decided to perform a combined cataract extraction with a sector iridectomy to remove the nodule and acquire a definitive diagnosis. Pathology observed perivascular fibrosis, necrotic tissue, and stromal infiltration of inflammatory cells consistent chronic iritis and a sterile iris abscess secondary to HZO. Therefore, we present the first case with histopathologic analysis supportive of HZO induced iris abscess.
There are various treatment modalities regarding primary and acquired cysts. Congenital iris stromal cysts in children under ten usually require surgical intervention as cysts can rapidly enlarge and rupture, resulting in iritis and glaucoma.9 However, congenital cysts in adolescents or adults only require intervention in about 25% of cases.6 Other primary cysts such as dislodged IPE cysts are generally benign and can be managed with simple observation. Conversely, acquired cysts can be more invasive due to rapid enlargement and destruction of neighboring tissue. Complications of these rapidly progressing cysts may include pain, reduction in visual acuity, iris bombe, corneal decompensation, cataract formation, uveitis, or glaucoma, necessitating urgent surgical intervention.6, 7, 8
Yet, when surgical intervention is deemed necessary, a wide variety of approaches exists. Conservative techniques involve cyst puncture and ablation via Argon laser photocoagulation or Nd:YAG laser iridocystotomy.10, 11, 12 Cyst puncture with careful aspiration, injection of sclerosing or cytotoxic agents, and irrigation can also be performed. Intracystic injection of saline, ethanol, or mitomycin-C have been effective in some instances to stop growth and promote involution of cysts.13, 14, 15 Lasers, combined with intracystic injections, can further facilitate the destruction and shrinkage of the cyst epithelial wall.
However, more invasive surgical excision may be required in the setting of recurrent cysts with significant sequelae. Surgical excision can range from viscoelastic dissection of the cyst,16 direct cauterization,17 sector iridectomy,18 and en bloc resection involving corneoscleral iridocyclectomy with subsequent corneoscleral graft.19,20 Although, iridectomy is not suitable for all symptomatic cysts, it may be a viable option when associated with cataract extraction, as seen in our patient, as it provides a definitive diagnosis and solution for recurrent cysts.
4. Conclusion
Herpes zoster commonly affects the aging population with ocular involvement in up to 20% of individuals. HZO is associated with a range of ocular sequelae with acquired iris nodules being a very rare complication. Only one previous case documented an iris nodule secondary to HZO. However, no definitive biopsy was obtained. Thus, we present the first case with histopathologic analysis supportive of an HZO induced iris abscess.
Patient consent
The patient consented to publication of the Case in writing. This report does not contain any personal information that could lead to the identification of the patient.
Funding
No funding or grant support.
Authorship
All authors attest that they meet the current ICMJE criteria for Authorship.
Conflict of interest
a. We wish to conform that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced the outcome.
Funding
a. No funding was received for this work.
Intellectual Property
a. We confirm that we have given due consideration to the protection of intellectual property associated with this work and that there are no impediments to publications, including the timing of publication, with respect to intellectual property. In so doing, we confirm that we have followed the regulations of our institutions concerning intellectual property.
Research Ethics
a. Written consent to publish potentially identifying information, such as details or the Case and photographs, was obtained from the patients(s) or their legal guardian(s).
Declaration of competing interest
None of the authors have any financial disclosures or potential conflicts of interest.
Authorship
a. All listed authors meet the ICMJE criteria. We attest that all authors contributed significantly to the creation of this manuscript, each having fulfilled criteria as established by the ICMJE.
b. We confirm that the manuscript has been read and approved by all named authors.
c. We confirm that the order of authors listed in the manuscript has been approved by all named authors.
Declaration of competing interest
The following authors have no financial disclosures: SAL, LP, NA, MB, EM, HG, ME.
Acknowledgments
SUNY Downstate pathology department for analysis of histological samples.
