SUMMARY
Background
Lateral intercostal artery perforator (LICAP) flap for breast volume augmentation provides the benefits of addressing axillary tissue excess and avoiding intramuscular dissection. Previous experience with the LICAP flap in patients with prior breast conservation therapy (BCT) has led to the development of an extended version for massive weight loss (MWL) patients as well.
Methods
A retrospective review of all cases of LICAP flaps was performed by a single surgeon. Data were subsequently extracted and analyzed including patient demographics, indication and timing of volume augmentation, complications, and follow-up length.
Results
From 2016 to 2020, 12 patients underwent 16 LICAP flaps for volume augmentation. Indications for volume augmentation included deficits from prior oncologic surgery (ten patients) and loss of volume due to MWL (two patients). The average BMI was 29.9 kg/m2. Among the oncologic group, eight patients had delayed reconstruction, while two were immediate. Nine patients underwent radiation prior to volume augmentation. Eight of the 14 patients simultaneously received fat grafting. There were 4 cases of delayed wound healing that improved with local wound care. There were no statistically significant differences in complication rates between the oncologic and MWL groups. The average length of follow-up was 11.4 months.
Conclusions
This study supports that the application of the LICAP flap can be effectively broadened from the oncologic population to the MWL population. If needed, extending the flap provides an option to simultaneously address excess axillary and back tissue.
Keywords: Breast reconstruction, Autoaugmentation, Massive weight loss, Oncoplastic
Introduction
Breast volumization is frequently sought in improving breast appearance in both reconstructive and aesthetic surgery. Current options for augmentation are vast and include implant-based techniques, locoregional tissue rearrangement, free tissue transfer, and fat grafting. Though popular forms of volumizing the breast, implants and free tissue transfer methods are fraught with widely known complications and lengthy recovery times, respectively. As such, this study presents the lateral intercostal artery perforator (LICAP) flap, a compelling option that repurposes local tissue to augment the breast, while maintaining a reliable source of blood supply. Versatility of the LICAP flap is demonstrated by its usage across several patient populations. Here, we highlight how prior experience with the LICAP flap for reconstruction in an oncologic population has translated to applications for volumizing the deflated breast in the massive weight loss (MWL) population.
Patients with breast malignancy who undergo breast conservation therapy (BCT) (i.e., lumpectomy +/- radiation therapy) are often left with decreased volume and contour deformities that lead to dissatisfaction in appearance.1 This has led to the development of oncoplastic techniques to optimize cosmetic outcomes, including both volume displacement and volume replacement techniques. While volume displacement methods involve modified breast reduction or parenchymal reshaping from within the breast, volume replacement techniques utilize locoregional flaps from outside the breast parenchyma (i.e., axillary region, upper abdomen, etc.) to add volume to lumpectomy defects.2,3 For women with small breasts and adequate local tissue, autologous volume augmentation is often required when a large amount of gland is removed.4 Current options for autologous volume augmentation include the thoracodorsal artery perforator (TDAP) flap, the spiral flap, and serial fat grafting. Among the current techniques, the LICAP flap provides benefits of repurposing local tissue by addressing axillary tissue excess, while maintaining a reliable tissue source due to its robust blood supply.
Prior success of the LICAP flap to address excess local tissue made it an ideal augmentation technique for the MWL population. Our well-established experience with smaller volume breast autoaugmentation in the BCT population encouraged our application of this approach to MWL patients for several reasons. The breasts of MWL patients often have significant ptosis, loss of skin elasticity, and volume depletion making breast contouring a challenge.5 Oftentimes the lateral portion of the breast blends into the axillae, arms, and back, causing the area to be treated as a unit.6 The LICAP flap simultaneously addresses the axillary and back tissue excess in these patients, while augmenting the breast. Although previous techniques using lateral chest wall fasciocutaneous flaps to augment the breast have been described, limiting the length of the flap often fails to address circumferential excess tissue and may not achieve a sufficient degree of autoaugmentation. Here, we describe the use of extended LICAP flaps to provide both a bra-line back lift and breast auto-augmentation with mastopexy in the MWL population.
Patients and methods
A retrospective review was performed with the approval of the Institutional Review Board (IRB) to identify patients who underwent volume augmentation with a lateral chest wall fasciocutaneous flap at Stanford Medical Center by a single surgeon from 2016 to 2020. Twelve patients representing a total of 16 LICAP flaps were identified (Table 1). Data including demographic details, BMI, history of radiation, timing of reconstruction, additional fat grafting, complications, and follow-up were documented. Statistical analysis was performed using RStudio Version 1.2.5033 (Boston, MA). Independent samples t tests, chi-square test, and Fisher's exact test were used to analyze summary data when appropriate. The study followed the STROBE guidelines.
Table 1.
Patient Demographics
| Oncologic Patient Population | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Patient | Unilateral or Bilateral | Age (yr) | Gender | BMI (kg/m2) | Oncologic Diagnosis | Radiation Therapy | Oncologic Procedure | Immediate or Delayed | Complications |
| 1 | Bilateral | 73 | F | 27.1 | ILC on L, remote hx of R breast CA | Y | Lumpectomy on L, (R mastectomy with TRAM years prior) | Delayed | |
| 2 | Unilateral | 65 | F | 24.7 | IDC | Y | Lumpectomy | Delayed | |
| 3 | Unilateral | 64 | F | 22.5 | IDC | Y | Lumpectomy | Delayed | Small incisional dehiscence |
| 4 | Unilateral | 87 | F | 22.7 | ILC | Y | Nipple sparing mastectomy | Immediate | Small incisional dehiscence |
| 5 | Unilateral | 44 | F | 25.3 | DCIS | Y | Lumpectomy | Immediate | |
| 6 | Unilateral | 50 | F | 28.4 | ILC | Y | Lumpectomy | Delayed | |
| 7 | Unilateral | 48 | F | 29.3 | IDC | Y | Lumpectomy | Delayed | |
| 8 | Bilateral | 70 | F | 30.6 | ILC | N | Lumpectomy | Delayed | Small incisional dehiscence |
| 9 | Unilateral | 56 | F | 41.0 | IDC | Y | Lumpectomy | Delayed | |
| 10 | Unilateral | 56 | F | 45.5 | IDC | Y | Nipple sparing mastectomy | Delayed | |
| Massive Weight Loss Patient Population | |||||||||
| Patient | Unilateral or Bilateral | Age (yr) | Gender | Pre op BMI (kg/m2) | BMI pre weight loss (kg/m2) | Duration of stable weight | Extended LICAP? | Complications | |
| 1 | Bilateral | 69 | F | 30.1 | 46.1 | Several years | Yes | Small triple point dehiscence | |
| 2 | Bilateral | 58 | F | 31.0 | 60.2 | Several years | No | ||
ILC invasive lobular carcinoma; IDC invasive ductal carcinoma; DCIS ductal carcinoma in situ; TRAM transverse rectus abdominis muscle
At our institution, the LICAP flap has previously been used to address volume deficits in patients after oncologic surgery, typically BCT. Our subsequent application of this approach to the MWL population arises from our well-established experience in smaller volume augmentations.
Surgical Technique
Table 2 Summarizes key points of the surgical technique for each patient population.
Table 2.
Comparison of Techniques.
| Oncologic Patient Population | Massive Weight Loss Patient Population | |
|---|---|---|
| Reconstructive Goals | Address volume deficits from prior BCT or mastectomy, especially useful for superior or lateral quadrant defects | Mastopexy of ptotic breasts while simultaneously addressing axillary excess – If bothered by “back rolls” may perform an extended LICAP |
| Markings | Consider size and location of tumor defect, utilize prior mastectomy/lumpectomy incision with lateral extension | Wise pattern mastopexy, possible to extend the flap to encompass excess back tissue circumferentially |
| Dissection | Position supine Start lateral to medial, suprafascial plane toward anterior axillary line, careful to preserve 5th and 6th intercostal artery perforators |
If extended, start prone then switch to supine Start lateral to medial, suprafascial plane toward anterior axillary line, careful to preserve 5th and 6th intercostal artery perforators |
| Inset | Tack to chest wall within the tumor defect | Rotate, transpose and tack underneath inferior pedicle |
Standard LICAP in the Oncologic Population
To design the flap, the patient is marked in the pre-operative area, taking into consideration the breast size and anticipated size and location of the defect. Understanding the tumor extent and area of resection is critical for planning reconstruction. Excess skin of the axilla and back are assessed with a pinch test. Potential perforators are located using a Doppler probe, and the flap is subsequently designed to incorporate one or several localized perforators. The width of the flap is determined by several factors including the estimated defect size and necessity for primary closure of the donor site. The design is oriented such that the scar remains well-hidden within the bra line.