References
- 1.Harpaz R., Ortega-Sanchez I.R., Seward J.F. Advisory committee on immunization practices (ACIP) centers for disease control and prevention (CDC). Prevention of herpes zoster: recommendations of the advisory committee on immunization practices (ACIP) MMWR Recomm Rep (Morb Mortal Wkly Rep) 2008;57(RR-5):1–30. quiz CE2-4. [PubMed] [Google Scholar]
- 2.Liesegang T.J. Herpes zoster ophthalmicus natural history, risk factors, clinical presentation, and morbidity. Ophthalmology. 2008;115(2 Suppl):S3–S12. doi: 10.1016/j.ophtha.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 3.Yawn B.P., Wollan P.M., St Sauver J.L., Butterfield L.C. Herpes zoster--eye complications: rates and trends. Mayo Clin Proc. 2013;88(6):562–570. doi: 10.1016/j.mayocp.2013.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Karlin J.D. Herpes zoster ophthalmicus and iris cysts. Ann Ophthalmol. 1990;22(11):414–415. [PubMed] [Google Scholar]
- 5.Shields C.L., Kancherla S., Patel J. Clinical survey of 3680 iris tumors based on patient age at presentation. Ophthalmology. 2012;119(2):407–414. doi: 10.1016/j.ophtha.2011.07.059. [DOI] [PubMed] [Google Scholar]
- 6.Shields C.L., Shields P.W., Manalac J., Jumroendararasame C., Shields J.A. Review of cystic and solid tumors of the iris. Oman J Ophthalmol. 2013;6(3):159–164. doi: 10.4103/0974-620X.122269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rao A., Gupta V., Bhadange Y., Sharma R., Shields J.A. Iris cysts: a review. Semin Ophthalmol. 2011;26(1):11–22. doi: 10.3109/08820538.2010.541319. [DOI] [PubMed] [Google Scholar]
- 8.Georgalas I., Petrou P., Papaconstantinou D., Brouzas D., Koutsandrea C., Kanakis M. Iris cysts: a comprehensive review on diagnosis and treatment. Surv Ophthalmol. 2018;63(3):347–364. doi: 10.1016/j.survophthal.2017.08.009. [DOI] [PubMed] [Google Scholar]
- 9.Lois N., Shields C.L., Shields J.A., Mercado G., De Potter P. Primary iris stromal cysts. A report of 17 cases. Ophthalmology. 1998;105(7):1317–1322. doi: 10.1016/S0161-6420(98)97041-5. [DOI] [PubMed] [Google Scholar]
- 10.Xiao Y., Wang Y., Niu G., Li K. Transpupillary argon laser photocoagulation and Nd:YAG laser cystotomy for peripheral iris pigment epithelium cyst. Am J Ophthalmol. 2006;142(4):691–693. doi: 10.1016/j.ajo.2006.04.050. [DOI] [PubMed] [Google Scholar]
- 11.Sugar J., Jampol L.M., Goldberg M.F. Argon laser destruction of anterior chamber implantation cysts. Ophthalmology. 1985;92(2):306–307. doi: 10.1016/s0161-6420(85)34040-x. [DOI] [PubMed] [Google Scholar]
- 12.Kuchenbecker J., Motschmann M., Schmitz K., Behrens-Baumann W. Laser iridocystotomy for bilateral acute angle-closure glaucoma secondary to iris cysts. Am J Ophthalmol. 2000;129(3):391–393. doi: 10.1016/s0002-9394(99)00392-x. [DOI] [PubMed] [Google Scholar]
- 13.Kawaguchi K., Yamamoto S., Nagae Y., Okada A., Iwasaki N., Tano Y. Treatment of recurrent giant iris cyst with intracyst administration of mitomycin C. Br J Ophthalmol. 2000;84(7):800–801. doi: 10.1136/bjo.84.7.799b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shields C.L., Arepalli S., Lally E.B., Lally S.E., Shields J.A. Iris stromal cyst management with absolute alcohol-induced sclerosis in 16 patients. JAMA Ophthalmol. 2014;132(6):703–708. doi: 10.1001/jamaophthalmol.2014.160. [DOI] [PubMed] [Google Scholar]
- 15.Hong E.S., Burden J.H., Alward W.L.M. Intralesional ethanol for an unresectable epithelial inclusion cyst. JAMA Ophthalmol. 2013;131(2):262–263. doi: 10.1001/2013.jamaophthalmol.113. [DOI] [PubMed] [Google Scholar]
- 16.Al-Ghadeer H., Al-Towerki A.E., Al-Rajhi A., Al-Awad A. Long-term follow-up and visual outcome after excision of a traumatic iris cyst by viscoelastic dissection. Int Ophthalmol. 2011;31(6):529–531. doi: 10.1007/s10792-011-9498-9. [DOI] [PubMed] [Google Scholar]
- 17.Wilson W. Iris cyst treated CY electrolysis. Br J Ophthalmol. 1964;48:45–49. doi: 10.1136/bjo.48.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Venkateswaran N., Ching S.S.T., Fischer W., Lee F., Yeaney G., Hindman H.B. The diagnostic and therapeutic challenges of posttraumatic Iris implantation cysts: illustrative Case presentations and a review of the literature. Case Rep Ophthalmol Med. 2015;2015 doi: 10.1155/2015/375947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Naumann G.O., Rummelt V. Block excision of cystic and diffuse epithelial ingrowth of the anterior chamber. Report on 32 consecutive patients. Arch Ophthalmol. 1992;110(2):223–227. doi: 10.1001/archopht.1992.01080140079031. [DOI] [PubMed] [Google Scholar]
- 20.Forster R.K. Corneoscleral block excision of postoperative anterior chamber cysts. Trans Am Ophthalmol Soc. 1995;93:83–97. discussion 97-104. [PMC free article] [PubMed] [Google Scholar]