After intubation, patients are positioned supine with both arms abducted. Using incisions based on previous surgery (i.e., prior mastectomy vs. lumpectomy incision), the breast skin is then elevated off of the underlying breast tissue to allow for adequate mobility for rotation and advancement. If reconstruction is immediate, coordination between the plastic surgeon and the breast surgeon is preferred to plan an incision that not only provides optimal access to the tumor, but also considers aesthetics. After elevating the skin flaps, the breast parenchyma is then rotated to partially fill in the previous defect. Attention is then turned to the lateral chest.
In the supine position, the dissection begins from the most distal flap tip. The superior skin edge is incised using a #15 blade. Dissection is then carried down to the chest wall. The lateral border of the latissimus is identified, and dissection continues above the muscle fascia from lateral to medial. Next, the inferior skin edge is incised. The fasciocutaneous flaps are then carefully elevated off of the chest wall in the suprafascial plane toward the anterior axillary line. Careful attention is made to preserve the 5th and 6th intercostal artery perforators within 6-8 cm from the anterior axillary line. The flap is inspected for bright red bleeding from the edges. The flap is then rotated and transposed into the breast pocket. Tacking sutures are placed to secure the flap to the anterior chest wall with interrupted 3-0 Vicryl sutures. The flap is then completely de-epithelialized. The breast skin flap is re-draped over the fasciocutaneous flap. (Figure 1). A #15- French Blake drain is used to drain the axillary pocket. Skin incisions are then closed with interrupted dermal sutures and a running subcuticular stitch. Figure 2 provides an example of pre- and post-operative results.
Figure 1.
Intraoperative photos demonstrating the use of the LICAP for BCT reconstruction. 1A Completed LICAP elevation off of the left chest wall prior to flap inset. The flap has been de-epithelialized and demonstrates healthy punctate bleeding. 1B The flap has been rotated and transposed into the defect pocket and demonstrates satisfactory breast contour.
Figure 2.
Pre- and postoperative photos of a patient with previous breast conservation therapy with subsequent LICAP flap reconstruction. 2A Patient with obvious left breast contour deformity with volume deficit after remote history of left sided lumpectomy. 2B Postoperative improved breast contour with a hidden incision along the lateral and posterior bra line.
An additional consideration in this patient population is the importance of achieving negative surgical margins during resection. Often times, intra-operative assessments using frozen section analysis may be used to minimize the likelihood of delayed re-excision of margins. However, if full processing of the tumor specimen warrants re-excision, the perforator flaps can be retracted to re-expose the original tumor defect.
Modifications for the Extended LICAP in the MWL Population
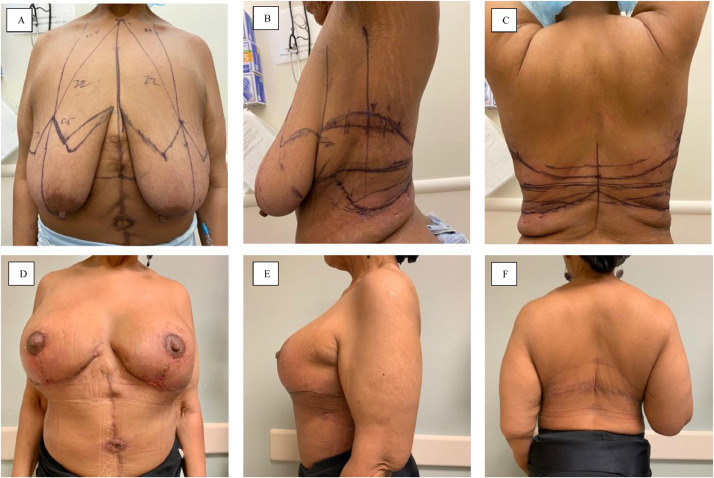
The patient is marked in the pre-operative area with standard Wise-pattern mastopexy markings. Additional focus is required on the back to address excess skin and subcutaneous tissue in this region. The pinch test is applied circumferentially to estimate the width of the flap and feasibility of primary closure of the donor site. The patient can be evaluated in her bra to ensure hidden placement of the final scar within the bra line. The lateral markings are then extended into the Wise pattern markings anteriorly to complete flap design (Figure 3A-C).
Figure 3.
Pre- and postoperative photos of a MWL patient undergoing LICAP flap for breast volume autoaugmentation and back lift. 3A-C Preoperative markings demonstrating standard Wise pattern mastopexy lines with incorporation of axillary and back excess tissue circumferentially. 3D-F Postoperative photos demonstrating significant improvement in breast shape and improved axillary and back definition.
After intubation, patients are initially placed in a prone position. The redundant skin on the back is incised while keeping the fasciocutaneous skin flaps attached laterally. After elevation of the flaps off of the back using electrocautery, a #15-Frech Blake drain is placed on each side. The skin flaps are advanced, and medially rotated to close the back. The lateral fasciocutaneous skin flaps are then wrapped in a moist laparotomy pad and covered with Ioban dressing, while the patient is gently repositioned supine.
A bilateral Wise pattern mastopexy is then performed. A cookie cutter device is used for the nipple-areola complex (NAC). After de-epithelializing an inferior pedicle breast mound, minimal tissue is resected medially, laterally, and superiorly to allow for re-shaping of the breast. The skin flaps are also elevated off of the chest wall to allow smooth re-draping of the inferior pedicle. The fasciocutaneous flaps on each side are then carefully elevated off of the chest wall in the suprafascial plane toward the anterior axillary line. Careful attention is made to preserve the 5th and 6th intercostal artery perforators within 6-8 cm from the anterior axillary line (Figure 4). The flaps are evaluated for bright red blood bleeding from the edges. The perforators are examined with Doppler to ensure robust blood flow. The flaps are then rotated and transposed in the breast pocket to center under the inferior pedicle (Figure 5). The flap is then completely de-epithelialized and secured to the chest wall with interrupted 3-0 Vicryl sutures. The skin flap is then re-draped over the fasciocutaneous flaps. #15-French Blake drains are placed to drain the axillary pockets. The incisions are closed, and the NAC is brought out through a keyhole incision and secured in place. Figure 3D-F demonstrates an example of post-operative results.
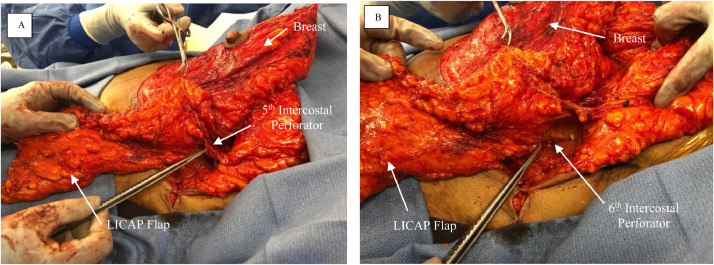
Figure 4.
The fasciocutaneous flap of the left side has been carefully elevated off of the chest wall in the suprafascial plane toward the anterior axillary line. Careful attention is made to preserve the fifth (4A) and sixth (4B) intercostal artery perforators during the dissection.
Figure 5.
Inset of the LICAP flap. 5A Complete dissection of the LICAP flap off of the chest wall prior to de-epithelialization. 5B-C Transposition of the flap to center under the inferior pedicle. 5D Securing the inferior pedicle after de-epithelializing and insetting the flap.
A standard LICAP dissection can also be applied to MWL patients who wish to undergo breast autoaugmentation and reduction of axillary tissue excess without backlift. Figure 6 demonstrates the dissection, and Figure 7 shows intra-operative results after inset.
Figure 6.
Standard LICAP dissection for breast autoaugmentation and excess axillary tissue reduction. 6A Wise pattern incisions used. 6B-C Elevation of the flap off of the chest wall. Arrow in 6C indicates the 5th lateral intercostal artery perforator. 6D Inset of the flap under the inferior pedicle.
Figure 7.
Intra-operative photos after flap inset. Lateral and anterior views of the breast demonstrating improved contour and lift of the right breast compared to left.
Post‐Operative Management
This procedure is performed as an outpatient surgery. Patients are instructed on home management of drains, and the drains are removed at the first post-operative visit one week later. Patients are instructed to avoid heavy lifting (> 10 lbs) or strenuous activity until incisions are healed, usually for 2-3 weeks after surgery.
Results
Between 2016 and 2020, 12 patients underwent 16 LICAP flaps for volume augmentation (Table 1). The average age of the patient population was 63 years old, and the average BMI was 29.9 kg/m2.
Ten patients had a prior history of breast malignancy and resection, and two patients underwent MWL. A majority of oncologic patients underwent delayed LICAP flap for reconstruction with a mean time between BCT and reconstruction of 4.7 years. However, 2 patients underwent immediate reconstruction with LICAP flaps after BCT. Nine of 10 patients had undergone radiation therapy as part of their oncologic treatment.
Table 3 demonstrates that there were no statistically significant differences between the oncologic and MWL groups in terms of average age, BMI at time of surgery, or complication rate. Three of the ten oncologic patients experienced minor wound healing issues that resolved with local wound care, and one of the two MWL patients experienced a small area of dehiscence at the triple point. Among all patients, there were no major complications that required operative intervention.
Table 3.
Comparative Data.
| Oncologic Patients | MWL Patients | p value | |
|---|---|---|---|
| Number of Patients | 10 | 2 | - |
| Number of Flaps | 12 | 4 | - |
| Average Age (yr) | 61.3±13.3 | 63.5± 7.8 | 0.829 |
| Average BMI (kg/m2) | 29.7± 7.7 | 30.5± 0.6 | 0.891 |
| Received XRT Prior to Reconstruction | 9 | 0 | 0.041 |
| Complications | 3 | 1 | 0.469 |
Overall, all patients were satisfied with the results of surgery. One patient underwent placement of sub-glandular implants for additional volume at a later date. The average length of follow-up was 11.4 months.
Discussion
Surgical techniques for breast reconstruction are vast and ever-changing as surgeons continue to develop new and innovative alternatives. When choosing among techniques, many women prefer rapid recovery times and wish to avoid the morbidity of muscle flap dissections. Since 1995, when Fisher et al. reported similar recurrence rates and survival in patients with tumors < 4-cm without lymphatic invasion who underwent either mastectomy or BCT with radiation, an even further increase in the BCT population highlighted the need for options for volume augmentation in this group.7
Various donor sites have been explored as options for breast autoaugmentation. In addition to the spiral flap, Hurwitz et al. described using the upper abdominal tissue during the body lift as an epigastric source of autoaugmentation.8 With this technique, the reverse abdominoplasty epigastric flap is flipped upward to augment the inferior pole of the breast. However, this procedure requires a large area of de-epithelialization that can be tedious. Vindigni et al. described the use of a pedicled posterior arm flap based on the brachial artery for simultaneous autoaugmentation mastopexy and brachioplasty.9 For this procedure, the risk of subsequent lymphedema remains unknown and scar placement on the arm must be acceptable to the patient. Overall, these techniques demonstrate that use of autologous tissue from various portions of the body requires a degree of creative artistry.
The use of pedicled perforator flaps in breast reconstruction was introduced in the literature by Hamdi et al in 2004.10 In his series, they investigated several chest wall perforator flaps and noted the utility of the LICAP flap specifically for patients with superior and lateral quadrant defects. The ideal patient population was noted to be those with small- to moderate-sized breasts with large tumors, thus requiring volume augmentation to achieve symmetry. In addition, he noted that the perforator flaps serve as a vascularized tissue matrix for subsequent fat grafting.11 Similarly, we have focused this technique primarily toward those with small to moderate breasts with large defects. In this patient group, the option of the LICAP flap often arises for several reasons: hesitancy toward sacrifice of the latissimus muscle, medical co-morbidities making long free tissue transfer cases risky, and avoidance of long recovery time.
Additional follow-up studies of the anatomy of the intercostal vessels demonstrated that a “dominant perforator” is frequently identified with higher concentrations in the sixth and seventh intercostal spaces (average of 3.5 cm from the anterior border of the latissimus muscle).12 With a new appreciation for the reliable perforator dissection and small case series demonstrating success, the use of the LICAP flap for volume augmentation began to expand. Kim et al. presented a series of 33 patients who underwent breast reconstruction using TDAP (n=14) or LICAP (n=19) flaps and reported that 46% of patients reported satisfaction to be “excellent” and an additional 36% reported “good” satisfaction.13 Five patients in the series developed complications that required additional intervention (2 within the LICAP group). An additional series by Kim et al. included 40 patients that underwent LICAP flap by either the propeller method (if resection involved significant skin excision) or the turnover method (skin excision minimal or not needed during the tumor excisions). Three cases required treatment for fat necrosis, and venous congestion occurred in two cases of the propeller method. Cosmetic satisfaction was 90% or greater for both techniques.14 In our series, 4 cases of wound healing issues resolved with local wound care without the need for surgical intervention, further supporting the reliability of the flap.
The increasing data in support of the LICAP flap in terms of reliability and patient satisfaction encouraged use of the flap for an alternative group, the MWL population. While certain fundamentals of the perforator dissection remain the same, there are several notable differences when applying this flap to the MWL population. In the MWL population, the axillary excess is more prominent than in the oncologic group and often forms “back rolls” circumferentially. As a result, an extended version of the LICAP flap with a donor site that spans the back can be used to address the circumferential excess tissue. In addition, loss of breast volume and skin laxity contribute to significant ptosis that is best addressed with a wise pattern mastopexy.15 The wise pattern mastopexy in this group creates a new breast pocket in which the flap can be positioned to create a suitable lateral breast contour.15
Several small studies have reported the use of LICAP in the MWL population, but only one study by Patel et al. has attempted an extended version.16 Kwei et al. provided the first multi-patient series using the LICAP flap for MWL breast autoaugmentation and reported no complications in all five patients.15 However, these short fasciocutaneous flaps limit the volume used for autoaugmentation and fail to address the circumferential excess tissue. In the first series to attempt an extended LICAP flap, Patel et al. reported 7 patients with 3 wound healing issues that resolved with nonoperative management and 1 hematoma requiring surgical intervention.16 Similarly, our study noted a total of 4 cases of minor wound healing issues that resolved with local wound care. The donor scars of our extended LICAP have the advantage of lying along the bra-line resulting in an aesthetically acceptable scar.
An additional benefit of this technique is the avoidance of implant-based complications. Though implant-based techniques may be appealing for volume replacement due to shorter operative times and minimal donor site morbidity, patients often remain dissatisfied with their long-term sequelae and failure to address other areas of redundant skin. Calvert et al. described a technique utilizing autologous tissue in combination with an implant by performing a superomedial pedicle mastopexy, de-epithelializing the lateral portion of the Wise pattern incision as a spiral flap for autoaugmentation, and submuscular implant placement.17 In the 20-patient study, five patients experienced small wound breakdown at the T-point and one patient developed early capsular contracture six months postoperatively requiring surgical revision. Though combining autologous tissue and implant techniques may initially achieve desirable volume, patients may require additional surgeries to address excess axillary or back folds and are still at risk for implant-related complications, including breast implant-associated anaplastic large cell lymphoma.
Ultimate choice of the source of autologous tissue relies on several factors including the locations of redundancy on an individual patient (i.e., the arm, “back roll,” upper abdominal tissue) and the location of scars that a patient considers acceptable. This study promotes the use of an extended LICAP as a reliable and satisfactory option for patients whose priority is volume autoaugmentation with simultaneous reduction of axillary and back tissue, but it also highlights the room for future creativity of potential autologous donor sites in this group.
Conclusions
The LICAP flap has been well established as a reliable method of volume augmentation in the breast conservation population. Our experience in this group has resulted in the use of an extended LICAP flap for the MWL group as well. Though the perforator dissection in these two groups remains similar, there are key differences in patient selection and operative technique, which are highlighted above. By demonstrating a creative use of autologous tissue for autoaugmentation in MWL patients, the extended LICAP flap encourages surgeons to consider additional donor sites of autologous tissue based on patient-specific body habitus and preference.
Ethical Approval
A retrospective review was performed with the approval of the IRB to identify patients who underwent volume augmentation with a lateral chest wall fasciocutaneous flap at Stanford Medical Center by a single surgeon from 2016 to 2020
Conflict of Interest Statement
The authors do not have any conflicts of interest to declare. The authors do not have any financial or personal relationships with other individuals or organizations that could inappropriately influence the work.
Funding
None
References
- 1.Clough KB, Cuminet J, Fitoussi A. Cosmetic sequelae after conservative treatment for breast cancer: classification and results of surgical correction. Ann Plast Surg. 1998;41:471–481. doi: 10.1097/00000637-199811000-00004. [DOI] [PubMed] [Google Scholar]
- 2.Yang JD, Lee JW, Cho YK. Surgical techniques for personalized oncoplastic surgery in breast cancer patients with small- to moderate-sized breasts (part 1): volume displacement. J Breast Cancer. 2012 Mar;15(1):1–6. doi: 10.4048/jbc.2012.15.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schaverien MV, Kuerer HM, Caudle AS, Smith BD, Hwang RF, Robb GL. Outcomes of Volume Replacement Oncoplastic Breast-Conserving Surgery Using Chest Wall Perforator Flaps: Comparison with Volume Displacement Oncoplastic Surgery and Total Breast Reconstruction. Plast Reconstr Surg. 2020 Jul;146(1):14–27. doi: 10.1097/PRS.0000000000006911. [DOI] [PubMed] [Google Scholar]
- 4.Schwartz JD. New Approach to Oncoplastic Breast Conservation: Combining Autologous Volume Replacement and the Wise-pattern Mammaplasty. Plast Reconstr Surg Glob Open. 2018 Oct 16;6(10):e1987. doi: 10.1097/GOX.0000000000001987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hurwitz DJ, Golla D. Breast reshaping after massive weight loss. Semin Plast Surg. 2004;18(3):179–187. doi: 10.1055/s-2004-831905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Losken A. Breast reshaping following massive weight loss: principles and techniques. Plast Reconstr Surg. 2010;126(3):1075–1085. doi: 10.1097/PRS.0b013e3181e60580. [DOI] [PubMed] [Google Scholar]
- 7.Fisher B, Anderson S, Redmond CK. Reanalysis and results after 12 years of follow-up in a randomized clinical trial comparing total mastectomy with lumpectomy with or without irradiation in the treatment of breast cancer. N Engl J Med. 1995;333:1456–1461. doi: 10.1056/NEJM199511303332203. [DOI] [PubMed] [Google Scholar]
- 8.Hurwitz DJ, Agha-Mohammadi S. Postbariatric surgery breast reshaping: the spiral flap. Ann Plast Surg. 2006;56:481–486. doi: 10.1097/01.sap.0000208935.28789.2d. [DOI] [PubMed] [Google Scholar]
- 9.Vindigni V, Marchica P, Pagani A, Bassetto F, Brambullo T. The Posterior Arm Flap for Reshaping the Postbariatric Breast. Plast Reconstr Surg Glob Open. 2019;7(9):e2434. doi: 10.1097/GOX.0000000000002434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hamdi M, Van Landuyt K, Monstrey S, Blondeel P. Pedicled perforator flaps in breast reconstruction: a new concept. Br J Plast Surg. 2004;57(6):531–539. doi: 10.1016/j.bjps.2004.04.015. [DOI] [PubMed] [Google Scholar]
- 11.Hamdi M, Rasheed MZ. Advances in autologous breast reconstruction with pedicled perforator flaps. Clin Plast Surg. 2012;39(4):477–490. doi: 10.1016/j.cps.2012.07.016. [DOI] [PubMed] [Google Scholar]
- 12.Hamdi M, Spano A, Van Landuyt K, D'Herde K, Blondeel P, Monstrey S. The lateral intercostal artery perforators: anatomical study and clinical application in breast surgery. Plast Reconstr Surg. 2008;121(2):389–396. doi: 10.1097/01.prs.0000298317.65296.cf. [DOI] [PubMed] [Google Scholar]
- 13.Kim JB, Kim DK, Lee JW. The usefulness of pedicled perforator flap in partial breast reconstruction after breast conserving surgery in Korean women. Arch Plast Surg. 2018;45(1):29–36. doi: 10.5999/aps.2017.01200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim JB, Eom JR, Lee JW, Lee J, Park HY, Yang JD. Utility of Two Surgical Techniques Using a Lateral Intercostal Artery Perforator Flap after Breast-Conserving Surgery: A Single-Center Retrospective Study. Plast Reconstr Surg. 2019;143(3):477e–487e. doi: 10.1097/PRS.0000000000005374. [DOI] [PubMed] [Google Scholar]
- 15.Kwei S, Borud LJ, Lee BT. Mastopexy with autologous augmentation after massive weight loss: the intercostal artery perforator (ICAP) flap. Ann Plast Surg. 2006;57(4):361–365. doi: 10.1097/01.sap.0000222569.59581.d9. [DOI] [PubMed] [Google Scholar]
- 16.Patel NB, Wong MS. Extended fasciocutaneous flaps for autologous augmentation mastopexy with upper body lift after massive weight loss: an early experience. Ann Plast Surg. 2015;74(1):S41–S45. doi: 10.1097/SAP.0000000000000413. Suppl. [DOI] [PubMed] [Google Scholar]
- 17.Calvert JW, Dickinson BP, Patel A, Brenner K. Lateral breast flap with superomedial pedicle breast lift. Aesthet Surg J. 2011;31(6):658–666. doi: 10.1177/1090820X11415241. [DOI] [PubMed] [Google Scholar]